Abstract
Methylmercury (CH3Hg+) is an environmental toxicant that may lead to significant pathologies in exposed individuals. The current study assessed the disposition and toxicological effects of 2.5 or 7.5 mg kg−1 CH3Hg+, conjugated to cysteine (Cys; Cys-S-CH3Hg) and administered orally to pregnant and non-pregnant Wistar and TR− rats. Rats were euthanized on gestational day 20 and the content of mercury in each fetus, amniotic sac, and placenta was determined. The brain, liver, and kidneys were removed from each fetus for estimation of mercury content. From the dams, a sample of blood, kidneys, liver, and brain were removed at the time of euthanasia. The findings from this study indicate that pregnancy leads to significant changes in the handling of mercuric ions, particularly in the liver. Furthermore, there are significant differences in the handling of non-nephrotoxic and nephrotoxic doses of Cys-S-CH3Hg by maternal and fetal organs.
Keywords: Methylmercury, Mercury, Placenta, Fetus, Metals
1. Introduction
Methylmercury (CH3Hg+) is an environmental toxicant that may lead to significant pathological effects in exposed individuals. In the United States, exposure to CH3Hg+ occurs primarily via the consumption of contaminated fish, although other routes of exposure have also been documented [1]. Despite guidelines from the United States Environmental Protection Agency (USEPA), certain populations continue to consume more than the recommended amount of fish [2–5]. Indeed, a recent study found that Asians, males, and older individuals tend to have blood concentrations of CH3Hg+ that meet or exceed the maximum blood concentration of CH3Hg+ recommended by the USEPA [6–8]. Consumption of more than the recommended amount of fish may be due to the absence of fish consumption advisories for contaminated waters [9] and/or a lack of awareness regarding fish consumption advisories [10]. Pregnant women, in particular, should be aware of guidelines regarding fish consumption in that exposure to methylmercury during pregnancy can lead to significant detrimental effects in the fetus [1].
CH3Hg+ has been shown accumulate in and exert significant toxicological effects in multiple organs [1]. In the brain, CH3Hg+ appears to readily cross the blood-brain barrier and can lead to neurotoxicological effects [11–14]. Owing to the role of the kidney in the excretion of metabolic wastes and xenobiotics, renal cells are also sites of CH3Hg+ accumulation [15]. In addition, mercuric ions have been shown to cross the placenta and accumulate in fetal organs following prenatal exposure to CH3Hg+ [16–18]. Interestingly, the concentration of mercuric ions has been shown to be greater in cord blood and the placenta than in maternal blood [19,20].Thus, the concentration of CH3Hg+ presented to the fetus is likely higher than that present in the maternal circulation. Indeed, CH3Hg+-induced fetotoxicity results from plasma concentrations of CH3Hg+ that do not lead to pathological alterations in the mother [19]. Prenatal exposure of fetuses to CH3Hg+ has been linked to numerous neurological impairments such as reduced cognitive function, reduced motor activity, speech disorders, and cerebral palsy [1,21,22]. Since the developing fetus is especially sensitive and susceptible to the effects of CH3Hg+ [21,23], understanding the way in which this metal is transported by placental syncytiotrophoblasts and fetal organs is particularly important.
Despite the clinical importance of this area, little is known about the molecular mechanisms involved in the handling of CH3Hg+ by placental and fetal tissues. Recently, the multidrug resistance-associated protein (Mrp) 2 was implicated in the transport of mercuric ions from the fetal circulation into maternal blood following intravenous administration of a non-toxic dose of CH3Hg+ [17]. This previous study did not provide data regarding the handling of mercuric ions following oral exposure to CH3Hg+, which is the primary route of human exposure. Furthermore, the previous study did not assess the effects of exposure to a potentially toxic dose of CH3Hg+. Therefore, the current study was designed to test the hypothesis that oral administration of a toxic dose of CH3Hg+ alters the transport and disposition of mercuric ions in dams and fetuses.
2. Materials and methods
2.1. Animals
Female Wistar and TR− rats, weighing 250–275 g, were obtained from our colony in the Mercer University School of Medicine animal facility. Breeder pairs of TR− rats were originally obtained from the laboratory of Dr. Kim Brouwer at the University of North Carolina. TR− rats, which are in a Wistar background, possess a spontaneous mutation that results in an early stop codon in the Mrp2 (Abcc2) transcript. Therefore, Mrp2 in these rats is nonfunctional [24]. There were no significant differences in body weight among the animals used for these studies. Female rats of each strain were mated for 40 h with male rats of the same strain from our colony. Pregnancy was verified by weight gain and abdominal palpation.
Animals were provided a commercial laboratory diet (Tekland 6% rat diet, Harlan Laboratories) and water ad libitum throughout all aspects of experimentation. All animals were maintained on a 12 h dark: 12 h light cycle. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. All protocols using animals were approved by the Mercer University Institutional Animal Care and Use Committee.
2.2. Oral exposure to methylmercury
A study using X-ray absorption spectroscopy showed that the primary form of CH3Hg+ found in muscle of fish is a conjugate of cysteine (Cys; Cys-S-CH3Hg) [25]. Therefore, in the current study, rats were exposed orally to Cys-S-CH3Hg in order to mimic the ingestion of fish tissue. Beginning on gestational day (GD) 10, rats were exposed daily to 2.5 or 7.5 mg kg−1 10 mL−1 Cys-S-CH3Hg (in normal saline). Based on unpublished findings from our laboratory, we conclude that the 2.5-mg kg−1 dose is not nephrotoxic to dams while the 7.5-mg kg−1 dose is expected to lead to nephrotoxic effects. Cys-S-CH3Hg was formed by mixing CH3Hg+ (Sigma) with Cys in a 1:1.25 ratio. Radioactive CH3Hg+ (CH3[203Hg]) was added to the solution so that each rat received a total of 2 μCi CH3[203Hg]. CH3[203Hg] (6–12 mCi/mg) was generated at the University of Missouri Research Reactor using a method described previously [26]. Cys-S-CH3Hg, containing CH3[203Hg], was administered orally to rats using 15 gauge × 100 mm disposable plastic feeding tubes (Instech, Plymouth Meeting, PA). On GD 10, rats were placed in individual plastic metabolic cages. Feces and urine were collected daily from GD 10 until GD 20, at which time rats were euthanized.
Following oral exposure to Cys-S-CH3Hg, it should be considered that thiol exchange likely occurs whereby the mercuric ion releases Cys and becomes bound to other available thiol-containing molecules [27,28]. In addition, previous studies have shown that following absorption/ingestion of CH3Hg+, a fraction of CH3Hg+ is biotransformed to inorganic mercury (Hg2+) [29,30]. Identification of the exact forms of mercury taken up by various organs and tissues was outside the scope of the current study. Therefore, in the current study, we use “Hg” as a generalized term to represent the various potential species of CH3Hg+ and Hg2+ that may be taken up into cells.
