Abstract
BACKGROUND
Imatinib, a selective BCR-ABL1 kinase inhibitor, improved the prognosis for patients with chronic myeloid leukemia (CML). We conducted efficacy and safety analyses on the basis of more than 10 years of follow-up in patients with CML who were treated with imatinib as initial therapy.
METHODS
In this open-label, multicenter trial with crossover design, we randomly assigned patients with newly diagnosed CML in the chronic phase to receive either imatinib or interferon alfa plus cytarabine. Long-term analyses included overall survival, response to treatment, and serious adverse events.
RESULTS
The median follow-up was 10.9 years. Given the high rate of crossover among patients who had been randomly assigned to receive interferon alfa plus cytarabine (65.6%) and the short duration of therapy before crossover in these patients (median, 0.8 years), the current analyses focused on patients who had been randomly assigned to receive imatinib. Among the patients in the imatinib group, the estimated overall survival rate at 10 years was 83.3%. Approximately half the patients (48.3%) who had been randomly assigned to imatinib completed study treatment with imatinib, and 82.8% had a complete cytogenetic response. Serious adverse events that were considered by the investigators to be related to imatinib were uncommon and most frequently occurred during the first year of treatment.
CONCLUSIONS
Almost 11 years of follow-up showed that the efficacy of imatinib persisted over time and that long-term administration of imatinib was not associated with unacceptable cumulative or late toxic effects. (Funded by Novartis Pharmaceuticals; IRIS ClinicalTrials.gov numbers, NCT00006343 and NCT00333840.)
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that is characterized by the Philadelphia (Ph) chromosome and driven by its product, the BCR-ABL1 tyrosine kinase.1 In 2001, imatinib was introduced as a BCR-ABL1 tyrosine kinase inhibitor and was approved for the treatment of CML on the basis of a high level of activity in phase 2 studies.2 Early results from the phase 3 International Randomized Study of Interferon and STI571 (IRIS) showed that imatinib at a dose of 400 mg once daily was more active and was associated with fewer side effects than interferon alfa plus cytarabine in patients with newly diagnosed CML in the chronic phase.3 At 18 months, the estimated rate of complete cytogenetic response (0% Ph-positive metaphases) was 76.2% (95% confidence interval [CI], 72.5 to 79.9) in the imatinib group, as compared with 14.5% (95% CI, 10.5 to 18.5) in the group that received interferon alfa plus cytarabine (nominal P<0.001), and the estimated rate of freedom from progression to the accelerated phase or blast crisis of CML was 96.7% versus 91.5% (nominal P<0.001).3
As a result, the majority of patients who had been randomly assigned to interferon alfa plus cytarabine crossed over to imatinib early in the trial, and the focus shifted from between-group comparisons to in-depth, exploratory analyses of outcomes with imatinib.4–6 A retrospective analysis that compared patients in the imatinib group of IRIS with those in a historical cohort of patients who had been treated with interferon alfa plus cytarabine in an earlier trial showed that imatinib therapy resulted in a higher rate of overall survival.7
This trial fundamentally changed CML treatment and led to marked improvements in prognosis for patients.8 In the United States, the annual age-adjusted mortality rate among patients with CML decreased from 0.9 deaths per 100,000 persons in 1996 to 0.4 deaths per 100,000 persons in 2006.9 Similar improvements have been observed in patients with CML in other regions of the world,10,11 and survival among patients is now considered to be driven by coexisting conditions rather than by CML.12 Here we report the final analysis of IRIS.
METHODS
TRIAL DESIGN
We have described the design of the trial previously.3 In brief, eligible patients were 18 to 70 years of age and had previously untreated (except with hydroxyurea or anagrelide), Ph-positive CML in the chronic phase that had been diagnosed within 6 months before trial entry. Patients were randomly assigned to receive imatinib (at an oral dose of 400 mg per day) or interferon alfa (administered subcutaneously at a dose of 5 million IU per square meter of body-surface area daily) plus cytarabine (administered subcutaneously for 10 days every month at a dose of 20 mg per square meter daily) (see the protocol, available with the full text of this article at NEJM.org).
Crossover was allowed for lack of response (defined as no complete hematologic response by 6 months or no major cytogenetic response by 12 months; response definitions are provided in the Methods section in the Supplementary Appendix, available at NEJM.org), disease progression (white-cell count, >20×109 per liter), loss of complete hematologic response or major cytogenetic response, unacceptable side effects, or reluctance to continue taking interferon alfa plus cytarabine after the trial results were released. After 7 years, the trial was extended for imatinib only. Patients in the group that received interferon alfa plus cytarabine were eligible to continue in the trial if they crossed over to imatinib.
