Abstract
Background:
Postoperative nausea and vomiting (PONV) is a common problem causing distress to patients in the postoperative period. Younger age, gynecological surgeries, laparoscopic surgeries, female gender, volatile anesthetics, increased duration of anesthesia, and postoperative opioid use are associated with increased incidence of PONV.
Aim:
The present study was conducted to study the efficacy of ramosetron and its comparison with ondansetron in the prevention of PONV in patients undergoing pyelolithotomy, pyeloplasty, and upper ureterolithotomy.
Methods:
One hundred patients with physical status American Society of Anesthesiologists I and II, aged 20–60 years were enrolled in the study. Patients were randomly distributed to receive either injection ramosetron 0.3 mg intravenously (IV) or injection ondansetron 6 mg IV just before extubation.
Results:
There was no significant difference between the groups in age, gender, weight, duration of anesthesia, and duration of surgery. In the patients, who received ramosetron, it was observed that incidence of the episodes of nausea and vomiting increased with time after surgery. Each patient had an episode of nausea and an episode of vomiting during the 6–12 h interval. Similarly, two patients had episodes of nausea and two patients had episodes of vomiting at 18–24 h. This necessitated the increased need for rescue antiemetics with a total of four patients needing rescue antiemetics at 18–24 h. In patients receiving ondansetron, the episodes of nausea were more in number when compared with the ramosetron group. Twelve patients complained of nausea and thirteen patients had episodes of vomiting with the needfor rescue antiemetic in 14 patients. Both genders had a comparable incidence of nausea and vomiting.
Conclusion:
A single dose of IV ramosetron (0.3 mg) is more effective when compared with a single dose IV ondansetron (6 mg) in the prevention of PONV. We observed that the benefit was more in the later stages of the postoperative period (12–24 h).
Keywords: Ondansetron, postoperative nausea and vomiting, ramosetron
INTRODUCTION
Postoperative nausea and vomiting (PONV) is common with an incidence of 30%[1,2,3] in the postoperative period and is very distressing to patients. In some high-risk patients, the incidence of PONV has been reported to be as high as 80%.[1,2,3] Although PONV is almost always self-limiting, it causes significant morbidity including dehydration, electrolyte imbalance, wound dehiscence, aspiration of vomitus, and raised intracranial pressure. Each vomiting episode delays discharge from the recovery room by approximately 20 min[4] increasing the medical costs.[5,6,7] Younger age group (<50 years), gynecological surgeries, laparoscopic surgeries, female gender, volatile anesthetics, increased duration of anesthesia, and postoperative opioid use have been found to be associated with increased incidence of PONV.[8,9]
Four major neurotransmitter systems (dopaminergic, histaminic, cholinergic, and 5-hydroxytryptaminergic) play an important role in mediating the emetic response. Multiple drugs are used for the control of PONV. However, because of the multireceptor origin of PONV, combination therapy is more widely employed.[10]
Using a decision analysis treatment model, it has been suggested that prophylactic antiemetic therapy may be better and cost-effective when compared with treatment of established symptoms in patients who are at high risk of emesis.[11,12] Ramosetron, a newer antiemetic drug, is a 5-hydroxytryptamine type 3 blocker. Various studies have shown that ramosetron has a longer duration of action and least unwanted side effects when compared to other antiemetics for the prevention of PONV.[13,14,15,16,17,18]
The present study was conducted to study the efficacy of ramosetron and its comparison with ondansetron in the prevention of PONV in patients undergoing pyelolithotomy, pyeloplasty, and upper ureterolithotomy under general anesthesia.
METHODS
The study was conducted in a tertiary care hospital. One hundred patients with physical status American Society of Anesthesiologists I and II, aged 20–60 years, undergoing elective urological procedures including pyelolithotomy, pyeloplasty, and upper ureter lithotomy, under general anesthesia were taken up for the study in a double-blind randomized fashion. The following groups of patients were excluded from the study:
Patients with a history of motion sickness, migraine, muscular dystrophy, or any other neurological problems
Patients at particular risk for developing torsade de pointes including those with underlying heart conditions, such as congenital long QT syndrome
Patients who were predisposed to low levels of potassium and magnesium in the blood
Patients on other medications that lead to QT prolongation
Patients who received antiemetics within 48 h before surgery
Patients with a history of recurrent vomiting in the postoperative period
Pregnant/lactating females.
After taking informed written consent and approval of the Institutional Ethics Committee, patients were randomly allocated to one of the following two groups to receive either injection ramosetron 0.3 mg IV or injection ondansetron 6 mg IV just before extubation.
The sample size for the study was calculated from the software G power 3.1.5. G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) is a stand-alone power analysis program for many statistical tests commonly used in the social, behavioral, and biomedical sciences. It is available free of charge via the Internet for both Windows and Mac OS X platforms. For an α value of 5% and keeping the power of the study (1−β) equal to 80%, it was found that a minimum of fifty patients are required to be recruited in each group. It was found that the expected proportion for one group is 31% and another group is 60% which depicts the moderate effect “d” size exists between the groups.
Hence, the ramosetron group comprised fifty patients who received injection ramosetron (0.3 mg), while ondansetron group again comprised fifty patients who received injection ondansetron (6 mg).
To ensure blindness, either of the study drugs was diluted in 0.9% normal saline to a volume of 4 ml and coded by the technologist in charge of operation theater who did not reveal the nature of drug to anyone till the completion of the study. He also maintained a register documenting the particulars of each patient and code of the drug. Decoding was done at the completion of the study and patients were allocated to their respective groups.
General anesthesia technique was standardized. On arrival in the operation theater, an IV line was established with dextrose in saline using 18/20 FG cannula. All patients received morphine 0.1 mg/kg IV just before induction of general anesthesia. General anesthesia was induced using injection propofol 2 mg/kg body weight. Tracheal intubation was facilitated using injection atracurium besylate 0.5 mg/kg body weight. Anesthesia was maintained with 66% nitrous oxide in oxygen and isoflurane 0.6–1%. Ventilation was controlled to end-tidal carbon dioxide of 30–35 mmHg, and neuromuscular blockade was maintained using supplementary injection atracurium besylate 0.2 mg/kg IV. At the end of the operation, residual neuromuscular block was reversed with injection neostigmine 80 µ/kg and injection atropine 40 µ/kg body weight.
After surgery, all the patients were observed for 24 h. Throughout the study period, vital signs such as pulse rate, blood pressure, and respiratory rate were monitored and recorded every 6 h, except during sleep.
The incidence of nausea and vomiting was recorded every 6 hourly for a period of 24 h after the surgery by direct questioning to the patient or his/her attendants. Nausea and vomiting were regarded as separate events and evaluated on a three-point scale [Table 1]. However, no distinction was made between vomiting and retching (retching event was considered as vomiting event) rescue antiemetic medication was administered as and when required in the form of injection metoclopramide (10 mg) and dose repeated, if the patient experienced severe nausea; there were more than three emetic episodes within a period of 15 min or the patient asked for it.
Table 1.
Gan and Alexander scale for assessment of nausea and vomiting