2.3. Collection of fetuses, tissues, organs, and urine
Pregnant dams were euthanized on GD 20. At the time of euthanasia, dams were anesthetized with an intraperitoneal (i.p.) dose of ketamine and xylazine (70/30 mg kg−1 in 2 mL saline). Each fetus and placenta was removed carefully from the uterus. The amniotic sac was punctured and the amniotic fluid was collected on a piece of Whatman paper (Thermo Fisher), which was placed in a tube to measure the content of CH3[203Hg]. Each placenta was separated from the fetus, weighed, and placed in a tube for estimation of CH3[203Hg] content. In addition, each fetus was weighed, decapitated, and placed in 3 mL of 80% EtOH in a glass scintillation vial. The whole fetus was counted in a gamma counter, following which the brain, kidneys, and liver were dissected from each fetus. Each organ was weighed and placed in separate tubes for the determination of CH3[203Hg] content. After each placenta and fetus was removed from the dam, the uterus was removed, placed in a scintillation vial and counted in a gamma counter. The number of fetuses and placentas harvested from each dam was 11–18.
Following removal of placentas and fetuses, a 1-mL sample of blood was obtained from the inferior vena cava of dams and was saved for estimation of CH3[203 Hg] content. Total blood volume was estimated to be 6% of body weight [31]. The liver and kidneys were also removed from each dam. Each kidney was weighed and cut in half along a transverse plain. One-half of the right kidney was frozen immediately in liquid nitrogen for future PCR analyses. The remaining half of the right kidney was utilized for estimation of CH3[203Hg] content. One-half of the left kidney was placed in fixative (40% formaldehyde, 50% glutaraldehyde in 96.7 mM NaH2PO4 and 67.5 mM NaOH) as preparation for histological analyses. A 3-mm transverse slice of the remaining half was utilized for separation of cortex, outer stripe of outer medulla, inner stripe of outer medulla and inner medulla. Each zone of the kidney was weighed and placed in a separate tube for estimation of CH3[203Hg] content. The liver was then excised, weighed, and a 1-g section of liver was removed for determination of CH3[203Hg] content. The brain was also removed, weighed, and placed in a glass scintillation vial for estimation of CH3[203Hg] content.
Urine was collected daily, beginning on GD 11. At the time of collection, a 1-mL sample of urine was weighed and placed in a tube for estimation of CH3[203Hg] content. Samples were counted in a Wallac Wizard 3 automatic gamma counter (Perkin Elmer, Boston, MA) in order to estimate the content of CH3[203Hg] in each sample.
2.4. Quantitative PCR
At the time of euthanasia, one-half of the right kidney from each Wistar and TR− rat was cut into sections and frozen in liquid nitrogen. In order to isolate RNA, frozen kidney sections were pulverized with a mortar and pestle, TRIzol Reagent (Life Technologies, Grand Island, NY) was added, and RNA was extracted according to the manufacturer’s protocol.
Reverse transcription of 1 μg of RNA was carried out using reverse transcriptase and random hexamers (Life Technologies). For real-time PCR analyses, 2 μL of the reverse transcriptase reaction were utilized. Analysis of kidney injury molecule-1 (Kim-1) and breast cancer resistance protein (Bcrp) was performed with an ABI Prism 7000 detection system using a Gene Expression Assay (Rn00597701_m1 and Rn00710585_m1, respectively, Life Technologies). Glyceraldehyde 3-phosphate dehydrogenase (Gapdh; Rn01775763_g1) was used as a reference gene.
2.5. Histology
One-half of the left kidney from each rat was fixed in 40% formaldehyde, 50% glutaraldehyde in 96.7 mM NaH2PO4 and 67.5 mM NaOH for 48 h at 4 °C. Following fixation, kidneys were washed twice with normal saline and placed in 70% ethanol. Tissues were processed in a Tissue-Tek VIP processor using the following sequence: 95% ethanol for 30 min (twice); 100% ethanol for 30 min (twice); 100% xylene (twice). Tissue was subsequently embedded in POLY/Fin paraffin (ThermoFisher). Five-μm sections were cut using a Leitz 1512 microtome, mounted on glass slides, and were stained with hematoxylin and eosin (H
2.6. Measurement of plasma creatinine and blood urea nitrogen
Plasma creatinine was measured in order to estimate alterations in renal function. Following separation of plasma from cellular components of blood, samples were stored at −20°C. For determination of plasma creatinine, 30 μL of plasma was utilized and the concentration of creatinine was assessed using the QuantiChrome creatinine assay (BioAssay). Similarly, using a 5 μL sample of plasma, the concentration of BUN was determined using the QuantiChrome urea assay (BioAssay).
2.7. Data analyses
Data for each experiment were analyzed first with the Kolmogorov-Smirnov test for normality and then with Levene’s test for homogeneity of variances. Data were then analyzed using a 2 × 2 two-way analysis of variance (ANOVA) to assess differences among the means. When statistically significant F-values were obtained with ANOVA, the data were analyzed using Tukey’s post hoc multiple comparison test. A p-value of <0.05 was considered statistically significant. Each group of animals contained 5 rats, with 11–18 fetuses per rat.
3. Results
3.1. Disposition of CH3Hg± in fetal organs
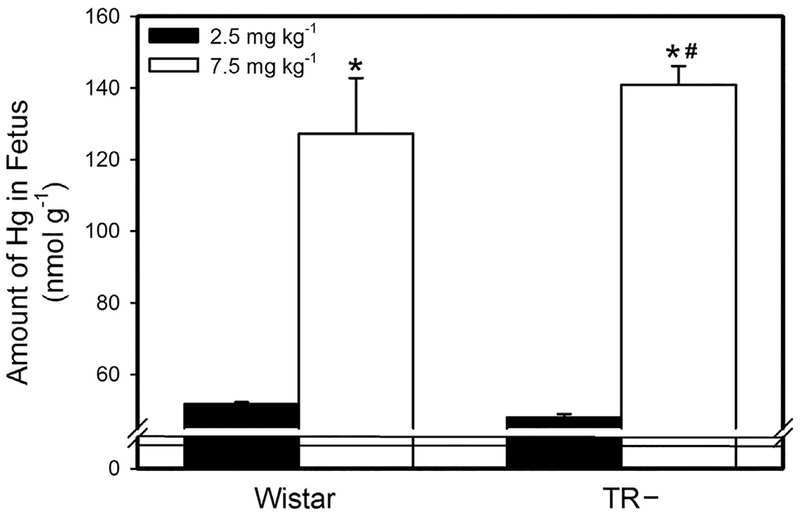
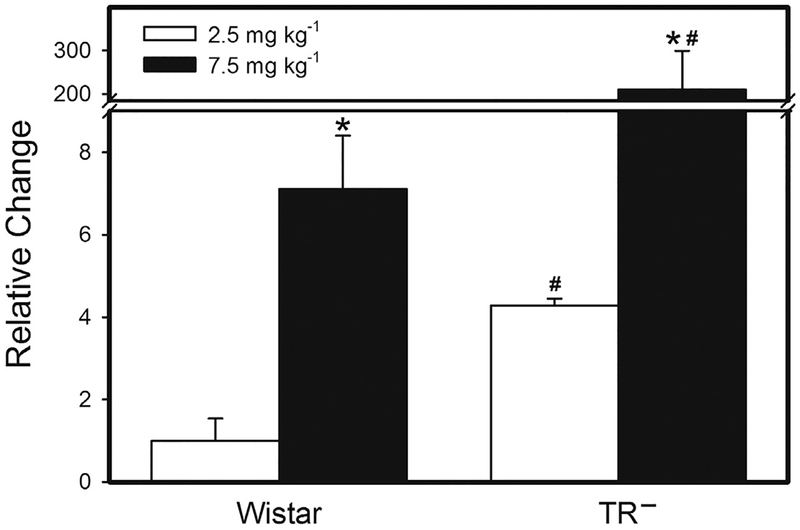
Following the exposure of dams to 2.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in each fetus was significantly greater in TR− than in Wistar rats (Fig. 1). Likewise, when dams were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the fetal burden of Hg was greater in TR− rats than in Wistar rats. Exposure of dams to 7.5 mg kg−1 Cys-S-CH3Hg enhanced the fetal burden of Hg by 1.75 fold in Wistar rats and 2.4 fold in TR− rats.