END POINTS
The initial primary end point was event-free survival (defined as survival without progression to accelerated phase or blast crisis, loss of complete hematologic response, loss of major cytogenetic response, or death from any cause during treatment),3 and the long-term primary end point was overall survival in the imatinib group. Secondary end points included response rates, time to response, disease progression, safety, and side-effect profile. Assessments of the patients’ overall prognosis were made with the use of the scoring system devised by Sokal et al.13 The Sokal score is based on age, spleen size, peripheral-blood platelet count, and blast count. A Sokal score of less than 0.8 indicates low risk, a score of 0.8 to 1.2 intermediate risk, and a score of more than 1.2 high risk. Additional information about the end points and assessments is provided in the Methods section in the Supplementary Appendix.
TRIAL OVERSIGHT
All the patients provided written informed consent. The trial was approved by the institutional review board at each participating institution and was conducted in accordance with the provisions of the Declaration of Helsinki. All the authors analyzed the trial data, were involved in the development of the manuscript, and had responsibility for the decision to submit the manuscript for publication. The trial sponsor (Novartis Pharmaceuticals), in collaboration with the trial investigators, participated in the design of the trial and analyzed the data. The sponsor and the authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol. Financial support for medical editorial assistance was provided by the sponsor.
STATISTICAL ANALYSIS
Because of the high rate of crossover and the subsequent closing of the group of patients receiving interferon alfa plus cytarabine, most long-term analyses (including safety, response rates, and landmark analyses) included only patients who had been randomly assigned to imatinib. Cytogenetic and molecular response rates in the imatinib group at each time point were calculated in the intention-to-treat population and among the patients who could be evaluated (i.e., patients with cytogenetic or quantitative reverse transcrip-tase–polymerase chain reaction [RT-PCR] assessments that could be evaluated) at the indicated time point.
Exploratory molecular monitoring of BCR-ABL1 transcript levels in peripheral blood used quantitative RT-PCR assessments that were performed at reference laboratories as described in the Methods section in the Supplementary Appendix. This analysis formed the basis for the development of the International Scale: major molecular response (now represented by a BCR-ABL1 level of ≤0.1% on the International Scale) was defined as a reduction of at least 3 log in the BCR-ABL1 value from the standardized baseline level on the International Scale, and molecular response 4.5 was defined as an undetectable level of BCR-ABL1 in a sample tested with the sensitivity to detect a reduction of at least 4.5 log from the standardized baseline value that was confirmed in a second assessment or a BCR-ABL1 value of 0.0032% or less on the International Scale with at least 32,000 copies of ABL1 or at least 400,000 copies of BCR; these definitions are consistent with the definitions proposed by the European Leukemia-Net14 and with those used in other studies.15,16
Serious adverse events and reasons for the discontinuation of study treatment were documented throughout follow-up. Information about other adverse events, biochemical evaluations, and concomitant medication use was not collected after 5.5 years.
RESULTS
ENROLLMENT AND FOLLOW-UP OF THE PATIENTS
We enrolled 1106 patients at 177 centers in 16 countries; 553 patients were assigned to each group. The first patient was enrolled in June 2000, and the last visit of the last patient was in January 2012. The characteristics of the patients at baseline were reported previously and were similar between groups.3 The median duration of follow-up was 10.9 years (range, 0 to 11.7, including follow-up after the discontinuation of study treatment).
Among patients who had been randomly assigned to imatinib, 267 (48.3%) completed treatment with the assigned study drug; 7 patients (1.3%) who had been randomly assigned to interferon alfa plus cytarabine completed treatment with that regimen (Fig. 1 and Table 1, and Table S1 in the Supplementary Appendix). Among the patients who had been randomly assigned to interferon alfa plus cytarabine, 363 (65.6%) crossed over to imatinib because of disease progression or lack or loss of response (31.5%), unacceptable side effects (26.2%), or reluctance to continue taking interferon alfa plus cytarabine (8.0%). The median duration of first-line therapy with interferon alfa plus cytarabine before crossover was 0.8 years (range, <0.1 to 8.0). By contrast, 15.9% of the patients in the imatinib group discontinued the study treatment because of an unsatisfactory therapeutic effect, and 6.9% discontinued because of adverse events (Table S2 in the Supplementary Appendix). The median duration of first-line imatinib therapy was 8.9 years (range, <0.1 to 11.7).
Figure 1.
