Abstract
Domoic acid is a potent marine algal toxin produced by diatomic genus of Pseudo-nitzschia causing amnesic shell fish poisoning. Domoic acid toxicosis mainly involves excitotoxic effects coupled with oxidative stress. The present study was aimed to evaluate the protective effects of hydro-alcoholic extract of Terminalia arjuna (TA) against domoic acid induced toxic effects in Caco-2 cell line. It was observed that the toxicity induced by domoic acid in Caco-2 cells was mediated by oxidative insult leading to morphological changes, DNA damage and apoptosis. In our study pre-treatment of the cells with TA (10, 20 and 30 μg/ml) showed significant protection against domoic acid induced morphological, oxidative and apoptotic damages in a dose dependent manner. The effect of phytocompounds present in TA viz., kaempferol and arjungenin showed significant protection against domoic acid induced toxicity in Caco-2 cell line. Hence, it could be inferred that the protective effect of TA extract against domoic acid induced toxicity could be due to the individual or synergistic effects of kaempferol and argungenin. However, further clinical studies are warranted to consider TA as a natural remedy to prevent amnesic shell fish poisoning.
Keywords: Domoic acid, Oxidative stress, Terminalia arjuna, Apoptosis, Morphological changes, Phytocompounds
Introduction
Domoic acid belonging to kainoid group of bio-toxins is produced by the diatomic species of Pseudo-nitzschia causes amnesic shell fish poisoning. The structure of domoic acid closely resembles glutamic acid and kainic acid. Due to the rigid structure of domoic acid, it binds with higher affinity to glutamate receptors, thereby causing neuro-excitatory behavioural effects in humans, marine mammals and birds (Pulido 2008). Domoic acid is reported to interact with various receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainic acid, N-methyl-D-aspartate (NMDA) and activate different subtypes of voltage-dependant Ca2+ channels to promote influx of Ca2+, which causes neuronal degeneration (Berman and Murray 1997; Hampson and Manalo 1998). In humans, an oral exposure to domoic acid leads to a variety of gastro-intestinal and neurological disorders including nausea, vomiting, diarrhoea, abdominal cramps, excitotoxicity, seizures and memory impairment (Lefebvre and Robertson 2010).
Memory impairment and associated neuropathology are the prominent effects of domoic acid toxicity (Sutherland et al. 1990). The toxicosis of domoic acid is associated with generation of large amount of reactive oxygen species which in turn causes, tissue damage, impairment of mitochondrial potential, apoptosis and leads to cell death (Giordano et al. 2008). Oxidative stress is the imbalance between the oxidants and antioxidants in the biological system that leads to accumulation of reactive oxygen species (ROS) in the body (Buttke and Sandstrom 1992). Accumulated ROS adversely affects cell homeostasis by oxidising vital components of the cell and leads to many metabolic disorders. Thus toxic insult disturbs the antioxidant defense system of the body resulting in damage to all macromolecules such are DNA, protein, lipid etc. These adverse effects in the body could be neutralized by providing supplementary antioxidants from natural sources (Uttara et al. 2009).
Herbal drugs make headway in modern research because of growing public acceptance due to their lesser side effects and increased effectiveness. A recent survey reveals that about 63.9% of the studied population endorse the use of alternative medicine (including herbal) and this trend is similar throughout the world Oluwatoyin et al. (2007). Terminalia arjuna (Roxb.) Wight and Arn. belongs to the family Combretaceae and is known as Arjuna in Ayurveda, the traditional Indian medicine. Bark of the plant is well known for its medicinal importance as cardio protective agent (Jain et al. 2009). Terminalia arjuna bark is frequently used to treat hypertension, ischemic heart diseases, hypercholesterolemia and it is known to possess anti-microbial, anti-mutagenic, anti-helmintic, anti-fertility and anti-HIV properties (Dwivedi 2007). Terminalia arjuna has shown in vitro antioxidant potential and DNA damage protective activity in PC12 cells (Kumar et al. 2013).
The aim of the present study was to evaluate modulatory effects of hydro-alcoholic extract of Terminalia arjuna bark (TA) against domoic acid induced toxicity in Caco-2 cells, the human epithelial colorectal adenocarcinoma cells. Cell morphology, cell viability, mitochondrial membrane potential and other oxidative stress markers were targeted to study the effect of TA pre treatment on domoic acid induced cytotoxicity.
Materials and methods
Chemicals and reagents
Domoic acid, ursolic acid, kaempferol, arjungenin, arjunolic acid, ellagic acid, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), 2′,7′-Dichloro dihydrofluorescein diacetate (H2 DCFDA), rhodamine 123, DAPI, Dulbecco’s modified Eagle’s medium (DMEM), l-glutamine, trypsin, protease and phosphatase inhibitor cocktail, luminol and p-coumaric acid, primary antibodies for catalase, glutathione reductase, glyceraldehyde 3-phosphate dehydrogenase, anti-mouse polyvalent secondary antibody were obtained from Sigma (St. Louis, MO, USA). Foetal bovine serum was obtained from HyClone™ (Logan, UT, USA).
