Abstract
The adipocyte-derived hormone leptin is an important regulator of body weight and metabolism through activation of brain leptin receptors expressed in regions such as the hypothalamus. Beyond these well described and characterized activities of leptin in the hypothalamus, it is becoming increasingly clear that the central activities of leptin extend to the hippocampus. Indeed, leptin receptors are expressed in the hippocampus where these receptors are proposed to mediate various aspects of hippocampal synaptic plasticity that ultimately impact cognitive function. This concept is supported by studies demonstrating that leptin promotes hippocampal-dependent learning and memory, as well as studies indicating that leptin resistance is associated with deficits in hippocampal-dependent behaviors and in the induction of depressive-like behaviors. The effects of leptin on cognitive/behavioral plasticity in the hippocampus may be regulated by direct activation of leptin receptors expressed in the hippocampus; additionally, leptin-mediated activation of synaptic networks that project to the hippocampus may also impact hippocampal-mediated behaviors. In view of these previous observations, the goal of this review will be to discuss the mechanisms through which leptin facilitates cognition and behavior, as well as to dissect the loci at which leptin resistance leads to impairments in hippocampal synaptic plasticity, including the development of cognitive deficits and increased risk of depressive illness in metabolic disorders such as obesity and type 2 diabetes mellitus (T2DM).
1. Leptin synthesis and signaling
Leptin is predominantly synthesized and released by adipocytes in proportion to body adiposity [1] and once released into circulation leptin can act in the periphery to play roles in immunity [2], regulation of insulin secretion [3], sex hormone release [4,5] and lipolysis in adipocytes [6,7]. Leptin is also synthesized and secreted by the gastrointestinal tract where it is proposed to participate in both endocrine and exocrine activities [8]. These actions of leptin are mediated through activation of the short form of the leptin receptor (ObRa). Leptin can also cross the blood-brain barrier (BBB) through a saturable transport system [9] and activate the long form of the leptin receptor (ObRb), a class 1 cytokine receptor encoded by the diabetes (db) gene [10]. This isoform of the leptin receptor activates several signaling cascades, most notably the JAK/STAT pathway. Activation of the JAK/STAT pathway is initiated by a conformational change in the ObRb that results in phosphorylation of tyrosine residues on JAK2, which leads to phosphorylation of tyrosine residues on the ObRb. These phosphorylation events ultimately result in phosphorylation of signal transducer and activator of transcription 3 (STAT3). Phosphorylated STAT3 serves as transcription factor to regulate the expression of genes such as suppressor of cytokine signaling 3 (SOCS3), which mediates feedback inhibition of the leptin receptor. In addition, leptin stimulates the PI3-kinase and MAPK (Erk 1/2) pathways through activation of the ObRb. For a more comprehensive overview of leptin signaling, see [11]. Leptin resistance can occur at various points in these signaling pathways, thereby significantly impacting both synaptic plasticity and behavior. Among the brain regions that are adversely affected by leptin resistance is the hippocampus.
2. The hippocampus, leptin and synaptic plasticity
The hippocampus is part of the limbic system and is comprised of the Cornu Ammonis regions 1–4 (CA1–4) and the dentate gyrus. From a functional/anatomical perspective, information enters the hippocampus from the entorhinal cortex through the perforant path to the dentate gyrus. Excitatory mossy fiber projections originating in dentate gyrus granule neurons project onto glutamatergic pyramidal neurons in the CA3 region and these excitatory CA3 neurons project to the CA1 pyramidal neurons via the Schaffer collaterals. Additionally, CA3 neurons send axon collaterals to other CA3 pyramidal neurons, thereby significantly enhancing the anatomical and functional capacity of the hippocampal network. Beyond these excitatory networks, GABAergic interneurons are expressed throughout the hippocampus and functionally these inhibitory networks help to coordinate excitatory activity in the hippocampus. It should be noted that an anatomical characterization of the hippocampus must also include descriptions of the functional distinctions between the dorsal hippocampus (proposed to be more involved in spatial cognitive function) and the ventral hippocampus (proposed to be more involved in emotional processing); for review, see [12]. Leptin receptors are expressed in the hippocampus [13,14] and leptin has been shown to directly impact electrophysiological and anatomical plasticity of the hippocampus. In this regard, leptin receptor activation promotes a variety of measures of synaptic plasticity that may ultimately be responsible for the pro-cognitive effects of leptin. For example, leptin administration to ex vivo brain slices prepared from control rodents enhances long-term potentiation (LTP) in the hippocampus [15–17], which is proposed to be a cellular correlate of learning and memory in the mammalian brain. Leptin-mediated alterations in electrophysiological plasticity are proposed to involve functional interactions with the hippocampal glutamatergic system, including trafficking of glutamate receptor subunits [18–20]. From a morphological perspective, leptin promotes motility of dendritic filipodia and increases synaptic density in hippocampal primary cultures [21] and also increases cell proliferation/neurogenesis in the dentate gyrus [22]. From a molecular perspective, signaling pathways activated by leptin, such as SOCS and STAT-3, indirectly mediate synaptic plasticity through transcription of a variety of genes, including genes which regulate cytokine production (e.g. as RANTES, IL6, IL8, MET, and MRAS) [23], neurite growth [24], and mitochondrial oxidative stress (e.g. Bcl-xL) [25]. However, few of these studies have specifically examined how leptin activation of hippocampal SOCS and STAT-3 pathways leads to the transcription of genes which control structural or cellular plasticity. Collectively, these results support the concept that leptin-mediated signaling promotes hippocampal synaptic plasticity.
Conversely, decreases in synaptic plasticity are likely to contribute to learning and memory deficits and depressive-like behaviors observed in experimental models of leptin resistance. In this regard, stimulus-evoked LTP is reduced in the hippocampus of rodents with leptin resistance [26–34]. Morphological alterations in hippocampal neurons are also observed in rodents with leptin resistance, including decreases in spine density [35], decreases in cell proliferation/neurogenesis [36–40] and deleterious alterations in synaptic organization and dendritic architecture [27,41,42]. Morphological deficits in the hippocampus of rodents with leptin resistance extend beyond neurons and include alterations in astroglial elements [42,43] and structural changes in microglia suggestive of microglial activation [42,44]. Blood-brain barrier integrity is also compromised in leptin resistant states [45–47], which may contribute to or exacerbate CNS leptin resistance. As discussed below, these measures of synaptic plasticity are the foundational substrates of leptin-mediated effects on cognitive/behavioral function.
3. Leptin & behavior
3.1. Leptin enhancement of learning and memory
One of the first studies to examine the effects of leptin on behavior failed to identify any pro-cognitive effects of leptin administration. Specifically, bilateral injection of leptin into the CA1 region of the hippocampus did not modulate learning and memory in the radial arm maze test in Wistar rats [48]. However, subsequent studies determined that increasing brain concentrations of leptin positively impacted learning and memory, often in a dose-dependent manner. For example, studies by Farr and coworkers determined that bilateral intrahippocampal injections of leptin dose-dependently improved performance in the T-maze and the step down inhibitory avoidance test [49]. Interestingly, the highest doses of leptin tested did not affect behavior in the step down avoidance test, thereby possibly providing insight into the lack of effect of intrahippocampal leptin effects reported in Wistar rats [48]. Subsequent studies revealed similar dose-response effects of leptin on behavior [50], in that peripheral administration of leptin dose-dependently enhanced behavioral performance in the passive avoidance task and the water maze. Interestingly, the highest doses of leptin tested actually impaired learning in these tests [16]. Studies by Kanoski, Hayes and coworkers differentiated between dorsal and ventral activities of leptin in hippocampal-dependent behaviors. In this regard, bilateral ventral hippocampal injection of leptin inhibited conditioned place preference appetitive behaviors and food-related spatial memory consolidation, while dorsal hippocampal leptin administration did not impact these behaviors [51]. Importantly, in these studies leptin did not impact locomotor performance in the elevated plus maze [52,53], the open field test [54,55], the operant runway [51] or the water maze [26]. Such results suggest that leptin does not ubiquitously impact behavior, but rather more specifically modulates plasticity in learning and memory-based behavioral tasks.