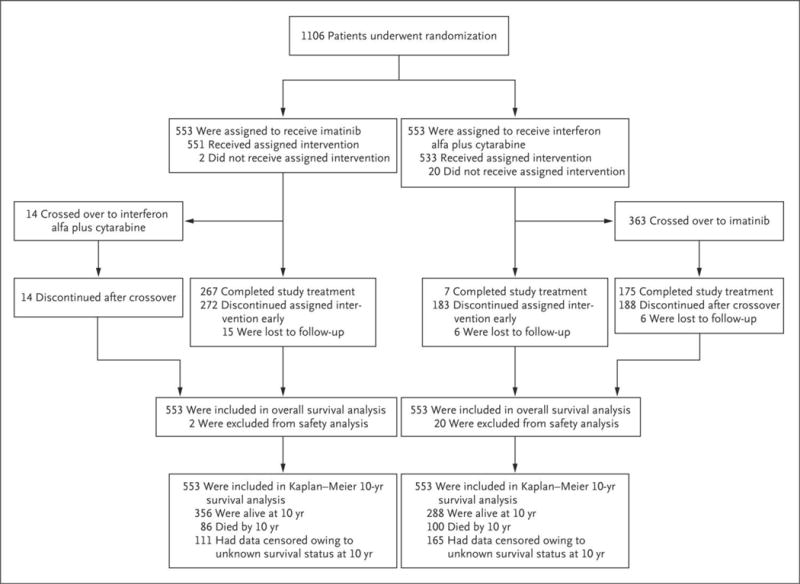
Enrollment and Randomization of Patients and Censoring of Data for the Analysis of Overall Survival at 10 Years.
Table 1.
Characteristics of the Patients, Trial Completion, Discontinuations, Crossovers, and Study-Drug Exposure in the Imatinib Group.*
| Variable | All Patients (N = 553) |
|---|---|
| Median age at baseline (range) — yr | 50 (18-70) |
| Male sex — no. (%) | 341 (61.7) |
| Median duration since diagnosis (range) — mo | 2.1 (0-10.4) |
| Geographic region — no. (%)† | |
| Europe | 277 (50.1) |
| North America | 245 (44.3) |
| Oceania | 31 (5.6) |
| Completed study treatment — no. (%) | 267 (48.3) |
| Up to end of the core trial in 2006 | 11 (2.0) |
| Up to 2007-2008 | 13 (2.4) |
| Up to closure of the trial in 2011-2012 | 243 (43.9) |
| Discontinued treatment — no. (%) | 272 (49.2) |
| Unsatisfactory therapeutic effect | 88 (15.9) |
| Withdrawal of consent | 57 (10.3) |
| Adverse events | 38 (6.9) |
| No longer required study drug owing to bone marrow transplant | 21 (3.8) |
| Death | 19 (3.4) |
| Protocol violation | 17 (3.1) |
| Loss to follow-up | 15 (2.7) |
| Administrative problems | 12 (2.2) |
| Abnormal laboratory values | 3 (0.5) |
| Abnormal procedure | 2 (0.4) |
| Crossed over to interferon alfa plus cytarabine — no. (%) | 14 (2.5) |
| Imatinib exposure during the trial‡ | |
| Duration of exposure — yr | |
| Mean | 7.5±4.0 |
| Median (range) | 8.9 (<0.1–11.7) |
| Duration of total exposure — patient-yr | 4129 |
| Median actual-dose intensity (range) — mg/day | 400 (114-770) |
Plus–minus values are means ±SD. Trial completion, discontinuation, and crossover were evaluated in the intention-to-treat population (553 patients).
The geographic region of Europe included Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Norway, Spain, Sweden, Switzerland, and the United Kingdom; North America included Canada and the United States; and Oceania included Australia and New Zealand.
Imatinib exposure during the trial was evaluated in the safety population (551 patients).
SAFETY
A total of 51 of 551 patients (9.3%) receiving first-line therapy with imatinib had a serious adverse event (most frequently abdominal pain, in 4 patients [0.7%]) that was considered by the investigators to be related to the study drug. The frequency of such events was highest during the first year of treatment and declined over time. Cardiac serious adverse events of any cause were reported in 39 patients (7.1%), and serious adverse events of a second neoplasm (benign or malignant) were reported in 62 (11.3%). No new safety signals were observed since the 5-year analysis.4 (Details are provided in Tables S3 through S6 in the Supplementary Appendix.)
PROGRESSION AND SURVIVAL
In the imatinib group in the intention-to-treat population, 38 of 553 patients (6.9%) had progression to the accelerated phase or blast crisis17 during study treatment, and the estimated rate of freedom from progression to the accelerated phase or blast crisis at 10 years was 92.1% (95% CI, 89.6 to 94.5). A total of 71 patients (12.8%) in the group that received interferon alfa plus cytarabine had progression to the accelerated phase or blast crisis during study treatment. In the two trial groups, most events of disease progression (in 34 of 38 patients in the imatinib group and in 64 of 71 in the group that received interferon alfa plus cytarabine) occurred during the first 4 years of study treatment. The estimated rate of event-free survival at 10 years was 79.6% (95% CI, 75.9 to 83.2) among the patients randomly assigned to imatinib, as compared with 56.6% (95% CI, 51.5 to 61.6) among those assigned to interferon alfa plus cytarabine (Table S7 in the Supplementary Appendix).
