Abstract
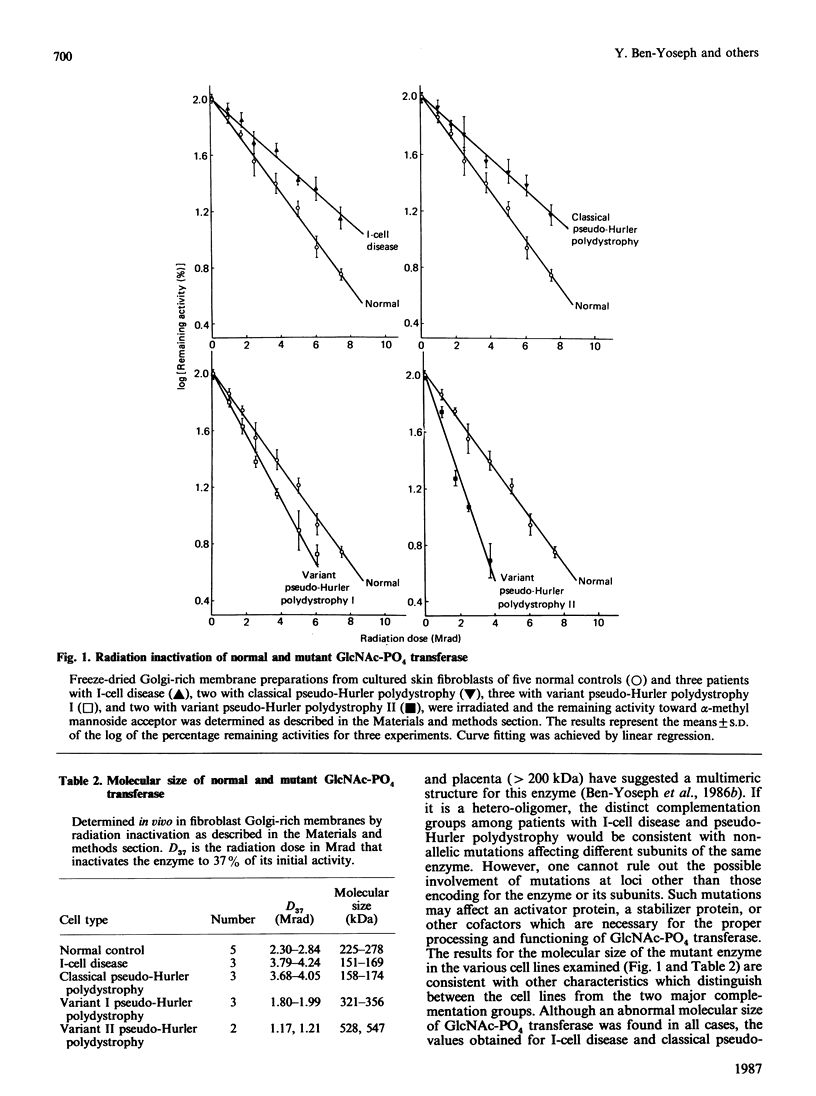
The size of the mutant N-acetylglucosamine 1-phosphotransferase in Golgi membranes from fibroblasts of patients with I-cell disease and classical pseudo-Hurler polydystrophy, which comprised one complementation group characterized by deficiency towards both artificial and natural acceptor substrates, was significantly smaller than the normal enzyme, 151-174 kDa compared with 225-278 kDa. The size of the mutant enzyme from cell lines of patients with variant forms of pseudo-Hurler polydystrophy, which comprised another complementation group characterized by normal activity towards mono- and oligo-saccharide substrates, was significantly larger than the normal enzyme, ranging from 321 to 356 kDa in two families and from 528 to 547 kDa in a third family. These findings suggest that the mutations in I-cell disease and classical pseudo-Hurler polydystrophy result in a missing enzyme component, which renders the enzyme catalytically inefficient toward any type of acceptor substrate. In contrast, the mutations in the variant forms of pseudo-Hurler polydystrophy produce a larger enzyme molecule which is active toward small substrates but is incapable of binding natural lysosomal glycoprotein substrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauregard G., Giroux S., Potier M. Target size analysis by radiation inactivation: a large capacity tube rack for irradiation in a Gammacell 220. Anal Biochem. 1983 Jul 15;132(2):362–364. doi: 10.1016/0003-2697(83)90021-0. [DOI] [PubMed] [Google Scholar]
- Beauregard G., Potier M. Radiation inactivation of enzymes at low dose rates: identical molecular weights of rat liver cytosolic and lysosomal neuraminidases. Anal Biochem. 1982 May 15;122(2):379–384. doi: 10.1016/0003-2697(82)90299-8. [DOI] [PubMed] [Google Scholar]
- Beauregard G., Potier M. Temperature dependence of the radiation inactivation of proteins. Anal Biochem. 1985 Oct;150(1):117–120. doi: 10.1016/0003-2697(85)90448-8. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Baylerian M. S., Momoi T., Nadler H. L. Thermal activation of hexosaminidase A in a genetic compound with Tay-Sachs disease. J Inherit Metab Dis. 1983;6(3):95–100. doi: 10.1007/BF01800733. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Baylerian M. S., Nadler H. L. Radiometric assays of N-acetylglucosaminylphosphotransferase and alpha-N-acetylglucosaminyl phosphodiesterase with substrates labeled in the glucosamine moiety. Anal Biochem. 1984 Nov 1;142(2):297–304. doi: 10.1016/0003-2697(84)90468-8. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Pack B. A., Mitchell D. A., Elwell D. G., Potier M., Melançon S. B., Nadler H. L. Characterization of the mutant N-acetylglucosaminylphosphotransferase in I-cell disease and pseudo-Hurler polydystrophy: complementation analysis and kinetic studies. Enzyme. 1986;35(2):106–116. doi: 10.1159/000469330. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Potier M., Pack B. A., Mitchell D. A., Melançon S. B., Nadler H. L. Molecular size of N-acetylglucosaminylphosphotransferase and alpha-N-acetylglucosaminyl phosphodiesterase as determined in situ in Golgi membranes by radiation inactivation. Biochem J. 1986 May 1;235(3):883–886. doi: 10.1042/bj2350883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Reid J. E., Shapiro B., Nadler H. L. Diagnosis and carrier detection of Tay-Sachs disease: direct determination of hexosaminidase A using 4-methylumbelliferyl derivatives of beta-N-acetylglucosamine-6-sulfate and beta-N-acetylgalactosamine-6-sulfate. Am J Hum Genet. 1985 Jul;37(4):733–740. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Choy F. Y., Woo M., Potier M. In situ radiation-inactivation size of fibroblast membrane-bound acid beta-glucosidase in Gaucher type 1, type 2 and type 3 disease. Biochim Biophys Acta. 1986 Mar 7;870(1):76–81. doi: 10.1016/0167-4838(86)90010-5. [DOI] [PubMed] [Google Scholar]
