Abstract
This special feature results from the symposium ‘Ants 2016: ant interactions with their biotic environments’ held in Munich in May 2016 and deals with the interactions between ants and other insects, plants, microbes and fungi, studied at micro- and macroevolutionary levels with a wide range of approaches, from field ecology to next-generation sequencing, chemical ecology and molecular genetics. In this paper, we review key aspects of these biotic interactions to provide background information for the papers of this special feature. After listing the major types of biotic interactions that ants engage in, we present a brief overview of ant/ant communication, ant/plant interactions, ant/fungus symbioses, and recent insights about ants and their endosymbionts. Using a large molecular clock-dated Formicidae phylogeny, we map the evolutionary origins of different ant clades' interactions with plants, fungi and hemiptera. Ants' biotic interactions provide ideal systems to address fundamental ecological and evolutionary questions about mutualism, coevolution, adaptation and animal communication.
Keywords: ants, interactions, insects, plants, fungi, bacteria
1. Introduction
With over 13 000 named species [1], ants (Formicidae) are the largest group of eusocial insects. Like other eusocial organisms, ant societies have cooperative brood care, overlapping generations living in the same nest and a division of labour with reproductive and non-reproductive individuals (workers). With an estimated 10 000 trillions of individuals, they equal the global human biomass, and constitute most of the animal biomass of rainforests [2]. Key to their success are the myriad of interactions ants engage in with members of their own colony, other insects, fungi, microbes and plants (figure 1 and table 1). These interactions have long been used as systems in which to address evolutionary questions about mutualism, coevolution, adaptation and animal communication. In this review, we highlight some of the most important interactions between ants and their biotic environment, centred on four points relevant to this special feature: (i) ant/ant communication, (ii) interactions between ants and plants, (iii) ant/fungus symbioses, and (iv) ants and their endosymbionts. We also provide a timeline for the evolution of some of the major ant biotic interactions.
Figure 1.
The diversity of ant biotic interactions. (a,b) Ant/ant interactions. (a) Slavemaking ant Protomognathus americanus (black) and its host Temnothorax longispinosus. (b) Parabiosis between Crematogaster modiglianii (small, left) and Camponotus rufifemur (large, right). (c,d) Ant/other arthropod interactions. (c) ant/lycaenid interaction. (d) The rove beetle Diploeciton nevermanni is a social parasite of the army ant Neivamyrmex pilosus. (e–k) Ant/plant interactions. (e) Ant foraging on Senna scabriuscula extrafloral nectary. (f) Pheidole pallidula ant dispersing a Borderea chouardii seed. (g) Philidris nagasau ant farm of Squamellaria plants in Fiji. (h) Pseudomyrmex concolor living in Tachigali domatium and cultivating Chaetothyriales fungi inside the domatium (black patches). (i–k) Ant/pitcher plant interactions (Nepenthes). (i) Ant foraging on peristome nectaries. When it rains, the peristome undulates with raindrops, acting as a mechanism to catch ant prey. (j,k) Mutualistic interaction between Camponotus schmitzi and Nepenthes bicalcarata. (j) Camponotus schmitzi ants live inside the hollow petiole of N. bicalcarata (arrowhead) and are able to walk inside the pitcher and swim to steal Nepenthes prey (k). (l,m) Ant/fungus interaction (see also (l) Trachymyrmex ants farm fungus cultivar). (m) Allomerus ants cultivate fungi to make carton scaffold to catch insect prey, here a horsefly. (n) Ant/microorganism interaction. Blochmannia endosymbionts in bacteriocytes (green) in the midgut tisue of a pupa (shortly after pupation) of Camponotus floridanus ants. Red cells are midgut cells that do not (yet) contain any bacteria. Photo credit: (a) Susanne Foitzik. (b) Florian Menzel. (c) School of Ecology and Conservation, UAS Bangalore, India. (d) Christoph von Beeren. (e) Brigitte Marazzi. (f) María García, Xavier Espadaler, Jens Olesen. (g) Guillaume Chomicki. (h) Rumsais Blatrix. (i,k) Ulrike Bauer. (l) Scott Solomon. (m) Claude Delhaye. (n) Sascha Stoll.
Table 1.
Main types of ant biotic interactions.
| partners involved | type of interaction | references |
|---|---|---|
| ant/ant interactions | ||
| within colony interactions | cooperative | [3] |
| between colony interactions | antagonistic | [3] |
| between species interactions | mutualistic (parabiosis); parasitic (social parasitism) | [4,5]; figure 1a,b |
| ant/other arthropod interactions | ||
| ant/hemipteran interactions | mutualistic to parasitic | [6,7] |
| ant/butterfly interactions | mutualistic to parasitic | [8]; figure 1c |
| ant/mollusc interactions | parasitic | [9] |
| ant/diptera interactions | parasitic | [10] |
| ant/spider interactions | parasitic | [11] |
| ant/beetle interactions | parasitic | [12–14]; figure 1d |
| ant/wasp interactions | parasitic | [15] |
| ant/silverfishes interactions | parasitic | [16] |
| ant/plant interactions | ||
| extrafloral nectary/ant | mutualistic | [17]; figure 1e |
| elaiosome-based dispersal by ants | mutualistic | [18]; figure 1f |
| seed predation (harvester ants) | parasitic | [19] |
| domatium-based symbioses | mutualistic (rarely parasitic) | [20,21]; figure 1h |
| plant farming | mutualistic | [22,23]; figure 1g |
| ant/pitcher plant interactions | mutualistic or parasitic | [24,25]; figure 1i–k |
| ant/fungus interactions | ||
| attine-fungus farming | mutualistic (parasitic [Escovopsis]) | [26,27]; figure 1l |
| ant-Chaetothyriales-domatium interactions | mutualistic | [28,29]; figure 1h |
| ant–Chaetothyriales–carton interactions | mutualistic | [30]; figure 1m |
| ant/microorganism interactions | ||
| ant/bacterial endosymbionts | mutualistic or parasitic | [31–33]; figure 1n |
| ant/bacterial gut symbionts | mutualistic (but also likely commensal and parasitic) | [6] |
| ant/fungal gut symbionts | mutualistic | [6] |
2. Ant communication with other ants
Ants interact with a wide range of the organisms that are part of their biotic environment. These interactions are mediated by semiochemicals, including cuticular hydrocarbons (CHCs), chemical footprints, trail pheromones and alarm pheromones, and these chemicals are central to cooperation and conflict at distinct scales. This section and two papers in this special feature [4,34] focus on chemical communication.
