Visual Abstract

Key Words: alpha adrenergic receptors, anthracyclines, cardioprotection, catecholamines, heart failure
Abbreviations and Acronyms: α1-AR, alpha-1 adrenergic receptor; AKO, alpha-1A adrenergic receptor knockout; ATP, adenosine triphosphate; BP, blood pressure; DOX, doxorubicin; EC50, half-maximal effective concentration; ERK, extracellular signal-regulated kinase; HF, heart failure; HR, heart rate; IP, intraperitoneal; NRVM, neonatal rat ventricular myocyte; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; qRT-PCR, quantitative reverse transcription polymerase chain reaction; TBARS, thiobarbituric acid reactive substances; WT, wild type
Highlights
-
•
There are 2 α1-ARs on cardiac myocytes: α1A and α1B. α1A adrenergic receptors serve important cardioprotective roles and do not mediate cardiac hypertrophy.
-
•
Dabuzalgron, an oral α1A-AR agonist developed for the treatment of urinary incontinence and tolerated well in Phase 2 clinical trials, protects against doxorubicin-induced cardiotoxicity in vivo. Dabuzalgron enhances contractile function, regulates transcription of genes related to energy production and mitochondrial function, and preserves myocardial ATP content after doxorubicin.
-
•
Activation of α1A-ARs on cardiomyocytes protects against doxorubicin cytotoxicity and enhances mitochondrial function in vitro. These cytoprotective effects likely are mediated by activation of ERK 1/2.
-
•
Future studies will explore whether dabuzalgron, a well-tolerated oral α1A-AR agonist, might be repurposed to treat heart failure.
Summary
Alpha-1 adrenergic receptors (α1-ARs) play adaptive and protective roles in the heart. Dabuzalgron is an oral selective α1A-AR agonist that was well tolerated in multiple clinical trials of treatment for urinary incontinence, but has never been used to treat heart disease in humans or animal models. In this study, the authors administered dabuzalgron to mice treated with doxorubicin (DOX), a widely used chemotherapeutic agent with dose-limiting cardiotoxicity that can lead to heart failure (HF). Dabuzalgron protected against DOX-induced cardiotoxicity, likely by preserving mitochondrial function. These results suggest that activating cardiac α1A-ARs with dabuzalgron, a well-tolerated oral agent, might represent a novel approach to treating HF.
Evidence from studies in cells and animals indicates that alpha-1 adrenergic receptors (α1-ARs) play numerous protective roles in the heart (reviewed in O’Connell et al. [1]). There are 3 α1-AR subtypes: α1A, α1B, and α1D. In rodent and human myocardium, the α1A and α1B predominate, and there is no measurable α1D. The α1D is the major α1-AR subtype in human and mouse coronary arteries, where its activation promotes vasoconstriction 2, 3. The role of the myocardial α1B remains unclear, but multiple lines of evidence suggest that the cardioprotective effects of nonselective α1-AR agonists are mediated by the α1A. Mice overexpressing the α1A have increased contractility (4) and are protected from ischemia-reperfusion injury (5), myocardial infarction 6, 7, and transverse aortic constriction (8). Abrogation of these adaptive processes may also account for the 2-fold increase in incident heart failure (HF) in hypertensive patients treated with the non-selective α1-AR antagonist, doxazosin, in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) (9). These findings and other evidence from animal and human studies suggest that activating myocardial α1A-ARs could be therapeutically effective in HF.
In this study, we used the oral selective α1A agonist dabuzalgron (Ro 115-1240) to test our hypothesis that stimulation of myocardial α1As could confer cardioprotection without increasing blood pressure (BP) through vascular α1-AR activation. Roche developed dabuzalgron for the treatment of urinary incontinence. It showed excellent α1A selectivity in preclinical testing (10) and was well tolerated by a total of 1,223 women in a Phase 1 trial (11); 2 Phase 2 randomized multicenter trials (Roche NN16378 and NN16691); and a subsequent open-label study (Roche NN16586). Importantly, there were no significant changes in BP in the subjects who received dabuzalgron in any of these trials, suggesting that the chosen dose did not affect vascular tone. When interim analysis of the Phase 2 trials revealed no clinically meaningful difference in urinary incontinence between the dabuzalgron and placebo groups, Roche decided to close trial enrollment and halt further development of dabuzalgron. The drug never has been used either clinically or experimentally to treat heart disease.
We chose to test the therapeutic efficacy of dabuzalgron in preventing heart injury using an anthracycline cardiotoxicity model, given previous evidence demonstrating α1A-mediated cytoprotection after doxorubicin (DOX) treatment 12, 13, 14. Anthracyclines, including DOX, are highly effective and commonly used chemotherapeutic agents, but have dose-limiting cardiotoxicity. Although the incidence of anthracycline-induced cardiomyopathy has declined with contemporary dosing regimens, left ventricular dysfunction still occurs in 20% to 30% of anthracycline recipients 15, 16 and remains an important cause of systolic HF. Numerous mechanisms contribute to cardiomyocyte injury after anthracycline administration, but mitochondrial dysfunction and broad deficits in cardiomyocyte energy production are central to the pathogenesis (reviewed in Tokarska-Schlattner et al. [17]).
Here, we show that dabuzalgron protects against the cardiotoxic effects of DOX in vitro and in vivo by activating the α1A-AR, and we demonstrate that preservation of mitochondrial function is one novel mechanism underlying this benefit.
Methods
Dabuzalgron was synthesized by Angene (Hong Kong) per published chemical structure (18), and its purity and identity were confirmed. Mice were 8- to 12-week-old males: C57Bl6J wild-type (WT) or α1A-AR knockout (AKO) mice, which were congenic on a C57Bl6J background. Animal care and experimental protocols were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee and complied with “Guide for the Care and the Use of Laboratory Animals.” Tail cuff BP and heart rate (HR) were obtained on awake and trained mice by at least 20 repeated measurements. DOX was administered by single intraperitoneal (IP) injection, and dabuzalgron was gavaged twice daily. Echocardiograms were performed on awake, loosely restrained mice. The echocardiogram reader was blind to treatment condition. Mice were sacrificed by cervical dislocation after an overdose of isoflurane, and heart tissue was immediately removed, flash frozen, and then processed for quantitative reverse transcription polymerase chain reaction (qRT-PCR), RNAseq, adenosine triphosphate (ATP) assay, and immunoblotting. Fibrosis was analyzed in 3 Masson Trichrome-stained sections of 4 or 5 hearts from each treatment group using Aperio ImageScope software (ImageScope 11.1, Leica Biosystems, Buffalo Grove, Illinois). RNAseq was performed at the Carolina Center for Genome Sciences High Throughput Sequencing Facility. Gene-level differential expression testing was implemented in the R package DESeq2 (version 3.1, R Foundation for Statistical Computing, Vienna, Austria).
