Abstract
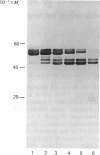
The latent forms of stromelysin and collagenase from human gingival fibroblasts were purified to homogeneity. These latent proenzymes underwent serial small reductions in Mr upon activation by treatment with either 4-aminophenylmercuric acetate or trypsin. Similar shifts in Mr and activation kinetics were observed upon identical treatments of either recombinant prostromelysin or procollagenase. Prostromelysin showed a lag between activation and Mr fall, suggesting an initial activation by conformational change. Collagenase activity was enhanced up to 12-fold by either natural or recombinant stromelysin in the presence of trypsin or 4-aminophenylmercuric acetate. Stromelysin caused a further apparent decrease in the Mr of procollagenase. Since these important connective-tissue-degrading enzymes are usually co-ordinately produced by cells, a cascade mechanism is proposed in which collagenase is activated by stromelysin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Clark E. J., Werb Z. Coordinate regulation of stromelysin and collagenase genes determined with cDNA probes. Proc Natl Acad Sci U S A. 1987 May;84(9):2600–2604. doi: 10.1073/pnas.84.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovíc J., Reynolds J. J., Murphy G. Inhibition of type I collagen film degradation by tumour cells using a specific antibody to collagenase and the specific tissue inhibitor of metalloproteinases (TIMP). Cell Biol Int Rep. 1985 Dec;9(12):1097–1107. doi: 10.1016/s0309-1651(85)80007-2. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Reynolds J. J. Immunolocalization of tissue inhibitor of metalloproteinases (TIMP) in human cells. Characterization and use of a specific antiserum. J Cell Sci. 1985 Feb;73:105–119. doi: 10.1242/jcs.73.1.105. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Ito A., Sakyo K., Mori Y. Procollagenase activator produced by rabbit uterine cervical fibroblasts. Biochem J. 1987 Jan 15;241(2):527–534. doi: 10.1042/bj2410527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- Nagase H., Brinckerhoff C. E., Vater C. A., Harris E. D., Jr Biosynthesis and secretion of procollagenase by rabbit synovial fibroblasts. Inhibition of procollagenase secretion by monensin and evidence for glycosylation of procollagenase. Biochem J. 1983 Aug 15;214(2):281–288. doi: 10.1042/bj2140281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J., Meikle M. C. Neutral metallo-proteinases of rabbit bone. Separation in latent forms of distinct enzymes that when activated degrade collagen, gelatin and proteoglycans. Biochem J. 1978 May 1;171(2):493–496. doi: 10.1042/bj1710493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklin G. P., Jeffrey J. J., Roswit W. T., Eisen A. Z. Human skin fibroblast procollagenase: mechanisms of activation by organomercurials and trypsin. Biochemistry. 1983 Jan 4;22(1):61–68. doi: 10.1021/bi00270a009. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Nagase H., Harris E. D., Jr Purification of an endogenous activator of procollagenase from rabbit synovial fibroblast culture medium. J Biol Chem. 1983 Aug 10;258(15):9374–9382. [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]