At the end, all the data collected were decoded and subjected to statistical analysis using unpaired Student's t-test, Chi-square test, and Fisher's exact test. The observations thus made and inferences drawn were highlighted.
RESULTS
Demographic data
There was no significant difference between the groups in age, gender, weight, duration of anesthesia, and duration of surgery [Table 2]. The average age in ramosetron and ondansetron groups was 38.44 years and 38.50 years, respectively, while the average weight in these two groups was 63.0 kg and 63.74 kg, respectively. With respect to gender distribution in ramosetron group, ratio of males to females was 30:20, while in the ondansetron group, it was 26 males to 24 females. The average duration of surgery in ramosetron group was 55.28 h while in the ondansetron group, it was 56.42 h. The two groups were similar in age, weight, duration of anesthesia, and duration of surgery with P = 0.976, P = 0.487, P = 0.298, and P = 0.407, respectively.
Table 2.
Demographic characteristics of patients in ramosetron and ondansetron groups

Nausea
In the patients who received ramosetron, it was observed that incidence of the episodes of nausea increased with time after surgery. One patient had an episode of nausea during the 6–12 h interval. Similarly, two patients had episodes of nausea at intervals 18–24 h. This necessitated the increased need for rescue antiemetics with a total of four patients needing rescue antiemetics at 18–24 h [Figure 1].
Figure 1.