Neonatal rat ventricular myocytes (NRVMs) were isolated as previously described (19). Experiments including immunoblotting, Annexin V-FLUOS (Sigma-Aldrich, St. Louis, Missouri), and JC-1 staining were performed after 36 to 96 h in serum-free medium with insulin, transferrin, and bromodeoxyuridine. Fluorescence was quantified using a plate reader.
All results are presented as mean ± SEM. Comparisons were made using the Student t test (groups of 2) or 1-way analysis of variance (groups of 3) with the Tukey post hoc analysis (GraphPad Prism, version 5.0, GraphPad Software, La Jolla, California). EC50 for extracellular signal-regulated kinase (ERK) activation was calculated using sigmoidal dose-response analysis (Prism).
Complete experimental details are available in the Supplemental Methods.
Results
Selective α1A-AR activation with dabuzalgron does not affect HR, BP, or heart size in WT mice
Given that nonselective α1-AR agonists such as phenylephrine can increase BP and cause cardiomyocyte hypertrophy, we sought to determine whether the selective α1A agonist, dabuzalgron, would have similar effects. Untreated mice were trained on the tail cuff apparatus daily for 5 days. On Days 6 to 10, mice received dabuzalgron (1 to 100 μg/kg/day) or water by gavage twice daily for 5 days with daily BP measurements. After 5 days, no difference in HR or BP could be found when comparing WT mice treated with dabuzalgron and vehicle (Figure 1A).
Figure 1.
Dabuzalgron Does Not Affect BP or Cause Myocardial Hypertrophy in Uninjured WT Mice
(A) Blood pressure (BP) and heart rate (HR) were measured noninvasively in male mice for 10 days. All daily values represent the average of at least 20 cuff inflations. Mice were trained on the apparatus for the first 5 days, during which no drug was administered. On Days 6 to 10, mice were gavaged with dabuzalgron (100 ng/kg to 100 μg/kg) or water twice daily. (B) Male mice were treated with dabuzalgron 10 μg/kg by gavage twice daily for 7 days. Heart weight (HW) (in mg) was indexed to tibia length (TL) (in mm). (C) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using heart tissue snap frozen at the time of sacrifice. ANP = atrial natriuretic peptide; d = day; Dabuz = dabuzalgron; MHCβ = myosin heavy chain beta; NS = nonsignificant; skAct = skeletal actin; WT = wild-type.
To test the effect of an α1A agonist on cardiac hypertrophy, we administered dabuzalgron (1 to 100 μg/kg/day) or vehicle by gavage twice daily for 7 days. There was no measurable change in body weight or heart weight at any dose (Table 1), and no difference in heart weight indexed to tibia length could be found when comparing WT mice treated with dabuzalgron and water (Figure 1B). We used qRT-PCR to assay traditionally accepted molecular markers of hypertrophy in the hearts of mice treated with dabuzalgron. There was no change in the transcript abundance of atrial natriuretic peptide, beta myosin heavy chain, or alpha-skeletal actin (Figure 1C).
Table 1.
Indexed Heart Weight After 7-Day Gavage Treatment With Dabuzalgron in Uninjured WT Mice
| Dabuzalgron, μg/kg/day (n) | Body Weight, Initial (g) | Body Weight, Final (g) | Tibia Length (mm) | Heart Weight (mg) | Heart/Body Weight (%) | Heart Weight/Tibia Length (mg/mm) |
|---|---|---|---|---|---|---|
| Vehicle (12) | 27.2 ± 1.0 | 26.9 ± 0.8 | 17.5 ± 0.2 | 109 ± 4 | 0.40 ± 0.01 | 6.2 ± 0.2 |
| 0.2 (3) | 25.5 ± 0.3 | 25.3 ± 0.4 | 17.3 ± 0.1 | 106 ± 4 | 0.42 ± 0.01 | 6.1 ± 0.3 |
| 2 (6) | 26.0 ± 0.8 | 25.9 ± 0.8 | 17.5 ± 0.1 | 106 ± 4 | 0.41 ± 0.02 | 6.1 ± 0.2 |
| 20 (12) | 27.9 ± 1.2 | 27.3 ± 1.1 | 17.5 ± 0.1 | 110 ± 4 | 0.41 ± 0.01 | 5.9 ± 0.2 |
| 200 (7) | 28.4 ± 1.8 | 27.4 ± 1.6 | 17.6 ± 0.2 | 113 ± 6 | 0.41 ± 0.01 | 6.1 ± 0.3 |
Values are mean ± SEM unless otherwise indicated.
WT = wild-type.
Collectively, these findings suggest that the chosen doses of dabuzalgron do not increase vascular tone or promote cardiac hypertrophy, 2 properties attributed to nonselective α1-AR activation.
Dabuzalgron protects against DOX cardiotoxicity by activating the α1A-AR
To test whether therapeutic activation of the α1A could prevent DOX-induced cardiac injury, we treated WT mice and mice lacking the α1A (AKO mice) with DOX 20 mg/kg IP injection followed by 7 days gavage with either water or dabuzalgron 10 μg/kg twice daily (Figure 2A). There was no difference in baseline heart weight in WT and AKO mice (Table 2). All animals treated with DOX lost 10% to 15% of their body weight. Raw heart weight and heart weight indexed to tibia length were lower in mice treated with DOX than in vehicle-treated WT and AKO controls (Table 2). Survival was 78% in WT mice treated with DOX and 86% (p = NS by Fisher exact test) in mice treated with DOX and gavaged with dabuzalgron. Survival in 16 AKO mice treated with DOX was 38% (p = 0.08 vs. DOX-treated WT mice by Fisher exact test) and unaffected by dabuzalgron administration (Table 2).
Figure 2.
Dabuzalgron Protects Mice Against DOX Cardiotoxicity by Activating the α1A-AR
(A) WT mice and knockout mice lacking the α1A-AR (AKO) underwent baseline awake echocardiography, received either doxorubicin (DOX) 20 mg/kg or vehicle control (VC) by intraperitoneal (IP) injection, then 7 days of treatment with either dabuzalgron 10 μg/kg or water by gavage twice daily. On Day 7, the mice underwent awake echocardiography before sacrifice. All analyses included only mice that survived to Day 7. (B) Fractional shortening, a measure of contractile function, with representative M-mode echocardiogram images. Results for mice that survived to Day 7 were compared in indicated groups using the Student t test, assuming normal distribution of values. (C) Day 7 heart sections stained with Masson Trichrome. Fibrosis (weighted average collagen content) was quantified using Aperio ImageScope software. Results were compared across treatment conditions by analysis of variance. Abbreviations as in Figure 1.
Table 2.