3.2. Leptin resistance and learning and memory impairments
Studies that have examined behavioral performance in rodents with leptin resistance provide further support for the concept that leptin receptor activation plays a critical role in hippocampal synaptic plasticity. These studies are based on several experimental models, including rodents with genetic mutations in the leptin gene (ob/ob mice), mutations that result in non-functional leptin receptors (db/db mice, Zucker rats) or experimental manipulations that induce leptin resistance (e.g. high fat diet models or through the use of virus-mediated gene transfer). For instance, db/db mice [26,39] and Zucker rats [26,31] exhibit spatial learning and memory deficits in the water maze that were not attributable to deficits in locomotor activity. Our previous studies also determined that Zucker rats exhibit deficits in hippocampal-dependent learning. Using the variable interval delayed alternation task, we reported that Zucker rats learned the go-no go task as effectively as their lean counterparts when there was no inter-trial interval (ITI) or when the was the ITI was short. However when the ITI was increased, which is more dependent on structural and functional plasticity in the hippocampus, Zucker rats performed more poorly when compared to lean control rats [56]. In addition to genetic models, rodents provided high fat diets that develop leptin resistance also exhibit deficits in hippocampal-dependent behaviors [29,57–62]. Our prior studies using a lentivirus packaged with an antisense sequence selective for the insulin receptor (LV-IRAS) determined that hypothalamic-specific insulin resistance (Hypo-IRAS) elicits deficits in hippocampal-dependent behaviors that are associated with leptin resistance. Specifically, downregulation of hypothalamic insulin receptors induces a metabolic syndrome (MetS) phenotype that includes leptin resistance [27,63] and also elicits decreases in contextual fear condition that were associated with structural and functional deficits in the hippocampus [27]. Nonetheless, it is important to emphasize that these models of leptin resistance share common endocrine and metabolic changes that results in a complex phenotype that does not exclusively include leptin resistance. Indeed, metabolic and endocrine changes of peripheral origin are likely contributing to the deficits in hippocampal synaptic plasticity observed in these studies. As such, in the broader context of the complex endocrine milieu associated with metabolic disorders, an important challenge for investigators is to determine the relative contribution of leptin resistance in the development of hippocampal neuroplasticity deficits.
3.3. Leptin, leptin resistance and depressive-like behaviors
In addition to enhancement of learning and memory, leptin administration also elicits antidepressant-like effects in control rodents. In this regard, peripheral administration of leptin dose-dependently decreases immobility time in the tail suspension test and forced swim test (FST) [55] and also elicits anxiolytic effects in the elevated plus maze [50, 55]. While these behavioral tests unequivocally involve multiple brain regions and activation of different neuronal circuitry, these results suggest that activation of hippocampal leptin receptors is associated with an enhancement of behavior in tests that are traditionally used to measure depressive-like and anxiety-like behaviors in rodents. In further support of this hypothesis, mice with leptin receptors selectively knocked out in glutamatergic neurons in the hippocampus and prefrontal cortex (PFC) exhibit depressive like behaviors, indicating that leptin mediation of these forebrain excitatory networks are important mediators of depressive-like behaviors [54]. Moreover, the antidepressant activities of leptin are abolished in these mice. Subsequent studies from this same group revealed an important role for hippocampal NMDA receptors in the antidepressant activities of leptin [64]. Therefore, while leptin receptor activity beyond the hippocampus is likely to be involved in these complex behaviors, these studies illustrate the pivotal role of hippocampal leptin activity in the antidepressant activities of leptin.
Studies using rodent models of leptin resistance further support this relationship between leptin and depressive-like behaviors. In this regard, ob/ob mice [65,66] and db/db mice [52] exhibit behavioral despair in the FST and anhedonia in the sucrose preference test. While locomotor activity (as measured by distance traveled) does not change in the elevated plus maze, db/db mice spend less time in the open arms and make fewer entries in the open arms of the maze, behavior which is indicative of an anxiety-like behavioral phenotype [52]. Rodents provided a high fat diet that elicits an obesity phenotype that includes leptin resistance also exhibit behavioral despair, anhedonia and anxiety-like behaviors [65,67]. Additionally, we have previously reported that leptin resistant Hypo-IRAS rats also exhibit depressive-like and anxiety-like behaviors [53]. Interestingly, dietary restriction or return to a normal chow diet reversed depressive-like behaviors in Hypo-IRAS rats [68] and diet-induced obese (DIO) mice [65], respectively. From a translational perspective, these results from experimental models reflect what is seen in the clinical setting in that obese individuals have a greater risk of developing depressive illness [69–74] and that reductions in body weight (as achieved through bariatric surgery) are associated with an elevation in mood [75–79]. Nonetheless, these clinical and pre-clinical studies suffer from the same limitation noted above, namely what is the relative contribution of leptin resistance in the development of neuropsychiatric disorders in MetS, obesity and T2DM?
4. Strategies to more selectively target leptin receptors in the CNS
4.1. Pegylated leptin receptor antagonist
As noted above, one of the limitations associated with these studies is that it is difficult to disentangle the relative contribution of peripheral endocrine abnormalities from CNS deficits in the development of neurobehavioral impairments in metabolic disorders [80]. Indeed, these experimental models exhibit a variety of changes that may include insulin resistance, hyperglycemia, hypothalamic-pituitary-adrenal (HPA) axis dysfunction, increases in pro-inflammatory cytokines and decreases in the expression of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) [81]. Moreover, CNS leptin resistance involves both a decrease in leptin transport across the BBB and decreases in leptin receptor signaling [82,83]. As such, differentiating cause from consequence in the development of neurobehavioral deficits in metabolic disorders that involve peripheral endocrine abnormalities and CNS deficits represents a major obstacle in the development of strategies to effectively manage cognitive deficits and neuropsychiatric disorders in obesity, MetS and T2DM.
