ABSTRACT
We developed mammary imaging windows (MIWs) to evaluate leukocyte infiltration and cancer cell dissemination in mouse mammary tumors imaged by confocal microscopy. Previous techniques relied on surgical resection of a skin flap to image the tumor microenvironment restricting imaging time to a few hours. Utilization of mammary imaging windows offers extension of intravital imaging of the tumor microenvironment. We have characterized strengths and identified some previously undescribed potential weaknesses of MIW techniques. Through iterative enhancements of a transdermal portal we defined conditions for improved quality and extended confocal imaging time for imaging key cell-cell interactions in the tumor microenvironment.
KEYWORDS: breast cancer, cell migration; intravital imaging, mammary imaging windows
Introduction
Breast cancer cells disseminate from the primary tumor and spread to lung, liver, bone marrow1,2 and lymph nodes.3-6 These metastatic disseminations are the main cause of death in breast cancer patients. Metastasis is a multi-step process that requires growth of the primary tumor, detachment of tumor cells, invasion into vascular or lymphatic vessels, tumor cell homing, adherence to distant organs, and survival and growth in the new environment.7 The ability to image the dynamic behavior of tumor cells and stromal cell types within tumors and tissue of mice has been under intense investigation.8-18 In vivo imaging of the tumor microenvironment allows assessment of tumor cell intravasation, leukocyte extravasation/intravasation, rate and direction of cell migration, and cell-cell interactions not captured by routine FACS (fluorescence activated cell sorting) and immunohistochemistry. With sophisticated imaging methods such as multi-photon, second harmonic generation, and spinning disk or laser-scanning confocal microscopy, intravital imaging has become a popular tool to study the behavior of fluorescently labeled cells in vivo.19,20 It has been difficult to image the tumor microenvironment, as the organs under study have to be surgically exposed to be anatomically accessible to the microscope.21 To image the tumor microenvironment in the mouse mammary gland, imaging techniques have relied on surgical resection of the skin to expose the tumor (surgical skin flap). While this procedure allows high-resolution visualization of the tumor microenvironment, there are several limitations for short-term imaging experiments: 1) this is a terminal experiment and conditions within the same tumor cannot be imaged for more than 5-6 hours; 2) there are side effects of prolonged anesthesia; and 3) tissue dehydration. Long-term intravital imaging of tumors in mice has been achieved with optimized imaging conditions21,22 (up to 40 hr), while imaging over several days has been achieved with the dorsal skinfold chamber,23,24 and other types of chambers to study tumors in brain25 and mammary tissues.14,26 The limitations for long-term imaging experiments are: 1) side effects of prolonged anesthesia, and 2) wounding response that leads to tissue granulation, collagen formation and re-epithelialization of the skin under the window.14,26
Building on previous efforts, we sought to develop a mammary imaging window (MIW) that will enable imaging of breast tumors in the mammary gland of a mouse for prolonged periods of time. Since tumor cell migration and invasion take place over several days, long-term intra-vital imaging is needed to capture valuable information regarding this process. The purpose of this paper is to report an evaluation of not only the capabilities but also the limitations (tissue granulation, wounding response, collagen buildup, re-epithelialization of the skin) of different MIWs fabricated from diverse biocompatible materials. We designed several types of MIWs in an attempt to minimize the deposition of collagen on the window and to prevent regrowth of the skin over the imaging area to extend the imaging time over the course of the metastatic process. Herein, we show further complications of the MIWs, which include: 1) exudate from the surgical procedure in placing the MIW over the tumor; 2) wound healing response in the tissue adjacent to the MIW based upon recruitment of leukocytes; 3) collagen formation under the window; and 4) tissue granulation and re-epithelialization of the epidermis of the skin 9 days after surgery over the wounded tumor. These complications interfere with long term intravital imaging. Kedrin and colleagues reported that the surgical implantation of the MIW requires removal of the skin, which elicits an inflammatory response, initially described as a “mild inflammatory reaction with a thin layer of fibrin and collagen deposition” by Shan et al.26 Tumor associated neutrophils and macrophages play an important role in wound repair and healing and can either promote or inhibit tumor growth.27-29 Given this information, the wound healing response must be taken into consideration when evaluating early leukocyte recruitment using intravital imaging techniques with inserted mammary windows. While the inflammatory, proliferative and remodeling/maturation phases of wound healing are evoked over the several days, it is possible to visualize and compare each of these components in reference to imaging of tumor-bearing versus non-tumor-bearing mice and to compare the inflammatory component of tumors with or without MIWs. Our data show that within the tumors tissue, there are few differences in the leukocyte composition of tumors with vs. without the MIW. These data suggest the MIW offers promise for studying the tumor microenvironment over time using intravital imaging for 2-5 days, after which there are some issues with collagen deposition and re-epithelialization that impair intravital imaging with a confocal microscope.
Results
Experimental approach and characterization of mammary imaging windows (MIWs)
To image breast tumors and leukocyte behavior, the MIWs were placed over the mammary gland in tumor-bearing mice. Experiments were performed in mice that develop mammary tumors due to the directed expression of polyoma middle T antigen (PyMT) to the mammary epithelium by the mouse mammary tumor virus long-terminal repeat (MMTV-LTR) promoter.30 For the experiments described herein, we utilized MMTV-PyMT mice with cyan fluorescent protein (CFP)-labeled mammary epithelial cells; MMTV-PyMT;ACTB-ECFP (expression of CFP is driven by a β-actin promoter in epithelial cells in the mammary gland).13 We crossed the MMTV-PyMT;ACTB-ECFP mice with the c-fms-EGFP (enhanced green fluorescence protein) mouse line, which expresses EGFP under the c-fms promoter, thus targeting the myeloid lineage.13,31 This allowed use of the MIW and intravital imaging to monitor behavior of the CFP-tumor cells and GFP-myeloid cells in the vasculature and tumor in real time.