Indexed HW After 7-Day Doxorubicin Treatment With or Without Dabuzalgron 20 μg/kg/day
| Treatment (n) | 7-Day Survival | Body Weight Day 0 (g) | Body Weight Day 7 (g) | Tibia Length (mm) | Heart Weight (mg) | Heart Weight/Body Weight (%) | Heart Weight/Tibia Length (mg/mm) | Lung Weight/Tibia Length (mg/mm) |
|---|---|---|---|---|---|---|---|---|
| Wild type | ||||||||
| Vehicle (12) | 100% | 27.3 ± 0.6 | 27.8 ± 0.6 | 17.2 ± 0.1 | 126 ± 3 | 0.45 ± 0.01 | 7.3 ± 0.2 | 7.1 ± 1.2 |
| Doxorubicin + vehicle (14) | 78% | 28.1 ± 0.7 | 25.1 ± 1.2∗ | 17.9 ± 0.2 | 104 ± 7∗ | 0.41 ± 0.01∗ | 5.8 ± 0.4∗ | 8.2 ± 0.3 |
| Doxorubicin + dabuzalgron (14) | 86% | 27.3 ± 0.5 | 24.3 ± 0.6∗ | 17.6 ± 0.1 | 97 ± 5∗ | 0.41 ± 0.02∗ | 5.5 ± 0.3∗ | 7.7 ± 0.4 |
| α1A-KO | ||||||||
| Vehicle (3) | 100% | 26.7 ± 0.9 | 26.7 ± 0.9 | 17.0 ± 0.0 | 118 ± 7 | 0.44 ± 0.02 | 6.9 ± 0.4 | 5.2 ± 0.3 |
| Doxorubicin + vehicle (3) | 38% | 29.9 ± 0.7 | 26.8 ± 1.2 | 17.5 ± 0.3 | 101 ± 9 | 0.42 ± 0.02 | 5.8 ± 0.3∗ | 5.0 ± 0.3 |
| Doxorubicin + dabuzalgron (4) | 50% | 29.3 ± 0.6 | 26.4 ± 1.2 | 17.4 ± 0.1 | 101 ± 5∗ | 0.39 ± 0.01∗ | 5.6 ± 0.2∗ | 5.1 ± 0.2 |
Values are mean ± SEM. Anatomic data are included only for mice that survived 7 days.
p < 0.05 vs. genotype vehicle.
Previous studies in rodents (20) and humans (21) have demonstrated that α1-AR activation increases inotropy in failing heart tissue, though it has minimal effects on contractility of the uninjured heart. Conscious echocardiography on Day 7 after DOX treatment in WT mice revealed a decrease in contractile function that was prevented by administration of dabuzalgron (Figure 2B, Table 3). Fractional shortening and left ventricular end-systolic volume both were preserved in animals that received dabuzalgron after DOX (Table 3), though dabuzalgron had no effect on echocardiographic parameters in uninjured mice (data not shown).
Table 3.
Echocardiographic Parameters After Doxorubicin Treatment With or Without Dabuzalgron 20 μg/kg/day
| HR | LVIDd | LVIDs | FS | LVd vol | LVs vol | IVSd | PWd | |
|---|---|---|---|---|---|---|---|---|
| Wild type (n) | ||||||||
| Doxorubicin + vehicle (14) | ||||||||
| Day 0 | 658 ± 30 | 2.9 ± 0.1 | 1.4 ± 0.1 | 54 ± 2 | 34 ± 4 | 5 ± 1 | 0.9 ± 0.0 | 0.8 ± 0.0 |
| Day 7 | 613 ± 23 | 2.8 ± 0.1 | 1.5 ± 0.1 | 46 ± 2 | 31 ± 3 | 7 ± 2 | 0.9 ± 0.0 | 0.8 ± 0.0 |
| Doxorubicin + dabuzalgron (14) | ||||||||
| Day 0 | 630 ± 59 | 3.0 ± 0.2 | 1.4 ± 0.1 | 50 ± 3 | 36 ± 6 | 5 ± 1 | 0.9 ± 0.1 | 0.9 ± 0.0 |
| Day 7 | 667 ± 10∗ | 2.8 ± 0.1 | 1.3 ± 0.0∗ | 53 ± 1∗ | 31 ± 2 | 5 ± 0∗ | 0.9 ± 0.0 | 0.9 ± 0.0 |
| α1A-KO (n) | ||||||||
| Doxorubicin + vehicle (3) | ||||||||
| Day 0 | 679 ± 38 | 3.0 ± 0.1 | 1.3 ± 0.0 | 55 ± 1 | 34 ± 2 | 5 ± 0 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Day 7 | 661 ± 21 | 3.0 ± 0.2 | 1.7 ± 0.1 | 43 ± 2 | 34 ± 4 | 9 ± 2 | 1.2 ± 0.0 | 1.1 ± 0.0 |
| Doxorubicin + dabuzalgron (4) | ||||||||
| Day 0 | 703 ± 13 | 2.8 ± 0.1 | 1.3 ± 0.1 | 54 ± 1 | 30 ± 3 | 4 ± 1 | 1.1 ± 0.0 | 1.1 ± 0.1 |
| Day 7 | 618 ± 19 | 2.8 ± 0.1 | 1.6 ± 0.0 | 42 ± 2 | 29 ± 2 | 8 ± 1 | 1.1 ± 0.0 | 1.1 ± 0.1 |
Values are mean ± SEM. Echocardiography was performed on unanesthetized mice. Data are included only for mice that survived to Day 7, n given in parentheses.
FS = fractional shortening (%); HR = heart rate (beats/min); IVSd = interventricular septal thickness, diastole (mm); LVd vol = left ventricular diastolic volume (μl); LVIDd = left ventricular internal diameter, diastole (mm); LVIDs = left ventricular internal diameter, systole (mm); LVm = left ventricular mass, calculated; LVs vol = left ventricular systolic volume (μl); PWd = posterior wall, diastole (mm).
p < 0.05 versus doxorubicin + vehicle.
There was no difference in baseline contractile function of WT and AKO mice (Figure 2B). However, the surviving DOX-treated AKO mice had significantly lower fractional shortening than DOX-treated WT mice (p < 0.01) (Figure 2B). This profound reduction in contractile function was not rescued by dabuzalgron (Figure 2B). The burden of fibrosis as detected by Masson Trichrome increased significantly after DOX (Figure 2C), but treatment with dabuzalgron mitigated this increase.
In summary, treatment with dabuzalgron preserved contractile function and reduced fibrosis after DOX administration. AKO mice treated with DOX had worse survival and more profoundly impaired contractile function than WT mice. Neither parameter was affected by dabuzalgron in AKOs, indicating that the beneficial effects of dabuzalgron require the presence of the α1A.