While solitary insects mostly communicate for mating, eusocial insects have self-organized societies, where individuals communicate local information to mediate tasks, such as the division of labour, collective resource utilization and collective defensive actions [35]. CHCs are present on the cuticle of virtually all insects, and play central roles as waterproofing agents, but also function as semiochemicals. Because CHCs primarily serve a waterproofing role, one can wonder about the extent to which climate, especially air humidity and rainfall, shapes CHC evolution. Menzel et al. [4] address this question and find that the amount of rainfall in an ant's environment indeed influences CHC profiles.
Particularly important to ant colonies is queen signalling, which indicates the queen's presence and fertility, and signals workers to abandon their own reproduction and to help with brood care [3,34,36]. If the queen's fertility diminishes, non-sterile workers or subordinate reproductives can take over her function [36]. Queen signalling is mediated either by CHC compounds [37–39] or volatile pheromones, as in the fire ant Solenopsis invicta [40]. Caste influences the CHC profiles [34,41,42], thereby facilitating the self-organization of the division of labour [43]. Another crucial type of signal for the survival of the colony is nest-mate recognition, highlighted in this special feature [4].
Ant communication among nest-mates also concerns food sources: ant workers alert and motivate their nest-mates, and direct them to the food source using tandem running. Ant trail pheromones originate from several glands (ventral venom gland, poison gland, Dufour's gland, sternal gland or hindgut), and the origin of trail pheromones is often subfamily-specific [44–47]. Quality of the food source, and its quantity and distance from the nest, can be reflected either in the strength of the trail [48] or in the abundance of chemicals produced by distinct glands [44]. Finally, alarm pheromones occur in all ant species studied [2], with the responses depending on colony size and ant species. In the ant genus Cardiocondyla, when sexual conflict occurs, winged males mimic the CHC profile of females to protect themselves against wingless males attacks [49,50].
The interactions of ants with other ant species are of three main types, mutualistic, parasitic or competitive. In this special feature, two studies focus on the evolution of chemicals that mediate mutualistic (parabiotic) [4] and parasitic ant/ant interactions [34]. Parabiosis ([51]; figure 1b) is a mutualistic symbiosis between different ant species that involves nest sharing, joint foraging and aphid tending, whereas brood are kept separate [5,52]. The relation is asymmetric in that only one of the species locates the pheromone trails of the other [53,54]. This asymmetry is reminiscent of parasitic ant/ant interactions (see below) and thus suggests that parabiosis might have evolved from parasitic associations [55–57]. Two factors appear to maintain parabiosis as mutualistic and prevent aggression: distinct CHC profiles with long carbon chains (more than C35; [4], this special feature) allow an ant colony to differentiate its parabiont from other ant species, and appeasement pheromones on the cuticle suppress aggressiveness, as shown for a Camponotus/Crematogaster parabiosis [58]. Non-parabiotic ants, which have different CHC profiles and lack appeasement pheromones, are attacked as intruders [59,60].
About 220 ant species (out of more than 13 000) are parasites of other ant species, often living in the same nest as the ‘host’ ([61], figure 1a). Such parasites either exploit other species by using their pheromone trails to find the discovered food source [62] or use ‘eavesdropping’—using auditory cues as information on food sources [63]. Trail following is always unilateral [62]. Distinct classes of social parasites exist, depending on whether or not they keep their brood separate from the host, and whether or not they kill the queen (see [61] for a review). When parasites locate the host nest, they need to prevent attacks from the host. This can be achieved by parasites: (i) mimicking the CHC signature of their hosts [61]; (ii) acquiring it via wiping themselves against the host's surface (allogrooming) [64]; (iii) secreting a substance to appease the host [65]; (iv) chemically inducing fights among host workers to ease nest takeover [66,67]; and/or (v) reducing the CHC recognition cue so they become ‘invisible’ [34]. A particular type of social parasitism is slavemaking in which ants capture broods in raids to increase the worker force of their colony. In this special feature, Kleeberg et al. [34] show that ‘chemical camouflage’ evolved several times in slavemaking ants (figure 1a) and that the shifts in the CHC profiles that mediated the evolution of parasitism affected mostly the worker caste, but hardly the reproductive caste.
3. Interactions between ants and plants
Ants engage in a range of interactions with plants, including non-symbiotic defence mutualisms mediated by extrafloral nectaries (EFNs) ([17]; figure 1e), spore, seed or fruit dispersal (myrmecochory) ([18]; figure 1f), plant farming ([22,23,68]; figure 1g) and ant/plant symbioses ([20,21]; figure 1h). Ants are prey for carnivorous plants, such as Nepenthes, but at least one carnivorous species has domatia and houses ants (figure 1i–k and table 1). Defence mutualism and dispersal by ants are widespread both in the tropics and temperate regions, whereas ant/plant symbioses and plant farming are restricted to the tropics. Dispersal by ants involves lipid- and protein-rich appendages on seeds that serve as rewards for the ants that disperse the seeds. Ant/plant symbioses involve plants that provide nesting spaces (domatia) to ants in return for extra nutrients brought in by the ants and the killing of insect herbivores and vines that shade, and directly or indirectly damage, the ant-hosting plant [21]. Plant farming occurs in so-called ant-gardens, where ants plant the seeds of selected epiphytes inside their carton nest in return for nest stabilization and food, and an extreme case of plant farming occurs in Fiji, where the ant Philidris nagasau obligately farms six species of the Rubiaceae genus Squamellaria [23]. These ant/plant interactions must involve chemical communication in: (i) the selection of the particular seed species farmed in the gardens; (ii) the detection of host plants by founding queens; (iii) the discrimination of plants to prune from or near the host (but obviously not the host itself), (iv) the selective patrolling on young developing host shoots (but not other shoots); and (v) host damage-induced more active protection [69].
Ant-garden ants are able to identify their host plant's seeds by chemical cues present on the seed coat [70], and seeds from distantly related species that may be part of the same ant gardens have evolved convergent seed chemical signatures [70,71]. One of the key compounds (6-MMS) is also found on the ant head [71], suggesting that ant-gardens might involve chemical mimicry, as initially hypothesized by Ule [72]. Such seed-collecting behaviour occurs in southeast Asian ant gardens [73]. In Fiji the dolichoderine ant Philidris nagasau obligately farms not only by planting, but also fertilizing, six Squamellaria species (Rubiaceae) by defecating in the plant-provided nesting side (domatium) of these plants from the seedling stage on, in return for nesting space and food rewards ([23]; figure 1g). The chemical basis of the latter interaction is as yet unknown. In this issue, Chomicki et al. [68] reveal new aspects of the macroevolution of southeast Asian and Australasia ant gardens, including the independent origin of 13 ant-garden fern and flowering plant lineages, and the acquisition during evolution of further host lineages by the ants' broadening of their gardens' diversity.
