Abstract
Stimulation of growth of the rat parotid gland by repeated injection of the beta-agonist isoprenaline led to a significant decrease in the activity of cyclic AMP-dependent protein kinases. Immunochemical quantification of the catalytic (C) and regulatory (RI and RII) subunits of the cyclic AMP-dependent protein kinases type I and type II revealed a loss of 65% of the immunochemically measurable amount of catalytic subunit C. The amount of the regulatory subunits, however, remained constant. The observed decrease in C-subunit was not due to a translocation of the molecule to cellular membranes or to an inhibiting effect of the heat-stable inhibitor of cyclic AMP-dependent protein kinases. A selective decrease in only the C-subunit was also observed after a brief exposure to isoprenaline leading to the stimulation of DNA synthesis. Under these conditions, the decrease was observed at the onset of DNA synthesis (17 h after injection), but not at the the time of an earlier small cyclic AMP peak (13 h after injection) or at the time of maximal DNA synthesis (24 h after injection). The results indicate that the amount of the catalytic subunit of cyclic AMP-dependent protein kinases can be regulated independently from that of the regulatory subunits. The time-limited occurrence of the specific change in the amount of the C-subunit suggests that such a regulation is of physiological significance and that it may participate in cyclic AMP-mediated events involved in the control of cellular proliferation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhanaty E., Tauber-Finkelstein M., Schmeeda H., Shaltiel S. cAMP-triggered proteolysis of cAMP-dependent protein kinase in brush border membranes. Curr Top Cell Regul. 1985;27:267–278. doi: 10.1016/b978-0-12-152827-0.50030-x. [DOI] [PubMed] [Google Scholar]
- BARKA T. INDUCED CELL PROLIFERATION: THE EFFECT OF ISOPROTERENOL. Exp Cell Res. 1965 Mar;37:662–679. doi: 10.1016/0014-4827(65)90214-4. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Byus C. V., Hayes J. S., Brendel K., Russell D. H. Regulation of glycogenolysis in isolated rat hepatocytes by the specific activation of type I cyclic AMP-dependent protein kinase. Mol Pharmacol. 1979 Nov;16(3):941–949. [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Doskeland S. O., Ueland P. M. Binding proteins for adenosine 3':5'-cyclic monophosphate in bovine adrenal cortex. Biochem J. 1977 Sep 1;165(3):561–573. doi: 10.1042/bj1650561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evain D., Gottesman M., Pastan I., Anderson W. B. A mutation affecting the catalytic subunit of cyclic AMP-dependent protein kinase in CHO cells. J Biol Chem. 1979 Aug 10;254(15):6931–6937. [PubMed] [Google Scholar]
- Fiszer-Szafarz B., Szafarz D. DNA and protein content as cellular biochemical parameters. A discussion with two examples: acid phosphatase and cathepsin D in rat liver and hepatoma and acid phosphatase in human breast normal tissue and adenocarcinoma. Anal Biochem. 1984 Apr;138(1):255–258. doi: 10.1016/0003-2697(84)90799-1. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A. cAMP mediated proteolysis of the catalytic subunit of cAMP-dependent protein kinase. FEBS Lett. 1986 Feb 3;196(1):126–130. doi: 10.1016/0014-5793(86)80226-5. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Hunzicker-Dunn M., Lorenzini N. A., Lynch L. L., West D. E. Coelution of the type II holoenzyme form of cAMP-dependent protein kinase with regulatory subunits of the type I form of cAMP-dependent protein kinase. J Biol Chem. 1985 Oct 25;260(24):13360–13369. [PubMed] [Google Scholar]
- Jahn R., Unger C., Söling H. D. Specific protein phosphorylation during stimulation of amylase secretion by beta-agonists or dibutyryl adenosine 3',5'-monophosphate in the rat parotid gland. Eur J Biochem. 1980 Nov;112(2):345–352. doi: 10.1111/j.1432-1033.1980.tb07211.x. [DOI] [PubMed] [Google Scholar]
- Keely S. L., Jr, Corbin J. D., Park C. R. On the question of translocation of heart cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1501–1504. doi: 10.1073/pnas.72.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Gani V., Jiménez J. S., Shaltiel S. Affinity labeling of the catalytic subunit of cyclic AMP-dependent protein kinase by N alpha-tosyl-L-lysine chloromethyl ketone. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3073–3077. doi: 10.1073/pnas.76.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y., PasMantier R., Fleischer N., Erlichman J. Hormonal activation of the cAMP-dependent protein kinases in AtT20 cells. Preferential activation of protein kinase I by corticotropin releasing factor, isoproterenol, and forskolin. J Biol Chem. 1984 Aug 25;259(16):10296–10302. [PubMed] [Google Scholar]
- Liu A. Y., Chang Z. F., Chen K. Y. Increased level of cAMP-dependent protein kinase in aging human lung fibroblasts. J Cell Physiol. 1986 Aug;128(2):149–154. doi: 10.1002/jcp.1041280203. [DOI] [PubMed] [Google Scholar]