Development and characterization of a polydimethylsiloxane (PDMS) double-flanged mammary imaging window
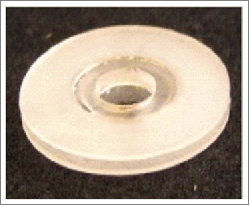
The mouse mammary gland presents a challenge for imaging with MIWs due to the proximity of the gland to the leg. The MIW must be small enough to remain fixed to the mouse and allow the mouse movement to walk, but large enough to present an area for imaging the vital space. We developed a double-flanged polydimethylsiloxane (PDMS) mammary imaging window to image the tumor microenvironment for long-term purposes. PDMS is used for biological studies in the fabrication of microfluidic devices and offers several advantages as a polymer for the MIW: it is biocompatible32 and inexpensive, has low auto-fluorescence and is easily moldable.33 PDMS is flexible, which permits easier movement of the leg around the MIW implanted in the fourth mammary gland. The PDMS MIW is fabricated with a double flange, allowing for retraction of the skin to prevent/retard regrowth over the MIW, and a mount for a glass coverslip (Fig. 1A). The PDMS MIW was first tested in a non-tumor-bearing c-fms-EGFP mouse. Figure 1B depicts surgical implantation of the PDMS MIW over the fourth mammary gland. The skin over the mammary gland is removed to expose the mammary gland, and the MIW is placed over the mammary gland and secured to the skin with sutures. We show that the PDMS MIW retracts the skin for up to 4 days, but growth of collagen (collagen fibers are shown being lifted by the forceps) into the wounded area and under the coverslip hinders the field of view for imaging by day 4 (Fig. 1C).
Figure 1.
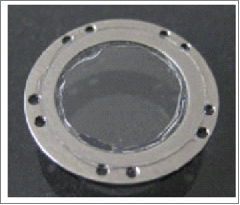
Polydimethylsiloxane (PDMS) double-flanged mammary imaging window (MIW) and stainless steel mammary imaging window (SS MIW) in FVB-c-fms-EGFP mice. (A) A PDMS MIW designed to be surgically implanted over the fourth mammary gland in an FVB-c-fms-EGFP mouse. The MIW contains 2 PDMS double-flanged rings that form a mount for a glass coverslip, shown in the oblique photograph on the left. A 6 mm-diameter No. 1 coverglass is affixed with silicone adhesive to a PDMS double-flange configuration. The PDMS flanges can be trimmed to appropriate size and shape. The double flanges, seen clearly in the lateral photograph on the right, allow for retraction of the skin to prevent skin growth over the MIW. (B) Surgical implantation of a PDMS MIW in an FVB-c-fms-EGFP mouse. The MIW is placed above the mammary gland and skin is tucked between 2 flanges of the MIW and secured with sutures. Although the MIW retracts the skin for up to 4 days, the growth of collagen by day 4 (C) hinders the field of view for imaging. (D) To image myeloid cells in vivo, a stainless steel MIW is placed over the mammary gland in an FVB-c-fms-EGFP mouse and secured with sutures (E). A 6 mm-diameter No. 1 coverglass is affixed with silicone adhesive to a steel substructure. Holes around the periphery allow the MIW to be sutured in place. (F) Intravital image at day 0 of migration of c-fms+ myeloid cells in a c-fms-EGFP mouse through the stainless steel MIW. Images were acquired with an LSM META 510 inverted confocal microscope with a 10X/0.5 Plan Neofluar objective. Bars, 100 µm. The panel shows an image of GFP-expressing myeloid cells in the mammary fat pad; the vasculature is labeled with Rhodamine Dextran (red). (G) By day 5, there is collagen deposition and increased migration of myeloid cells into the wounded area. Bars, 100 µm. (H and I) By day 9, there is tissue granulation, re-epithelialization and closure of the skin.
Development and characterization of a stainless steel mammary imaging window
To address the issues of re-epithelialization of the epidermis of the skin and collagen buildup over the wounded tumor, we designed a stainless steel (SS) MIW that is engineered with a stainless steel washer with holes drilled around its rim for sutures, and an 8 mm glass coverslip glued to the middle of the washer (Fig. 1D). We chose to fabricate the window with stainless steel as it is biologically compatible and thinner than the PDMS version. The SS MIW was first tested in a non-tumor-bearing c-fms-EGFP mouse. Figure 1E depicts surgical implantation of the SS MIW in the fourth mammary gland. The skin over the mammary gland was removed to expose the mammary gland, and the SS MIW was placed over the mammary gland and secured to the skin with sutures using horizontal mattress stitch-like sutures allowing distribution of the suture-generated tension among more stitch points so each stitch generates less tension to skin tissue with less ischemia, damage and inflammation at each stitch site.The mouse was placed in a custom-designed imaging box that fits the stage on the confocal microscope (Fig. S1). Confocal intravital images taken at day 0 (Fig. 1F, Supplementary Movie 1) and day 5 (Fig. 1G) are representative images of GFP-expressing myeloid cells, with the vasculature labeled with Rhodamine Dextran (red). The myeloid cells (c-fms-EGFP green) that have ingested the Rhodamine Dextran appear yellow (Fig. 1F). The images depict GFP-myeloid cells migrating under the SS MIW. Unfortunately, by day 5, collagen has been deposited in the surgical area, hindering the field of view for imaging (Fig. 1G). Also, there was an increased number of GFP + myeloid cells in the wounded area by day 5 (Fig. 1G) and re-epithelialization of the skin under the MIW by day 9 (Fig. 1H and 1I). To further characterize the buildup of collagen ECM under the SS MIW in the tumor microenvironment, we implanted the SS MIW in MMTV-PyMT x c-fms EGFP tumor bearing mice, removed tumors and adjacent skin at days 2 and 7 post MIW implantation, and stained fixed tissue with Picrosirius Red. Tumor samples from mice without the SS MIW implantation were used as controls. Increased polarized intensity of Picrosirius Red-stained tissue suggested higher content of total and fibrillary collagen in the tumors at day 7 as compared to day 2 or mice without the SS MIW (Fig. S2).