Plant material
Termimalia arjuna bark was collected from Mysore, India and authenticated by the Department of Botany, University of Mysore, Mysore, India (Voucher number: AND 1357). The shade dried bark material was defatted with petroleum ether and then extracted with 70% ethyl alcohol for 72 h by using soxhlet apparatus. The T. arjuna bark extract (TA) was concentrated under reduced pressure (Heidolph, Schwabach, Germany) and lyophilised for further studies.
Phytochemical studies
LC-ESI–MS/MS analysis of TA
The LC-ESI–MS/MS analysis of TA was performed using 6520 Accurate Q-TOF (Agilent Santa Clara, CA, USA) mass spectrometer coupled to HPLC equipped with a UV–VIS detector. Zorbax SBC18 Rapid resolution column of 4.6 × 150 mm, 3.5 μ particle size was used under the following conditions: (A) formic acid (0.1%v/v) and 10 mM ammonium fluoride and (B) acetonitrile +0.1% Formic acid; gradient (in solvent B): (i) 30%, from 0 to 15 min, (ii) 55%, from 15 min, (iii) 95%, from 25 to 45 min, and (iv) 35%, at 45–48 min; flow rate: 0.2 ml/min; injection volume was 5 μL. Both negative and positive ion modes of ESI parameters was used; mass range 100–1200 m/z; 4 kV spray voltage; gas temperature 325 °C; gas flow10 L/min; and nebulizer 40 psi.
Estimation of total polyphenols and flavonoids
Total polyphenolic content present in TA was estimated using Folin-Ciocalteu reagent as mentioned by Slinkard and Singleton (1977) and expressed as mg/g gallic acid equivalents (GAE). The quantification of flavonoids was carried out as described by Ordon et al. (2006) and was expressed as mg/g rutin equivalents.
Free radical scavenging activity
DPPH radical scavenging activity
The free radical scavenging activity of TA was measured using DPPH (1, 1-diphenyl 2- picryl hydrazyl) assay. Briefly, 100 µl of different concentrations of extract was added to 2.9 ml of DPPH (0.1 mM). The tubes were incubated for 30 min and OD was taken at 515 nm. The results were then expressed as percentage inhibition of sample.
Hydrogen peroxide (H2O2) radical scavenging activity
The H2O2 radical scavenging activity assay was performed as mentioned by Ruch et al. (1989). A solution of hydrogen peroxide (2 mmol/l) was prepared in phosphate buffer (pH 7.4). TA was added to hydrogen peroxide solution (0.6 ml) in different concentrations and the absorbance was read at 230 nm.
2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity
The working reagent was prepared by mixing of 7 mM ABTS and 2.4 mM potassium persulfate (K2S2O8) in equal quantities and then incubated for 12 h at room temperature in dark. 1 ml of the working reagent was added to different concentrations of TA extract and absorbance was measured at 734 nm.
Ferrous reducing antioxidant power assay
Briefly, to TA (100 µl, various concentrations) 2.9 ml of FRAP reagent (a mixture of 25 ml acetate buffer (300 mM; pH 3.6), 2.5 ml TPTZ (2, 4, 6-tripyridyl-s-triazine; 10 mM) in HCl (40 mM), 2.5 ml FeCl3 (20 mM)) was added. The mixture was then incubated for 15 min in dark and then absorbance was measured at 593 nm for the coloured product of ferrous tripyridyltriazine complex; FeSO4 was used as the standard.
Cell culture and maintenance
Human colorectal adenocarcinoma cell line (Caco-2) was obtained from National Centre for Cell Sciences (NCCS), Pune, India. The cells were grown in culture plates (24 well and 96 well plates) and culture flasks (25 and 75 cm2) with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and l-glutamine, and anti-mycotic and antibiotic solution (Sigma) at 37 °C in a humidified atmosphere of 5% CO2. The medium was replaced every alternate day and 80% confluent cells were used for all assays.
Cytotoxicity by MTT assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was done to determine cell viability, since it is widely accepted and reliable cytotoxicity assay (Fotakis and Timbrell 2006). The Caco-2 cells were seeded in 96 wells plate. To observe the effects of domoic acid, TA and phytocompounds viz., ursolic acid, ellagic acid, kaempferol, arjunolic acid and arjungenin on cell viability, individual treatment was done at varying concentrations. After 24 h of individual treatments (10–100 ng ml−1 of domoic acid and TA), 100 μl of MTT (0.7 mg/ml) was added and incubated for 4 h at 37 °C. The formazan crystals formed after incubation were dissolved in DMSO and the absorbance was read at 570 nm using VERSA max Hidex plate chameleon™V (Turku, Finland). Based on the above results, the toxin (IC50) was added to the plates, which were 1 h prior incubated with different concentrations of TA (10, 20 and 30 µg/ml) or the phytocompounds. The protective effect of plant extract and the phytocompounds against domoic acid induced toxicity on Caco-2 was determined. The cells without any treatments served as control. Results were expressed as percentage viability, where absorbance of untreated well (control) was taken as 100%.