Fig. 1.
Fetal Burden of Hg. The burden of Hg was measured in fetuses from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Fetuses were harvested on gestational day 20 and the total burden of mercury was measured. *, significantly different (p < 0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
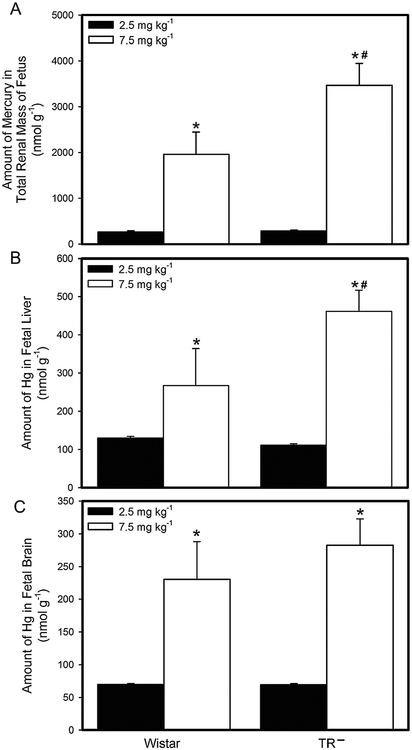
When dams were exposed to 2.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in the total renal mass of fetuses from Wistar dams was not significantly different from that of fetuses from TR− dams (Fig. 2a). However, when dams were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in the total renal mass of TR− fetuses was significantly greater than that of Wistar fetuses. The amount of Hg in the total renal mass of fetuses from Wistar and TR− dams exposed to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that of fetuses from corresponding dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg.
Fig. 2.
Burden of Hg in Fetal Organs. The burden of Hg was measured in fetuses from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Fetuses were harvested on gestational day 20 and the amount of mercury was measured in the total renal mass (A), liver (B), and brain (C) of each fetus. *, significantly different (p < 0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
The distribution of Hg in the fetal liver (Fig. 2b) was similar to that of the fetal kidneys. The hepatic burden of Hg in fetuses from Wistar dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg was not significantly different from that of fetuses from TR− dams. In contrast, when pregnant dams were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in the liver of TR− fetuses was significantly greater than that in the liver of Wistar fetuses. In addition, the fetal burden of Hg in both groups of fetuses from dams exposed to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that of fetuses from corresponding dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg.
The amount of Hg in the brain of fetuses from Wistar rats was not significantly different from that of fetuses from TR− rats at either dose of Cys-S-CH3Hg (Fig. 2c). The amount of Hg in the brains of Wistar and TR− fetuses from dams exposed to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that of corresponding fetuses from dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg.
3.2. Burden of CH3Hg± in amniotic fluid, placenta & uterus
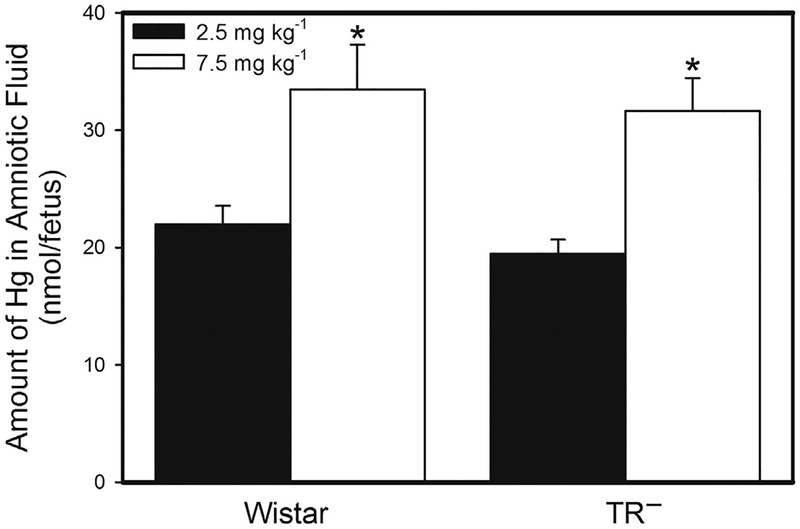
The amount of Hg in amniotic fluid of Wistar and TR− dams is shown in Fig. 3. There was no significant difference in the amount of Hg in the amniotic fluid of Wistar and TR− dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg. Similarly, the burden of Hg in the amniotic fluid of Wistar dams exposed to 7.5 mg kg−1 Cys-S-CH3Hg was not significantly different from that of corresponding TR− dams. However, the amount of Hg in the amniotic fluid of Wistar and TR− dams exposed to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that of corresponding dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg.
Fig. 3.
Amount of Hg in Amniotic Fluid. The burden of Hg was measured in amniotic fluid from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Rats were euthanized on gestational day 20 and the amniotic fluid surrounding each fetus was collected. *, significantly different (p<0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. n = 5 dams.
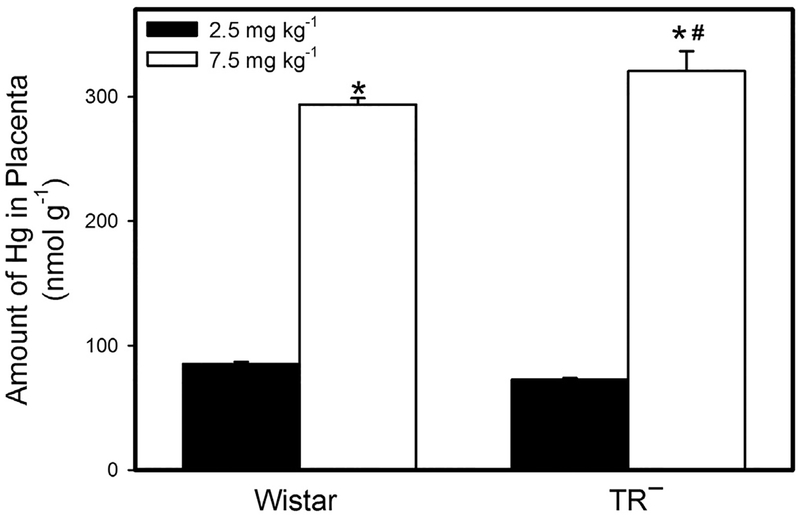
The amount of Hg in the placentas of Wistar dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg was not significantly different from that of corresponding TR− dams (Fig. 4). However, when dams were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in the placentas of TR− dams was significantly greater than that of Wistar dams. In both strains of rats, the placental burden of Hg following exposure to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that following exposure to 2.5 mg kg−1 Cys-S-CH3Hg.
Fig. 4.