- Livesey S. A., Kemp B. E., Re C. A., Partridge N. C., Martin T. J. Selective hormonal activation of cyclic AMP-dependent protein kinase isoenzymes in normal and malignant osteoblasts. J Biol Chem. 1982 Dec 25;257(24):14983–14987. [PubMed] [Google Scholar]
- Lohmann S. M., Schwoch G., Reiser G., Port R., Walter U. Dibutyryl cAMP treatment of neuroblastoma-glioma hybrid cells results in selective increase in cAMP-receptor protein (R-I) as measured by monospecific antibodies. EMBO J. 1983;2(2):153–159. doi: 10.1002/j.1460-2075.1983.tb01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- Malkinson A. M., Beer D. S., Wehner J. M., Sheppard J. R. Elution of the regulatory subunit of cAMP-dependent protein kinase Type I isozyme derived from epididymal fat within the type II isozyme chromatographic peak. Biochem Biophys Res Commun. 1983 Apr 15;112(1):214–220. doi: 10.1016/0006-291x(83)91818-1. [DOI] [PubMed] [Google Scholar]
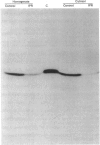
- Mednieks M. I., Hand A. R. Cyclic AMP-dependent protein kinase in stimulated rat parotid gland cells: compartmental shifts after in vitro treatment with isoproterenol. Eur J Cell Biol. 1982 Oct;28(2):264–271. [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 3. Purification and properties of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969 Dec 10;244(23):6395–6402. [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Pawelek J. M. Evidence suggesting that a cyclic AMP-dependent protein kinase is a positive regulator of proliferation in Cloudman S91 melanoma cells. J Cell Physiol. 1979 Mar;98(3):619–625. doi: 10.1002/jcp.1040980320. [DOI] [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Richards J. S., Rolfes A. I. Hormonal regulation of cyclic AMP binding to specific receptor proteins in rat ovarian follicles. Characterization by photoaffinity labeling. J Biol Chem. 1980 Jun 10;255(11):5481–5489. [PubMed] [Google Scholar]
- Roskoski R., Jr, Ngan P., Mettenburg R., Lund D. D. Isoproterenol injection alters protein kinase DEAE-cellulose profiles in selected rat tissues. Biochem Biophys Res Commun. 1978 May 30;82(2):641–647. doi: 10.1016/0006-291x(78)90923-3. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Type I cyclic AMP-dependent protein kinase as a positive effector of growth. Adv Cyclic Nucleotide Res. 1978;9:493–506. [PubMed] [Google Scholar]
- SELYE H., VEILLEUX R., CANTIN M. Excessive stimulation of salivary gland growth by isoproterenol. Science. 1961 Jan 6;133(3445):44–45. doi: 10.1126/science.133.3445.44. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Schäfer G., Lichtenberg V., Hilz H. Maximal steroidogenic capacity of mouse leydig cells. Kinetic analysis and dependence on protein kinase activation and cAMP accumulation. FEBS Lett. 1979 Nov 15;107(2):398–402. doi: 10.1016/0014-5793(79)80416-0. [DOI] [PubMed] [Google Scholar]
- Schwoch G. Differential activation of type-I and type-II adenosine 3':5'-cyclic monophosphate-dependent protein kinases in liver of glucagon-treated rats. Biochem J. 1978 Mar 15;170(3):469–477. doi: 10.1042/bj1700469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Hamann A. Determination and comparative analysis of the catalytic subunit of adenosine 3',5'-cyclic phosphate-dependent protein kinase by an enzyme-linked immunosorbent assay. Biochem J. 1982 Oct 15;208(1):109–117. doi: 10.1042/bj2080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Hamann A., Hilz H. Antiserum against the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Reactivity towards various protein kinases. Biochem J. 1980 Oct 15;192(1):223–230. doi: 10.1042/bj1920223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Lohmann S. M., Walter U., Jung U. Determination of cyclic AMP-dependent protein kinase subunits by an immunoassay reveals a different subcellular distribution of the enzyme in rat parotid than does determination of the enzyme activity. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(3):247–258. [PubMed] [Google Scholar]
- Singh T. J., Roth C., Gottesman M. M., Pastan I. H. Characterization of cyclic AMP-resistant Chinese hamster ovary cell mutants lacking type I protein kinase. J Biol Chem. 1981 Jan 25;256(2):926–932. [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Turnover of regulatory subunit of cyclic AMP-dependent protein kinase in S49 mouse lymphoma cells. Regulation by catalytic subunit and analogs of cyclic AMP. J Biol Chem. 1981 Nov 10;256(21):10731–10734. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang B. K., Rixon R. H., Whitfield J. F. A possible role for cyclic AMP in the initiation of DNA synthesis by isoproterenol-activated parotid gland cells. J Cell Physiol. 1980 Jan;102(1):19–26. doi: 10.1002/jcp.1041020104. [DOI] [PubMed] [Google Scholar]
- Weber W., Hilz H. cAMP-dependent protein kinases I and II: divergent turnover of subunits. Biochemistry. 1986 Sep 23;25(19):5661–5667. doi: 10.1021/bi00367a047. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Sikorska M., Tsang B. K. The regulation of cell proliferation by calcium and cyclic AMP. Mol Cell Biochem. 1979 Nov 1;27(3):155–179. doi: 10.1007/BF00215364. [DOI] [PubMed] [Google Scholar]