Postoperative nausea at different time intervals in Group A (ramosetron group).
In patients receiving ondansetron, the episodes of nausea were more in number when compared with the ramosetron group. Twelve patients complained of nausea with the need for rescue antiemetic in 14 patients [Figure 2].
Figure 2.
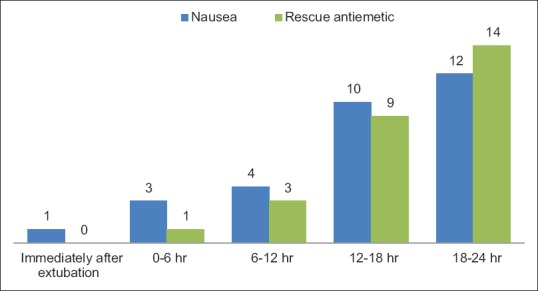
Postoperative nausea at different time intervals in Group B (ondansetron group).
When the two groups were compared there was no difference in nausea between the ramosetron and ondansetron groups immediately after extubation (P = 1.00), 0–6 h after extubation (P = 0.61), and at 6–12 h after extubation (P = 0.36). Both genders had a comparable incidence of nausea [Table 3]. However, the patients who received ramosetron had a statistically lower incidence of nausea at 12–18 h after extubation (P = 0.02). Similar results were seen at 18–24 h with just two patients having nausea in the ramosetron group, while the 12 patients had nausea in ondansetron group (P = 0.0076). There was no difference between the two genders in the incidence of nausea and vomiting in ramosetron group (P = 0.17) and ondansetron group (P = 0.57) [Tables 4 and 5].
Table 3.
Postoperative nausea and vomiting score at different time intervals between the two groups

Table 4.
Comparison of postoperative nausea and vomiting scores between male and female patients in Group A (ramosetron group)

Table 5.
Comparison of postoperative nausea and vomiting scores between male and female patients in Group B (ondansetron group)

Vomiting
In the patients who received ramosetron, it was observed that incidence of the episodes of vomiting increased with time after surgery. One patient had an episode of vomiting during the 6–12 h interval. Similarly, two patients had episodes of vomiting at 18–24 h. This necessitated the increased need for rescue antiemetics with a total of four patients needing rescue antiemetics at 18–24 h [Figure 3]. There was no statistical difference in the incidence of vomiting between the patients receiving either ondansetron or ramosetron at (a) immediately after extubation (P = 1.00), (b) at 6 h after extubation (P = 0.49), or (c) at 6–12 h after extubation (P = 0.36). However, patients who received ramosetron had a lower incidence of vomiting when compared to those who received ondansetron at 12–18 h after extubation (P = 0.14) and at 18–24 h after extubation (P = 0.003).
Figure 3.

Postoperative vomiting at different time intervals in Group A (ramosetron group).
In patients receiving ondansetron, thirteen patients had episodes of vomiting with the need for rescue antiemetic in 14 patients [Figure 4]. It was seen that the patients between the two groups did not have any difference in the incidence of rescue antiemetics used at immediately after extubation (P = 1.00), 0–6 h after extubation (P = 1.00), and 6–12 h after extubation (P = 1.00). However, there was a decreased use of antiemetics in the patients who received ramosetron at 6–18 h after extubation (P = 0.05) and 18–24 h after extubation (P = 0.01) [Figures 5 and 6].
Figure 4.

Postoperative vomiting at different time intervals in Group B (ondansetron group).
Figure 5.

Postoperative nausea and vomiting score at different time intervals in ramosetron group.
Figure 6.