An implicit consequence of specialized farming mutualisms, such as those between Squamellaria and Philidris nagasau in Fiji, is a structured population (with high relatedness on both the ant and the plant side) [23]. In this special feature, a theoretical study [74] reveals that population structure reduces the benefits from partner choice (a mechanism that stabilizes mutualisms by allowing active partner discrimination), as long as the benefits to symbionts are undirected. The Squamellaria/P. nagasau mutualism, however, shows population structure and partner choice with rewards directed to a specific partner, namely P. nagasau [23,75], suggesting that population structure and the specificity of benefits indeed need to be modelled together.
The efficiency of herbivore protection by plant-nesting ants (in the context of local herbivore pressure) plays a central role in the stability of ant/plant and ant/plant/fungus symbioses. In this special feature, Orivel et al. [76] reveal trade-offs in ant–plant–fungus mutualisms as a result of the same traits being simultaneously involved in two mutualisms. Ants nest in domatia, but cultivate fungi in a carton scaffold that workers use to hide and trap insects (figure 1m). More investment in fungi leads to less plant defence, and species that provide too little defence should be displaced in the long term [76]. Reduction in plant defence can also occur as a result of abiotic stress, such as that experienced at high altitudes [77]. An example of this is provided by the results of Plowman et al. [78], who report on ant/plant mutualistic networks along an elevation gradient in Papua New Guinea and find that the benefits for the plant are reduced at high altitudes.
Convergent evolution is frequent in species interactions [79], probably because of similar biotic, abiotic and phylogenetic constraints. A particular form of convergence is parallel evolution, wherein similar traits evolved in related species but from different lineages. Phylogenetic analysis revealed such parallel evolution in the iconic ant/plant symbiosis involving Pseudomyrmex ants that protect Vachellia trees [80], and in this special feature, Ward & Branstetter [81] now use a phylogenomic approach to show that two closely related Pseudomyrmex lineages independently evolved traits related to obligate domatium-living. Detecting such cases of independent evolution, impossible without abundant genomic data, is important for our understanding of the flexibility of specialization and generalization.
4. Ant interactions with fungi
A particularly fascinating mutualism is that between attine ants and fungi (figure 1l). Attine ants obligately depend on mutualistic fungi for food. Five types of fungal farming by attine ants are known, from the generalist agriculture practised by the basal-most attine lineages to the specialized agriculture of a single fungal clone practised by the higher attines (including leafcutter ants) (reviewed in [26]). Attine farming mutualisms involve two further protagonists, the parasitic fungi in the genus Escovopsis [27], which also coevolved as part of tripartite associations [82], and the mutualistic Pseudonocardia bacteria that densely cover the lower part of these ants' thorax and that produce an antibiotic effective against Escovopsis [83,84].
Ants also interact with Chaetothyriales fungi, either as part of the carton nest building process where arboreal ants use fungal hyphae to strengthen the nest or trap insects ([30,85], figure 1m) or inside plant domatia as part of an ant/plant symbiosis ([28,29], figure 1h). Some domatium-living Chaetothyriales seem to be cultivated by ants and are involved in a complex trophic mutualism where the ants (especially larvae) feed on the fungi, which they actively fertilize by defecation [86], and the fungi play a role in nutrient recycling as well as facilitating nutrient uptake by breaking down materials [87]. In this special feature, Vasse et al. [88] show that ant/Chaetothyriales interactions evolved multiple times and that carton nest fungi and domatium fungi form different lineages. Another breakthrough published in this special feature is the finding by Baker et al. [89], who report community-level differences in domatium fungi and evidence that ant queens transport fungal communities when colonizing new trees.
5. Ants and their endosymbionts
Ant endosymbionts are microorganisms living either in specialized intracellular structures or in the ant gut. Endosymbiotic microbiomes may be central to the success of ants in tropical canopies and in other areas depleted in proteins ([6,90], this special issue). The so far best-known (obligate) bacterial endosymbiosis with ants involves Camponotus (the largest ant genus with more than 1000 species) and Blochmannia bacteria living in structures called bacteriocytes ([91], figure 1n), and these endosymbionts are also found in related genera [31]. Bacteriocytes in ovules are transmitted maternally [32,33], which has led to cospeciation between Blochmannia and Camponotus [92]. The bacteria provide the ants with essential amino acids and potentially recycle nitrogen by using ant urea that they can break down with their functional urease [31,93]. Besides these intracellular endosymbionts, ants have extracellular bacterial endosymbionts in the gut. This has been documented in Tetraponera (Pseudomyrmecinae) ants, which have a dense aggregation of gut bacteria that fix nitrogen and supplement the ants' phloem-based diet [94]. In this special feature, Pringle & Moreau [6] reveal new parts of the endosymbiont puzzle: ants share gut endosymbionts with their hemipteran trophobionts (via the honeydew they feed on). Moreover, ants have many facultative symbionts, and for the first time, a fungal endosymbiosis is discovered in Azteca [6].