Placental Burden of Hg. The burden of Hg was measured in placentas from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Rats were euthanized on gestational day 20 and each placenta was collected and analyzed for Hg content. *, significantly different (p < 0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
Interestingly, following exposure to either dose of Cys-S-CH3Hg, the amount of Hg in the uterus of TR− dams was significantly lower than that in the uterus of Wistar dams (Fig. 5). Even so, exposure of Wistar and TR− dams to the 7.5-mg kg−1 dose of Cys-S-CH3Hg led to a uterine burden of Hg that was greater than that of corresponding rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg.
Fig. 5.
Uterine Burden of Hg. The burden of Hg was measured in the uterus of Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Rats were euthanized on gestational day 20 and each uterus was collected and analyzed for Hg content. *, significantly different (p < 0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
3.3. Renal burden of CH3Hg± in pregnant and non-pregnant wistar and TR− rats
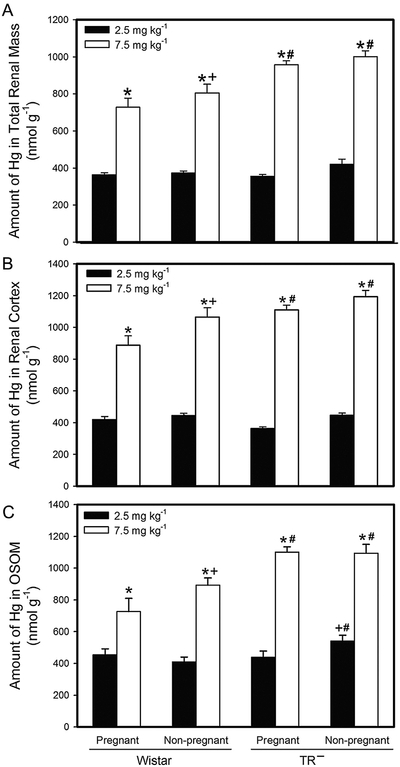
The renal burden of Hg in pregnant and non-pregnant Wistar and TR− female rats is shown in Fig. 6a. In each experimental group, the renal burden of Hg was significantly greater in rats exposed to 7.5 mg kg−1 Cys-S-CH3Hg than to 2.5 mg kg−1 Cys-S-CH3Hg. The renal burden of Hg was significantly greater in TR− rats than in Wistar rats at the corresponding dose.
Fig. 6.
Amount of Hg in Adult Kidneys. The burden of Hg was measured in kidneys from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Rats were euthanized on gestational day 20 and adult kidneys were removed for analysis of total Hg content (A). The content of Hg in individual sections of cortex (B) and outer stripe of the outer medulla (C) was also measured. *, significantly different (p<0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. +, significantly different (p < 0.05) from the mean of the corresponding group of pregnant rats of the same strain and dose. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
The amount of Hg that accumulated in the renal cortex of pregnant and non-pregnant Wistar and TR− female rats is shown in Fig. 6b. When pregnant and non-pregnant Wistar and TR− rats were exposed to 2.5 mg kg−1 Cys-S-CH3Hg, the accumulation of Hg in the renal cortex was found to be significantly greater in non-pregnant females (Wistar and TR−) than in corresponding pregnant rats. When pregnant and non-pregnant Wistar rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in the renal cortex of non-pregnant rats was significantly greater than that of pregnant Wistar rats. In contrast, when TR− rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the cortical burden of Hg in pregnant and non-pregnant TR− rats was not significantly different from each other. Not surprisingly, the amount of Hg in the renal cortex of Wistar and TR− rats following exposure to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that in the renal cortex of corresponding rats after exposure to 2.5 mg kg−1 Cys-S-CH3Hg.
The amount of Hg in the outer stripe of the outer medulla (OSOM) of pregnant and non-pregnant Wistar and TR− adult rats is shown in Fig. 6b. The amount of Hg in the OSOM of pregnant Wistar rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg was not significantly different from that of corresponding non-pregnant Wistar rats. In TR− rats, however, the amount of Hg in the OSOM of non-pregnant rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that of corresponding pregnant TR− rats. In addition, the amount of Hg in the OSOM of non-pregnant TR− rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that of corresponding non-pregnant Wistar rats. When Wistar rats were exposed to the 7.5-mg kg−1 dose of Cys-S-CH3Hg, the amount of Hg in the OSOM was significantly greater in non-pregnant rats than in corresponding pregnant rats. When TR− rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, there was no significant difference in the amount of Hg in the OSOM of pregnant and non-pregnant rats. However, following exposure to 7.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in the OSOM of both pregnant and non-pregnant TR− rats was significantly greater than that of corresponding Wistar rats.
3.4. Hepatic, hematologic & cerebral burden of CH3Hg± in pregnant and non-pregnant wistar and TR− rats
The hepatic burden of Hg was measured in adult Wistar and TR− rats (Fig. 7a). When Wistar and TR− rats were exposed to 2.5 mg kg−1 Cys-S-CH3Hg, the hepatic burden of Hg was significantly greater in pregnant rats than in non-pregnant rats. Interestingly, the hepatic burden of Hg in pregnant and non-pregnant Wistar rats was significantly greater than that of corresponding TR− rats. Likewise, when rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the hepatic burden of pregnant and non-pregnant Wistar rats was significantly greater than that of corresponding TR− rats. The hepatic burden of Hg was significantly greater in all groups of rats exposed to the 7.5-mg kg−1 dose of Cys-S-CH3Hg than in corresponding groups of rats exposed to the 2.5-mg kg−1 dose of Cys-S-CH3Hg.
Fig. 7.
Amount of Hg in Adult Liver and Blood. The burden of Hg was measured in liver (A) and blood (B) from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Rats were euthanized on gestational day 20 and the liver and a sample of blood were removed for analysis of Hg content. *, significantly different (p < 0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg.+, significantly different (p < 0.05) from the mean of the corresponding group of pregnant rats of the same strain and dose. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
The content of Hg in blood of pregnant and non-pregnant Wistar and TR− rats was also measured (Fig. 7b). In Wistar and TR− rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in blood was significantly greater in non-pregnant rats than in pregnant rats. The amount of Hg in blood of pregnant and non-pregnant Wistar rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg was not significantly different from that of corresponding TR− rats. When Wistar rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the amount of blood in pregnant and non-pregnant rats was not significantly different. A similar pattern of accumulation was observed in corresponding TR− rats. Interestingly, the amount of Hg in blood of pregnant and non-pregnant Wistar rats exposed to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that of corresponding TR− rats.