Postoperative nausea and vomiting score at different time intervals in ondansetron group.
Rationale of results
From our results, we observed that the incidence of nausea was very low immediately after extubation (P = 1.00) and at 0–6 h (P = 1.00), which could be due to high plasma concentration of the drug.
We noticed with increased duration of postoperative period, incidence of both nausea and vomiting increased with maximum incidence at 18–24 h. This could probably be due to decreasing plasma concentration of drug with time.
Moreover, we found that patients receiving ondansetron had a higher incidence of nausea and vomiting as compared to ramosetron group and as such more number of rescue antiemetics. It was found that 18% of patients received antiemetics in the ondansetron group compared to 4% patients in the ramosetron group at 12–18 h with a P = 0.05. Similarly, 28% of the patients receiving ondansetron needed rescue antiemetics when compared to 8% patients in the ondansetron group with a significant P = 0.017. This could possibly be due to increased potency of ramosetron as compared to ondansetron.
DISCUSSION
Despite the remarkable advances in medicine and development of newer anesthetics, PONV continues to be a major cause of morbidity, with an incidence of 30% in the postoperative period.[7]
The problem is multifactorial in origin, including patient characteristics, nature of underlying disease, the type of surgery, as well as the anesthetic agents and postoperative care. The main patient-related factors are age, gender, history of motion sickness, previous nausea and vomiting, and pregnancy. The incidence of PONV in females has been reported to be very high and two to three times more prevalent and more severe in adult women than in men.[5] Hormonal factors may lead to a higher incidence of emetic episodes, with an observed incidence of emesis around four times higher in menstrual age group as compared to the postmenopausal state.[19]
Several studies tried to see the comparison between ramosetron and granisetron and found that there was no difference between the two groups till 12 h in the postoperative period. However, patients who received ramosetron had a lower incidence of PONV after 12 h.[13,16,20,21]
Choi et al.[22] studied in 94 female nonsmoker patients, who were randomly allocated to receive either ondansetron or the ramosetron after lumbar spine surgery. The authors found that ramosetron was superior to ondansetron in terms of preventing vomiting and reducing the severity of nausea related to fentanyl-based IV patient-controlled analgesia. Hahm et al.[23] compared the prophylactic antiemetic efficacy of ramosetron and ondansetron in patients undergoing PONV after total knee replacement. The incidence of nausea was lower in the patients who received ramosetron group.
Kim et al.[24] compared the efficacy of ramosetron with ondansetron for the prevention of PONV in patients who underwent gynecological surgery. The incidence of vomiting was lower in both the ramosetron (17%) and the ondansetron (20%) groups than in the placebo group (44%) during the first 24 h after surgery (P < 0.05). However, the authors found that ramosetron 0.3 mg IV was as effective as ondansetron 8 mg IV in decreasing the incidence of PONV and reducing nausea severity in female patients during the first 24 h after gynecological surgery.
In our study, we had comparable results with the studies conducted by Choi et al.[22] and Hahm et al.[23] However, our results were not consistent with the study published by Kim et al.[24] who found that there was no significant difference between ondansetron and ramosetron in controlling PONV. The difference between the two studies could be a result of the nature of the surgical population with the gastrointestinal tract and reproductive tract handling in the former group of patients which was minimal in our group of population.
The same could be true for the observed differences between the work done by Kim et al.,[24] Choi et al.,[22] and Hahm et al.[23]
Limitations of the study
There were certain limitations of our study. First, there was a lack of a control group as we felt it would be unethical to withhold prophylaxis in these patients for PONV. Second, due to the limitation of our resources, we could not measure the biochemical parameters of nausea and vomiting such as C-reactive protein, aldehydes, and ketones. We used morphine and nitrous oxide in these patients. This was a major limitation of the study as both these agents cause nausea and vomiting. Unfortunately, we do not have the availability of medical air in our hospital. However, the use of nitrous oxide and morphine was a common denominator in both the groups which could have minimized the bias among the two groups.
CONCLUSION