Dabuzalgron preserves in vivo abundance of mitochondrial function transcripts, up-regulates PGC1α, and restores ATP synthesis after treatment with DOX
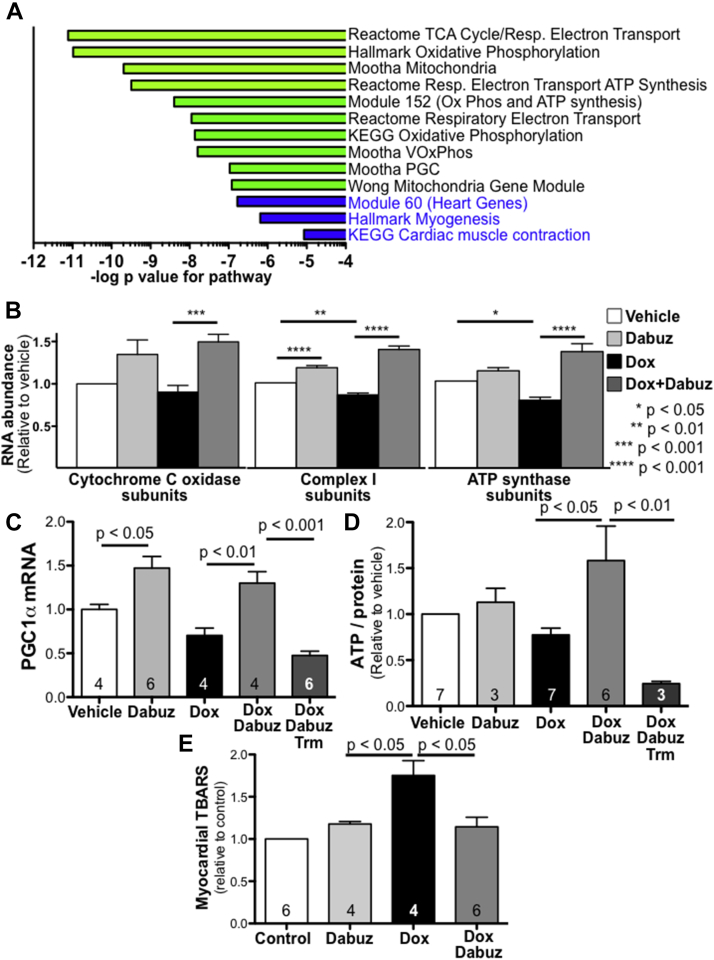
To investigate the mechanisms behind dabuzalgron’s cardioprotective effects after DOX, we used RNAseq to analyze heart tissue from mice treated with DOX with and without dabuzalgron. An omnibus test of transcript abundance across all groups was performed using DESeq2 with groups encoded as categorical variables. One hundred one genes were identified as significant by meeting the q < 0.05 threshold (the set of genes with a 5% false discovery rate) (Supplemental Table 1). Gene set analysis was performed based on the univariate statistics calculated from DESeq2 (Supplemental Tables 2 and 3). Marked differences were identified in numerous pathways related to mitochondrial function (Figure 3A).
Figure 3.
Dabuzalgron Augments Mitochondrial Transcript Expression and Function in Hearts From Mice Treated With DOX
Male mice were treated with either DOX 20 mg/kg or vehicle by IP injection followed by 7 days gavage with either dabuzalgron 10 μg/kg twice daily, water, or trametinib (Trm) (1 mg/kg daily). Heart tissue was collected and immediately flash frozen on Day 7. (A) RNAseq was performed using RNA from the hearts of 3 mice per group (PBS + water; PBS + dabuzalgron; DOX + water; DOX + dabuzalgron). Gene set analysis was performed on DESeq2-derived statistics across these four categories. The results were highly enriched in gene sets involved in mitochondrial processes, a selection of which is shown here. (B) RNA abundance for all sequenced cytochrome C oxidase subunits (25 genes), mitochondrial complex I subunits (42 genes), and ATP synthase subunits (17 genes) was normalized by individual gene to vehicle treatment, then aggregated by treatment group. (C) Quantitative reverse transcription polymerase chain reaction for peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) was performed on mouse heart tissue (n in individual bars) (D) ATP content was measured in freshly harvested mouse heart tissue (total n in individual bars), then quantified relative to protein content. Results are presented relative to vehicle treatment for 4 independent experiments. (E) Thiobarbituric acid reactive substances (TBARS) were assayed in mouse myocardium. Abbreviations as in Figures 1 and 2.
Further analysis of transcripts within these mitochondrial pathways revealed that DOX decreased the abundance of complex I (42 genes) and ATP synthase subunits (17 genes) (Figure 3B). Treatment with dabuzalgron restored normal expression of these gene sets and also increased expression of cytochrome c oxidase subunits (25 genes) after DOX. Treatment with dabuzalgron in the absence of DOX increased complex I subunit abundance, but had no significant effect on cytochrome c or ATP synthase (Figure 3B).
Many of the genes encoding electron transport and other key mitochondrial proteins are under transcriptional regulation by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) (22). We found that DOX decreased PGC1α abundance in vivo (Figure 3C), consistent with prior reports (23). Treatment with dabuzalgron increased PGC1α abundance in the hearts of mice treated with either DOX or vehicle control (Figure 3C).
To assess the functional effect of these transcriptional differences, we assayed ATP content in freshly harvested heart homogenates. DOX decreased ATP content by 23 ± 7% compared to untreated hearts, consistent with previous reports (17) (Figure 3D). Treatment with dabuzalgron restored ATP content in the hearts of DOX-treated mice, but did not affect ATP in uninjured mice. Using the highly selective MEK inhibitor, trametinib, we found that inhibiting activation of ERK1/2 abrogated dabuzalgron’s beneficial effect on ATP synthesis after DOX.
Oxidative stress is central to the pathobiology of DOX cardiotoxicity and arises in part from compromised mitochondrial function (24). To assess further the functional implications of these transcriptional findings, we measured thiobarbituric acid reactive substances (TBARS), in mouse heart tissue. TBARS, a measure of lipid peroxidation, were more abundant in the hearts of mice treated with DOX. Coadministration of dabuzalgron normalized TBARS content (Figure 3E).
In summary, dabuzalgron protected against the reduction in transcripts related to mitochondrial function, preserved ATP content, and reduced oxidative stress in the hearts of mice treated with DOX. These beneficial effects may be mediated by activation of ERK1/2 and up-regulation of PGC1α.