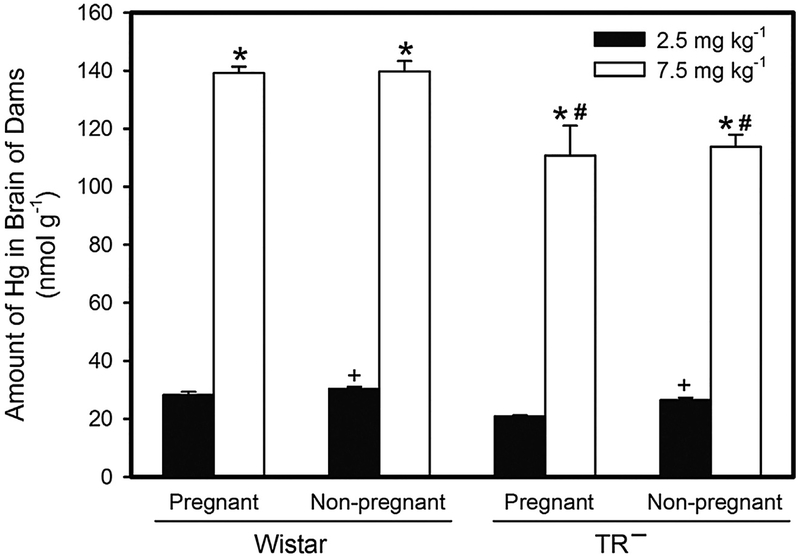
When the amount of Hg was measured in the brains of adult Wistar and TR− rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg, the content of Hg in brains of non-pregnant Wistar rats was found to be significantly greater than that in brains of pregnant Wistar rats (Fig. 8). When rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the amount of Hg in the brain of pregnant and non-pregnant Wistar rats was significantly greater than that of corresponding TR− rats. In all groups, the burden of Hg in the brain following exposure to 7.5 mg kg−1 Cys-S-CH3Hg was significantly greater than that in the brain of rats exposed to the 2.5-mg kg−1 dose.
Fig. 8.
Amount of Hg in Adult Brain. The burden of Hg was measured in brain from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Rats were euthanized on gestational day 20 and the brain was removed for analysis of Hg content. *, significantly different (p < 0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. +, significantly different (p < 0.05) from the mean of the corresponding group of pregnant rats of the same strain and dose. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
3.5. Urinary excretion of CH3Hg± in pregnant and non-pregnant wistar and TR− rats
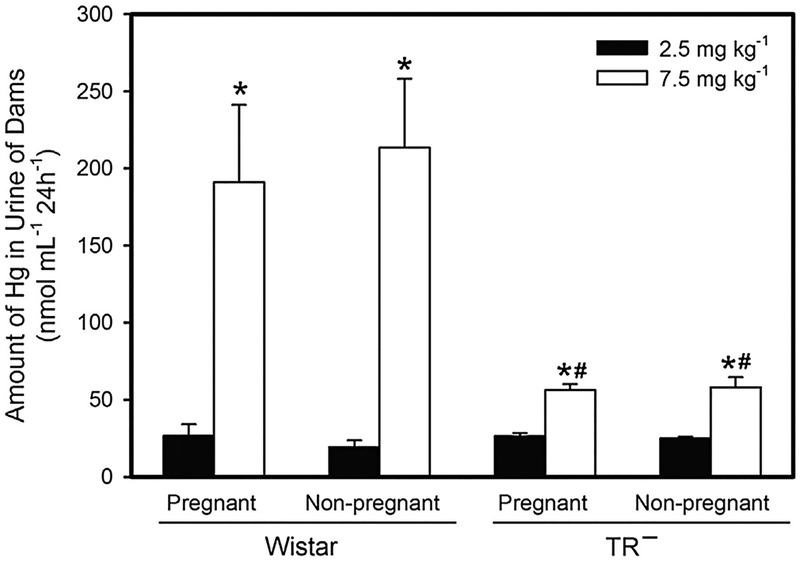
The amount of Hg in excreted in urine of pregnant and non-pregnant Wistar and TR− rats is shown in Fig. 9. When rats were exposed to 2.5 mg kg−1 Cys-S-CH3Hg, there was no significant difference in the urinary excretion of Hg among the groups of rats. However, when rats were exposed to the 7.5-mg kg−1 dose of Cys-S-CH3Hg, the amount of Hg excreted in urine of pregnant and non-pregnant Wistar rats was significantly greater than that of corresponding TR− rats. Pregnancy did not seem to significantly influence the urinary excretion of Hg.
Fig. 9.
Amount of Mercury Excreted in Urine. The burden of Hg was measured in urine from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Urine was collected after a 24-h period immediately following each of the three exposures. *, significantly different (p < 0.05) from the mean of the corresponding group of rats of the same strain exposed to 2.5 mg kg−1 Cys-S-CH3Hg. #, significantly different from the mean of the corresponding group of Wistar rats. n = 5 dams.
3.6. Toxicity of CH3Hg± in pregnant and non-pregnant wistar and TR− rats
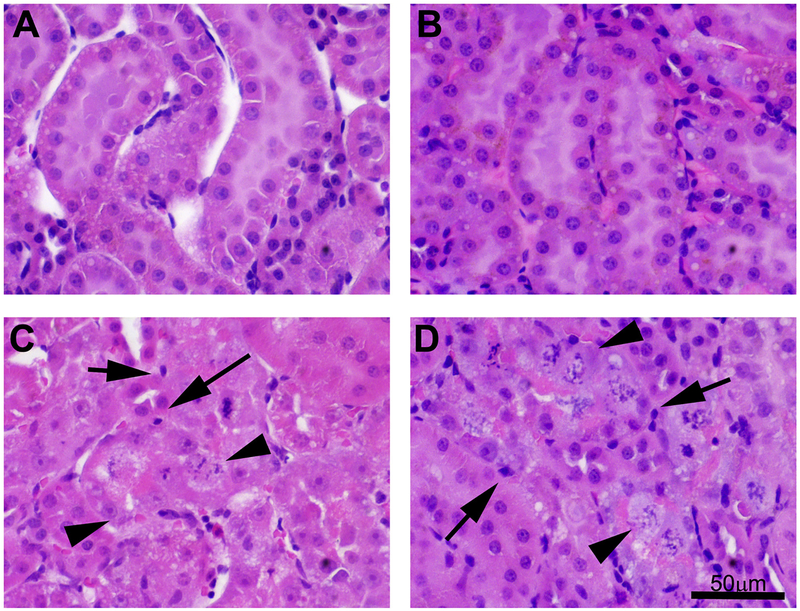
Morphological changes were assessed in the kidneys of pregnant and non-pregnant Wistar and TR− rats exposed to 2.5 or 7.5 mg kg−1 Cys-S-CH3Hg (Fig. 10). Representative sections of normal kidney from Wistar and TR− rats are shown in Fig. 10A and B, respectively. When rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, cytoplasmic vacuoles, pyknotic nuclei and cellular disintegration were apparent within proximal tubular cells of kidneys of Wistar (Fig. 10C) and TR− (Fig. 10D) rats.
Fig. 10.
Histological Analyses of Adult Kidneys. Histological analyses of kidneys from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. Rats were euthanized on gestational day 20 and adult kidneys were harvested. In rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg, no histological alterations or pathological changes were observed in kidneys of Wistar (A) or TR− rats (B). In rats exposed to 7.5 mg kg−1 Cys-S-CH3Hg, pyknotic nuclei (arrows) and cellular degeneration (arrowheads) were evident in kidneys of Wistar (C) and TR− rats (D). Bar = 50 μm.
Analyses of plasma creatinine and blood urea nitrogen (BUN) confirm the results of the histological analyses (Table 1). Plasma creatinine and BUN were significantly elevated in Wistar and TR− rats exposed to the 2.5- and 7.5-mg kg−1 doses of Cys-S-CH3Hg+.
Table 1.