The estimated overall survival rate at 10 years among patients receiving first-line imatinib treatment was 83.3% (95% CI, 80.1 to 86.6) (Fig. 2). A total of 260 patients (47.0%) were alive and still receiving study treatment at 10 years, 96 patients (17.4%) were alive and not receiving study treatment, 86 known deaths (15.6% of patients) had occurred, and 111 patients (20.1%) had unknown survival status (the age distribution and other characteristics at baseline were generally similar between patients with known survival status and those with unknown survival status) (Table S8 in the Supplementary Appendix). After we accounted for patients with unknown survival status, the estimated survival rates at 10 years ranged from 64.4% (assuming all 111 patients had died on the date of their last known followup) to 84.4% (assuming all 111 were alive).
Figure 2. Kaplan–Meier Estimated Overall Survival Rates at 10 Years in the Intention-to-Treat Population.

Shown is the overall survival over time among patients assigned to each trial group. For the curve for the group of patients who had been randomly assigned to receive interferon alfa plus cytarabine, data include survival among the 363 patients who crossed over to imatinib (65.6%). These patients crossed over to imatinib after a median of 0.8 years of receiving interferon alfa plus cytarabine. In patients with no reported death (whether because they were known to be alive or because their survival status was unknown), survival was censored (tick marks) at the date of last contact.
Patients with a high Sokal score had a worse estimated overall survival rate at 10 years (68.6%) than those with an intermediate score (80.3%) or a low score (89.9%), in an analysis that was not adjusted for patients with unknown survival status (Table S9 in the Supplementary Appendix). Overall, by the end of the trial, 89 patients (16.1%) in the imatinib group were known to have died from any cause; 37 of the deaths were related to CML in patients who had not undergone hematopoietic stem-cell transplantation (16 of these 37 deaths occurred in year 5 or later) (Table 2).
Table 2.
Deaths during the Trial among Patients Randomly Assigned to Imatinib.
| Variable | Patients (N = 553) |
|---|---|
| no. (%) | |
| Total deaths | 89 (16.1) |
|
| |
| Cause of death | |
|
| |
| Chronic myeloid leukemia | |
|
| |
| Without hematopoietic stem-cell transplantation | 37 (6.7) |
|
| |
| After hematopoietic stem-cell transplantation | 13 (2.4) |
|
| |
| Secondary malignant condition* | 11 (2.0) |
|
| |
| Cardiac disorder or cardiovascular disease | 7 (13) |
|
| |
| Infectious disease | 5 (0.9) |
|
| |
| Other | 16 (2.9) |
Reported causes of death due to secondary malignant condition were metastases to the liver (in two patients) and BCR-ABL1–negative acute myelogenous leukemia, bronchial carcinoma, esophageal carcinoma, lung cancer, prostate cancer, rectal cancer, renal-cell carcinoma, sarcoma, and transitionalcell carcinoma (in one patient each).
In the group that received interferon alfa plus cytarabine in the intention-to-treat population, 105 patients (19.0%) were known to have died from any cause by the end of the trial; 48 deaths were related to CML in patients who had not undergone hematopoietic stem-cell transplantation. A total of 165 patients (29.8%) had an unknown survival status at 10 years. The high rate of crossover precluded a direct comparison of overall survival between the imatinib group and the group that received interferon alfa plus cytarabine. However, a hazard ratio of 0.74 (95% CI, 0.56 to 0.99) indicated a 26% lower risk of death with first-line imatinib therapy than with interferon alfa plus cytarabine (nominal P = 0.04 by the log-rank test).
CYTOGENETIC AND MOLECULAR RESPONSES
In the imatinib group, the cumulative rate of major cytogenetic response at the end of the trial was 89.0%, and the rate of complete cyto-genetic response at the end of the trial was 82.8% (Table S10 in the Supplementary Appendix). The median time to response was 3.0 months (interquartile range, 2.9 to 5.7) among patients who had a major cytogenetic response and 5.8 months (interquartile range, 3.0 to 11.1) among those who had a complete cytogenetic response. An estimated 49.1% of all the patients in the imatinib group had a complete cytogenetic response at 6 months. A total of 66 of 416 patients (15.9%) who had had a confirmed complete cytogenetic response at any time during the trial no longer had that response by the time of data cutoff, including 9 patients who had progression to the accelerated phase or blast crisis.
Among 134 patients with cytogenetic assessments at 10 years, 123 (91.8%) had a complete cytogenetic response, whereas 11 did not (a lack of response was confirmed by means of molecular assessments in 4 of these 11 patients) (Table 3, and Fig. S1 in the Supplementary Appendix). Among 204 patients who had molecular assessments that could be evaluated at 10 years, 190 (93.1%) had a major molecular response and 129 (63.2%) had molecular response 4.5. Some of these patients may have been eligible for studies investigating treatment-free remission, but this was not prospectively investigated within the present trial.
Table 3.