In view of these limitations, we recently completed a study to examine whether induction of a CNS leptin-deficient state would elicit depressive-like behaviors in rats. To accomplish this goal, rats were treated with a pegylated leptin receptor antagonist (Peg-LRA) that blocks BBB transport of leptin and thereby creates a leptin-deficient state in the CNS [84]. In support of this approach, administration of the Peg-LRA elicited the expected decreases in BBB transport of leptin, as evidenced by decreases in leptin receptor signaling in response to peripherally administered leptin [85]. From a behavioral perspective, Peg-LRA administration did not elicit anhedonia in the sucrose preference test, unlike observations in DIO mice [65] and Hypo-IRAS rats [53]. However, Peg-LRA-treated rats exhibited increases in immobility behaviors and corresponding decreases in active behaviors in the FST, consistent with other experimental models of leptin resistance [52, 65–67], including our prior studies in Hypo-IRAS rats [53]. Closer examination of the behaviors in Peg-LRA rats in the FST revealed that the deficits in active behaviors were due exclusively to deficits in swimming behavior, whereas climbing behaviors were similar in Peg-LRA rats compared to control rats (Fig. 1, Panel A). Conversely, Hypo-IRAS rats exhibited non-significant decreases in both swimming and climbing behaviors compared to Hypo-Con rats (Fig. 1, Panel B), which when combined resulted in significant decreases in active behaviors [53]. Such results provide insight into the neurotransmitter systems that may be affected by leptin resistance elicited by Peg-LRA administration when compared with leptin resistance observed in Hypo-IRAS rats. In this regard, prior studies by Cryan and Lucki and coworkers determined that serotonin-selective reuptake inhibitors (SSRIs) preferentially affect swimming behaviors in the FST while drugs that primarily affect norepinephrine preferentially impact climbing behaviors (For review see [86]). Since the raphe nucleus is the primary site of synthesis of 5-HT in the brain and leptin receptors are expressed in this brain region [13, 87], leptin resistance in the raphe nucleus may diminish the synthesis of 5-HT and thereby be a critical site for the neurochemical deficits that drive some components of depressive illness in obesity. In this context, it is interesting to speculate that Peg-LRA administration preferentially impacts the serotonergic system while Hypo-IRAS rats develop an endocrine and metabolic phenotype that affects a number of neurotransmitters systems that includes the serotonergic system. These results from Peg-LRA-treated rats also support the concept that distinct endocrine or immune features of the obesity profile might underlie specific neurochemical and depressive-like behavioral consequences. As such, an interesting future direction would be to perform a comprehensive neurochemical evaluation of serotonin, norepinephrine and dopamine to determine how changes in these neurotransmitters contribute to the more selective behavioral deficits in Peg-LRA rats vis-à-vis other models of leptin resistance.
Fig. 1.
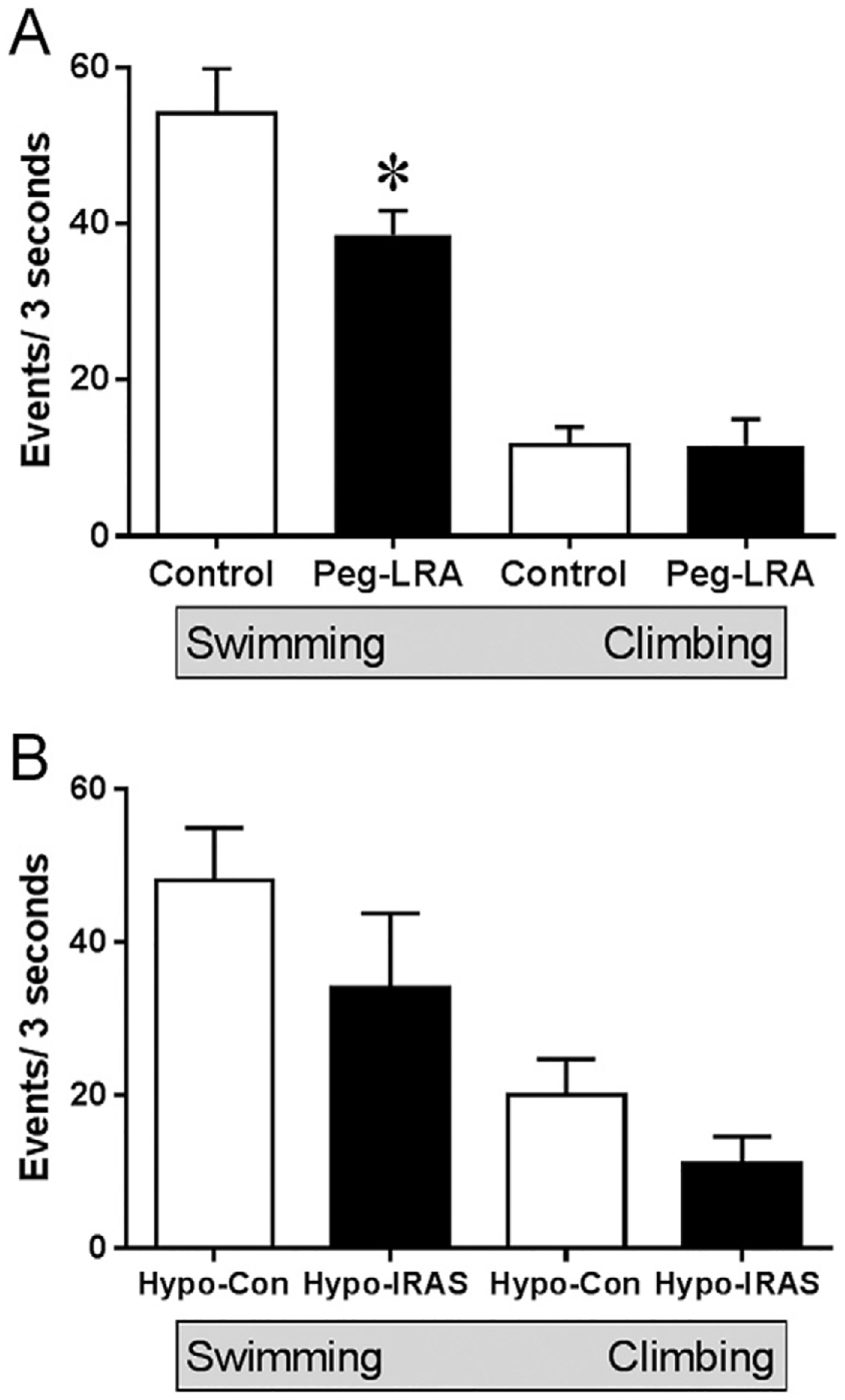
Behavioral despair is differentially affected in models of leptin resistance. Panel A: In the forced swim test, administration of a pegylated leptin receptor antagonist (Peg-LRA) significantly reduces swimming behaviors while climbing behaviors are unaffected compared to vehicle-treated control rats. Panel B: Virus-mediated downregulation of hypothalamic insulin receptors (Hypo-IRAS) elicits an obesity/MetS phenotype that is associated with depressive-like behaviors, including behavioral despair in the forced swim test [53]. However, both climbing and swimming behaviors are non-significantly reduced in Hypo-IRAS compared to Hypo-Control (Hypo-Con) rats. These differential effects upon ‘active’ behaviors in the FST provide insight into the neurotransmitter systems and circuits that may regulate these behaviors in leptin resistant rodents. See text for details. [* = p < 0.05].