Plasma creatinine and blood urea nitrogen (BUN) in Wistar and TR− rats exposed orally to 2.5 or 7.5 mg kg−1 methylmercury(CH3Hg+) as a conjugate of cysteine. Data represent mean ± SE. n = 5 dams.
| Dose of CH3Hg+ | Rat | Creatinine (mg/dL) | BUN (mg/dL) |
|---|---|---|---|
| Control | Wistar | 0.48 ± 0.06 | 5.34 ± 0.11 |
| TR- | 0.39 ± 0.04 | 8.28 ± 0.55 | |
| 2.5 mg kg−1 | Wistar | 0.69 ± 0.02 | 10.53 ± 0.35 |
| TR- | 0.80 ± 0.03 | 12.28 ± 0.27 | |
| 7.5 mg kg−1 | Wistar | 0.99 ± 0.02 | 14.97 ± 0.88 |
| TR- | 1.0 ± 0.04 | 16.55 ± 0.58 |
Quantitative PCR was utilized to analyze renal expression of kidney injury molecule-1 (Kim-1) in kidneys of Wistar and TR− rats exposed to either 2.5 or 7.5 mg kg−1 Cys-S-CH3Hg (Fig. 11). Kim-1, which is also known as hepatitis A virus cellular receptor 1 (Havcr 1) is a transmembrane protein that is expressed at high levels in proximal tubular epithelial cells following ischemic or toxic injury [32]. Expression of mRNA encoding Kim-1 was significantly greater in TR− rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg than in corresponding Wistar rats exposed to the same dose (Fig. 11). When rats were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the expression of mRNA encoding Kim-1 increased significantly in Wistar and TR− rats. At this dose, expression of Kim-1 mRNA was significantly greater in TR− rats than in corresponding Wistar rats.
Fig. 11.
Quantitative PCR Analysis of Adult Kidneys. The expression of mRNA encoding kidney injury molecule-1 (Kim-1) was analyzed in kidneys from Wistar and TR− rats exposed orally to daily doses of 2.5 or 7.5 mg kg−1 CH3Hg+, as a conjugate of cysteine (Cys-S-CH3Hg), beginning on gestational day 10. *, significantly different (p < 0.05) from the mean of the corresponding group of rats exposed of the same strain to 2.5 mg kg−1 Cys-S-CH3Hg. +, significantly different (p < 0.05) from the mean of the corresponding group of pregnant rats of the same strain and dose. n = 3.
The renal expression of mRNA encoding Bcrp was also measured. No significant differences were detected among the groups of rats (data not shown).
4. Discussion
The current study was designed to assess the disposition and toxicological effects of a non-nephrotoxic or nephrotoxic dose of orally administered Cys-S-CH3Hg on maternal and fetal organs in pregnant and non-pregnant Wistar rats. The current data show that there are significant differences in the handling and accumulation of Hg between the two doses of Cys-S-CH3Hg.
Interestingly, the accumulation of Hg in fetuses from Wistar dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg was similar to that of corresponding TR− dams. Since TR− rats lack functional Mrp2 protein, which mediates the excretion of mercuric species [17,26,33], one would expect the accumulation of Hg to be greater in fetuses of TR− rats. The current finding suggests that one or more additional transporters may be overexpressed in TR− rats in order to compensate for the loss of Mrp2. Possible candidates for this transport include the breast cancer resistance protein (Bcrp; Abcg2) and p-glycoprotein (P-gp; Abcb1), which are localized in the apical (maternal-facing) membrane of placental syncytiotrophoblasts [34,35]. The current PCR data show that mRNA expression of Bcrp is not upregulated in TR− rats and thus, this carrier does not appear to play a role in compensating for the absence of Mrp2. Alternatively, the expression of P-gp has been shown to be upregulated in TR− rats, [36] suggesting that this transporter may compensate, in part, for the absence of functional Mrp2. Currently, P-gp has not been implicated in the transport of Hg.
When the dose of Cys-S-CH3Hg was increased from 2.5 to 7.5 mg kg−1, the fetal burden of Hg increased by 1.75- and 2.4-fold in Wistar and TR− rats, respectively. The enhanced accumulation of Hg in fetuses of TR− rats is most likely due to the absence of Mrp2. Compensatory transport mechanisms, such as P-gp, may be saturated at the 7.5-mg kg−1 dose of Cys-S-CH3Hg and thus may be unable to reduce the fetal burden of Hg to levels similar to those in corresponding Wistar rats.
When the burden of Hg was measured in fetal organs, the greatest amount of Hg was detected in the total renal mass. This finding may be due largely to the role of the fetal kidney in the excretion of wastes. In addition, it is important to note that amniotic fluid, which is formed in part by fetal urine, is cycled through the fetus via swallowing and excretion. Consequently, fetal kidneys are exposed continuously to Hg throughout gestation. Therefore, it is logical that the burden of Hg is greater in fetal kidneys than in other fetal organs. Interestingly, the amount of Hg in kidneys of Wistar fetuses was similar to that of TR− fetuses, following exposure of dams to 2.5 mg kg−1 Cys-S-CH3Hg. One explanation for this similarity is the possibility that one or more additional transporters are compensating for the absence of Mrp2 in TR− rats. Interestingly, when the maternal dose increased from 2.5 to 7.5 mg kg−1 Cys-S-CH3Hg (a 3-fold increase), the renal burden of Hg increased 5-fold in Wistar fetuses and nearly 9-fold in TR− fetuses. In contrast, the amount of Hg in amniotic fluid of Wistar and TR− rats increased by only 50%. Together, these findings suggest that as amniotic fluid is cycled through the fetus and filtered by the kidney, the fetal kidney retains a fraction of Hg present in the amniotic fluid and thus, the fetal kidney is exposed to greater levels of Hg. Consequently, the fetal kidneys are likely more susceptible to Hg than adult kidneys because of the continuous exposure to Hg. It should also be noted that, at the higher dose, the renal accumulation of Hg in TR− fetuses was significantly greater than that of Wistar fetuses, suggesting that mechanisms compensating for the absence of Mrp2 are overwhelmed, which leads to enhanced renal accumulation of Hg.
The pattern of Hg accumulation in the fetal liver was similar to that in the fetal kidneys. The hepatic burden of Hg in fetuses from Wistar dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg was similar to that of fetuses from corresponding TR− dams. Similar to the kidney, this finding may be due to the enhanced expression of additional transport proteins that mediate the hepatobiliary export of Hg. When dams were exposed to 7.5 mg kg−1 Cys-S-CH3Hg, the hepatic burden was significantly greater in TR− fetuses than in Wistar fetuses. This enhanced burden is most likely due to the saturation of and/or lack of mechanisms (e.g, Mrp2) that mediate hepatobiliary export of Hg in TR− rats.
In the fetal brain, there was no difference in the accumulation of Hg between Wistar and TR− rats. This finding may be due to a slow rate of transport into neuronal cells and/or intracellular retention of Hg due to the binding of Hg to intracellular thiols. In particular, glutathione (GSH) and metallothionein (MT), specifically MT3, are abundant in the brain [37,38] and may form complexes with intracellular Hg. Binding of Hg to these thiols may impede the export of CH3Hg+ and consequently enhance retention of mercuric ions in the brain.
The disposition of Hg in the placenta was noteworthy since the placental accumulation of Hg following exposure of dams to 7.5 mg kg−1 Cys-S-CH3Hg was 11–12-fold greater than that following exposure of dams to the 2.5-mg kg−1 dose. The difference in magnitude of this accumulation may be due to the continuous exposure of placental syncytiotrophoblasts to high concentrations of Hg in blood, thereby facilitating the absorption of mercuric ions by syncytiotrophoblasts. This accumulation may also be related to the enhanced placental expression of MT following exposure to CH3Hg+ and the subsequent formation of MT-S-CH3Hg complexes that are not transported readily out of placental syncytiotrophoblasts [39]. In addition, binding of intracellular Hg to GSH, which is abundant within placental syncytiotrophoblasts [40], can create a GSH-S-CH3Hg complex that may not be a readily transportable form of Hg.