ERK1/2 activation contributes to the cardioprotective effects of dabuzalgron
NRVMs express the α1A and α1B subtypes, have been used extensively to assess the effects of non-selective α1-AR activation, and faithfully predict in vivo α1-AR biology 25, 26. To test the effect of an α1A agonist on uninjured NRVMs, we administered various concentrations of dabuzalgron. After 15 min of treatment, we blotted NRVM lysates for activation of ERK (Figure 4A), a canonical downstream signaling partner of the α1A that mediates the cytoprotective effects of α1A activation in vitro (13). Dabuzalgron increased ERK phosphorylation in a dose-dependent fashion with a half-maximal effective concentration (EC50) of 4.8 × 10−7 mol/l (Figure 4B). The pERK/ERK ratio was increased roughly 1.5-fold after treatment with dabuzalgron 10 μmol/l, an effect equivalent to norepinephrine 1 μmol/l (in the presence of the nonselective β-AR blocker, propranolol 1 μmol/l) (Figure 4C).
Figure 4.
Dabuzalgron Activates ERK, A Canonical Signaling Partner of the α1A-AR
(A) NRVMs were pre-treated with the β-AR antagonist propranolol then exposed for 15 minutes to dabuzalgron. The non-selective α1-AR agonist norepinephrine (NE) was a positive control. Lysates were blotted for total and phospho-ERK (pERK). (B) The EC50 was calculated from 4 concentrations of Dabuzalgron across 5 separate experiments. (C) Summary of pERK/ERK for experiments using 5 different NRVM isolations. The average pERK/ERK ratio (≥2 individual wells) for each experiment was normalized to the pERK/ERK ratio for vehicle-treated NRVMs. (D and E) Mice were treated with DOX, DOX and dabuzalgron, Trm, or DOX, dabuzalgron and Trm for 7 days. Heart lysates were blotted for pERK and ERK. Results were compared using 1-way analysis of variance with Tukey post-test. (F) Mice underwent conscious echocardiography after 7 days of treatment with Trm, DOX and Trm, or DOX, Trm, and dabuzalgron. EC50 = half-maximal effective concentration; NRVM = neonatal rat ventricular myocyte; other abbreviations as in Figures 1, 2, and 3.
We then tested the role of ERK activation in dabuzalgron’s cardioprotective effects in vivo using trametinib. Trametinib (1 mg/kg by gavage once daily) almost completely eliminated ERK activation (Figures 4D and 4E). DOX also reduced ERK activation, consistent with previous reports (27). Treatment with dabuzalgron partially mitigated that effect but could not restore ERK activation after trametinib (Figures 4D and 4E). Coadministration of trametinib with DOX and dabuzalgron abrogated dabuzalgron’s protective effect on contractile function (Figure 4F), suggesting that α1A-mediated positive inotropy requires ERK activation.
Dabuzalgron protects NRVMs from cell death due to DOX
Previous studies showed that adenoviral infection with the α1A is cytoprotective in vitro, but the effects of selective α1A activation have not been tested previously. To test the cytoprotective effects of an α1A agonist, we treated NRVMs with DOX 2 μmol/l in the presence and absence of dabuzalgron 10 μmol/l, then assayed apoptosis and cell death using Annexin V-FLUOS and propidium iodide (Figure 5A). Four-hour treatment with DOX increased apoptosis (Annexin V staining), and necrotic cell death (costaining with Annexin V and propidium iodide) (Figure 5B). Concomitant treatment with dabuzalgron abrogated these effects. Treatment with dabuzalgron in the absence of DOX did not change Annexin V or propidium iodide staining when compared with untreated cells.
Figure 5.
In NRVMs, Dabuzalgron Protects Against Apoptotic and Necrotic Cell Death Due to DOX
NRVMs were treated for 4 h with doxorubicin 2μM in the presence and absence of dabuzalgron 10 μmol/l. Apoptosis was detected using fluorescein isothiocyanate–labeled Annexin V, cell death was detected using propidium iodide (PI), and nuclei were labeled with Hoechst. (A) Representative epifluorescence microscopy for each treatment condition. (B) Fluorescent intensity was analyzed using ImageJ 1.41 software (NIH, Bethesda, Maryland) for 3 independent experiments, using at least 2 wells per condition for each experiment. An average of 352 nuclei were counted in an average of 6 microscopic fields per experiment. Abbreviations as in Figures 1, 2, 3, and 4.
Dabuzalgron regulates activators of apoptosis and mitochondrial membrane potential in NRVMs
In light of our findings that treatment with dabuzalgron preserved mitochondrial function in vivo and protected against cell death in vitro after DOX exposure, we sought to explore the effect of dabuzalgron on aspects of mitochondrial function in NRVMs. Maintenance of mitochondrial membrane potential is essential to ATP generation, and loss of membrane potential can contribute to apoptosis by increasing cytochrome c release (28), leading to activation of proapoptotic effectors. DOX interferes with the cellular capacity to maintain mitochondrial membrane potential and mitochondrial dysfunction contributes significantly to DOX cardiotoxicity (24).
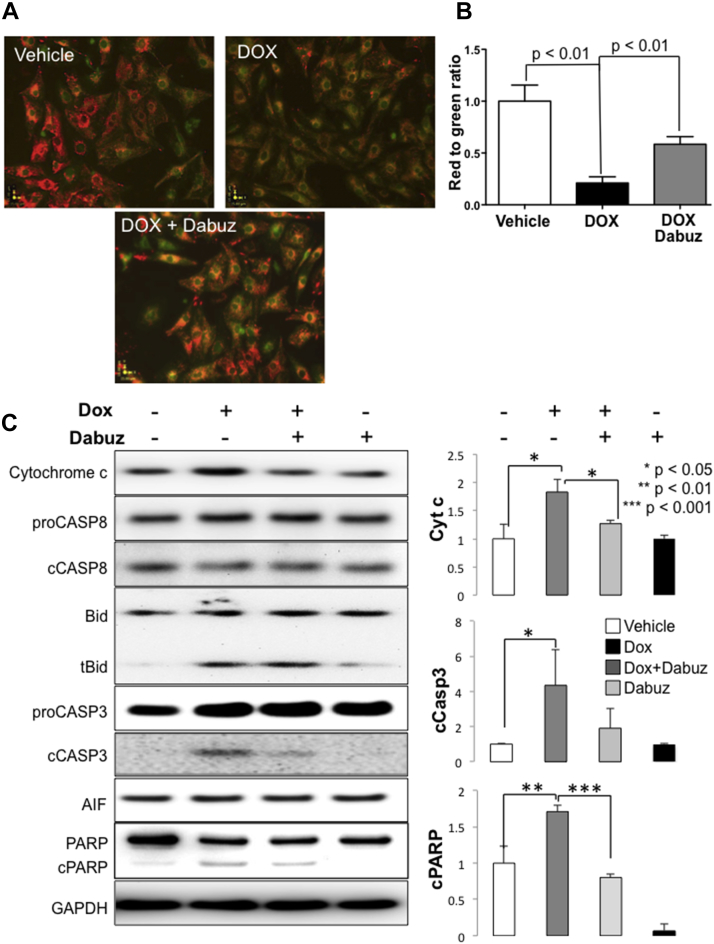
To test the effect of α1A activation on mitochondrial membrane potential, we treated NRVMs with DOX 2 μmol/l for 4 h in the presence or absence of dabuzalgron 10 μmol/l then stained with the membrane permeant dye, JC-1. JC-1 exists as a green fluorescent monomer at low mitochondrial membrane potential and a red fluorescent aggregate at high mitochondrial membrane potential. DOX led to a profound loss of mitochondrial membrane potential that was partially rescued by coadministration of dabuzalgron (Figures 6A and 6B).