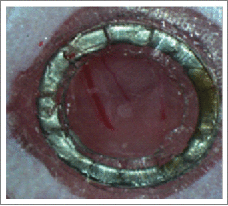
Since spontaneous tumors in this model may arise in all 8 mammary glands of the mouse, we tested the MIW in the third and fourth mammary gland in vivo. Figure 2 demonstrates implantation of a SS MIW over the third mammary gland of a 12-week-old MMTV-PyMT;ACTB-ECFP-c-fms-EGFP mouse (Fig. 2A) and the fourth mammary gland of an 8-week-old tumor-bearing c-fms-EGFP mouse (Fig. 2D). Confocal intravital images taken through the SS MIW in the third mammary gland at day 1 (Fig. 2B, Supplementary Movie 2) and day 2 (Fig. 2C, Supplementary Movie 3) are representative images of GFP-expressing myeloid cells migrating through the vasculature in the MMTV-PyMT tumor (labeled blue, ACTB-ECFP) under the SS MIW. The vasculature was labeled with Rhodamine Dextran (red). Figure 2E (and Supplementary Movie 4) and 2F (Supplementary Movie 5) are 2 representative panels of migration of GFP+ myeloid cells migrating through fourth mammary gland in the MMTV-PyMT tumor (ACTB-ECFP) and vasculature under the SS MIW on day 1. The SS MIW allowed us to obtain clear images of c-fms-EGFP-myeloid cells in the tumor and tumor vasculature; however, drawbacks associated with the SS MIW include: 1) the window is sutured to the skin, and the sutures create an uneven surface on the microscope stage for imaging; in addition, the mouse is able to chew the sutures in an attempt to remove the window; 2) the outer diameter of the MIW is too large for implantation over the third mammary gland; 3) due to the wound healing response there is collagen buildup under the MIW by day 5 (Fig. 1G) thus hindering the field of view during imaging, and 4) there is complete re-epithelialization of the skin under the MIW by day 9 (Figs. 1H and I).
Figure 2.
Test of stainless steel mammary imaging windows in MMTV-PyMT CFP x c-fms-EGFP mice that present with tumors in the third or fourth mammary gland. (A) The stainless steel mammary imaging window is implanted over the third mammary gland of a MMTV-PyMT-CFP x c-fms-EGFP mouse and imaged on day 1 (B and C). (B and C) The panels represent images of GFP-expressing myeloid cells in the mammary fat pad, CFP-expressing tumor cells, and the vasculature is labeled with Rhodamine Dextran (red). (D) The stainless steel mammary imaging window is implanted over the fourth mammary gland of a MMTV-PyMT-CFP x c-fms-EGFP mouse and imaged on day 1 (E and F). The panels represent images of GFP-expressing myeloid cells in the mammary fat pad, CFP-expressing tumor cells, and the vasculature is labeled with Rhodamine Dextran (red). Bars, 100 µm. Images were acquired with an LSM META 510 inverted confocal microscope with a10X/0.5 Plan Neofluar or a 20X/0.75 Plan apochromat objective. The images were processed with LSM Imaging software and Adobe Photoshop.
Development and characterization of a stainless steel double-flanged PDMS mammary imaging window
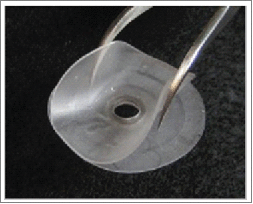
Due to the above-mentioned complications, for longer-term experiments we engineered a smaller stainless steel window that is encased in a double-flanged PDMS device to prevent regrowth of the skin. This SS-PDMS MIW contains an 8 mm coverslip and was implanted over the tumor in the fourth mammary gland in a c-fms-EGFP mouse. As an alternative to suturing the MIWs over the mammary gland, we attached the SS-PDMS MIW directly to the skin with Dermabond cyanoacrylate adhesive (Medex supply). The skin was tucked between the lower and upper PDMS flanges, and Dermabond adhesive is applied between the PDMS flanges to adhere the skin to the MIW (Fig. 3A). Dermabond is widely used by plastic surgeons as a waterproof dressing to replace the deep dermal sutures necessary to bring the edges of the skin into everted apposition to prevent scarring. This method of adhering the SS-PDMS MIW to the skin works well, as the mice were not bothered by the SS-PDMS MIW, did not chew on the window, and the MIW was in closer proximity to the tissue for imaging. This SS-PDMS MIW successfully remained in place for 11 days, but we continued to observe collagen deposition by day 5 and re-epithelialization of the skin by day 9 (Fig. 3A).
Figure 3.
Progression of collagen buildup and re-epithelialization of the skin under the stainless steel – double-flanged PDMS MIW in c-fms-EGFP mice in the fourth mammary gland. (A) An 8 mm-diameter No. 1 coverglass is affixed with silicone adhesive to a PDMS double-flange configuration. A central steel reinforcing ring is positioned between the flanges. The PDMS flanges can be trimmed to appropriate size and shape. The mammary imaging window is surgically implanted over the fourth mammary gland. The skin is adhered to the double-flanged PDMS with Dermabond adhesive, and progressive images of the MIW on day 0 (stereoscope image) and day 1 to day 11 (digital images) are shown. High Gelling Alginate Dressing (3M) is placed in the wound (B) to prevent collagen deposition under the mammary window in a c-fms-EGFP mouse and covered with sterile adhesive (C). The dressing was changed twice a day and monitored for collagen growth. The adhesive was removed at day 3 (D, E) and found to delay collagen formation. The arrow in (E) indicates the field of view for intravital imaging in the next panels. (F and G) Intravital images of migration of c-fms+ myeloid cells in the c-fms-EGFP mouse 3 days after wounding and placement of the alginate dressing. The alginate dressing was removed, and images were taken of GFP positive myeloid cells with 10x (F) and 20x Plan APO objectives (G). Bars, 100 µm. The images were processed with LSM Imaging software and Adobe Photoshop.