Patients Treated with First-Line Imatinib Therapy Who Had a Complete Cytogenetic Response, Major Molecular Response, or Molecular Response 4.5 at the Indicated Time Points.*
| Time Point | Complete Cytogenetic Response | Major Molecular Response | Molecular Response 4.5 | |||
|---|---|---|---|---|---|---|
| Patients Who Could Be Evaluated | Intention-to-Treat Population (N = 553) | Patients Who Could Be Evaluated | Intention-to-Treat Population (N = 553) | Patients Who Could Be Evaluated | Intention-to-Treat Population (N = 553) | |
| no./total no. (%) | % | no./total no. (%) | % | no./total no. (%) | % | |
| Baseline | 2/523 (0.4) | 0.4 | 1/153 (0.7) | 0.2 | 0/153 | 0 |
|
| ||||||
| 1 yr | 292/412 (70.9) | 52.8 | 153/305 (50.2) | 27.7 | 4/305 (1.3) | 0.7 |
|
| ||||||
| 4 yr | 315/348 (90.5) | 57.0 | 235/305 (77.0) | 42.5 | 50/305 (16.4) | 9.0 |
|
| ||||||
| 5 yr | 276/302 (91.4) | 49.9 | 278/316 (88.0) | 50.3 | 127/316 (40.2) | 23.0 |
|
| ||||||
| 6 yr | 244/257 (94.9) | 44.1 | 257/292 (88.0) | 46.5 | 122/292 (41.8) | 22.1 |
|
| ||||||
| 7 yr | 207/225 (92.0) | 37.4 | 227/247 (91.9) | 41.0 | 86/247 (34.8) | 15.6 |
|
| ||||||
| 8 yr | 150/161 (93.2) | 27.1 | 211/228 (92.5) | 38.2 | 77/228 (33.8) | 13.9 |
|
| ||||||
| 9 yr | 72/73 (98.6) | 13.0 | 218/233 (93.6) | 39.4 | 128/233 (54.9) | 23.1 |
|
| ||||||
| 10 yr | 123/134 (91.8) | 22.2 | 190/204 (93.1) | 34.4 | 129/204 (63.2) | 23.3 |
A complete cytogenetic response was defined as 0% Philadelphia (Ph) chromosome–positive cells in metaphase. Major molecular response was defined as a reduction of at least 3 log in the BCR-ABL1 value from the standardized baseline level on the International Scale, and molecular response 4.5 as an undetectable level of BCR-ABL1 in a sample tested with the sensitivity to detect a reduction of at least 4.5 log from the standardized baseline level that was confirmed in a second assessment or a BCR-ABL1 value of 0.0032% or less on the International Scale with at least 32,000 copies of ABL1 or at least 400,000 copies of BCR. Owing to protocol amendments and for practical reasons, fewer (and to some extent different) patients were analyzed for cytogenetic response at 8, 9, and 10 years than were analyzed for molecular response. Thus for these time points, the percentage of patients in the intention-to-treat population who had a complete cytogenetic response cannot be compared with that of patients who had a major molecular response or molecular response 4.5.
Among the 304 patients taking first-line imatinib who could be evaluated for a molecular response at 12 months, the estimated overall survival rate at 10 years was 91.1% among those with a major molecular response, as compared with 85.3% among those without a major molecular response at 12 months (Table 4). In an analysis that included only CML-related deaths, the estimated survival rate at 10 years was 97.8% among patients with a major molecular response, as compared with 89.4% among those without a major molecular response. Similar trends were observed in the comparison of survival rates at 10 years among patients with a major molecular response at 18 months and those without such a response (overall survival rate, 93.0% vs. 85.6%; rate of freedom from CML-related death, 100% vs. 90.5%).
Table 4.
Landmark Analysis of Outcomes at 10 Years According to Molecular Response Levels at 12 Months and 18 Months in Patients Treated with First-Line Imatinib Therapy Who Could Be Evaluated.*
| Variable | Major Molecular Response or Better | Lack of Major Molecular Response | P Value |
|---|---|---|---|
| At 12 mo | |||
| No. of patients who could be evaluated | 153 | 151 | |
| Death — no. (%) | 15 (9.8) | 22 (14.6) | |
| Not related to CML | 11 (7.2) | 7 (4.6) | |
| Related to CML | 4 (2.6) | 15 (9.9) | |
| Estimated 10-yr overall survival — % (95% CI) | 91.1 (86.5-95.7) | 85.3 (79.5-91.1) | 0.15 |
| Estimated 10-yr freedom from CML-related death — % (95% CI) | 97.8 (95.4-100) | 89.4 (84.3-94.5) | 0.007 |
| At 18 mo | |||
| No. of patients who could be evaluated | 164 | 89 | |
| Death — no. (%) | 12 (7.3) | 13 (14.6) | |
| Not related to CML | 12 (7.3) | 4 (4.5) | |
| Related to CML | 0 | 9 (10.1) | |
| Estimated 10-yr overall survival — % (95% CI) | 93.0 (89.0-97.0) | 85.6 (77.9-93.2) | 0.04 |
| Estimated 10-yr freedom from CML-related death — % (95% CI) | 100 (100-100) | 90.5 (84.1-96.8) | <0.001 |
A total of 305 patients were considered able to be evaluated for molecular response at 12 months; however, 1 patient discontinued study treatment at 11 months (the patient was considered able to be evaluated for molecular response at 12 months on the basis of an 11-month assessment) and was therefore excluded from the 12-month landmark analysis. Patients who died or who had data censored before each landmark analysis were excluded from that landmark analysis. The deaths reported here are those that occurred in patients with the indicated level of molecular response at 12 months or 18 months who died at some point after 12 months or 18 months, respectively. Two-sided P values were calculated with the use of the log-rank test. CML denotes chronic myeloid leukemia.