4.2. Molecular approaches to target leptin receptor expression
The studies using the Peg-LRA illustrates that the induction of leptin resistance initiates the development of an obesity phenotype and deficits in some but not all behaviors that have been traditionally used to measure ‘depressive-like’ behaviors. Nonetheless, similar to other models of leptin resistance, Peg-LRA rats exhibit a variety of endocrine and metabolic alterations including increases in body weight and adiposity, insulin resistance, increases in plasma triglyceride levels and increased indices of peripheral inflammation [85]. As an alternative, genetic targeting of leptin receptors may provide an approach to more accurately determine the contribution of CNS leptin resistance to the development of neurobehavioral deficits independent of peripheral endocrine and metabolic changes. As noted above, Guo and coworkers reported that forebrain knockout of leptin receptors in mice elicited behavioral despair and anhedonia in the absence of changes in body weight, body adiposity, plasma levels of insulin and plasma leptin levels [54]. Virus-mediated gene transfer also represents an approach to selectively target leptin receptor-containing populations in the CNS [88,89]. Using this strategy, we have developed a lentivirus packaged with an antisense sequence selective for the leptin receptor (LV-LepRAS). Rats that receive intra-hypothalamic injections of the LV-LepRAS construct exhibit significant increases in body weight compared to rats treated with the LV-Control construct (Fig. 2). In order to determine whether downregulation of hypothalamic leptin receptors would elicit deficits in leptin signaling, LV-LepRAS rats and LV-Control rats received an intraperitoneal injection of leptin (5 mg/kg) and were then processed for pSTAT3 immunoreactivity 60 min following leptin administration. As shown in Fig. 3, LV-Control rats exhibited the expected increases in pSTAT3 in the ventromedial nucleus of the hypothalamus, demonstrating that leptin receptor signaling was unaffected by administration of the control virus. Conversely, peripheral leptin administration failed to elicit a significant increase in pSTAT3 immunoreactivity in the hypothalamus of LV-LepRAS rats. These results demonstrate that the LV-LepRAS construct effectively induces hypothalamic leptin resistance and thereby may serve as an effective tool to examine the region specific activities of leptin receptors throughout the CNS. We have used a similar approach to examine the functional activities of insulin receptors in the hippocampus [90]. The results of this study illustrated that induction of hippocampal-specific insulin resistance elicited deficits in hippocampal synaptic plasticity and impairments in hippocampal-dependent spatial learning and memory independent of changes in peripheral insulin sensitivity or glucose homeostasis. Accordingly, an interesting future direction would be to determine whether intra-hippocampal administration of the LV-LepRAS construct similarly elicits deficits in hippocampal synaptic plasticity independent of peripheral endocrine or metabolic changes. Such results would more specifically determine the functional activities of leptin receptors expressed in the hippocampus. However, it is also likely that leptin receptor activation of neural circuits that project to the hippocampus modulate behavioral activities and virus-mediated gene transfer would be an effective way of identifying these circuits. In this regard, previous elegant studies by Davis et al. [88] and Hommel et al. [89] demonstrated that virus-mediated knockdown of midbrain leptin receptors regulate/activate reward pathways through the modulation of dopaminergic tone. Similarly, an interesting approach would be to determine whether leptin resistance restricted to the raphe nucleus elicits depressive-like behaviors and deficits in hippocampal serotonergic tone. Such results would support the concept that, similar to the insulin receptor system (see [80]), CNS leptin receptor activity acts independently of peripheral leptin signaling and moreover that leptin receptors exhibit region-specific functional activities.
Fig. 2.
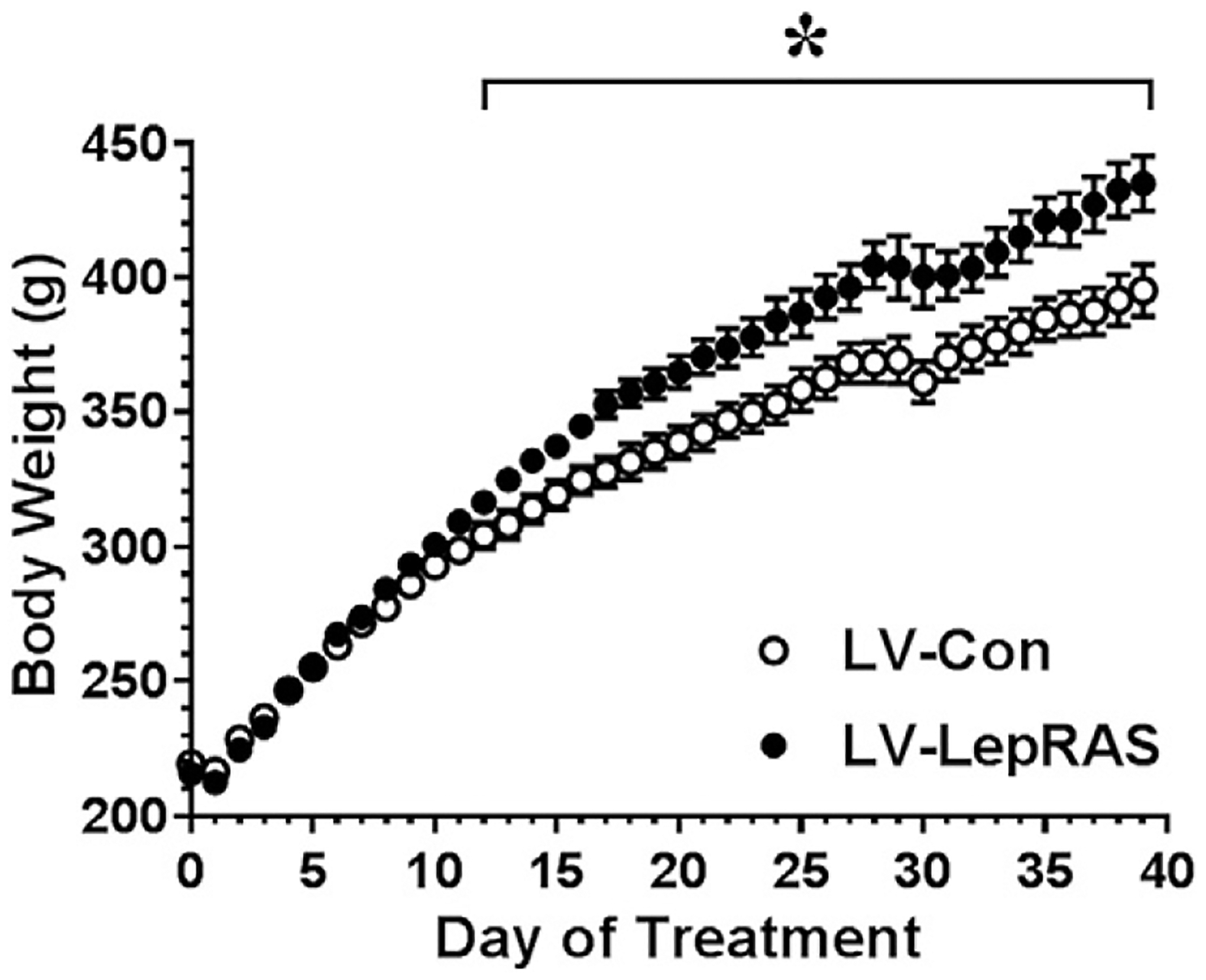
Lentivirus-mediated downregulation of hypothalamic leptin receptors significantly increases body weight. Rats received third ventricular injections of either a control lentivirus (LV-Con) or a lentivirus containing an antisense sequence selective for the leptin receptor (LV-LepRAS). Beginning approximately 14 days following virus administration, LV-LepRAS rats exhibit significant increases in body weight compared to LV-Con rats. [* = p < 0.05].
Fig. 3.