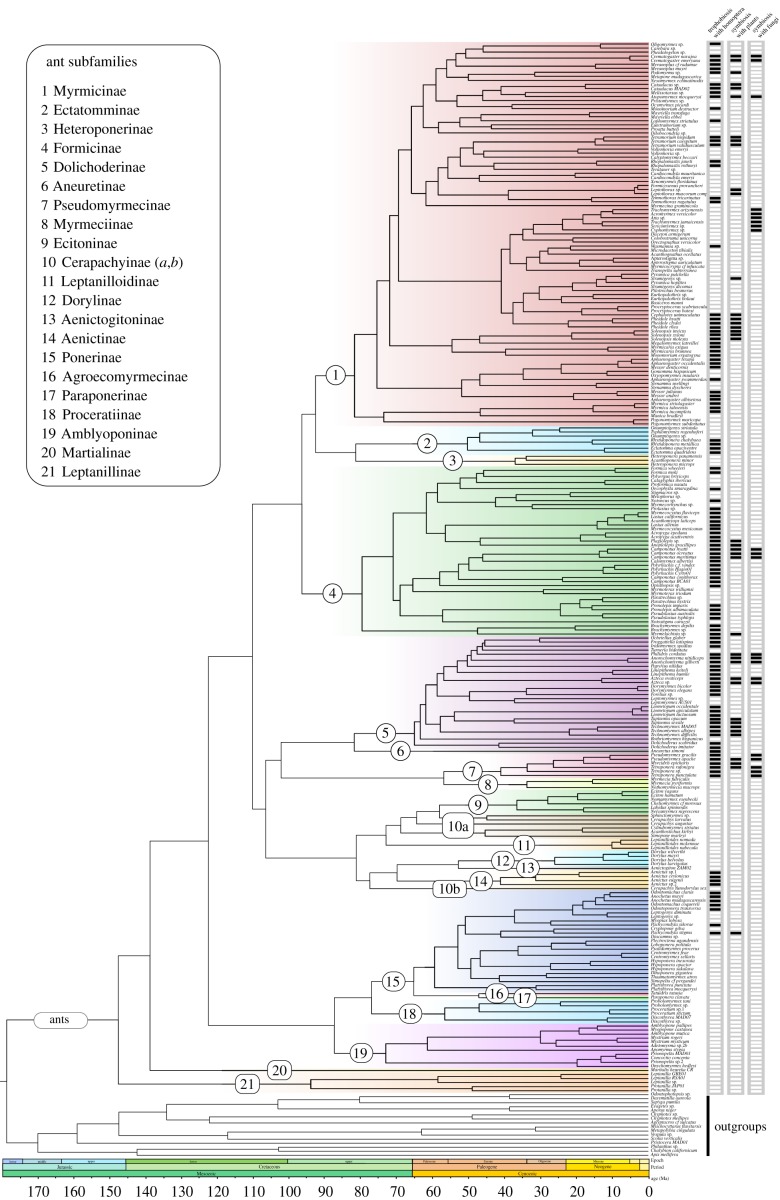
6. The timeline of ant interactions with fungi, hemiptera and plants
The first unambiguous ant fossils date from the Lower Cretaceous [95,96], and molecular clock analyses have dated the ant crown group to 120–160 million years ago (Ma) [97–99]. Attine fungiculture is 50–60 million years old [100,101] and has a single evolutionary origin, but leafcutter ants appear to have evolved only 8–12 Ma [100]. However, several other ant lineages also use and farm fungi (figure 2). Interactions between ants and hemiptera (a suborder of Rhynchota) vary from generalist and facultative to specialized and obligate, and involve a wide range of aphids that provide honeydew or ‘meat’ [7]. They have evolved multiple times during ant evolutionary history (figure 2), and dating associations with hemipterans thus will require densely sampled phylogenies for both groups. Fossils in amber have revealed ant/hemipteran associations in the Eocene (34–55 Ma) [102] and Early Miocene (15–20 Ma) [103,104], but comparative phylogenetic analyses are still lacking.
Figure 2.
Phylogenetic distribution of ant symbioses with hemipterans, plants, and fungi. The tree from Moreau & Bell [99] was kindly provided by Corrie Moreau.
Regarding interactions with plants, phylogenetic dating and trait analyses suggest that elaiosomes (fatty seed appendages) that mediate plant dispersal by ants evolved as early as 75 Ma [105]. Non-symbiotic defence mutualisms based on EFNs are documented in the fossil record since the Oligocene (23–34 Ma; [106]), and evolved over 450 times in vascular plants [17], with the earliest EFNs originating at least 35–40 Ma [107–109]. Ant/plant symbioses mediated by plant-formed nesting sites (domatia) have at least 158 independent origins, starting some 15 Ma in the Neotropics and Australasia, and as recently as 5 Ma in Africa [21].
7. Conclusion
In organizing the symposium ‘Ants 2016: ant interactions with their biotic environments’ and this special feature issue, our aim was to bring together workers in this field to foster exchange and encourage integrative approaches. This Introduction has highlighted some of the ways through which ants interact with their biotic environment, and we also offer a new timeline (figure 2) for the evolution of interactions with hemipterans, plants and fungi. Studying interactions between ants and other species provides an opportunity to address fundamental and timely ecological and evolutionary questions.
A central theme that remains poorly studied are the genetic bases of many ant biotic interactions, which will probably emerge in the next decade, with several ant genomes published (e.g. the fire ant: Solenopsis invicta [110]; the Argentine ant: Linepithema humile [111]; the red harvester ant: Pogonomyrmex barbatus [112]; the leafcutter ant Atta cephalotes [113] and Pseudomyrmex plant ants [114]) and ever faster and low-cost, next-generation sequencing approaches and gene expression studies for targeted tissues and genes. Genomic approaches will allow a new understanding of ant biotic interactions by revealing discrete or genome-wide selection, gene duplication or loss or gene expression changes linked to new interactions [8,115]. Another emerging theme is multi-partite interactions, how they evolve and how the benefits are negotiated between species. Unravelling these and other themes relevant to the interactions between ants and their biotic environment will require the integration of multiple approaches, as exemplified in this special feature.
Acknowledgements
We thank all participants to the symposium ‘Ants 2016: ant interactions with their biotic environments' held in Munich on 5–7 May 2016. We thank Ulrike Bauer, Christoph von Beeren, Rumsais Blatrix, Heike Feldhaar, Susanne Foitzik, Brigitte Marazzi, Florian Menzel, Jérôme Orivel and Scott Solomon for providing photographs for figure 1 and Corrie Moreau for sharing a large ant tree.
Competing interests
We declare we have no competing interests.
Funding
Our research is supported by the DFG grants RE 603/20-1 and -2, and we are also grateful to the DFG for funding the symposium Ants 2016 (grant no. RE 603/23-1).
References
- 1.Bolton B.2012. AntCat. An online catalog of the ants of the world. See http://www.antcat.org .