Morphological studies
Morphological studies under bright-field microscope
Approximately, 1 × 106 cells/ml were seeded in 25 cm2 cell culture. The cells were exposed with domoic acid (DA) (75 ng/ml) for 24 h with or without prior treatment for 1 h with different concentrations of TA (10, 20 and 30 µg/ml) or the phytocompounds such as ursolic acid, ellagic acid, kaempferol, arjunolic acid and arjungenin. The cells without any treatments were considered as control. After incubation period, cells were photographed (Olympus, Tokyo, Japan) to observe the morphological changes.
Morphological studies under scanning electron microscope
Approximately, 1 × 106 cells/ml were seeded on cover slip placed in 12-well cell culture plate and were exposed to domoic acid (75 ng/ml) for 24 h with or without pre-treatment of TA. After incubation period, medium was discarded and cells were fixed with 2.5% of glutaraldehyde and washed with gradient of ethanol (70–100%). The cover slip was pasted on dual side glue carbon tape and sputter-coated with gold. Cells without domoic acid exposure or TA pre-treatment were considered as control. The morphological features of cells were observed under SEM (FEI, Hillsboro, OR, USA) at 20 kV and, images were captured under 500× and 3000× magnification.
Estimation of apoptotic damage
Mitochondrial membrane potential (MMP)
MMP was determined using fluorescent probe rhodamine 123. Cells seeded in 24 wells plate were exposed to domoic acid (75 ng/ml) for 24 h with or without prior treatment of various concentrations of TA for 1 h. The medium was discarded and incubated with rhodamine 123 (10 µg/ml) for 1 h. The cells were then washed twice with PBS and fluorescence was read at excitation wavelength, 485 nm and an emission wavelength, 535 nm using Hidex plate chameleon™V.
DNA damage using DAPI staining
Caco-2 seeded on cover slips were left to adhere and were exposed to domoic acid (75 ng/ml) for 24 h with or without prior treatment of various concentrations of TA for 1 h. The medium was removed and cells were washed with phosphate buffer and fixed using 100% ethanol. To this, 100 µl of DAPI stain (1 µl/ml ethanol) was added and incubated for 5 min. The stain was removed and mounting solution was added to it before visualization. The fluorescence microscope was used to take the images of the treated cells to analyze the nuclear features.
Estimation of oxidative stress status
Reactive oxygen species (ROS) production
Caco-2 cells (80% confluent) grown in 24 well plates were exposed to domoic acid (75 ng/ml) for 24 h with or without prior treatment for 1 h with various concentrations of TA (10, 20 and 30 µg/ml) or phytocompounds viz., ursolic acid, ellagic acid, kaempferol, arjunolic acid and arjungenin. After incubation, the medium was removed and dichlorodihydrofluorescein diacetate (H2 DCFDA), at 5 mg/ml was added and incubated for 30 min. The cells were washed thrice with phosphate buffer and increase in fluorescence was measured at excitation wavelength of 485 nm and an emission wavelength of 535 nm using Hidex plate chameleon™V.
Nitric oxide (NO) production
Caco2 cells (80% confluent) grown in 24 well plates were exposed to domoic acid (75 ng/ml) for 24 h with or without prior treatment for 1 h with various concentrations of TA (10, 20 and 30 µg/ml) or phytocompounds viz., ursolic acid, ellagic acid, kaempferol, arjunolic acid and arjungenin. After incubation, an equal amount of Griess reagent was added to the supernatant and the pink colour developed was read at 540 nm.
Western blot analysis
Cells grown in 75 cm2 flasks were treated with domoic acid for 24 h with or without prior treatment with various concentrations of TA (10, 20 and 30 µg/ml) for 1 h, washed with phosphate buffer and collected in centrifuge tube. The proteins from the cells were extracted using HEPES buffer (pH 7.4) containing protease and phosphatase inhibitor cocktail (10 µl/ml) and estimated using the method published by Lowry et al. (1951). Equal amount of protein was loaded to each well in SDS-PAGE gel and separated. The protein bands were then transferred on to a nitrocellulose membrane (Pall Scientific Pvt. Ltd., Mumbai, India) and blocked with 5% non-fat milk at 4 °C for 24 h. Primary antibodies to CAT and GR (1:1000) were individually added to the blot and kept during shaking at room temperature for 3 h. The membranes were washed with Tris-buffered saline with Tween 20 for 15 min each and HRP conjugated anti-mouse secondary antibody (1:10,000) was added. Following incubation for 2 h the membranes were washed and the blot was developed by chemiluminescence method using luminol and p-coumaric acid. The bands developed were transferred on to an x-ray film to analyse the band intensity (Jayaraj et al. 2006).