Figure 6.
In NRVMs, Dabuzalgron Regulates Activators of Apoptosis and Protects Mitochondrial Membrane Potential After Treatment With DOX
NRVMs were treated for 4 h with doxorubicin 2 μmol/l in the presence and absence of dabuzalgron 10 μmol/l. (A and B) Mitochondrial membrane potential was assessed using JC-1, and fluorescent intensity was quantified using a plate reader. Red indicates intact mitochondrial membrane potential; green indicates compromised mitochondrial membrane potential. Representative images (A) and summary findings (B) are presented. (C) NRVM lysates were blotted for selected regulators of apoptosis and mitochondrial cell death effectors. Representative Western blots and summary findings from 3 independent experiments with at least 2 wells per condition in each experiment are shown. Abbreviations as in Figures 1, 2, 3, and 4.
To examine the role of α1A-mediated mitochondrial protection on DOX-induced apoptosis, we immunoblotted NRVM lysates for cytochrome c and downstream apoptosis effectors. DOX increased cytochrome c release and caused cleavage of caspases and PARP, suggesting that mitochondrial damage induced activation of the intrinsic apoptosis pathway, consistent with previous characterizations of DOX cytotoxicity (29). Coadministration of dabuzalgron abrogated these changes (Figure 6C).
In summary, activation of the α1A-AR with dabuzalgron mitigated the detrimental effects of DOX on mitochondrial membrane potential and abrogated the activation of important elements of the apoptotic response to mitochondrial damage. These findings suggest that preservation of mitochondrial function may underlie the cytoprotective effects of α1A activation.
Discussion
The central novel finding of this study is that the oral selective α1A-AR agonist, dabuzalgron, is protective against anthracycline-induced cardiotoxicity. Though dabuzalgron has not been tested previously in animal models of heart injury, it was well tolerated in 2 large randomized clinical trials for treatment of urinary incontinence. We found that its cardioprotective effect is mediated in part through preservation of mitochondrial function, an adaptive mechanism that has not been attributed previously to activation of cardiac α1A-ARs.
α1-ARs are best known as vascular receptors, where α1-AR activation promotes vasoconstriction. At high doses, nonselective α1-AR agonists such as phenylephrine increase BP experimentally and clinically. In this study, we found no effect on BP or HR in mice treated with a range of dabuzalgron doses. We chose to use 10 μg/kg for subsequent experiments because Roche studied this dose in pigs and rabbits (10). Our findings mirror the published human experience with dabuzalgron as a treatment for urinary incontinence, wherein administration of 1.5 mg by mouth twice daily did not alter BP or HR (11).
Though cardiac α1-ARs are a minor AR subpopulation relative to β1-ARs, they contribute to numerous important processes in the heart (26). Subpressor doses of nonselective α1-AR agonist also can cause cardiac hypertrophy, indicating a direct and load-independent effect on the heart (30). We found that activation of the α1A did not cause myocardial hypertrophy, consistent with the fact that heart size is normal in mice with global and cardiac-specific α1A overexpression 4, 5, 6. α1AKO mice on a congenic C57Bl6 background also have normal heart size and BP (data not shown). Mice lacking both myocardial α1 subtypes (α1ABKO) have small hearts (31). Collectively, these findings suggest the α1B subtype mediates cardiomyocyte hypertrophy induced by non-selective α1-AR agonists.
We found that oral administration of a subpressor dose of dabuzalgron protected WT mice against DOX cardiotoxicity. This beneficial effect was absent in AKO mice, indicating that dabuzalgron’s adaptive effects result from on-target activation of the α1A. High mortality and very poor contractile function in DOX-treated AKO mice further reinforce the cardioprotective function of the α1A-AR. Though other labs have used transgenic overexpression of the α1A to identify cardioprotective effects, ours is the first study to our knowledge to demonstrate greater susceptibility to cardiac injury in AKO mice. As such, we present evidence supporting adaptive functions for cardiac α1A-ARs using both novel pharmacological gain-of-function and novel genetic loss-of-function approaches.
The function of α1-ARs in cardiomyocyte mitochondria has not been explored to any significant extent previously. In our study, dabuzalgron protected against DOX-induced apoptosis and necrosis in NRVMs and decreased levels of intrinsic apoptotic effectors, suggesting that this benefit may be associated with preservation of mitochondrial integrity and function. Analysis of our RNAseq results showed rescue of pathways associated with mitochondrial function and metabolism after therapeutic α1A activation, a previously unrecognized mechanism for α1A activity. Treatment with DOX diminished transcript abundance within these pathways, whereas coadministration of dabuzalgron restored expression of complex I, cytochrome c oxidase, and ATP synthase genes. Treatment with dabuzalgron abrogated the DOX-induced reduction in myocardial ATP levels, indicating functional significance of the transcriptional changes. Though we cannot exclude a contribution from other cell types to these findings, they seem most likely to represent changes in cardiomyocytes because the α1A is not expressed on nonmyocytes in the heart (32).
We show that dabuzalgron activates ERK, a canonical downstream signaling partner of the α1A in NRVMs, and partially restores ERK activation in the hearts of mice treated with DOX. Using the highly selective MEK inhibitor, trametinib, we demonstrate that ERK phosphorylation is necessary for dabuzalgron’s protective effects on inotropy and ATP synthesis. ERK activation was found to be critical to α1A-mediated cytoprotection in previous work using adenoviral constructs in vitro (13), but our experiments are the first to show ERK activation in vivo by an α1A agonist. Interestingly, dabuzalgron-mediated cardioprotection does not require full restoration of ERK activation to levels seen in uninjured heart. Given the broad cellular effects of DOX, it is possible that DOX impairs ERK activation through multiple pathways, not all of which are modified by α1A activation. α1-ARs can activate ERK through multiple pathways, both PKC-dependent (33) and PKC-independent (34), suggesting signaling resilience. Furthermore, α1A activation might mitigate the adverse effects of DOX on abundance of activated ERK by targeting activated ERK to caveolae, where its function is enhanced, as shown previously in vitro 35, 36.