- 2.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Leonhardt SD, Menzel F, Nehring V, Schmitt T. 2016. Ecology and evolution of communication in social insects. Cell 164, 1277–1287. ( 10.1016/j.cell.2016.01.035) [DOI] [PubMed] [Google Scholar]
- 4.Menzel F, Blaimer BB, Schmitt T. 2017. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzel F, Blüthgen N. 2010. Parabiotic associations between tropical ants: equal partnership or parasitic exploitation? J. Anim. Ecol. 79, 71–81. ( 10.1111/j.1365-2656.2009.01628.x) [DOI] [PubMed] [Google Scholar]
- 6.Pringle E, Moreau CS. 2017. Community analysis reveals microbial sharing and specialization in a Costa Rican ant-plant-hemipteran symbiosis. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadler B, Dixon AF. 2005. Ecology and evolution of aphid-ant interactions. Annu. Rev. Ecol. Evol. Syst. 36, 345–372. ( 10.1146/annurev.ecolsys.36.091704.175531) [DOI] [Google Scholar]
- 8.Pierce NE, Braby MF, Heath A, Lohman DJ, Mathew J, Rand DB, Travassos MA. 2002. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Ann. Rev. Entomol. 47, 733–771. ( 10.1146/annurev.ento.47.091201.145257) [DOI] [PubMed] [Google Scholar]
- 9.Witte V, Janssen R, Eppenstein A, Maschwitz U. 2002. Allopeas myrmekophilos (Gastropoda, Pulmonata), the first myrmecophilous mollusc living in colonies of the ponerine army ant Leptogenys distinguenda (Formicidae, Ponerinae). Insect. Soc. 49, 301–305. ( 10.1007/PL00012646) [DOI] [Google Scholar]
- 10.Elmes GW, Barr B, Thomas JA, Clarke RT. 1999. Extreme host specificity by Microdon mutabilis (Diptera: Syrphidae), a social parasite of ants. Proc. R. Soc. Lond. B 266, 447–453. ( 10.1098/rspb.1999.0658) [DOI] [Google Scholar]
- 11.von Beeren C, Hashim R, Witte V. 2012. The social integration of a myrmecophilous spider does not depend exclusively on chemical mimicry. J. Chem. Ecol. 38, 262–271. ( 10.1007/s10886-012-0083-0) [DOI] [PubMed] [Google Scholar]
- 12.von Beeren C, Maruyama M, Kronauer DJ. 2016. Community sampling and integrative taxonomy reveal new species and host specificity in the army ant-associated beetle genus Tetradonia (Coleoptera, Staphylinidae, Aleocharinae). PLoS ONE 11, e0165056 ( 10.1371/journal.pone.0165056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoeffler M, Maier TS, Tolasch T, Steidle JLM. 2007. Foreign-language skills in rove-beetles? Evidence for chemical mimicry of ant alarm pheromones in myrmecophilous Pella beetles (Coleoptera: Staphylinidae). J. Chem. Ecol. 33, 1382–1392. ( 10.1007/s10886-007-9315-0) [DOI] [PubMed] [Google Scholar]
- 14.Fikáček M, Maruyama M, Komatsu T, von Beeren C, Vondráček D, Short AE. 2015. Protosternini (Coleoptera: Hydrophilidae) corroborated as monophyletic and its larva described for the first time: a review of the myrmecophilous genus Sphaerocetum. Invert. Syst. 29, 23–36. ( 10.1071/IS14026) [DOI] [Google Scholar]
- 15.Gómez Durán J-M, van Achterberg C. 2011. Oviposition behaviour of four ant parasitoids (Hymenoptera, Braconidae, Euphorinae, Neoneurini and Ichneumonidae, Hybrizontinae), with the description of three new European species. ZooKeys 106, 59–106. ( 10.3897/zookeys.125.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Beeren C, Schulz S, Hashim R, Witte V. 2011. Acquisition of chemical recognition cues facilitates integration into ant societies. BMC Ecol. 11, 30 ( 10.1186/1472-6785-11-30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber MG, Keeler KH. 2013. The phylogenetic distribution of extrafloral nectaries in plants. Ann. Bot. 111, 1251–1261. ( 10.1093/aob/mcs225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengyel S, Gove AD, Latimer AM, Majer JD, Dunn RR. 2010. Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: a global survey. Perspect. Plant Ecol. 12, 43–55. ( 10.1016/j.ppees.2009.08.001) [DOI] [Google Scholar]
- 19.Taber SW. 1999. The world of the harvester ants. College Station, TX: Texas A&M University Press. [Google Scholar]
- 20.Davidson DW, McKey D. 1993. The evolutionary ecology of symbiotic ant/plant relationships. J. Hymenopt. Res. 2, 13–83. [Google Scholar]
- 21.Chomicki G, Renner SS. 2015. Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytol. 207, 411–424. ( 10.1111/nph.13271) [DOI] [PubMed] [Google Scholar]
- 22.Davidson DW. 1988. Ecological studies of neotropical ant gardens. Ecology 69, 1138–1152. ( 10.2307/1941268) [DOI] [Google Scholar]
- 23.Chomicki G, Renner SS. 2016. Obligate plant farming by a specialized ant. Nat. Plants 2, 16181 ( 10.1038/nplants.2016.181) [DOI] [PubMed] [Google Scholar]
- 24.Bohn HF, Federle W. 2004. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl Acad. Sci. USA 101, 14 138–14 143. ( 10.1073/pnas.0405885101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scharmann M, Thornham DG, Grafe TU, Federle W. 2013. A novel type of nutritional ant–plant interaction: ant partners of carnivorous pitcher plants prevent nutrient export by dipteran pitcher in fauna. PLoS ONE 8, e63556 ( 10.1371/journal.pone.0063556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehdiabadi NJ, Schultz TR. 2010. Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecol. News 13, 37–55. [Google Scholar]
- 27.Currie CR, Mueller UG, Malloch D. 1999. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA 96, 7998–8002. ( 10.1073/pnas.96.14.7998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Defossez E, Selosse MA, Dubois MP, Mondolot L, Faccio A, Djieto-Lordon C, McKey D, Blatrix R. 2009. Ant-plants and fungi: a new threeway symbiosis. New Phytol. 182, 942–949. ( 10.1111/j.1469-8137.2009.02793.x) [DOI] [PubMed] [Google Scholar]
- 29.Mayer VE, Frederickson ME, McKey D, Blatrix R. 2014. Current issues in the evolutionary ecology of ant–plant symbioses. New Phytol. 202, 749–764. ( 10.1111/nph.12690) [DOI] [PubMed] [Google Scholar]