- Geiger B., Ben-Yoseph Y., Arnon R. Purification of human hexosaminidases A and B by affinity chromatography. FEBS Lett. 1974 Sep 1;45(1):276–281. doi: 10.1016/0014-5793(74)80861-6. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Waheed A., von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981 Feb 12;98(3):761–767. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- Honey N. K., Miller A. L., Shows T. B. The mucolipidoses: identification by abnormal electrophoretic patterns of lysosomal hydrolases. Am J Med Genet. 1981;9(3):239–253. doi: 10.1002/ajmg.1320090310. [DOI] [PubMed] [Google Scholar]
- Honey N. K., Mueller O. T., Little L. E., Miller A. L., Shows T. B. Mucolipidosis III is genetically heterogeneous. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7420–7424. doi: 10.1073/pnas.79.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang L., Kornfeld S. A simplified procedure for synthesizing large quantities of highly purified uridine [beta-32P]diphospho-N-acetylglucosamine. Anal Biochem. 1984 Jul;140(1):264–269. doi: 10.1016/0003-2697(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Little L. E., Mueller O. T., Honey N. K., Shows T. B., Miller A. L. Heterogeneity of N-acetylglucosamine 1-phosphotransferase within mucolipidosis III. J Biol Chem. 1986 Jan 15;261(2):733–738. [PubMed] [Google Scholar]
- Maret A., Potier M., Salvayre R., Douste-Blazy L. Modification of subunit interaction in membrane-bound acid beta-glucosidase from Gaucher disease. FEBS Lett. 1983 Aug 22;160(1-2):93–97. doi: 10.1016/0014-5793(83)80943-0. [DOI] [PubMed] [Google Scholar]
- Mueller O. T., Honey N. K., Little L. E., Miller A. L., Shows T. B. Mucolipidosis II and III. The genetic relationships between two disorders of lysosomal enzyme biosynthesis. J Clin Invest. 1983 Sep;72(3):1016–1023. doi: 10.1172/JCI111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Kato T., Oshima T., Yutaka T., Yabuuchi H. Heterogeneity in mucolipidosis II (I-cell disease). Clin Genet. 1983 Feb;23(2):155–159. doi: 10.1111/j.1399-0004.1983.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Reitman M. L., Kornfeld S. Lysosomal enzyme targeting. N-Acetylglucosaminylphosphotransferase selectively phosphorylates native lysosomal enzymes. J Biol Chem. 1981 Dec 10;256(23):11977–11980. [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg P. O., Marzella L., Glaumann H. A method for rapid isolation of rough and smooth microsomes and Golgi apparatus from rat liver in the same sucrose gradient. Exp Cell Res. 1980 Dec;130(2):393–400. doi: 10.1016/0014-4827(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Mueller O. T., Honey N. K., Wright C. E., Miller A. L. Genetic heterogeneity of I-cell disease is demonstrated by complementation of lysosomal enzyme processing mutants. Am J Med Genet. 1982 Jul;12(3):343–353. doi: 10.1002/ajmg.1320120312. [DOI] [PubMed] [Google Scholar]
- Varki A. P., Reitman M. L., Kornfeld S. Identification of a variant of mucolipidosis III (pseudo-Hurler polydystrophy): a catalytically active N-acetylglucosaminylphosphotransferase that fails to phosphorylate lysosomal enzymes. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7773–7777. doi: 10.1073/pnas.78.12.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. UDP-N-acetylglucosamine:lysosomal enzyme precursor N-acetylglucosamine-1-phosphotransferase. Partial purification and characterization of the rat liver Golgi enzyme. J Biol Chem. 1982 Oct 25;257(20):12322–12331. [PubMed] [Google Scholar]