To evaluate the possibility of absorbing exudate from the wounded site and thereby preventing collagen deposition, we utilized initial test experiments without a MIW gelling alginate material (Tegaderm Alginate, 3M) which is a commonly used primary wound dressing. The gelling alginate material is a gel pad that absorbs fluid and swells in size. Drainage is contained within the gel and doesn't leak out beyond the gel pad. To utilize the gelling alginate dressing to remove exudate from the wound and collagen produced during the wounded response, the gel pad was inserted in the wound (Fig. 3B) and sealed with sterile adhesive (Fig. 3C). The alginate is a biocompatible material and does not directly affect the tumor microenvironment when placed over a wound). 34-36 By day 1 the gel pad is saturated and has absorbed the exudate (Fig. 3D). The gel pad was changed twice a day and removed on day 3. Intravital images were taken of migration of c-fms-EGFP expressing myeloid cells on day 3 (Figs. 3F and 3G). We did not find evidence for wound exudate or collagen buildup at day 3 after placement of the gelling material.
Development and characterization of a PDMS double-flange “Open” MIW with removable glass coverslip
Due to the difficulty of imaging the tumor environment through tissue exudate and collagen that adheres to the MIW by day 5, we developed an open-able MIW glass coverslip that allows use of the gelling alginate material after surgery to absorb wound exudate. The removable glass coverslip can be replaced during and after imaging the tumor. This MIW consists of a PDMS double flange with an 5 mm inner diameter opening in which an 8 mm stainless steel washer is embedded (Fig. 4A). Separately, an 8 mm glass coverslip is bonded to a stainless-steel washer (Fig. 4B), which can be attached to the 8 mm washer in the PDMS window with adhesive tape prior to imaging. The PDMS double-flange MIW was implanted in the fourth mammary gland of a MMTV-PyMT β-actin CFP x c-fms EGFP mouse and adhered with Dermabond. The gelling alginate material (Tegaderm Alginate, 3M) was placed through the opening of the MIW, as a primary wound dressing and to absorb the wound exudate, and sealed with sterile adhesive (Fig. 4C). By day 1, the gelling alginate material has become saturated with exudate from the area over the tumor (Fig. 4D). The gelling alginate material is easily removed prior to imaging, and the glass coverslip-stainless steel washer was placed over the PDMS double-flange MIW with adhesive and imaged on day 1. Intravital images were taken of migration of c-fms-EGFP-expressing myeloid cells in the MMTV-PyMT tumor (blue) (Fig. 4E). Using this technique we were able to successfully image myeloid cell migration within the tumor microenvironment. However, the gelling alginate material did not prevent the collagen deposition, granulation and re-epithelialization of the skin in the wounded environment underneath the window at later time points.
Figure 4.
A PDMS double-flange “open” MIW with removable glass coverslip is adhered to a stainless steel washer and placed in MMTV-PyMT CFP x c-fms-EGFP mice that present with tumors in the fourth mammary gland. (A) A PDMS double-flange configuration with central steel reinforcing ring positioned between the flanges. An 8 mm cover glass assembly (see Fig. 4B) may be temporarily affixed to the MIW. The flanges can be trimmed to appropriate size and shape. (B) The 8 mm-diameter No. 1 coverglass is affixed with silicone adhesive to a steel substructure. (C) The 8 mm-diameter No. 1 coverglass is affixed with silicone adhesive to a steel substructure (see Fig. 4B). This assembly can be temporarily affixed to a PDMS double-flange configuration with a central steel reinforcing ring positioned between the flanges, as pictured. (C and D) Digital images of surgical implantation of the mammary imaging window over the fourth mammary gland. The skin is adhered to the double-flanged PDMS with Dermabond adhesive, and the opening of the MIW is filled with a gelling alginate dressing on day 0. (D) At day 1, the gelling alginate material has become saturated with exudate from the wounded area. (E) Image of GFP-expressing myeloid cells in the mammary fat pad and CFP-expressing tumor cells, and the vasculature is labeled with Rhodamine Dextran (red). To acquire these images, the PDMS double-flange mammary imaging window is implanted over the fourth mammary gland of a MMTV-PyMT-CFP x c-fms-EGFP mouse and imaged on day 1 after the gelling alginate material was removed. Bars, 100 µm. Images were acquired with an LSM META 510 inverted confocal microscope with a 20x Plan APO objective. The images were processed with LSM Imaging software and Adobe Photoshop.
Characterization of the inflammatory response in tumors and in the tissue immediately under the MIW
We tested 4 generations of MIWs to monitor tumor cell and leukocyte behavior in mammary tumors. A potential limitation of the device is the presence of an inflammatory response after surgical implantation of the window. Since the effects of surgery have a direct effect on leukocyte infiltration, we hypothesized this would be reflected in observable differences in leukocyte populations of tumor bearing mice with MIWs compared to mice not implanted with MIWs. To test this hypothesis, we implanted the 4 different MIWs in MMTV-PyMT x c-fms EGFP tumor bearing mice, removed tumors and adjacent skin at days 2 and 7 post MIW implantation, and performed immunohistochemical staining to examine leukocyte populations in the tumor and in the skin tissue adjacent to the MIW. Since Shan et.al reported that implantation of MIWs was associated with a mild inflammatory reaction that resolved after 3-4 days,26 we evaluated leukocyte recruitment early after MIW implantation (day 2) and later (day 7). Serial sections of tumor and skin adjacent to the MIW were analyzed with immunohistochemistry for T cells (CD3+), macrophages (F4/80+), and neutrophils (neut). Tumor samples from mice without MIW implantation were used as controls. Immunostaining and quantification of the average percentage of positive nuclei (DAB or brown staining) are summarized in Table 1 for T cells, macrophages, and neutrophils in the tumor (Fig. S3) and the adjacent skin of mice with MIWs at day 2 and day 7 post MIW implantation (Fig. S4). Analysis of the CD3, F4/80 and neutrophils present at day 2 and day 7 showed similar percentages of T cells, macrophages and neutrophils in tumors of mice with MIWs compared to mice without MIWs (tumor only) with 2 exceptions. On day 7, the tumor under the PDMS double-flanged/SS MIW and the stainless steel MIW showed a trend toward more F4/80 positive cells compared to tumors from mice without the MIW (20% and 26% compared to 14%) (Table 1 and Fig. S3). Moreover, for all 4 MIW designs we observed considerably more neutrophils in the skin adjacent to the MIW than in the tumor itself (identified as wound area in Fig. S4), and the % neutrophils tended to decline by day 7 post implantation of the MIW for 2 of the 4 MIW designs (PDMS double flanged/SS MIW and Stainless Steel MIW) (Table 1, Fig. S4). In areas where there was necrosis within the tumor under the MIW, neutrophil content was often greatly increased. At post MIW implantation day 7, there was an increased percentage of cells in the skin adjacent to the MIW (wound area) that stained positive for F4/80 in mice with PDMS double-flanged “open” MIW (23%) and the Stainless Steel MIW (23%), compared to other MIWs (10%, and 0%) and the percentage of neutrophils decreased in mice with PDMS double-flanged/stainless steel MIW (13%) compared to other MIWs (38%, 49%, and 70%) while the percentages of T cells were similar among the groups (Table 1, Fig. S4). In skin tissue adjacent to the MIW neutrophils were present in large percentages at both time points compared to F4/80 and CD3 positive cells.