DISCUSSION
With more than 10 years of follow-up in IRIS, the long-term outcomes in imatinib-treated patients that we describe here confirm and extend earlier findings. No new safety signals and few drug-related serious adverse events were observed during the later years of follow-up, and molecular and cytogenetic response rates were high among the patients who could be evaluated. The estimated overall survival rate at 10 years with first-line imatinib therapy was 83.3%, which is similar to the rate (84%) reported among patients who were treated with imatinib-based regimens in the CML-IV study, which was initiated shortly after IRIS to further evaluate alternative dosing strategies and drug combinations in patients with newly diagnosed CML in the chronic phase.18
There are several caveats to these long-term data, including the large number of patients who had an unknown survival status (approximately 20% of the patients in the imatinib group) or who did not have molecular or cytogenetic assessments that could be evaluated (approximately half the patients who completed the trial while taking imatinib had cytogenetic assessments at 10 years that could be evaluated) and the limited collection of long-term safety information. Nonetheless, these results highlight the safety and efficacy of imatinib therapy, with a clear improvement over the outcomes that were expected in patients who received a diagnosis of CML before the introduction of tyrosine kinase inhibitor therapy, when interferon alfa and hematopoietic stem-cell transplantation were the standard therapies.19,20
The ability of imatinib to reduce rates of disease progression and CML-related death (and the resulting increase in the rate of overall survival) has made it a model for targeted cancer therapy.21 Although high rates of response have now also been observed with targeted therapies in patients who have other cancers with well-characterized molecular abnormalities, including BRAF-driven melanoma and epidermal growth factor receptor (EGFR)–mutated lung cancer, the durability of responses observed with targeted therapies for these cancers is much less impressive (and similar to the results with imatinib in patients with CML in blast crisis).21 These findings are likely to be attributable to CML in the chronic phase being driven solely by BCR-ABL1, whereas solid tumors and the advanced phases of CML may be driven by multiple pathways and complex genomic abnormalities,21 which further underscores the importance of initiating treatment early in the course of the disease.
While the IRIS trial was under way, new recommendations for CML treatment22 and new BCR-ABL1 inhibitors23–26 were developed. Each of the newer agents has a distinct safety and efficacy profile,23–26 and two (nilotinib and dasatinib) have been approved as first-line therapies in patients with CML in the chronic phase on the basis of results from phase 3 trials in which they were associated with higher response rates than imatinib (although a higher dose of imatinib may also increase the rates of response).15,16,23,24,27 Furthermore, nilotinib resulted in lower rates of progression to the accelerated phase and blast crisis and CML-related death than imatinib.16 However, despite the better early control of disease observed with second-generation tyrosine kinase inhibitors than with imatinib, it remains to be seen whether they will have similarly favorable long-term safety. Given the long-term safety and efficacy results with imatinib and the increasing availability of generic imatinib, comparative analyses evaluating the available tyrosine kinase inhibitors for first-line therapy are likely to be forthcoming.
As experience with imatinib accrued throughout the course of this trial, the treatment of patients who were taking a tyrosine kinase inhibitor improved over time. For example, after issues related to poor adherence to the oral-drug regimens became apparent (more so in routine clinical practice than among patients enrolled in clinical trials), physicians learned to conduct more specific consultations with patients to ensure that they were taking their medication as prescribed.