Interestingly, the uterine content of Hg was lower in TR− rats than in Wistar rats. Since the endometrial lining of the rat uterus is not shed through regular menstrual cycles and the transport activity is minimal, it appears that there are few opportunities for removal of Hg from the uterus. Given than Mrp2 has not been detected in the uterus, it is logical that the uterine accumulation of Hg is similar in Wistar and TR− rats. It is unclear at this time why the uterine accumulation of Hg was lower in TR− rats than in Wistars.
The disposition of Hg was also analyzed in organs of pregnant and non-pregnant adult Wistar and TR− rats following exposure to 2.5 or 7.5 mg kg−1 Cys-S-CH3Hg. Pregnancy did not appear to alter the renal disposition of Hg. As expected, the renal accumulation of Hg was greater in TR− rats than in Wistars, which corresponded to previous studies [41]. Renal accumulation of Hg was greater in dams exposed to 7.5 mg kg−1 Cys-S-CH3Hg than in those exposed to 2.5 mg kg−1 Cys-S-CH3Hg, indicating that the uptake and accumulation of mercuric ions in the kidney are dose-dependent processes. Of the four renal zones examined, Hg was found to accumulate primarily in the cortex and the OSOM. It is important to note that all three segments of the proximal tubule are located within these two zones.
When the hepatic burden of Hg was assessed, significant changes were noted between pregnant and non-pregnant rats. In Wistar and TR− rats exposed to 2.5 mg kg−1 Cys-S-CH3Hg, the hepatic burden of Hg was greater in pregnant rats than in non-pregnant rats. This finding may be related to how mercuric ions are handled following oral exposure. Following ingestion of CH3Hg+, a large fraction of Hg is likely taken up by enterocytes in the small intestine, where it is transported subsequently into blood for delivery to the liver. During pregnancy, blood flow and volume increase significantly [42,43], which promotes absorption of nutrients and xenobiotics from the intestinal lumen. We propose that under these conditions, Hg delivery to the liver is enhanced, resulting in a greater hepatic burden of Hg in pregnant animals. Interestingly, when Wistar and TR− dams were exposed to the 7.5-mg kg−1 dose of Cys-S-CH3Hg, the hepatic burden of Hg in pregnant rats was only 10–15% greater than that of non-pregnant rats. This small increase suggests that at this dose, transporters on the sinusoidal membrane of hepatocytes are saturated by the elevated burden of CH3Hg+ in blood.
As expected, the amount of Hg in blood correlated negatively with the disposition of Hg in liver. The amount of Hg in maternal brain correlated positively with the amount of Hg in blood, suggesting that the burden of mercuric ions via blood may be an important predictor of organ accumulation.
The amount of Hg in urine of pregnant and non-pregnant Wistar and TR− dams exposed to 2.5 mg kg−1 Cys-S-CH3Hg was similar across all groups. One would expect that the absence of Mrp2 in kidneys of TR− rats would lead to a reduction in the urinary excretion of mercuric ions. However, it should be considered that upregulation of transport proteins, as mentioned previously, may facilitate the urinary excretion of Hg by TR− rats exposed to a low dose of Cys-S-CH3Hg. When rats were exposed to the 7.5-mg kg−1 dose of Cys-S-CH3Hg, urinary excretion of Hg in TR− rats was found to be significantly lower than that in Wistar rats, confirming that Mrp2 plays a major role in the urinary excretion of mercuric ions.
In summary, the current data show that pregnancy leads to significant changes in the maternal handling of mercuric ions, particularly in the liver. These data also show that there are significant differences in the maternal and fetal handling of nontoxic and toxic doses of Cys-S-CH3Hg. Following prenatal exposure to Cys-S-CH3Hg, mercuric ions appear to concentrate in fetal kidneys, which may make these kidneys more susceptible to injury and can lead to delayed nephrogenesis and/or altered renal function. A thorough understanding of how mercuric ions are taken up and retained in target organs may lead to more effective therapeutic strategies for mercury poisoning and/or better education for avoiding exposure to mercury.
Acknowledgements
This work was supported by the National Institutes of Health (National Institute of Environmental Health Sciences) grants awarded to Dr. Bridges (ES015511, ES019991). In addition, funding from the National Institutes of Health, R01GM41935 (to KR Brouwer), supports the maintenance of the TR- colony from which rats were obtained. The authors would like to thank Ms. Allison Stahl for technical assistance and Dr. Rudolfs K. Zalups for assistance with the design of the study.
Abbreviations:
- CH3Hg+
methylmercury
- Cys-S-CH3Hg
cysteine conjugate of methylmercury Cys cysteine
- USEPA
United States Environmental Protection Agency
- GSH
glutathione
- Mrp
multidrug resistance-associated protein
- Bcrp
breast cancer resistance protein
- P-gp
P-glycoprotein
- OSOM
outer stripe of the outer medulla
References
- [1].ATSDR(Agency for Toxic Substance and Disease Registry), in: U.S. Department of Health, Human Services PHS (Eds.), Toxicological Profile for Mercury, Centers for Disease Control, Atlanta, GA, 2008. [Google Scholar]
- [2].Nair A, Jordan M, Watkins S, Washam R, DuClos C, Jones S, et al. , Fish consumption and hair mercury levels in women of childbearing age, martin county, Florida, Maternal Child Health J. 18 (2014) 2352–2361. [DOI] [PubMed] [Google Scholar]
- [3].Soon R, Dye TD, Ralston NV, Berry MJ, Sauvage LM, Seafood consumption and umbilical cord blood mercury concentrations in a multiethnic maternal and child health cohort, BMC Pregnancy Childbirth 14 (2014) 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xu X, Newman MC, Mercury exposure as a function of fish consumption in two asian communities in coastal virginia, USA, Arch. Environ. Contam. Toxicol 68 (April (3)) (2015) 462–475. [DOI] [PubMed] [Google Scholar]
- [5].Burch JB, Wagner Robb S Puett R, Cai B, Wilkerson R, Karmaus W, et al. , Mercury in fish and adverse reproductive outcomes: results from South Carolina, Int. J. Health Geogr 13 (2014) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mortensen ME, Caudill SP, Caldwell KL, Ward CD, Jones RL, Total and methyl mercury in whole blood measured for the first time in the U: S. population: NHANES 2011–2012, Environ. Res 134 (2014) 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schaefer AM, Jensen EL, Bossart GD, Reif JS, Hair mercury concentrations and fish consumption patterns in Florida residents, Int. J. Environ. Res. Public Health 11 (2014) 6709–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Laks DR, Mercury rising: response to the EPA assessment of mercury exposure, Biometals 27 (2014) 1–4. [DOI] [PubMed] [Google Scholar]