Table 1.
Expression of T cells, macrophages, and neutrophils in tumors of MMTV-PyMT x c-fms EGFP mice with or without MIWs and wounds of mice with MIWs.
| Group | CD3 Tumor Percent Positive Nuclei | F480 Tumor Percent Positive Nuclei | Neut Tumor Percent Positive Nuclei | CD3 Wound Percent Positive Nuclei | F480 Wound Percent Positive Nuclei | Neut Wound Percent Positive Nuclei | |
|---|---|---|---|---|---|---|---|
 |
PDMS double-flanged/SS MIW : Day 2 | 2% +/− .13% | 15% +/− 20% | 9% +/− .05% | 5% +/− 3% | 28% +/− 20% | 47% +/− 2% |
| PDMS double-flanged/SS MIW : Day 7 | 3% +/− .62% | 20% +/− 4% | 2% +/− 3% | 5% +/− 2% | 10% +/− 6% | 13% +/− 2% | |
 |
Stainless Steel MIW: Day 2 | 2% +/− 1.6% | 8% +/− 4% | 4% +/− 3% | 8% +/− 2% | 6% +/− 6% | 57% +/− 2% |
| Stainless Steel MIW: Day 7 | 2% +/− .13% | 26% +/− 10% | 2% +/− 6% | 5.0% +/− 2% | 23% +/− 6% | 39% +/− 13% | |
 |
Stainless Steel-double flanged PDMS MIW Day 2 | 3% +/− .24% | 13% +/− .72% | 5% +/− 3% | 11% +/− 8% | 4% +/− 4% | 40% +/− 16% |
| Stainless Steel-double flanged PDMS MIW Day 7 | 5% +/− .42% | 10% +/− 9% | 2% +/− 1% | 13% +/− 8% | 0% +/−0.11% | 49% +/− 5% | |
 |
PDMS-double flange "open" MIW: Day 2 | 2% +/− .13% | 10% +/− 10% | 3% +/− 6% | 7% +/− 2% | 7% +/− 6% | 40% +/− 13% |
| PDMS-double flange "open" MIW: Day 7 | 3% +/− 3% | 15% +/− 5% | 3 % +/− 2% | 6% +/− 5% | 23% +/− 10% | 70% +/− 14% | |
| Tumor only | 3% +/− 3% | 14% +/− 9% | 3% +/− 4% | N/A | N/A | N/A |
The 4 different MIWs were implanted in MMTV-PyMT x c-fms EGFP mice (n=2 per group). Serial sections were immunostained and the table represents quantification of the average percentage of positive nuclei (brown staining) for T cells (CD3+), macrophages (F4/80+), and neutrophils (neut) in the tumor of unwounded or wounded tissue was performed at indicated time points post-injury.
Discussion
Tumor progression is influenced by the tumor microenvironment. Our objective was to image the dynamic behavior of tumor cells and stromal cell types within a murine mammary tumor for prolonged periods of time with intravital confocal microscopy. An ideal model to study mammary carcinoma would utilize a mammary imaging window that allows repeated, continuous, and non-invasive imaging of cellular behavior in the tumor microenvironment. In this study, we developed several MIWs to overcome limitations of the skin flap method and collagen growth under previously described tissue windows. We tested 4 generations of MIWs to monitor tumor cell and leukocyte behavior in mammary tumors. It is our opinion that the stainless steel, double-flanged PDMS MIW adhered to the skin with Dermabond (Fig. 3) was the best model in terms of ease of surgical implantation, distress for the mouse, and imaging. The procedure reported here allows repeated imaging over a period of 4-5 days using confocal microscopy. While the MIW remains securely fastened to the skin for 11 days, collagen growth by day 5 and re-epithelialization of the skin by day 9 limit the longer-term imaging. Multi-photon imaging may allow imaging through the deposited collagen, allowing analysis of cell motility. This re-epithelialization occurs even though the skin is placed between the 2 flanges of the MIW, presumably due to the migration of the epithelial cells under the device allowing re-surfacing of the wound.