Survival rates in our trial were especially high in certain subgroups of patients, including those who had a major molecular response at 12 months or 18 months and those with low Sokal scores. These results are consistent with previous reports from IRIS and other studies showing that early responses to tyrosine kinase inhibitor therapy are valuable prognostic markers for long-term outcomes and that patients with low risk scores (according to Sokal,13 Hasford,28 or European Treatment and Outcome Study29 scores) at baseline typically have high rates of complete cytogenetic response and overall survival while taking any of the available tyrosine kinase inhibitors.4,6,16,27,30,31 The risk profile of our trial cohort, according to Sokal score, was similar to that in other studies.23,32
Imatinib treatment has also allowed for the successful stopping of therapy (for >5 years) in small subgroups of patients with a sustained deep molecular response (molecular response 4 [defined as a reduction of 4 log in the BCR-ABL1 value from the standardized baseline level on the International Scale], molecular response 4.5, or undetectable BCR-ABL1 transcripts), which promotes treatment-free remission as a treatment goal.33 Approximately 39 to 45% of patients who attempt treatment-free remission after having a durable deep molecular response with imatinib therapy can remain in remission for 3 years or longer.33 The eligibility rate for an attempt at treatment-free remission has been estimated to be 21.6% after 6 years of imatinib therapy (on the basis of maintenance of a stable deep molecular response for ≥12 months).34 Thus, the total percentage of imatinib-treated patients who may have a stable treatment-free remission is approximately 10%. Because a greater percentage of patients have deep molecular responses with second-generation tyrosine kinase inhibitors than with imatinib across all risk strata, second-generation agents may enable a larger proportion of patients in all Sokal-score risk groups to be eligible to attempt treatment-free remission.16,27
Although long-term survival data were incomplete, the observation that many of the reported deaths in the imatinib group were unrelated to CML is in line with published data showing that patients who have a response to imatinib have a survival rate that is equivalent to that in the general population and are unlikely to die from CML.35 This trend also highlights the importance of monitoring and managing coexisting conditions in patients with CML in the chronic phase. As the prognosis that is associated with CML in the chronic phase improves and as patients can be expected to live for years or decades, an increasing number of patients will be at risk for death from general health conditions or coexisting conditions rather than from CML.31,35 In particular, of the few deaths reported in the low-risk Sokal-score group, most were unrelated to CML (and were likely to be related to the coexisting-condition profile that is typical of this age group12). In contrast, patients with a high Sokal score typically died from CML. As is consistent with a previous analysis of the outcomes in patients who crossed over from interferon alfa plus cytarabine to imatinib,36 the long-term overall survival rate in the group that received interferon alfa plus cytarabine was high, which suggests that second-line imatinib was an effective therapy in many of these patients.
Several new questions have arisen, such as the relative benefits and risks of imatinib versus newer tyrosine kinase inhibitors and the role and effect of second-line inhibitor therapy. Approximately half the patients in the imatinib group discontinued the trial early, which suggests that the high rate of overall survival in the imatinib group must be attributed to the use of commercially available imatinib or effective second-line therapies in these patients.25,26,37,38 Despite these unanswered questions, the long-term results presented here highlight the clinical benefits observed in patients with CML over the past 15 years.
Supplementary Material
Acknowledgments
Supported by Novartis Pharmaceuticals.
Dr. Hochhaus reports receiving fees for serving on advisory boards and travel support from Novartis Pharmaceuticals, Bristol-Myers Squibb, Pfizer, and Ariad Pharmaceuticals and grant support from Novartis Pharmaceuticals, Bristol-Myers Squibb, Pfizer, Ariad Pharmaceuticals, and Merck Sharp & Dohme; Dr. Larson, receiving consulting fees from Novartis Pharmaceuticals; Dr. Guilhot, receiving fees for serving on advisory boards from Novartis Pharmaceuticals and Celgene and lecture fees from Novartis Pharmaceuticals; Dr. Radich, receiving fees for serving on advisory boards from Novartis Pharmaceuticals, Bristol-Myers Squibb, and Ariad Pharmaceuticals; Dr. Branford, receiving travel support from Bristol-Myers Squibb and grant support from Bristol-Myers Squibb and Ariad Pharmaceuticals; Dr. Hughes, receiving lecture fees and fees for serving on advisory boards from Novartis Pharmaceuticals, Bristol-Myers Squibb, and Ariad Pharmaceuticals; Dr. Baccarani, receiving lecture fees and fees for serving on an advisory board from Novartis Pharmaceuticals; Dr. Deininger, receiving fees for serving on advisory boards from Ariad Pharmaceuticals, CTI BioPharma, Incyte, Novartis Pharmaceuticals, and Pfizer, consulting fees from Ariad Pharmaceuticals, Incyte, Novartis Pharmaceuticals, and Pfizer, and grant support from Bristol-Myers Squibb, Celgene, Gilead Sciences, Novartis Pharmaceuticals, and Pfizer; Dr. Cervantes, receiving fees for serving on advisory boards from Novartis Pharmaceuticals and Pfizer and lecture fees from Novartis Pharmaceuticals, Bristol-Myers Squibb, Ariad Pharmaceuticals, and Pfizer; Dr. Fujihara, Ms. Ortmann, and Dr. Menssen, being employees of Novartis Pharmaceuticals; Dr. Kantarjian, receiving grant support from Amgen, Pfizer, Bristol-Myers Squibb, Novartis Pharmaceuticals, and Ariad Pharmaceuticals; and Dr. Druker, being scientific founder of and receiving consulting fees and stock options from MolecularMD, serving as an investigator in trials funded by Bristol-Myers Squibb and Ariad Pharmaceuticals, and receiving royalties from a patent related to the treatment of gastrointestinal stromal tumors (U.S. patent number, 6958335 B2, licensed to Novartis Pharmaceuticals).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank John Goldman, D.M., now deceased, for contributions to the trial and advice during the early stages of manuscript development; Josy Reiffers, M.D., now deceased, for contributions to the trial; and Karen Kaluza, Ph.D., and Staci Heise, Ph.D., of ArticulateScience, for medical editing assistance with an earlier version of the manuscript, funded by Novartis Pharmaceuticals.