- [9].Adams KJ, Drenner RW, Chumchal MM, Donato DI, Disparity between state fish consumption advisory systems for methylmercury and US Environmental Protection Agency recommendations: a case study of the south central United States, Environ. Toxicol. Chem 35 (2016) 247–251. [DOI] [PubMed] [Google Scholar]
- [10].Johnston JE, Hoffman K, Wing S, Lowman A, Fish consumption patterns and mercury advisory knowledge among fishers in the haw river basin, N. C. Med. J 77 (2016) 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diez S, Human health effects of methylmercuty exposure, Rev. Environ. Contam. Toxicol 198 (2009) 111–132. [DOI] [PubMed] [Google Scholar]
- [12].Huang CF, Hsu CJ, Liu SH, Lin-Shiau SY, Neurotoxicological mechanism of methylmercury induced by low-dose and long-term exposure in mice: oxidative stress and down-regulated Na+/K(+)-ATPase involved, Toxicol. Lett 176 (2008) 188–197. [DOI] [PubMed] [Google Scholar]
- [13].Kerper LE, Ballatori N, Clarkson TW, Methylmercury transport across the blood-brain barrier by an amino acid carrier, Am. J. Physiol 262 (1992) R761–5. [DOI] [PubMed] [Google Scholar]
- [14].Aschner M, Aschner JL, Mercury neurotoxicity: mechanisms of blood-brain barrier transport, Neurosci. Biobehav. Rev 14 (1990) 169–176. [DOI] [PubMed] [Google Scholar]
- [15].Bridges CC, Zalups RK, Mechanisms involved in the transport of mercuric ions in target tissues, Arch. Toxicol 91 (January (1)) (2017) 63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bridges CC, Joshee L, Zalups RK, Effect of DMPS and DMSA on the placental and fetal disposition of methylmercury, Placenta 30 (2009) 800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bridges CC, Joshee L, Zalups RK, Placental and fetal disposition of mercuric ions in rats exposed to methylmercury: role of Mrp2, Reprod. Toxicol 34 (2012) 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kajiwara Y, Yasutake A, Adachi T, Hirayama K, Methylmercury transport across the placenta via neutral amino acid carrier, Arch. Toxicol 70 (1996) 310–314. [DOI] [PubMed] [Google Scholar]
- [19].Ong CN, Chia SE, Foo SC, Ong HY, Tsakok M, Liouw P, Concentrations of heavy metals in maternal and umbilical cord blood, Biometals 6 (1993) 61–66. [DOI] [PubMed] [Google Scholar]
- [20].Yang J, Jiang Z, Wang Y, Qureshi IA, Wu XD, Maternal-fetal transfer of metallic mercury via the placenta and milk, Ann. Clin. Lab. Sci 27 (1997) 135–141. [PubMed] [Google Scholar]
- [21].Antunes Dos Santos A, Appel Hort M, Culbreth M, Lopez-Granero C, Farina M, Rocha JB, et al. , Methylmercury and brain development: a review of recent literature, J. Trace Elem. Med. Biol 38 (December) (2016) 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Diav-Citrin O, Prenatal exposures associated with neurodevelopmental delay and disabilities, Dev. Disabil. Res. Rev 17 (2011) 71–84. [DOI] [PubMed] [Google Scholar]
- [23].Ceccatelli S, Bose R, Edoff K, Onishchenko N, Spulber S, Long-lasting neurotoxic effects of exposure to methylmercury during development, J. Intern. Med 273 (2013) 490–497. [DOI] [PubMed] [Google Scholar]
- [24].Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, et al. , Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene, Science 271 (1996) 1126–1128. [DOI] [PubMed] [Google Scholar]
- [25].Harris HH, Pickering IJ, George GN, The chemical form of mercury in fish, Science 301 (2003) 1203. [DOI] [PubMed] [Google Scholar]
- [26].Bridges CC, Joshee L, Zalups RK, Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid, J. Pharmacol. Exp. Ther 324 (2008) 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Keith RL, Setiarahardjo I, Fernando Q, Aposhian HV, Gandolfi AJ, Utilization of renal slices to evaluate the efficacy of chelating agents for removing mercury from the kidney, Toxicology 116 (1997) 67–75. [DOI] [PubMed] [Google Scholar]
- [28].Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, et al. , Mobilization of heavy metals by newer, therapeutically useful chelating agents, Toxicology 97 (1995) 23–38. [DOI] [PubMed] [Google Scholar]
- [29].Norseth T, Clarkson TW, Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats, Arch. Environ. Health 21 (1970) 717–727. [DOI] [PubMed] [Google Scholar]
- [30].Norseth T, Clarkson TW, Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury, Biochem. Pharmacol 19 (1970) 2775–2783. [DOI] [PubMed] [Google Scholar]
- [31].Lee HB, Blaufox MD, Blood volume in the rat, J. Nucl. Med 26 (1985) 72–76. [PubMed] [Google Scholar]
- [32].Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV, Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury, Am. J. Physiol. Renal. Physiol 286 (2004) F552–63. [DOI] [PubMed] [Google Scholar]
- [33].Bridges CC, Joshee L, Zalups RK, MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury, Toxicol. Sci 105 (2008) 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Young AM, Allen CE, Audus KL, Efflux transporters of the human placenta, Adv. Drug Deliv. Rev 55 (2003) 125–132. [DOI] [PubMed] [Google Scholar]
- [35].Mathias AA, Hitti J, Unadkat JD, P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages, Am. J. Physiol. Regul. Integr. Comp. Physiol 289 (2005) R963–9. [DOI] [PubMed] [Google Scholar]
- [36].Hoffmann K, Loscher W, Upregulation of brain expression of P-glycoprotein in MRP2-deficient TR(−) rats resembles seizure-induced up-regulation of this drug efflux transporter in normal rats, Epilepsia 48 (2007) 631–645. [DOI] [PubMed] [Google Scholar]
- [37].Hozumi I, Suzuki JS, Kanazawa H, Hara A, Saio M, Inuzuka T, et al. , Metallothionein-3 is expressed in the brain and various peripheral organs of the rat, Neurosci. Lett 438 (2008) 54–58. [DOI] [PubMed] [Google Scholar]
- [38].Mandal PK, Saharan S, Tripathi M, Murari Brain G, glutathione levels-a novel biomarker for mild cognitive impairment and Alzheimer’s disease, Biol. Psychiatry 78 (2015) 702–710. [DOI] [PubMed] [Google Scholar]
- [39].Benitez MA, Mendez-Armenta M, Montes S, Rembao D, Sanin LH, Rios C, Mother-fetus transference of lead and cadmium in rats: involvement of metallothionein, Histol. Histopathol 24 (2009) 1523–1530. [DOI] [PubMed] [Google Scholar]
- [40].Madazli R, Benian A, Aydin S, Uzun H, Tolun N, The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia, J. Obstet. Gynaecol 22 (2002) 477–480. [DOI] [PubMed] [Google Scholar]
- [41].Zalups RK, Bridges CC, MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA, Toxicol. Appl. Pharmacol 235 (2009) 10–17. [DOI] [PubMed] [Google Scholar]
- [42].Buelke-Sam J, Nelson CJ, Byrd RA, Holson JF, Blood flow during pregnancy in the rat: i. Flow patterns to maternal organs, Teratology 26 (1982) 269–277. [DOI] [PubMed] [Google Scholar]
- [43].Hytten F, Blood volume changes in normal pregnancy, Clin. Haematol 14 (1985) 601–612. [PubMed] [Google Scholar]