Implantation of the MIW requires surgical removal of the skin over the tumor in the mammary gland, and this in itself evokes a wounding response characterized by an influx of leukocytes and blood monocytes that differentiate into tissue macrophages. In a normal skin wound, neutrophils invade the wounded area within hours, and one day after tissue injury, neutrophils constitute 50% of all cells at the wounded site.37 Monocytes/macrophages invade the wounded area within the first 24 hours and reach the highest levels of the infiltrate at day 2.37 In this study we show that the skin adjacent to the MIW shows a greater content of leukocytes than the tumor itself after surgical implantation of the window. While this may complicate the interpretation of leukocyte involvement in the tumor microenvironment, it is well established that tissues adjacent to tumors often have considerably greater leukocyte infiltrate than the tumor tissue itself and this infiltrate can have prognostic significance, depending upon the anti-tumor versus pro-tumor properties of the leukocytes.38-42
Harold Dvorak characterized tumors as wounds that do not heal, since cancer and wound healing share cellular and molecular pathways.43 In the tumor microenvironment, CD4+ and CD8+ T lymphocytes, dendritic cells, tumor-associated macrophages and neutrophils are a major component of tumor infiltrate and influence many aspects of tumor growth and progression.44,45 Since it has been shown that the wounding response results in infiltration of neutrophils and macrophages that also plays a role in cancer progression, these leukocyte infiltrates should be taken into consideration when using intravital imaging to study the leukocyte infiltrate and cellular behavior in cancer models. Our studies show that the MIW does not markedly alter the leukocyte profile in the tumor proper (Table 1) and the leukocytes in the skin adjacent to the MIW were similar to that previously reported for normal breast or tumor adjacent tissue, with the exception of neutrophils which are very abundant in the tissue just under the MIW.46-49 Intravital imaging is a powerful tool for tracking cellular behavior in the tumor microenvironment, and cellular behavior is influenced by factors such as cytokine gradients. Despite the technical refinements we made in each generation of the MIW to obtain non-invasive continuous images of leukocyte and tumor cell behavior, the deposition of collagen and re-epithelialization of the tissue under the MIW remained problematic for long term imaging by confocal microscopy. However, the advantages of this type of window are that very clear, unobstructed confocal images can be obtained during the early phase of tumor development without the use of 2-photon-excited fluorescence microscopy. For longer imaging, 2-photon-excited fluorescence microscopy which may be able to penetrate the collagen and re-epithelized tissue may be required. These imaging data can be coupled with immunohistochemistry and/or FACS analysis of the leukocyte infiltrate of the tumor to better understand the involvement of specific populations of immune cells in tumor progression or regression.
Materials and methods
Animal models
All experiments were approved by IACUC at Vanderbilt University Medical Center, and all protocols were carried out in accordance with IACUC approved protocols. MMTV-PyMT-ACTB-ECFP and c-fms-EGFP mice were kind gifts from Zena Werb (University of California, San Francisco). The MMTV-PyMT-ACTB-ECFP male mice were crossed with female c-fms-EGFP mice to obtain female MMTV-PyMT;ACTB-ECFP;c-fms-EGFP mice that were used for intravital imaging.
Fabrication of mammary imaging windows
Polydimethylsiloxane (PDMS) MIW
A 30 mm-diameter circle was cut from a block of acrylic with an end mill. PDMS was then poured over the acrylic mold, leaving a thin (~300 μm) layer around the circular shape of the window. The PDMS was cured at 60o C overnight, removed from the mold, and trimmed to create a 3–4 mm flange around the circular window area. A lower flange was created by cutting a 3 X 6 mm window out of Nitex mesh and partially curing a thin (~300 μm) layer of PDMS over the mesh (3–4 hrs at 37°C). When the PDMS sheet was still slightly sticky, but not stringy to the touch, the circular window was pressed against the partially cured sheet. Curing was completed at 60°C for 6 hours. The window was removed and the lower flange was trimmed to 3-4 mm from the circular window. Four holes were placed on the flange area to allow subsequent securing of the window to the skin with sutures. A 12 mm diameter cover glass was then glued to the washer with Dow Corning 732 RTV food-grade sealant, which was allowed to cure for 24 hours. Windows were stored at room temperature in a sterile container prior to use. To sterilize the windows immediately before surgical implantation, they were briefly submerged in 70% ethanol and air dried for a few seconds.
Stainless steel MIW
Ten 1/32" diameter holes were drilled around the outer circumference of a 14 mm diameter, 0.4 mm thick stainless steel washer with an automated milling machine. Any burrs and sharp edges were removed manually with a countersink. Washers were inspected under a microscope to ensure that all burrs and sharp edges were removed. A 12 mm diameter cover glass was then glued to the washer with Dow Corning 732 RTV food-grade sealant, which was allowed to cure for 24 hours.
PDMS double-flange “open” MIW with removable glass coverslip
The mold is manufactured from a polyester film sandwiched between 2 acrylic sheets. If desired, steel rings may be pressed into holes in the polyester before voids machined in the acrylic are filled with liquid PDMS and the mold is assembled. Posts left in the machined regions may be utilized to exclude PDMS and yield through-holes in the MIW. Registration pins and holes in the corners of the layers allow repeatable alignment.
Surgical procedures and implantation of mammary imaging windows
Mice were anesthetized with 2.5% isoflurane inhalation anesthesia (Isothesia, Butler Animal Health Supply). Prior to surgery, buprenorphine (3 mg per mouse) was administered intramuscularly to provide analgesia. During surgery, body temperature was maintained at 32°C with a heating pad. Before surgery, the abdominal area of the mouse was shaved, and the skin was disinfected using 70% ethanol. An incision was made through the skin with sterilized scissors, and the mammary gland was surgically exposed. The MIW was placed over the mammary gland with the cover glass positioned over the center of the mammary gland. In some cases, the skin was tucked between the double flanges of the MIW, and the MIW was secured in place with either horizontal mattress stitch like sutures or Dermabond topical skin adhesive (Ethicon Dermabond topical skin adhesive DVH12 (2-Octyl Cyanoacrylate) Medex Supply). After surgery, the mice were kept at 32°C until fully recovered from anesthesia.
Intravital confocal imaging setup and equipment
During the imaging procedure, the mice were anesthetized with 2.5% isoflurane, and received 100 µl/hr of saline via intraperitoneal injection. Host vasculature was labeled with 30 µL of 20 mg/mL Rhodamine Dextran (2,000,000 MW; Invitrogen) via tail vein injection. Mice were placed in the imaging box (Fig. S1) and the MIW was secured onto the box, and then placed on the confocal microscope. A heating pad was placed over the imaging box during imaging. Isoflurane was delivered through a port on the imaging box and ventilated by an outlet port on the other side of the box into a charcoal filter. Images were acquired with an LSM 510 META inverted confocal microscope with a 10X/0.3 Plan Neofluar or a 20X/0.75 Plan apochromat objective. We used simultaneous imaging of ECFP (enhanced cyan fluorescent protein) and EGFP (enhanced green fluorescent protein) using excitation selectivity of 405 and 488 nm light. Experiments were typically 3 to 4 hours long, and time-lapse images were taken at 10-second intervals for 20 minutes. This allowed the imaging of numerous areas in the imaging field.