From our study, we conclude that a single dose of IV ramosetron (0.3 mg) is more effective when compared with a single dose IV ondansetron (6 mg) in the prevention of PONV. We observed that the benefit was more in the later stages of the postoperative period (12–24 h).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–9. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91:109–18. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–98. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 5.Evers AS, Maze M. Antiemetics. Anesthetic Pharmacology, Physiologic Principles and Clinical Practice, A Companion to Miller's Anesthesia. First ed. Ch. 45. Philadelphia: Churchill Livingstone; 2004. p. 777. [Google Scholar]
- 6.Ho KY, Chiu JW. Multimodal antiemetic therapy and emetic risk profiling. Ann Acad Med Singapore. 2005;34:196–205. [PubMed] [Google Scholar]
- 7.Kim EJ, Ko JS, Kim CS, Lee SM, Choi DH. Combination of antiemetics for the prevention of postoperative nausea and vomiting in high-risk patients. J Korean Med Sci. 2007;22:878–82. doi: 10.3346/jkms.2007.22.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apfel CC, Philip BK, Cakmakkaya OS, Shilling A, Shi YY, Leslie JB, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology. 2012;117:475–86. doi: 10.1097/ALN.0b013e318267ef31. [DOI] [PubMed] [Google Scholar]
- 9.Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–53. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- 10.Heffernan AM, Rowbotham DJ. Postoperative nausea and vomiting – Time for balanced antiemesis? Br J Anaesth. 2000;85:675–7. doi: 10.1093/bja/85.5.675. [DOI] [PubMed] [Google Scholar]
- 11.Watcha MF, Smith I. Cost-effectiveness analysis of antiemetic therapy for ambulatory surgery. J Clin Anesth. 1994;6:370–7. doi: 10.1016/s0952-8180(05)80006-6. [DOI] [PubMed] [Google Scholar]
- 12.Jolley S. Managing post-operative nausea and vomiting. Nurs Stand. 2001;15:47–52. doi: 10.7748/ns2001.06.15.40.47.c3044. [DOI] [PubMed] [Google Scholar]
- 13.Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Comparison of ramosetron and granisetron for preventing postoperative nausea and vomiting after gynecologic surgery. Anesth Analg. 1999;89:476–9. doi: 10.1097/00000539-199908000-00043. [DOI] [PubMed] [Google Scholar]
- 14.Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Ramosetron for preventing postoperative nausea and vomiting in women undergoing gynecological surgery. Anesth Analg. 2000;90:472–5. doi: 10.1097/00000539-200002000-00043. [DOI] [PubMed] [Google Scholar]
- 15.Fujii Y, Tanaka H, Ito M. A randomized clinical trial of a single dose of ramosetron for the prevention of vomiting after strabismus surgery in children: A dose-ranging study. Arch Ophthalmol. 2005;123:25–8. doi: 10.1001/archopht.123.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Feng FY, Zhang P, He YJ, Li YH, Zhou MZ, Cheng G, et al. Comparison of the selective serotonin 3 antagonists ramosetron and granisetron in treating acute chemotherapy-induced emesis, nausea, and anorexia: A single-blind, randomized, crossover study. Curr Ther Res. 2000;61:901–9. [Google Scholar]
- 17.Cheirsilpa A, Sinthusake T, Songsakkaesorn A, Visawaprasit S, Chulaka K, Changkuingdee N. Comparison of ramosetron and granisetron for the prevention of acute and delayed emesis in cisplatin-based chemotherapy: A randomized controlled trial. Jpn J Clin Oncol. 2005;35:695–9. doi: 10.1093/jjco/hyi192. [DOI] [PubMed] [Google Scholar]
- 18.Kim K, Chie EK, Jang JY, Kim SW, Oh DY, Im SA, et al. Ramosetron for the prevention of nausea and vomiting during 5-fluorouracil-based chemoradiotherapy for pancreatico-biliary cancer. Jpn J Clin Oncol. 2009;39:111–5. doi: 10.1093/jjco/hyn140. [DOI] [PubMed] [Google Scholar]
- 19.Beattie WS, Lindblad T, Buckley DN, Forrest JB. The incidence of postoperative nausea and vomiting in women undergoing laparoscopy is influenced by the day of menstrual cycle. Can J Anaesth. 1991;38:298–302. doi: 10.1007/BF03007618. [DOI] [PubMed] [Google Scholar]
- 20.Fuji Y, Saitoh Y, Tanaka H, Toyooka H. Ramosetron versus granisetron for prevention of post-operative nausea and vomiting after laparoscopic cholecystectomy. Can J Anesth. 1999;46:991–3. doi: 10.1007/BF03013138. [DOI] [PubMed] [Google Scholar]
- 21.Fujii Y, Tanaka H. Comparison of granisetron and ramosetron for the prevention of nausea and vomiting after thyroidectomy. Clin Ther. 2002;24:766–72. doi: 10.1016/s0149-2918(02)85150-5. [DOI] [PubMed] [Google Scholar]
- 22.Choi YS, Shim JK, Yoon DH, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: Comparison with ondansetron. Spine (Phila Pa 1976) 2008;33:E602–6. doi: 10.1097/BRS.0b013e31817c6bde. [DOI] [PubMed] [Google Scholar]
- 23.Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron, a newly developed 5-HT3 antagonist, and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anesthesia. 2010;65:500–4. doi: 10.1111/j.1365-2044.2010.06310.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim SI, Kim SC, Baek YH, Ok SY, Kim SH. Comparison of ramosetron with ondansetron for prevention of postoperative nausea and vomiting in patients undergoing gynaecological surgery. Br J Anaesth. 2009;103:549–53. doi: 10.1093/bja/aep209. [DOI] [PubMed] [Google Scholar]