Study limitations
One potential limitation of our study is the use of an acute DOX toxicity model. We administered 20 mg/kg of DOX intraperitoneally, a dose that allometrically scales to roughly 60 mg/m2 in humans. Though this scaled dose is at the upper limit of the typical range for treatment of breast cancer and lymphoma, the observed mortality in our studies is out of proportion to the insult to cardiac function, suggesting that mice may suffer noncardiac toxicities at this dose that are not fully representative of the human response. The pathogenesis and signaling associated with acute DOX cardiotoxicity likely are distinct from chronic DOX cardiomyopathy, and the contribution of oxidative stress in this model may be disproportionately represented. Chronic cardiomyopathy is the most significant source of DOX-associated cardiac morbidity; however, numerous studies indicate that acute DOX cardiotoxicity is more common than previously thought (11% [37] to 21% [38]) and predicts poor outcomes. In one recent study, 32% of subjects had elevated troponin I (TnI) acutely after DOX. Ejection fraction dropped measurably in most subjects by 3 months and early +TnI predicted durable reduction in ejection fraction (39). In a follow-up study, the authors found that early institution of evidence-based HF therapy protected against chronic anthracycline cardiomyopathy (40). Collectively, these findings suggest that acute DOX cardiotoxicity may be a clinically meaningful and actionable entity.
We have proposed previously that α1-AR agonists could be used to treat HF (25). Anthracycline-induced cardiac dysfunction is not wholly representative of the various causes of human HF, but there are some commonalities. In particular, mitochondrial dysfunction and impaired cardiomyocyte energetics are central to the pathobiology of HF regardless of etiology (41). Unlike β-ARs, the abundance of α1A is maintained or increased in failing human heart tissue 42, 43. One small study indicated a benefit from the use of the nonselective α1-AR agonist midodrine in patients with advanced HF (44). Long-term selective activation of the α1A for treatment of HF has not been tested therapeutically, though the present results suggest that this novel approach may have promise. Interestingly, long-term systemic 2-fold overexpression of the α1A actually is associated with prolonged lifespan, decreased cancer incidence (45), and improved cognition (46).
Conclusions
Future mechanistic work will examine the role of the α1A-AR in regulating mitochondrial function and cellular energy production. We also plan to test selective α1A-AR activation with dabuzalgron in other mouse models of HF. These studies will help to determine the therapeutic potential of repurposing this well-tolerated oral α1A-AR agonist for the treatment of HF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The detrimental role of chronic catecholaminergic hyperstimulation of cardiac β-ARs is a well-recognized aspect of HF pathobiology and antagonizing those effects with β-blockers is central to the treatment of HF. Cardiac α1-ARs are a smaller population of receptors that also are activated endogenously by the catecholamines, norepinephrine and epinephrine. Emerging data indicate that α1-ARs mediate adaptive, rather than toxic, effects in the heart. Here we use dabuzalgron, an oral α1A-AR agonist, to protect against DOX-induced cardiotoxicity and HF in mice. Our findings reinforce previous cell and animal data demonstrating cardioprotection through the α1A-AR, and suggest that dabuzalgron might be used to treat other forms of HF.
TRANSLATIONAL OUTLOOK: We chose to study dabuzalgron because it has a published record of safety and tolerability in previous clinical trials for treatment of urinary incontinence. Hence, developing dabuzalgron as a HF treatment would not require extensive preclinical toxicological testing. Indeed, many of the medications that currently are used to treat HF have been repurposed from other indications. Confirmation of the therapeutic potential of dabuzalgron will require demonstration of its efficacy in other animal models of HF. Although dabuzalgron was well tolerated by hundreds of women with urinary incontinence, its safety would need to be evaluated in Phase 1 studies of patients with heart disease.
Acknowledgments
The authors would like to thank Monte Willis and Tim O’Connell for critical appraisal of the manuscript; and the McAllister Heart Institute Animal Surgery Core Lab.
Footnotes
This work was funded by grants to Dr. Jensen from NC TraCS–National Center for Advancing Translational Sciences (NCATS) (K08 HL096836), National Institutes of Health through UL1TR001111, UAI Research Foundation, and Hugh A. McAllister; and to Dr. Jin from NC TraCS. Drs. Simpson and Jensen are involved in AdrenRx, a company studying the therapeutic potential of α1-AR agonists. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.O’Connell T.D., Jensen B.C., Baker A.J., Simpson P.C. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol Rev. 2014;66:308–333. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbull L., McCloskey D.T., O'Connell T.D., Simpson P.C., Baker A.J. Alpha 1-adrenergic receptor responses in alpha 1AB-AR knockout mouse hearts suggest the presence of alpha 1D-AR. Am J Physiol Heart Circ Physiol. 2003;284:H1104–H1109. doi: 10.1152/ajpheart.00441.2002. [DOI] [PubMed] [Google Scholar]
- 3.Jensen B., Swigart P., Laden M.-E., DeMarco T., Hoopes C., Simpson P. The alpha-1D is the predominant alpha-1-adrenergic receptor in human epicardial coronary arteries. J Am Coll Cardiol. 2009;54:1137–1145. doi: 10.1016/j.jacc.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin F., Owens W.A., Chen S. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. 2001;89:343–350. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- 5.Rorabaugh B.R., Ross S.A., Gaivin R.J. alpha1A- but not alpha1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovasc Res. 2005;65:436–445. doi: 10.1016/j.cardiores.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Du X.J., Gao X.M., Kiriazis H. Transgenic alpha1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res. 2006;71:735–743. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X., Balaji P., Pachon R. Overexpression of cardiomyocyte alpha1a-adrenergic receptors attenuates postinfarct remodeling by inducing angiogenesis through heterocellular signaling. Arterioscler Thromb Vasc Biol. 2015;35:2451–2459. doi: 10.1161/ATVBAHA.115.305919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du X.J., Fang L., Gao X.M. Genetic enhancement of ventricular contractility protects against pressure-overload-induced cardiac dysfunction. J Mol Cell Cardiol. 2004;37:979–987. doi: 10.1016/j.yjmcc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 9.ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 10.Blue D.R., Daniels D.V., Gever J.R. Pharmacological characteristics of Ro 115-1240, a selective alpha1A/1L-adrenoceptor partial agonist: a potential therapy for stress urinary incontinence. BJU Int. 2004;93:162–170. doi: 10.1111/j.1464-410x.2004.04577.x. [DOI] [PubMed] [Google Scholar]