Histology and immunostaining
Tissue sections (including wounds and tumor sections) were bisected and fixed overnight in 4% paraformaldehyde, embedded in paraffin and 5 μm sections were stained for hematoxylin and eosin, mouse anti-F4/80 (myeloid cell marker), mouse anti-CD3 (T cell marker), and mouse anti-neutrophil marker. Slides were incubated with anti-mouse secondary antibody for one hour followed by 30 minute incubation with ABC reagent (Vectastain ABC kit; Vector Laboratories). Color was developed with 3,3′-diaminobenzidine (DAB) using the DAB substrate Kit (Vector Laboratories). Histological analysis was performed on serial sections from the wound and tumor tissue. Immunostained tissue slides were imaged on a Leica SCN400 Slide Scanner automated digital image system (Leica Biosystems). Whole slides were imaged at 20X magnification to a resolution of 0.5 μm/pixel. Cells were identified utilizing standard Ariol® analysis scripts. (Leica) Upper and lower thresholds for color, saturation, intensity, size, roundness, and axis length were set for both blue Hematoxylin staining of nuclei and for brown DAB reaction products. Thus, brown (DAB) positive cells can be distinguished from blue (Hematoxylin only) negative cells. The area of positive staining per core was calculated as a percent of the total analyzed area divided by area of brown (DAB-positive) pixels. Positive staining was calculated separately in tumor and wound.
Five-micron sections of paraffin-embedded tissue sections (wounds and tumor) were stained with 0.1% Picrosirius Red (Direct Red 80; Sigma Aldrich) and imaged on a Zeiss Axiophot equipped with a cross-polarizer.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Linda Horton and Phil Owens for technical assistance. We thank Dr. Zena Werb for the MMTV-PyMT;ACTB-ECFP mice and the c-fms-EGFP mice. We are grateful to lab colleagues for helpful discussions on this project. We thank members of the Vanderbilt Cell Imaging Shared Resource for their assistance with confocal microscopy for intravital imaging; the VIIBRE Microfabrication Core for the design and fabrication of the MIWs and the imaging box; and the Vanderbilt Translational Pathology Shared Resource (supported by funding from the Cancer Center Support Grant 5P30 CA068485). Whole slide imaging and quantification of immunostaining were performed in the Digital Histology Shared Resource at Vanderbilt University Medical Center. We also thank Allison Price for critical editorial comments regarding this manuscript.
Funding
This work was funded by VA Career Scientist award to A.R., the Vascular Biology Training grant T32-HL0775, (P. Bock, PI), Vanderbilt-Ingram Cancer Center Support grant-CA68485, the CA34590 to A.R., Ingram Professorship to A.R., and ACS Postdoctoral award to T.S.-PF-11-092-01-CSM, NCI grant-CA143081 and NCI grant-CA120711 to AZ and the Vanderbilt Institute for Integrative Biosystems Research and Education (VIIBRE).
Author contributions
Designed experiments: TS, YS, DS, WA, JW, AZ, AR; wrote or edited paper: TS, JW, AR; designed and consulted for imaging studies: SW; performed experiments: TS, YS; analyzed data: TS.
References
- 1. Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410:50-6; PMID:11242036; http://dx.doi.org/ 10.1038/35065016 [DOI] [PubMed] [Google Scholar]
- 2. Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 2011; 11:597-606; PMID:21866172; http://dx.doi.org/ 10.1038/nri3049 [DOI] [PubMed] [Google Scholar]
- 3. Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res 2003; 5:R144-50; PMID:12927045; http://dx.doi.org/ 10.1186/bcr627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su YC, Wu MT, Huang CJ, Hou MF, Yang SF, Chai CY. Expression of CXCR4 is associated with axillary lymph node status in patients with early breast cancer. Breast 2006; 15:533-9; PMID:16239110; http://dx.doi.org/ 10.1016/j.breast.2005.08.034 [DOI] [PubMed] [Google Scholar]
- 5. Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The elevated level of CXCR4 is correlated with nodal metastasis of human breast cancer. Breast 2005; 14:360-7; PMID:16216737; http://dx.doi.org/ 10.1016/j.breast.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 6. Klevesath MB, Pantel K, Agbaje O, Provenzano E, Wishart GC, Gough P, Pinder SE, Duffy S, Purushotham AD. Patterns of metastatic spread in early breast cancer. Breast 2013; 22(4):449-54; PMID:23726130 [DOI] [PubMed] [Google Scholar]
- 7. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147:275-92; PMID:22000009; http://dx.doi.org/ 10.1016/j.cell.2011.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med 2001; 7:864-8; PMID:11433354; http://dx.doi.org/ 10.1038/89997 [DOI] [PubMed] [Google Scholar]
- 9. Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med 2003; 9:796-800; PMID:12754503; http://dx.doi.org/ 10.1038/nm879 [DOI] [PubMed] [Google Scholar]
- 10. Halin C, Mora JR, Sumen C, von Andrian UH. In vivo imaging of lymphocyte trafficking. Annu Rev Cell Dev Biol 2005; 21:581-603; PMID:16212508; http://dx.doi.org/ 10.1146/annurev.cellbio.21.122303.133159 [DOI] [PubMed] [Google Scholar]
- 11. Stroh M, Zimmer JP, Duda DG, Levchenko TS, Cohen KS, Brown EB, Scadden DT, Torchilin VP, Bawendi MG, Fukumura D, et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat Med 2005; 11:678-82; PMID:15880117; http://dx.doi.org/ 10.1038/nm1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med 2007; 204:345-56; PMID:17261634; http://dx.doi.org/ 10.1084/jem.20061890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech 2008; 1:155-67; discussion 65; PMID:19048079; http://dx.doi.org/ 10.1242/dmm.000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods 2008; 5:1019-21; PMID:18997781; http://dx.doi.org/ 10.1038/nmeth.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol 2008; 130:1147-54; PMID:18987875; http://dx.doi.org/ 10.1007/s00418-008-0529-1 [DOI] [PubMed] [Google Scholar]