- 30.Nepel M, Voglmayr H, Schönenberger J, Mayer VE. 2014. High diversity and low specificity of Chaetothyrialean fungi in carton galleries in a Neotropical ant–plant association. PLoS ONE 9, e112756 ( 10.1371/journal.pone.0112756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldhaar H, Straka J, Krischke M, Berthold K, Stoll S, Mueller MJ, Gross R. 2007. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 5, 48 ( 10.1186/1741-7007-5-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumann P, Baumann L, Lai CY, Rouhbakhsh D, Moran NA, Clark MA. 1995. Genetics, physiology, and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu. Rev. Microbiol. 49, 55–94. ( 10.1146/annurev.mi.49.100195.000415) [DOI] [PubMed] [Google Scholar]
- 33.Sauer C, Stackebrandt E, Gadau J, Hölldobler B, Gross R. 2000. Systematic relationships and cospeciation of bacterial endosymbionts and their carpenter ant host species: proposal of the new taxon Candidatus Blochmannia gen. nov. Int. J. Syst. Evol. Microbiol. 5, 1877–1886. ( 10.1099/00207713-50-5-1877) [DOI] [PubMed] [Google Scholar]
- 34.Kleeberg I, Menzel F, Foitzik S. 2017. The influence of slavemaking lifestyle, caste and sex on chemical profiles in Temnothorax ants: insights into the evolution of cuticular hydrocarbons. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyatt TD. 2014. Pheromones and animal behaviour: chemical signals and signatures. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Keller L, Nonacs P. 1993. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787–794. ( 10.1006/anbe.1993.1092) [DOI] [Google Scholar]
- 37.Hannonen M, Sledge MF, Turillazzi S, Sundström L. 2002. Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim. Behav. 64, 477–485. ( 10.1006/anbe.2002.4001) [DOI] [Google Scholar]
- 38.Endler A, Liebig J, Hölldobler B. 2006. Queen fertility, egg marking and colony size in the ant Camponotus floridanus. Behav. Ecol. Sociobiol. 59, 490–499. ( 10.1007/s00265-005-0073-0) [DOI] [Google Scholar]
- 39.Holman L, Jørgensen CG, Nielsen J, d'Ettorre P. 2010. Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. B. 277, 3793–3800. ( 10.1098/rspb.2010.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vargo EL, Hulsey CD. 2000. Multiple glandular origins of queen pheromones in the fire ant Solenopsis invicta. J. Insect Physiol. 46, 1151–1159. ( 10.1016/S0022-1910(99)00226-7) [DOI] [PubMed] [Google Scholar]
- 41.Pamminger T, Foitzik S, Kaufmann KC, Schützler N, Menzel F. 2014. Worker personality and its association with spatially structured division of labor. PLoS ONE 9, e79616 ( 10.1371/journal.pone.0079616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner D, Tissot M, Gordon D. 2001. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 27, 1805–1819. ( 10.1023/A:1010408725464) [DOI] [PubMed] [Google Scholar]
- 43.Greene MJ, Gordon DM. 2003. Social insects: cuticular hydrocarbons inform task decisions. Nature 423, 32 ( 10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 44.Chapman RF. 1998. The insects: structure and function. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Witte V, Abrell L, Attygalle AB, Wu X, Meinwald J. 2007. Structure and function of Dufour gland pheromones from the crazy ant Paratrechina longicornis. Chemoecology 17, 63–69. ( 10.1007/s00049-006-0365-5) [DOI] [Google Scholar]
- 46.Hölldobler B, Wilson EO. 2009. The Superorganism: the beauty, elegance, and strangeness of insect societies. New York, NY: W W Norton & Company. [Google Scholar]
- 47.Morgan DE. 2009. Trail pheromones of ants. Physiol. Entomol. 34, 1–17. ( 10.1111/j.1365-3032.2008.00658.x) [DOI] [Google Scholar]
- 48.Wilson EO, Pavan M. 1959. Source and specificity of chemical releasers of social behaviour in the dolichoderine ants. Psyche 65, 41–51. ( 10.1155/1958/57483) [DOI] [Google Scholar]
- 49.Cremer S, Sledge M, Heinze J. 2002. Chemical mimicry: male ants disguised by the queen's bouquet. Nature 419, 897 ( 10.1038/419897a) [DOI] [PubMed] [Google Scholar]
- 50.Heinze J. 2017. Life history evolution in ants: the case of Cardiocondyla. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forel A. 1898. La parabiose chez les fourmis. Bull. Soc. Vaud. Sc. Nat. 34, 380–384. [Google Scholar]
- 52.Vantaux A, Dejean A, Dor A, Orivel J. 2007. Parasitism versus mutualism in the ant garden parabiosis between Camponotus femoratus and Crematogaster levior. Insect. Soc. 54, 95–99. ( 10.1007/s00040-007-0914-0) [DOI] [Google Scholar]
- 53.Menzel F, Pokorny T, Blüthgen N, Schmitt T. 2010. Trail-sharing among tropical ants: interspecific use of trail pheromones? Ecol. Entomol. 35, 495–503. ( 10.1111/j.1365-2311.2010.01206.x) [DOI] [Google Scholar]
- 54.Menzel F, Orivel J, Kaltenpoth M, Schmitt T. 2014. What makes you a potential partner? Insights from convergently evolved ant-ant symbioses. Chemoecology 24, 105–119. ( 10.1007/s00049-014-0149-2) [DOI] [Google Scholar]
- 55.Bronstein JL. 2001. The exploitation of mutualisms. Ecol. Lett. 4, 277–287. ( 10.1046/j.1461-0248.2001.00218.x) [DOI] [Google Scholar]
- 56.Bronstein JL. 1994. Conditional outcomes in mutualistic interactions. Trends Ecol. Evol. 9, 214–217. ( 10.1016/0169-5347(94)90246-1) [DOI] [PubMed] [Google Scholar]
- 57.Ferriere R, Bronstein JL, Rinaldi S, Law R, Gauduchon M. 2002. Cheating and the evolutionary stability of mutualisms. Proc. R. Soc. Lond. B 269, 773–780. ( 10.1098/rspb.2001.1900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menzel F, Blüthgen N, Tolasch T, Conrad J, Beifuß U, Beuerle T, Schmitt T. 2013. Crematoenones: a novel substance class exhibited by ants functions as appeasement signal. Front. Zool. 10, 32 ( 10.1186/1742-9994-10-32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menzel F, Linsenmair KE, Blüthgen N. 2008. Selective interspecific tolerance in tropical Crematogaster–Camponotus associations. Anim. Behav. 75, 837–846. ( 10.1016/j.anbehav.2007.07.005) [DOI] [Google Scholar]