APPENDIX
The authors’ affiliations are as follows: Abteilung Hämatologie–Onkologie, Universitätsklinikum Jena, Jena, Germany (A.H.); the Department of Medicine, University of Chicago, Chicago (R.A.L.); INSERM Centre d’Investigation Clinique 1402, Centre Hospitalier Universitaire de Poitiers, Poitiers, France (F.G.); Fred Hutchinson Cancer Research Center, Seattle (J.P.R.); Centre for Cancer Biology, SA Pathology, University of South Australia and University of Adelaide (S.B.), and the South Australian Health and Medical Research Institute and University of Adelaide (T.P.H.), Adelaide, SA, Australia; University of Bologna, Bologna, Italy (M.B.); the University of Utah Huntsman Cancer Institute, Salt Lake City (M.W.D.); the Hematology Department, Hospital Clínic de Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona, Barcelona (F.C.); Novartis, Basel, Switzerland (S.F., C.-E.O., H.D.M.); M.D. Anderson Cancer Center, Houston (H.K.); the University of Newcastle, Newcastle, United Kingdom (S.G.O.); and Knight Cancer Institute, Oregon Health and Science University and Howard Hughes Medical Institute, Portland (B.J.D.).
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Rumpold H, Webersinke G. Molecular pathogenesis of Philadelphia-positive chronic myeloid leukemia — is it all BCR-ABL? Curr Cancer Drug Targets. 2011;11:3–19. doi: 10.2174/156800911793743619. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MH, Williams G, Johnson JR, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935–42. [PubMed] [Google Scholar]
- 3.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–61. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 6.Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116:3758–65. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials. Blood. 2006;108:1478–84. doi: 10.1182/blood-2006-02-001495. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119:1981–7. doi: 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fast Stats: an interactive tool for access to SEER cancer statistics. Silver Spring, MD: Surveillance, Epidemiology, and End Results Program; Sep 12, 2016. http://seer.cancer.gov/faststats. [Google Scholar]
- 10.Chihara D, Ito H, Matsuda T, et al. Decreasing trend in mortality of chronic myelogenous leukemia patients after introduction of imatinib in Japan and the U.S. Oncologist. 2012;17:1547–50. doi: 10.1634/theoncologist.2012-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 12.Saussele S, Krauss MP, Hehlmann R, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML Study IV. Blood. 2015;126:42–9. doi: 10.1182/blood-2015-01-617993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–99. [PubMed] [Google Scholar]
- 14.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hehlmann R, Müller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-Study IV. J Clin Oncol. 2014;32:415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 16.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilhot J, Baccarani M, Clark RE, et al. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: a proposal by the European LeukemiaNet. Blood. 2012;119:5963–71. doi: 10.1182/blood-2011-10-383711. [DOI] [PubMed] [Google Scholar]
- 18.Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-Study IV. Leukemia. 2015;29:1123–32. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 19.Hehlmann R, Berger U, Pfirrmann M, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109:4686–92. doi: 10.1182/blood-2006-11-055186. [DOI] [PubMed] [Google Scholar]
- 20.Gratwohl A, Pfirrmann M, Zander A, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016;30:562–9. doi: 10.1038/leu.2015.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westin JR, Kurzrock R. It’s about time: lessons for solid tumors from chronic myelogenous leukemia therapy. Mol Cancer Ther. 2012;11:2549–55. doi: 10.1158/1535-7163.MCT-12-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 23.Saglio G, Kim D-W, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 25.Gambacorti-Passerini C, Brümmen-dorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: minimum 24-month follow-up. Am J Hematol. 2014;89:732–42. doi: 10.1002/ajh.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. J Natl Cancer Inst. 1998;90:850–8. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 29.Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–92. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 30.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 31.Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56. doi: 10.1038/leu.2015.261. [DOI] [PubMed] [Google Scholar]
- 32.Hochhaus A, Rosti G, Cross NC, et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia. 2016;30:57–64. doi: 10.1038/leu.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638–47. doi: 10.1038/leu.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochhaus A, Saglio G, Hughes TP, et al. Impact of treatment with frontline nilotinib (NIL) vs imatinib (IM) on sustained deep molecular response (MR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) Blood. 2015;126:2781. abstract. [Google Scholar]
- 35.Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103:553–61. doi: 10.1093/jnci/djr060. [DOI] [PubMed] [Google Scholar]
- 36.Guilhot F, Druker B, Larson RA, et al. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009;94:1669–75. doi: 10.3324/haematol.2009.010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107–12. doi: 10.1038/leu.2012.181. [DOI] [PubMed] [Google Scholar]
- 38.Shah NP, Guilhot F, Cortes JE, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014;123:2317–24. doi: 10.1182/blood-2013-10-532341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