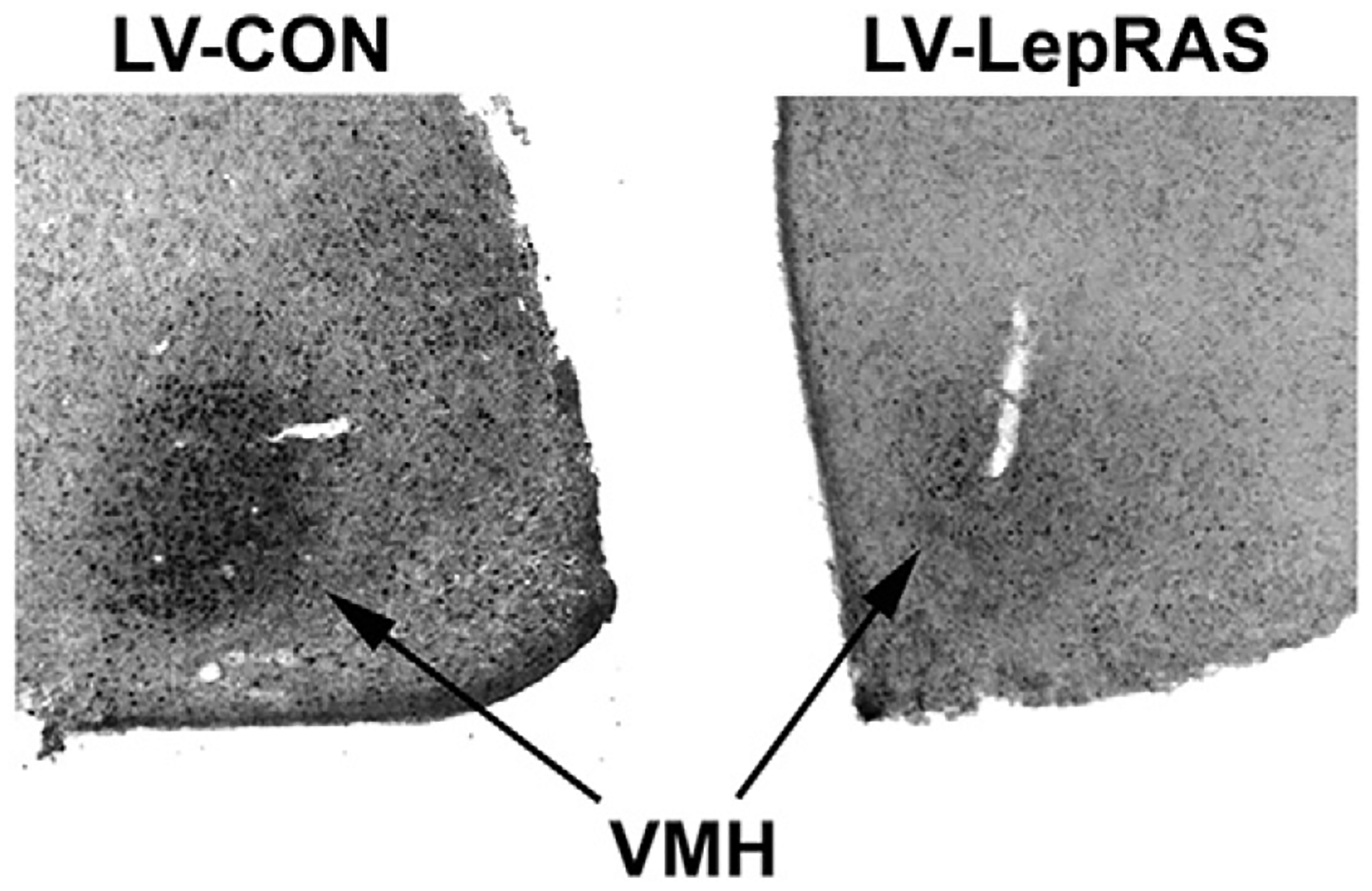
Peripheral leptin administration increases the phosphorylation of STAT3 (pSTAT3) in the hypothalamus of LV-Control rats but not in the hypothalamus of LV-LepRAS rats. Rats received an intraperitoneal injection of leptin (5 mg/kg) and brains were processed for pSTAT3 immunohistochemistry as described in our previous studies [27,85]. Left panel depicts representative bright-field micrograph of leptin-induced pSTAT3 immunoreactivity in the ventromedial nucleus of the hypothalamus (VMH) of rats that received third ventricular injections of the LV-Con construct. Right panel depicts representative bright-field micrograph in which leptin-stimulated pSTAT3 immunoreactivity is significantly reduced in the VMH of LV-LepRAS rats. Such results indicate that LV-LepRAS-treated rats develop leptin resistance.
5. Conclusions and perspectives
Collectively, the existing literature illustrates that different leptin receptor populations modulate diverse behavioral activities including feeding, motivated behaviors and cognition. Furthermore, these behavioral endpoints may be regulated by the local expression of leptin receptors, as well as by the stimulation of neuronal networks activated by leptin receptors expressed at more distal sites (Fig. 4). Another important, and perhaps underappreciated issue, relates to the temporal nature of the responses mediated by leptin under normal physiological conditions, as well as the temporal nature of the development of leptin resistance. For example, a key question is how accurately administration of leptin to an ex vivo preparation or intraventricular or intrahippocampal leptin administration mimics the CNS actions of leptin released from adipocytes or from other sources such the GI tract following a meal. Indeed, peripherally-derived peptides such as leptin do not act as rapidly or as dynamically as classic neurotransmitters (i.e. amino acids, catecholamines, acetylcholine) or neuropeptides released directly into the synaptic cleft. Therefore, leptin could be thought of more as a trophic factor that establishes a substrate upon which neuroplasticity can be facilitated by neurotransmitters and neuropeptides, as well as by peripherally-derived signals such as insulin or leptin itself. Under such circumstances, the trophic effects of leptin likely develop more slowly and involve structural and functional synaptic changes, as well as changes in gene expression. Similarly, the initial stages of leptin resistance involve decreases in leptin receptor signaling which are followed by impairments in BBB transport of leptin [91]. Additionally, leptin resistance could slowly and incrementally lead to the disintegration of the structural and functional substrates which are necessary for neuroplasticity. As such, one of the limitations of our current approaches is that we are often restricted to measuring leptin activity as a ‘snapshot’ rather than as a continuum of how leptin facilitates neuroplasticity or how leptin resistance impairs neuroplasticity. As we move forward, an important question that remains to be addressed is how integration of localized hippocampal leptin resistance, leptin resistance in circuits that project to the hippocampus, decreases in BBB leptin transport and peripheral leptin resistance ultimately result in cognitive deficits and neuropsychiatric disorders. Addressing these issues would represent a major advance and would hopefully assist in the development of novel treatment strategies for the neurological consequences elicited by metabolic disorders like obesity, MetS and T2DM.
Fig. 4.