- 60.Menzel F, Kriesell H, Witte V. 2014. Parabiotic ants: the costs and benefits of symbiosis. Ecol. Entomol. 39, 436–444. ( 10.1111/een.12116) [DOI] [Google Scholar]
- 61.Lenoir A, d'Ettorre P, Errard C, Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. ( 10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 62.Menzel F, Woywod M, Blüthgen N, Schmitt T. 2010. Behavioural and chemical mechanisms behind a Mediterranean ant-ant association. Ecol. Entomol. 35, 711–720. ( 10.1111/j.1365-2311.2010.01231.x) [DOI] [Google Scholar]
- 63.Powell S, Del-Claro K, Feitosa RM, Brandão CRF. 2014. Mimicry and eavesdropping enable a new form of social parasitism in ants. Am. Nat. 184, 500–509. ( 10.1086/677927) [DOI] [PubMed] [Google Scholar]
- 64.Lenoir A, Malosse C, Yamaoka R. 1997. Chemical mimicry between parasitic ants of the genus Formicoxenus and their host Myrmica (Hymenoptera, Formicidae). Biochem. Syst. Ecol. 25, 379–389. ( 10.1016/S0305-1978(97)00025-2) [DOI] [Google Scholar]
- 65.Witte V, Lehmann L, Lustig A, Maschwitz U. 2009. Polyrhachis lama, a parasitic ant with an exceptional mode of social integration. Insect. Soc. 56, 301–307. ( 10.1007/s00040-009-0024-2) [DOI] [Google Scholar]
- 66.Bauer S, Witte V, Böhm M, Foitzik S. 2009. Fight or flight? A geographic mosaic in host reaction and potency of a chemical weapon in the social parasite Harpagoxenus sublaevis. Behav. Ecol. Sociobiol. 64, 45–56. ( 10.1007/s00265-009-0817-3) [DOI] [Google Scholar]
- 67.Jongepier E, Kleeberg I, Foitzik S. 2015. The ecological success of a social parasite increases with manipulation of collective host behaviour. J. Evol. Biol. 28, 2152–2162. ( 10.1111/jeb.12738) [DOI] [PubMed] [Google Scholar]
- 68.Chomicki G, Janda M, Renner SS. 2017. The assembly of ant-farmed gardens: mutualism specialization following host broadening. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blatrix R, Mayer V. 2010. Communication in ant–plant symbioses. In Plant communication from an ecological perspective (eds F Baluška, V Ninkovic), pp. 127–158. Berlin, Germany: Springer. [Google Scholar]
- 70.Youngsteadt E, Nojima S, Häberlein C, Schulz S, Schal C. 2008. Seed odor mediates an obligate ant-plant mutualism in Amazonian rainforests. Proc. Natl Acad. Sci. USA 105, 4571–4575. ( 10.1073/pnas.0708643105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seidel JL, Epstein WW, Davidson DW. 1990. Neotropical ant gardens. I. Chemical constituents. J. Chem. Ecol. 16, 1791–1816. ( 10.1007/BF010204950) [DOI] [PubMed] [Google Scholar]
- 72.Ule E. 1906. Blumengarten der Ameisen am Amazonstrome. Vegetationsbilder 3, Heft 1. [Google Scholar]
- 73.Kaufmann E. 2002. Southeast Asian ant-gardens: diversity, ecology, ecosystematic significance, and evolution of mutualistic ant-epiphyte associations. PhD thesis, University Frankfurt, Germany.
- 74.Akçay E. 2017. Population structure reduces the benefits from partner choice in mutualism. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chomicki G, Staedler Y, Schönenberger J, Renner SS. 2016. Partner choice through concealed floral sugar rewards evolved with the specialization of ant/plant mutualisms. New Phytol. 211, 1358–1370. ( 10.1111/nph.13990) [DOI] [PubMed] [Google Scholar]
- 76.Orivel J, Malé P-J, Lauth J, Roux O, Petitclerc F, Dejean A, Leroy C. 2017. Trade-offs in mutualistic investment in a tripartite symbiosis. Proc. R. Soc. B. [Google Scholar]
- 77.Koptur S. 1985. Alternative defenses against herbivores in Inga (Fabaceae: Mimosoideae) over an elevational gradient. Ecology 66, 1639–1650. ( 10.2307/1938026) [DOI] [Google Scholar]
- 78.Plowman NS, Hoodc ASC, Moses J, Redmond C, Novotny V, Klimes P, Fayle TM. 2017. Network reorganisation and breakdown of an ant-plant protection mutualism with elevation. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bittleston LS, Pierce NE, Ellison AM, Pringle A. 2016. Convergence in multispecies interactions. Trends Ecol. Evol. 31, 269–280. ( 10.1016/j.tree.2016.01.006) [DOI] [PubMed] [Google Scholar]
- 80.Chomicki G, Ward PS, Renner SS. 2015. Macroevolutionary assembly of ant/plant symbioses: Pseudomyrmex ants and their ant-housing plants in the Neotropics. Proc. R. Soc. B 282, 20152200 ( 10.1098/rspb.2015.2200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ward P, Branstetter M. 2017. The acacia ants revisited: convergent evolution and biogeographic context in an iconic ant/plant mutualism. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Mueller UG, Sung GH, Spatafora JW, Straus NA. 2003. Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299, 386–388. ( 10.1126/science.1078155) [DOI] [PubMed] [Google Scholar]
- 83.Currie CR, Scott JA, Summerbell RC, Malloch D. 1999. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704. ( 10.1038/195190) [DOI] [Google Scholar]
- 84.Haeder S, Wirth R, Herz H, Spiteller D. 2009. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc. Natl Acad. Sci. USA 106, 4742–4746. ( 10.1073/pnas.0812082106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruiz-González MX, Malé PJG, Leroy C, Dejean A, Gryta H, Jargeat P, Quilichini A, Orivel J. 2011. Specific, non-nutritional association between an ascomycete fungus and Allomerus plant-ants. Biol. Lett. 7, 475–479. ( 10.1098/rsbl.2010.0920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blatrix R, Djiéto-Lordon C, Mondolot L, La Fisca P, Voglmayr H, McKey D. 2012. Plant-ants use symbiotic fungi as a food source: new insight into the nutritional ecology of ant–plant interactions. Proc. R. Soc. B 279, 3940–3947. ( 10.1098/rspb.2012.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Defossez E, Djiéto-Lordon C, McKey D, Selosse MA, Blatrix R. 2011. Plant-ants feed their host plant, but above all a fungal symbiont to recycle nitrogen. Proc. R. Soc. B 278, 1419–1426. ( 10.1098/rspb.2010.1884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vasse M, et al. 2017. A phylogenetic perspective of the association between ants (Hymenoptera: Formicidae) and black yeasts (Ascomycota: Chaetothyriales). Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baker C, Pelaez J, Billen J, Pringle A, Frederickson M, Pierce N. 2017. Fungal communities in an obligate African ant/plant mutualism. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davidson DW, Cook SC, Snelling RR, Chua TH. 2003. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972. ( 10.1126/science.1082074) [DOI] [PubMed] [Google Scholar]