- 16. Pittet MJ, Weissleder R. Intravital imaging. Cell 2011; 147:983-91; PMID:22118457; http://dx.doi.org/ 10.1016/j.cell.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel MF. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 2012; 21:402-17; PMID:22439936; http://dx.doi.org/ 10.1016/j.ccr.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ritsma L, Steller EJ, Beerling E, Loomans CJ, Zomer A, Gerlach C, Vrisekoop N, Seinstra D, van Gurp L, Schafer R, et al. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Sci Transl Med 2012; 4:158ra45. [DOI] [PubMed] [Google Scholar]
- 19. Lohela M, Werb Z. Intravital imaging of stromal cell dynamics in tumors. Curr Opin Genet Dev 2010; 20:72-8; PMID:19942428; http://dx.doi.org/ 10.1016/j.gde.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci 2011; 124:299-310; PMID:21242309; http://dx.doi.org/ 10.1242/jcs.072728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ewald AJ, Werb Z, Egeblad M. Preparation of mice for long-term intravital imaging of the mammary gland. Cold Spring Harb Protoc 2011; 2011:pdb prot5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ewald AJ, Werb Z, Egeblad M. Monitoring of vital signs for long-term survival of mice under anesthesia. Cold Spring Harb Protoc 2011; 2011:pdb prot5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol 1993; 143:1055-62; PMID:7692730 [PMC free article] [PubMed] [Google Scholar]
- 24. Schietinger A, Arina A, Liu RB, Wells S, Huang J, Engels B, Bindokas V, Bartkowiak T, Lee D, Herrmann A, et al. Longitudinal confocal microscopy imaging of solid tumor destruction following adoptive T cell transfer. Oncoimmunology 2013; 2:e26677; PMID:24482750; http://dx.doi.org/ 10.4161/onci.26677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res 1994; 54:4564-8; PMID:8062241 [PubMed] [Google Scholar]
- 26. Shan S, Sorg B, Dewhirst MW. A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc Res 2003; 65:109-17; PMID:12686168; http://dx.doi.org/ 10.1016/S0026-2862(02)00017-1 [DOI] [PubMed] [Google Scholar]
- 27. Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005; 15:599-607; PMID:16202600; http://dx.doi.org/ 10.1016/j.tcb.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 28. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184:3964-77; PMID:20176743; http://dx.doi.org/ 10.4049/jimmunol.0903356 [DOI] [PubMed] [Google Scholar]
- 29. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229:176-85; PMID:23096265; http://dx.doi.org/ 10.1002/path.4133 [DOI] [PubMed] [Google Scholar]
- 30. Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992; 12:954-61; PMID:1312220; http://dx.doi.org/ 10.1128/MCB.12.3.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 2003; 101:1155-63; PMID:12393599; http://dx.doi.org/ 10.1182/blood-2002-02-0569 [DOI] [PubMed] [Google Scholar]
- 32. Belanger MC, Marois Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: a review. J Biomed Mater Res 2001; 58:467-77; PMID:11505420; http://dx.doi.org/ 10.1002/jbm.1043 [DOI] [PubMed] [Google Scholar]
- 33. Piruska A, Nikcevic I, Lee SH, Ahn C, Heineman WR, Limbach PA, Seliskar CJ. The autofluorescence of plastic materials and chips measured under laser irradiation. Lab Chip 2005; 5:1348-54; PMID:16286964; http://dx.doi.org/ 10.1039/b508288a [DOI] [PubMed] [Google Scholar]
- 34. Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res 2009; 26:1739-44; PMID:19384466; http://dx.doi.org/ 10.1007/s11095-009-9884-4 [DOI] [PubMed] [Google Scholar]
- 35. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012; 37:106-26; PMID:22125349; http://dx.doi.org/ 10.1016/j.progpolymsci.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A 2009; 106:399-404; PMID:19126683; http://dx.doi.org/ 10.1073/pnas.0808932106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998; 153:1849-60; PMID:9846975; http://dx.doi.org/ 10.1016/S0002-9440(10)65699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015; 7(10):1120-34; PMID:25959051; http://dx.doi.org/ 10.1039/c5ib00040h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011; 71:5601-5; PMID:21846822; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1316 [DOI] [PubMed] [Google Scholar]
- 40. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat 2011; 127:99-108; PMID:20556505; http://dx.doi.org/ 10.1007/s10549-010-0987-8 [DOI] [PubMed] [Google Scholar]
- 41. Ni YH, Ding L, Huang XF, Dong YC, Hu QG, Hou YY. Microlocalization of CD68(+) tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patients. Tumour Biol 2015; 36:5291-8; PMID:25666753; http://dx.doi.org/ 10.1007/s13277-015-3189-5 [DOI] [PubMed] [Google Scholar]
- 42. Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 2012; 65:159-63; PMID:22049225; http://dx.doi.org/ 10.1136/jclinpath-2011-200355 [DOI] [PubMed] [Google Scholar]
- 43. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315:1650-9; PMID:3537791; http://dx.doi.org/ 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- 44. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23:549-55; PMID:12401408; http://dx.doi.org/ 10.1016/S1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 45. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39-51; PMID:20371344; http://dx.doi.org/ 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A 2012; 109:2796-801; PMID:21825174; http://dx.doi.org/ 10.1073/pnas.1104303108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 2013; 339:286-91; PMID:23329041; http://dx.doi.org/ 10.1126/science.1232227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer 2013; 81:130-7; PMID:23540719; http://dx.doi.org/ 10.1016/j.lungcan.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 49. McCormack DR, Walsh AJ, Sit W, Arteaga CL, Chen J, Cook RS, Skala MC. In vivo hyperspectral imaging of microvessel response to trastuzumab treatment in breast cancer xenografts. Biomed Opt Express 2014; 5:2247-61; PMID:25071962; http://dx.doi.org/ 10.1364/BOE.5.002247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