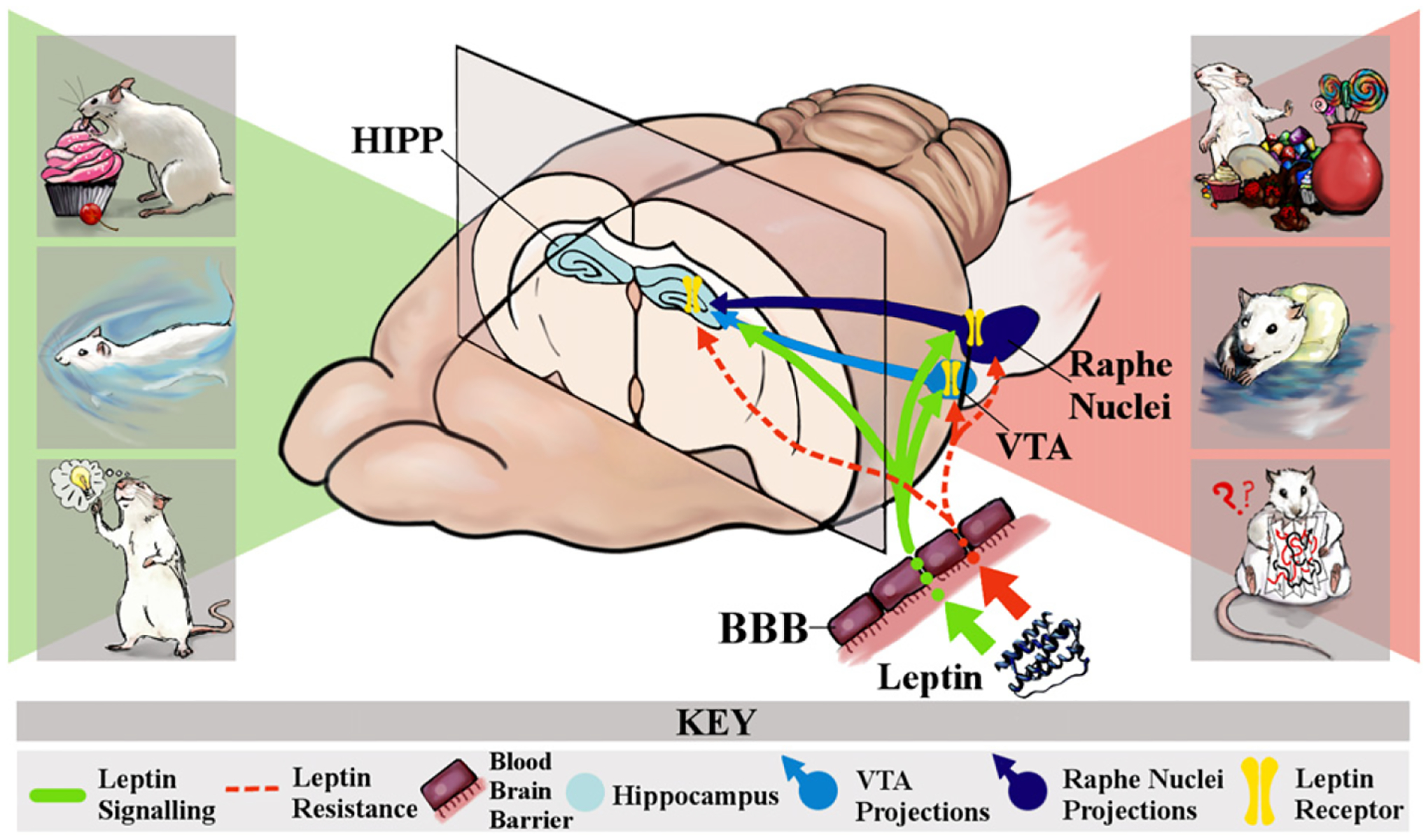
Direct and indirect mechanistic circuits through which leptin receptor activity modulates hippocampal-related behaviors. Under physiological conditions, leptin released from adipocytes crosses the blood-brain barrier via a facilitated transport system and activates leptin receptors expressed in the CNS (as depicted in the green arrows and yellow receptor symbols). Activation of hippocampal leptin receptors is proposed to contribute to enhancement of hippocampal-dependent behaviors such as spatial learning and memory. In combination with the activation of hippocampal leptin receptors, activation of leptin receptors in the VTA and the raphe nucleus may directly modulate motivated behaviors and elicit anti-depressant like effects via activation of neuronal circuits that project to the hippocampus (as depicted in the green shaded portion of the figure). While obesity is associated with increases in adiposity and plasma levels of leptin, a characteristic feature of obesity is CNS leptin resistance, which results from a combination of decreased blood-brain barrier transport of leptin and a decrease in leptin receptor signaling (as depicted in the dashed red lines). Under such conditions, the combination of hippocampal leptin resistance and reduced circuit activation from the raphe nucleus and the ventral tegmental area (VTA) would result in impairments in hippocampal-dependent learning and memory and the development of depressive-like behaviors (as depicted in the red-shaded portion of the figure). These observations from pre-clinical studies predict that CNS leptin resistance is a mechanistic link in the cognitive deficits and increased risk for neuropsychiatric disorders in obese individuals. See text for details.
A final note: this manuscript was submitted as part of a Special Issue for the 2016 Society for the Study of Ingestive Behaviors (SSIB) meeting. At the SSIB meeting, our laboratory participated in a symposium dedicated to our friend and colleague Dr. Randall Sakai. Words cannot truly encapsulate our appreciation to the Society for creating the time in the 2016 meeting for this session, and we were humbled by the invitation to participate. Randall was a guiding force in our lives (“Dude, are you going to fish or cut bait?”) and in the way we conducted our research (“The controls are the most important group!”). Every day we miss his scholarship, his wisdom, his friendship and his infectious laugh. While his legacy will continue on in our Society through the ways he influences the science of his colleagues and mentees, the science cannot fill the cavernous void we feel in our hearts and our souls and in our lives. For these reasons we are most grateful to the SSIB community for this opportunity and hope we were able to make him proud with our presentation and this review.
HIGHLIGHTS.
Hippocampal leptin receptor activation enhances behavior.
Leptin resistance elicits cognitive dysfunction and depressive-like behaviors.
Raphe nucleus leptin resistance may decrease hippocampal serotoninergic activity.
Molecular approaches can identify region-specific leptin activity in the CNS.
Acknowledgments:
Supported by the Department of Veterans Affairs (IO1 BX001804 and I21 BX002085; LPR), the Center for Targeted Therapeutics – COBRE (P20 GM109091-03, Pilot Grant to CAG), the National Science Foundation (IOS-1656626 to CAG), and South Carolina Translational Research Institute (NIH/NCATS Grant #UL1TR00062, Pilot Project Program to CAG).