- 91.Blochmann F. 1892. Über das Vorkommen von bakterie nähnlichen Gebilden in den Geweben und Eiern verschiedener Insekten. Zentralbl. Bakteriol. 11, 234–240. [Google Scholar]
- 92.Degnan PH, Lazarus AB, Brock CD, Wernegreen JJ. 2004. Host–symbiont stability and fast evolutionary rates in an ant–bacterium association: cospeciation of Camponotus species and their endosymbionts, Candidatus blochmannia. Syst. Biol. 53, 95–110. ( 10.1080/10635150490264842) [DOI] [PubMed] [Google Scholar]
- 93.Zientz E, Feldhaar H, Stoll S, Gross R. 2005. Insights into the microbial world associated with ants. Arch. Microbiol. 184, 199–206. ( 10.1007/s00203-005-0041-0) [DOI] [PubMed] [Google Scholar]
- 94.Van Borm S, Buschinger A, Boomsma JJ, Billen J. 2002. Tetraponera ants have gut symbionts related to nitrogen-fixing root-nodule bacteria. Proc. R. Soc. B 269, 2023–2027. ( 10.1098/rspb.2002.2101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agosti D, Grimaldi D, Carpenter JM. 1998. Oldest known ant fossils discovered. Nature 391, 447 ( 10.1038/35051) [DOI] [Google Scholar]
- 96.Nel A, Perrault G, Perrichot V, Néradeau D. 2004. The oldest ant in the lower Cretaceous amber of Charente-maritime (SW France) (Insecta: Hymenoptera: Formicidae). Geol. Acta 2, 23–30. ( 10.1344/105.000001630) [DOI] [Google Scholar]
- 97.Brady SG, Schultz TR, Fisher BL, Ward PS. 2006. Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc. Natl Acad. Sci. USA 103, 18 172–18 177. ( 10.1073/pnas.0605858103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moreau CS, Bell CD, Vila R, Archibald SB, Pierce NE. 2006. Phylogeny of the ants: diversification in the age of angiosperms. Science 312, 101–104. ( 10.1126/science.1124891) [DOI] [PubMed] [Google Scholar]
- 99.Moreau CS, Bell CD. 2013. Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution 67, 2240–2257. ( 10.1111/evo.12105) [DOI] [PubMed] [Google Scholar]
- 100.Schultz TR, Brady SG. 2008. Major evolutionary transitions in ant agriculture. Proc. Natl Acad. Sci. USA 105, 5435–5440. ( 10.1073/pnas.0711024105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mikheyev AS, Mueller UG, Abbot P. 2010. Comparative dating of attine ant and lepiotaceous cultivar phylogenies reveals coevolutionary synchrony and discord. Am. Nat. 175, E126–E133. ( 10.1086/652472) [DOI] [PubMed] [Google Scholar]
- 102.Dlussky GM. 1997. Genera of ants (Hymenoptera: Formicidae) from Baltic amber. Paleontol. J. 31, 616–627. [Google Scholar]
- 103.Johnson C, Agosti D, Delabie JH, Dumpert K, Williams DJ, Tschirnhaus MV, Maschwitz U. 2001. Acropyga and Azteca ants (Hymenoptera: Formicidae) with scale insects (Sternorrhyncha: Coccoidea): 20 million years of intimate symbiosis. Am. Mus. Novit. 3335, 1–18. ( 10.1206/0003-0082(2001)335) [DOI] [Google Scholar]
- 104.LaPolla JS. 2005. Ancient trophophoresy: a fossil Acropyga (Hymenoptera: Formicidae) from Dominican amber. T. Am. Entomol. Soc. 131, 21–28. [Google Scholar]
- 105.Dunn RR, Gove AD, Barraclough TG, Givnish TJ, Majer JD. 2007. Convergent evolution of an ant–plant mutualism across plant families, continents, and time. Evol. Ecol. Res. 9, 1349–1362. [Google Scholar]
- 106.Pemberton RW. 1992. Fossil extrafloral nectaries, evidence for the ant-guard antiherbivore defense in an Oligocene Populus. Am. J. Bot. 79, 1242–1246. ( 10.2307/2445051) [DOI] [Google Scholar]
- 107.Marazzi B, Sanderson MJ. 2010. Large-scale patterns of diversification in the widespread legume genus Senna and the evolutionary role of extrafloral nectaries. Evolution 64, 3570–3592. ( 10.1111/j.1558-5646.2010.01086.x) [DOI] [PubMed] [Google Scholar]
- 108.Muschner VC, Zamberlan PM, Bonatto SL, Freitas LB. 2012. Phylogeny, biogeography and divergence times in Passiflora (Passifloraceae). Genet. Mol. Biol. 35, 1036–1043. ( 10.1590/S1415-47572012000600019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spriggs EL, Clement WL, Sweeney PW, Madriñán S, Edwards EJ, Donoghue MJ. 2015. Temperate radiations and dying embers of a tropical past: the diversification of Viburnum. New Phytol. 207, 340–354. ( 10.1111/nph.13305) [DOI] [PubMed] [Google Scholar]
- 110.Wurm Y, et al. 2011. The genome of the fire ant Solenopsis invicta. Proc. Natl Acad. Sci. USA 108, 5679–5684. ( 10.1073/pnas.1009690108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith CD, et al. 2011. Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl Acad. Sci. USA 108, 5673–5678. ( 10.1073/pnas.1008617108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith CR, et al. 2011. Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc. Natl Acad. Sci. USA 108, 5667–5672. ( 10.1073/pnas.1007901108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suen G, et al. 2011. The genome sequence of the leaf-cutter ant Atta cephalotes reveals insights into its obligate symbiotic lifestyle. PLoS Genet. 7, e1002007 ( 10.1371/journal.pgen.1002007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rubin BE, Moreau CS. 2016. Comparative genomics reveals convergent rates of evolution in ant–plant mutualisms. Nat. Commun. 7, 12679 ( 10.1038/ncomms12679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nygaard S, et al. 2016. Reciprocal genomic evolution in the ant-fungus agricultural symbiosis. Nat. Commun. 7, 12233 ( 10.1038/ncomms12233) [DOI] [PMC free article] [PubMed] [Google Scholar]