- 11.Musselman D.M., Ford A.P., Gennevois D.J. A randomized crossover study to evaluate Ro 115-1240, a selective alpha1A/1L-adrenoceptor partial agonist in women with stress urinary incontinence. BJU Int. 2004;93:78–83. doi: 10.1111/j.1464-410x.2004.04560.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan T., Dash R., Simpson P. An alpha-1A-adrenergic receptor subtype agonist prevents cardiomyopathy without increasing blood pressure (abstr) Circulation. 2008;118:S533. [Google Scholar]
- 13.Huang Y., Wright C.D., Merkwan C.L. An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 14.Dash R., Chung J., Chan T. A molecular MRI probe to detect treatment of cardiac apoptosis in vivo. Magn Res Med. 2011;66:1152–1162. doi: 10.1002/mrm.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Pal H.J., van Dalen E.C., Hauptmann M. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 16.Bloom M.W., Hamo C.E., Cardinale D. Cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9:e002661. doi: 10.1161/CIRCHEARTFAILURE.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokarska-Schlattner M., Zaugg M., Zuppinger C., Wallimann T., Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol. 2006;41:389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Bishop M.J. Recent advances in the discovery of alpha1-adrenoceptor agonists. Curr Top Med Chem. 2007;7:135–145. doi: 10.2174/156802607779318217. [DOI] [PubMed] [Google Scholar]
- 19.Simpson P., Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 20.Cowley P.M., Wang G., Chang A.N. The alpha1A-adrenergic receptor subtype mediates increased contraction of failing right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2015;309:H888–H896. doi: 10.1152/ajpheart.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skomedal T., Borthne K., Aass H., Geiran O., Osnes J.B. Comparison between alpha-1 adrenoceptor-mediated and beta adrenoceptor-mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. J Pharmacol Exp Ther. 1997;280:721–729. [PubMed] [Google Scholar]
- 22.Scarpulla R.C., Vega R.B., Kelly D.P. Transcriptional integration of mitochondrial biogenesis. Trends in endocrinology and metabolism: Trends Endocrinol Metab. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moulin M., Piquereau J., Mateo P. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodeling. Circ Heart Fail. 2015;8:98–108. doi: 10.1161/CIRCHEARTFAILURE.114.001180. [DOI] [PubMed] [Google Scholar]
- 24.Varga Z.V., Ferdinandy P., Liaudet L., Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;309:H1453–H1467. doi: 10.1152/ajpheart.00554.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen B.C., O'Connell T.D., Simpson P.C. Alpha-1-adrenergic receptors: targets for agonist drugs to treat heart failure. J Mol Cell Cardiol. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen B.C., O'Connell T.D., Simpson P.C. Alpha-1-adrenergic receptors in heart failure: the adaptive arm of the cardiac response to chronic catecholamine stimulation. J Cardiovasc Pharmacol. 2014;63:291–301. doi: 10.1097/FJC.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou H., Danelisen I., Singal P.K. Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;288:H1925–H1930. doi: 10.1152/ajpheart.01054.2004. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb E., Armour S.M., Harris M.H., Thompson C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–717. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 29.Suliman H.B., Carraway M.S., Ali A.S., Reynolds C.M., Welty-Wolf K.E., Piantadosi C.A. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel M.B., Stewart J.M., Loud A.V. Altered function and structure of the heart in dogs with chronic elevation in plasma norepinephrine. Circulation. 1991;84:2091–2100. doi: 10.1161/01.cir.84.5.2091. [DOI] [PubMed] [Google Scholar]
- 31.O'Connell T.D., Ishizaka S., Nakamura A. The a1A/C- and a1B-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart A.F., Rokosh D.G., Bailey B.A. Cloning of the rat alpha 1C-adrenergic receptor from cardiac myocytes. alpha 1C, alpha 1B, and alpha 1D mRNAs are present in cardiac myocytes but not in cardiac fibroblasts. Circ Res. 1994;75:796–802. doi: 10.1161/01.res.75.4.796. [DOI] [PubMed] [Google Scholar]
- 33.Rao V.U., Shiraishi H., McDermott P.J. PKC-epsilon regulation of extracellular signal-regulated kinase: a potential role in phenylephrine-induced cardiocyte growth. Am J Physiol Heart Circ Physiol. 2004;286:H2195–H2203. doi: 10.1152/ajpheart.00475.2003. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Aso M., Segura V., Monto F. The three alpha1-adrenoceptor subtypes show different spatio-temporal mechanisms of internalization and ERK1/2 phosphorylation. Biochim Biophys Acta. 2013;1833:2322–2333. doi: 10.1016/j.bbamcr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Ogata T., Naito D., Nakanishi N. MURC/Cavin-4 facilitates recruitment of ERK to caveolae and concentric cardiac hypertrophy induced by alpha1-adrenergic receptors. Proc Natl Acad Sci U S A. 2014;111:3811–3816. doi: 10.1073/pnas.1315359111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright C.D., Chen Q., Baye N.L. Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ Res. 2008;103:992–1000. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luminari S., Montanini A., Caballero D. Nonpegylated liposomal doxorubicin (MyocetTM) combination (R-COMP) chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL): results from the phase II EUR018 trial. Ann Oncol. 2010;21:1492–1499. doi: 10.1093/annonc/mdp544. [DOI] [PubMed] [Google Scholar]
- 38.Tirelli U., Errante D., Van Glabbeke M. CHOP is the standard regimen in patients > or = 70 years of age with intermediate-grade and high-grade non-Hodgkin's lymphoma: results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. J Clin Oncol. 1998;16:27–34. doi: 10.1200/JCO.1998.16.1.27. [DOI] [PubMed] [Google Scholar]
- 39.Cardinale D., Sandri M.T., Martinoni A. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 40.Cardinale D., Colombo A., Bacchiani G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 41.Rosca M.G., Tandler B., Hoppel C.L. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2013;55:31–41. doi: 10.1016/j.yjmcc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen B.C., Swigart P.M., De Marco T., Hoopes C., Simpson P.C. {alpha}1-Adrenergic receptor subtypes in nonfailing and failing human myocardium. Circ Heart Fail. 2009;2:654–663. doi: 10.1161/CIRCHEARTFAILURE.108.846212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bristow M.R., Minobe W., Rasmussen R., Hershberger R.E., Hoffman B.B. Alpha-1 adrenergic receptors in the nonfailing and failing human heart. J Pharmacol Exp Ther. 1988;247:1039–1045. [PubMed] [Google Scholar]
- 44.Zakir R.M., Folefack A., Saric M., Berkowitz R.L. The use of midodrine in patients with advanced heart failure. Congest Heart Fail. 2009;15:108–111. doi: 10.1111/j.1751-7133.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 45.Collette K.M., Zhou X.D., Amoth H.M. Long-term alpha1B-adrenergic receptor activation shortens lifespan, while alpha1A-adrenergic receptor stimulation prolongs lifespan in association with decreased cancer incidence. Age. 2014;36:9675. doi: 10.1007/s11357-014-9675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doze V.A., Papay R.S., Goldenstein B.L. Long-term alpha1A-adrenergic receptor stimulation improves synaptic plasticity, cognitive function, mood, and longevity. Mol Pharmacol. 2011;80:747–758. doi: 10.1124/mol.111.073734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.