References
- [1].Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS, Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action, Nat. Med 1 (1995) 1311–1314. [DOI] [PubMed] [Google Scholar]
- [2].Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI, Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression, Nature 394 (1998) 897–901. [DOI] [PubMed] [Google Scholar]
- [3].Bjorbaek C, Kahn BB, Leptin signaling in the central nervous system and the periphery, Recent Prog. Horm. Res 59 (2004) 305–331. [DOI] [PubMed] [Google Scholar]
- [4].Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM, Role of leptin in hypothalamic-pituitary function, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS, Role of leptin in the neuroendocrine response to fasting, Nature 382 (1996) 250–252. [DOI] [PubMed] [Google Scholar]
- [6].Wang MY, Lee Y, Unger RH, Novel form of lipolysis induced by leptin, J. Biol. Chem 274 (1999) 17541–17544. [DOI] [PubMed] [Google Scholar]
- [7].Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, Cusin I, Rohner-Jeanrenaud F, Burger AG, Zapf J, Meier CA, Direct effects of leptin on brown and white adipose tissue, J. Clin. Invest 100 (1997) 2858–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M, Endocrine and exocrine secretion of leptin by the gastric mucosa, J. Histochem. Cytochem 53 (2005) 851–860. [DOI] [PubMed] [Google Scholar]
- [9].Banks WA, Kastin AJ, Huang WT, Jaspan JB, Maness LM, Leptin enters the brain by a saturable system independent of insulin, Peptides 17 (1996) 305–311. [DOI] [PubMed] [Google Scholar]
- [10].Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI, Identification and expression cloning of a leptin receptor, OB-R, Cell 83 (1995) 1263–1271. [DOI] [PubMed] [Google Scholar]
- [11].Flak JN, Myers MG Jr., Minireview: CNS mechanisms of leptin action, Mol. Endocrinol 30 (2016) 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fanselow MS, Dong HW, Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65 (2010) 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB, Distributions of leptin receptor mRNA isoforms in the rat brain, J. Comp. Neurol 395 (1998) 535–547. [PubMed] [Google Scholar]
- [14].Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L, Localization of leptin receptor mRNA expression in mouse brain, Neurol. Rep 7 (1996) 2635–2638. [DOI] [PubMed] [Google Scholar]
- [15].Shanley LJ, Irving AJ, Harvey J, Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity, J. Neurosci 21 (2001), art-RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K, Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats, Peptides 27 (2006) 2738–2749. [DOI] [PubMed] [Google Scholar]
- [17].Wayner MJ, Armstrong DL, Phelix CF, Oomura Y, Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo, Peptides 25 (2004) 991–996. [DOI] [PubMed] [Google Scholar]
- [18].Durakoglugil M, Irving AJ, Harvey J, Leptin induces a novel form of NMDA receptor-dependent long-term depression, J. Neurochem 95 (2005) 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moult PR, Cross A, Santos SD, Carvalho AL, Lindsay Y, Connolly CN, Irving AJ, Leslie NR, Harvey J, Leptin regulates AMPA receptor trafficking via PTEN inhibition, J. Neurosci 30 (2010) 4088–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moult PR, Harvey J, Regulation of glutamate receptor trafficking by leptin, Biochem. Soc. Trans 37 (2009) 1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J, Leptin promotes rapid dynamic changes in hippocampal dendritic morphology, Mol. Cell. Neurosci 35 (2007) 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garza JC, Guo M, Zhang W, Lu XY, Leptin increases adult hippocampal neurogenesis in vivo and in vitro, J. Biol. Chem 283 (2008) 18238–18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR, Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB, Genes Dev 21 (2007) 1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P, Richardson PM, Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons, J. Neurosci 26 (2006) 9512–9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guo Z, Jiang H, Xu X, Duan W, Mattson MP, Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization, J. Biol. Chem 283 (2008) 1754–1763. [DOI] [PubMed] [Google Scholar]
- [26].Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T, Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents, Neuroscience 113 (2002) 607–615. [DOI] [PubMed] [Google Scholar]
- [27].Grillo CA, Piroli GG, Junor L, Wilson SP, Mott DD, Wilson MA, Reagan LP, Obesity/hyperleptinemic phenotype impairs structural and functional plasticity in the rat hippocampus, Physiol. Behav 105 (2011) 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grillo CA, Piroli GG, Evans AN, Macht VA, Wilson SP, Scott KA, Sakai RR, Mott DD, Reagan LP, Obesity/hyperleptinemic phenotype adversely affects hippocampal plasticity: effects of dietary restriction, Physiol. Behav 104 (2011) 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP, Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats, Hippocampus 18 (2008) 1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karimi SA, Salehi I, Komaki A, Sarihi A, Zarei M, Shahidi S, Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study, Brain Res 1539 (2013) 1–6. [DOI] [PubMed] [Google Scholar]
- [31].Kamal A, Ramakers GM, Gispen WH, Biessels GJ, Hyperinsulinemia in rats causes impairment of spatial memory and learning with defects in hippocampal synaptic plasticity by involvement of postsynaptic mechanisms, Exp. Brain Res 226 (2013) 45–51. [DOI] [PubMed] [Google Scholar]
- [32].Gerges NZ, Aleisa AM, Alkadhi KA, Impaired long-term potentiation in obese Zucker rats: possible involvement of presynaptic mechanism, Neuroscience 120 (2003) 535–539. [DOI] [PubMed] [Google Scholar]
- [33].Alzoubi KH, Aleisa AM, Alkadhi KA, Impairment of long-term potentiation in the CA1, but not dentate gyrus, of the hippocampus in obese Zucker rats: role of calcineurin and phosphorylated CaMKII, J. Mol. Neurosci 27 (2005) 337–346. [DOI] [PubMed] [Google Scholar]
- [34].Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC, Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice, Obesity (Silver Spring) 18 (2010) 463–469. [DOI] [PubMed] [Google Scholar]
- [35].Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP, Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice, Hippocampus 19 (2009) 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK, Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet, Brain Res 1241 (2008) 1–6. [DOI] [PubMed] [Google Scholar]
- [37].Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C, High-fat diet impairs hippocampal neurogenesis in male rats, Eur. J. Neurol 13 (2006) 1385–1388. [DOI] [PubMed] [Google Scholar]
- [38].Park HR, Park M, Choi J, Park KY, Chung HY, Lee J, A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor, Neurosci. Lett 482 (2010) 235–239. [DOI] [PubMed] [Google Scholar]
- [39].Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP, Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons, Nat. Neurosci 11 (2008) 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Laye S, Ferreira G, Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice, Hippocampus 22 (2012) 2095–2100. [DOI] [PubMed] [Google Scholar]
- [41].Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF, High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice, Neurobiol. Dis 67 (2014) 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Freeman LR, Haley-Zitlin V, Stevens C, Granholm AC, Diet-induced effects on neuronal and glial elements in the middle-aged rat hippocampus, Nutr. Neurosci 14 (2011) 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tomassoni D, Nwankwo IE, Gabrielli MG, Bhatt S, Muhammad AB, Lokhandwala MF, Tayebati SK, Amenta F, Astrogliosis in the brain of obese Zucker rat: a model of metabolic syndrome, Neurosci. Lett 543 (2013) 136–141. [DOI] [PubMed] [Google Scholar]
- [44].Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM, Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity, J. Neurosci 34 (2014) 2618–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Freeman LR, Granholm AC, Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet, J. Cereb. Blood Flow Metab 32 (2012) 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kanoski SE, Zhang Y, Zheng W, Davidson TL, The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat, J. Alzheimers Dis 21 (2010) 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W, The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats, Physiol. Behav 107 (2012) 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Paulus K, Schulz C, Lehnert H, Central nervous effects of leptin and insulin on hippocampal leptin and insulin receptor expression following a learning task in Wistar rats, Neuropsychobiology 51 (2005) 100–106. [DOI] [PubMed] [Google Scholar]
- [49].Farr SA, Banks WA, Morley JE, Effects of leptin on memory processing, Peptides 27 (2006) 1420–1425. [DOI] [PubMed] [Google Scholar]
- [50].Haleem DJ, Haque Z, Inam QU, Ikram H, Haleem MA, Behavioral, hormonal and central serotonin modulating effects of injected leptin, Peptides 74 (2015) 1–8. [DOI] [PubMed] [Google Scholar]
- [51].Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, Grill HJ, Hippocampal leptin signaling reduces food intake and modulates food-related memory processing, Neuropsychopharmacology 36 (2011) 1859–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sharma AN, Elased KM, Garrett TL, Lucot JB, Neurobehavioral deficits in db/db diabetic mice, Physiol. Behav 101 (2010) 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, Reagan LP, Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats, Behav. Brain Res 222 (2011) 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, Lu B, Lu XY, Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression, Transl. Psychiatry 2 (2012), e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY, Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine, Psychopharmacology 207 (2010) 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS, Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity, Behav. Neurosci 119 (2005) 1389–1395. [DOI] [PubMed] [Google Scholar]
- [57].Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, Ferreira G, Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhancedhippocampal inflammation in rats, Brain Behav. Immun 40 (2014) 9–17. [DOI] [PubMed] [Google Scholar]
- [58].Kanoski SE, Meisel RL, Mullins AJ, Davidson TL, The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat, Behav. Brain Res 182 (2007) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kanoski SE, Davidson TL, Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet, J. Exp. Psychol. Anim. Behav. Process 36 (2010) 313–319. [DOI] [PubMed] [Google Scholar]
- [60].Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K, Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat, J. Alzheimers Dis 14 (2008) 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Greenwood CE, Winocur G, Learning and memory impairment in rats fed a high saturated fat diet, Behav. Neural Biol 53 (1990) 74–87. [DOI] [PubMed] [Google Scholar]
- [62].Greenwood CE, Winocur G, Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake, Behav. Neurosci 110 (1996) 451–459. [DOI] [PubMed] [Google Scholar]
- [63].Grillo CA, Tamashiro KL, Piroli GG, Melhorn S, Gass JT, Newsom RJ, Reznikov LR, Smith A, Wilson SP, Sakai RR, Reagan LP, Lentivirus-mediated downregulation of hypothalamic insulin receptor expression, Physiol. Behav 92 (2007) 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang X, Zhang D, Lu XY, Dentate gyrus-CA3 glutamate release/NMDA transmission mediates behavioral despair and antidepressant-like responses to leptin, Mol. Psychiatry 20 (2015) 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, Nakao K, Impaired CNS leptin action is implicated in depression associated with obesity, Endocrinology 152 (2011) 2634–2643. [DOI] [PubMed] [Google Scholar]
- [66].Collin M, Hakansson-Ovesjo ML, Misane I, Ogren SO, Meister B, Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse, Brain Res. Mol. Brain Res 81 (2000) 51–61. [DOI] [PubMed] [Google Scholar]
- [67].Sharma S, Fulton S, Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry, Int. J. Obes 37 (2013) 382–389. [DOI] [PubMed] [Google Scholar]
- [68].Grillo CA, Mulder P, Macht VA, Kaigler KF, Wilson SP, Wilson MA, Reagan LP, Dietary restriction reverses obesity-induced anhedonia, Physiol. Behav 128 (2014) 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC, Association between obesity and psychiatric disorders in the US adult population, Arch. Gen. Psychiatry 63 (2006) 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG, Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies, Arch. Gen. Psychiatry 67 (2010) 220–229. [DOI] [PubMed] [Google Scholar]
- [71].Fabricatore AN, Wadden TA, Obesity, Annu. Rev. Clin. Psychol 2 (2006) 357–377. [DOI] [PubMed] [Google Scholar]
- [72].McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB, Are mood disorders and obesity related? A review for the mental health professional, J. Clin. Psychiatry 65 (2004) 634–651 quiz. [DOI] [PubMed] [Google Scholar]
- [73].Andersen JR, Aasprang A, Bergsholm P, Sletteskog N, Vage V, Natvig GK, Anxiety and depression in association with morbid obesity: changes with improved physical health after duodenal switch, Health Qual. Life Outcomes 8 (2010) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Stunkard AJ, Faith MS, Allison KC, Depression and obesity, Biol. Psychiatry 54 (2003) 330–337. [DOI] [PubMed] [Google Scholar]
- [75].Burgmer R, Legenbauer T, Muller A, de Zwaan M, Fischer C, Herpertz S, Psychological outcome 4 years after restrictive bariatric surgery, Obes. Surg 24 (2014) 1670–1678. [DOI] [PubMed] [Google Scholar]
- [76].Hayden MJ, Dixon JB, Dixon ME, Shea TL, O’Brien PE, Characterization of the improvement in depressive symptoms following bariatric surgery, Obes. Surg 21 (2011) 328–335. [DOI] [PubMed] [Google Scholar]
- [77].White MA, Kalarchian MA, Levine MD, Masheb RM, Marcus MD, Grilo CM, Prognostic significance of depressive symptoms on weight loss and psychosocial outcomes following gastric bypass surgery: a prospective 24-month follow-up study, Obes. Surg 25 (2015) 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Thonney B, Pataky Z, Badel S, Bobbioni-Harsch E, Golay A, The relationship between weight loss and psychosocial functioning among bariatric surgery patients, Am. J. Surg 199 (2010) 183–188. [DOI] [PubMed] [Google Scholar]
- [79].Rutledge T, Braden AL, Woods G, Herbst KL, Groesz LM, Savu M, Five-year changes in psychiatric treatment status and weight-related comorbidities following bariatric surgery in a veteran population, Obes. Surg 22 (2012) 1734–1741. [DOI] [PubMed] [Google Scholar]
- [80].Biessels GJ, Reagan LP, Hippocampal insulin resistance and cognitive dysfunction, Nat. Rev. Neurosci 16 (2015) 660–671. [DOI] [PubMed] [Google Scholar]
- [81].Fadel JR, Jolivalt CG, Reagan LP, Food for thought: the role of appetitive peptides in age-related cognitive decline, Ageing Res. Rev 12 (2013) 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Banks WA, The many lives of leptin, Peptides 25 (2004) 331–338. [DOI] [PubMed] [Google Scholar]
- [83].Levin BE, Dunn-Meynell AA, Reduced central leptin sensitivity in rats with diet-induced obesity, Am. J. Phys. Regul. Integr. Comp. Phys 283 (2002) R941–R948. [DOI] [PubMed] [Google Scholar]
- [84].Elinav E, Niv-Spector L, Katz M, Price TO, Ali M, Yacobovitz M, Solomon G, Reicher S, Lynch JL, Halpern Z, Banks WA, Gertler A, Pegylated leptin antagonist is a potent orexigenic agent: preparation and mechanism of activity, Endocrinology 150 (2009) 3083–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Macht VA, Vazquez M, Petyak CE, Grillo CA, Kaigler K, Enos RT, McClellan JL, Cranford TL, Murphy EA, Nyland JF, Solomon G, Gertler A, Wilson MA, Reagan LP, Leptin resistance elicits depressive-like behaviors in rats, Brain Behav. Immun 60 (2016) 151–160. [DOI] [PubMed] [Google Scholar]
- [86].Cryan JF, Markou A, Lucki I, Assessing antidepressant activity in rodents: recent developments and future needs, Trends Pharmacol. Sci 23 (2002) 238–245. [DOI] [PubMed] [Google Scholar]
- [87].Finn PD, Cunningham MJ, Rickard DG, Clifton DK, Steiner RA, Serotonergic neurons are targets for leptin in the monkey, J. Clin. Endocrinol. Metab 86 (2001) 422–426. [DOI] [PubMed] [Google Scholar]
- [88].Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC, Leptin regulates energy balance and motivation through action at distinct neuralcircuits, Biol. Psychiatry 69 (2010) 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ, Leptin receptor signaling in midbrain dopamine neurons regulates feeding, Neuron 51 (2006) 801–810. [DOI] [PubMed] [Google Scholar]
- [90].Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP, Hippocampal insulin resistance impairs spatial learning and synaptic plasticity, Diabetes 64 (2015) 3927–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Levin BE, Dunn-Meynell AA, Banks WA, Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset, Am. J. Phys. Regul. Integr. Comp. Phys 286 (2004) R143–R150. [DOI] [PubMed] [Google Scholar]