Statistical analysis
The data are expressed as mean ± SEM for at least three independent experiments (triplicate). Statistical differences between treatment groups were determined by ANOVA test. Differences were considered significant at p < 0.05.
Results
In vitro free radical scavenging potential of TA
TA is found to be rich in polyphenols (47 ± 0.16%) and flavonoids (5.8 ± 0.05%). Scavenging activity of TA against various free radicals i.e. DPPH, H2O2, ABTS, NO and ferric reducing antioxidant power are shown in Table 1.
Table 1.
In vitro free radical scavenging activity of TA
| Parameters | TA |
|---|---|
| DPPH radical scavenging activitya | 85 ± 5 |
| H2O2 radical scavenging activitya | 180 ± 9 |
| ABTS radical scavenging activitya | 13.3 ± 2 |
| Nitric oxide radical scavenging activitya | 109 ± 5 |
| Ferric reducing antioxidant powerb | 683 ± 12 |
| Total polyphenolsc | 470 ± 16 |
| Total flavonoidsd | 58 ± 5 |
a Expressed in IC50 values in µg/ml
b Expressed in units of mmol Fe (II)/g
c Expressed in units of mg/g of gallic acid equivalents
d Expressed in units of mg/g of rutin equivalents
LC-ESI–MS/MS analysis of TA
LC-ESI–MS/MS analysis of TA was carried out and compounds were confirmed by mass fragmentation analysis. Some of the major phytochemicals identified are arjunolic acid, arjungenin, kaempferol 3-glucuronide, ursolic acid, ellagic acid and its derivatives etc. (Fig. 1).
Fig. 1.

LC–ESI–MS/MS chromatograms of some identified bioactive compounds from TA
Effect of TA and on domoic acid induced cytotoxicity
Caco-2 cells treated with TA (10–50 µg/ml) did not show any significant change in cell viability, when compared to the control cells (Fig. 2a). This result confirmed that TA alone did not cause any effect on cell viability. Caco-2 cells were exposed to different concentrations of domoic acid to observe the mortality of cell line (Fig. 2b). Domoic acid treatment markedly decreased cell viability in a dose dependent manner and IC50 was found to be 75 ± 3 ng/ml. Domoic acid exposed cells were significantly protected by pre-treatment with TA, and improved cell viability in increasing order from 10 µg/ml, to 20 µg/ml, and 30 µg/ml, respectively (Fig. 2c). Cells pre-treated with phytocompounds, kaempferol and arjungenin showed significant increase (p < 0.05) in cell viability with respect to cells exposed to domoic acid without any pre-treatment (Fig. 2d).
Fig. 2.
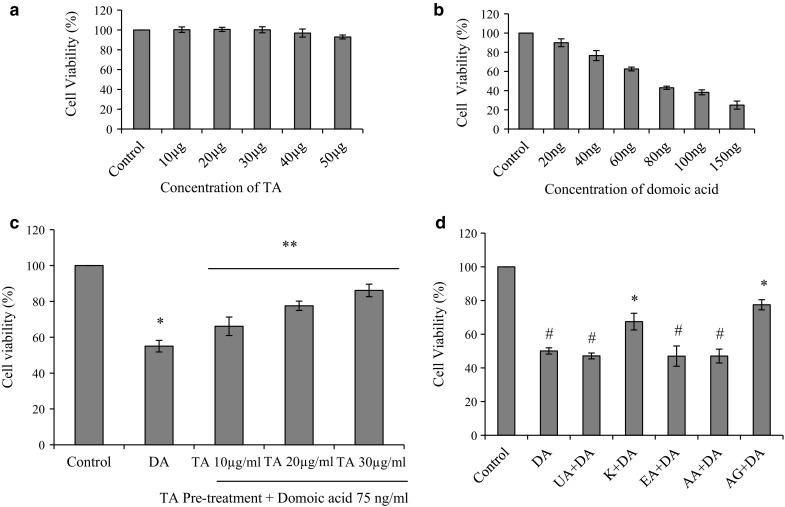
Protective effect of TA against domoic acid induced cytotoxicity in Caco2 cells. a Response to pre-treatment of TA. Caco-2 cells were treated with hydro-alcoholic extract of Terminalia arjuna at different concentrations (10–50 µg/ml) for 24 h. b Cytotoxicity of domoic acid. Cytotoxicity of domoic acid was assessed in various doses of domoic acid i.e. 20–150 ng/ml and 75 ± 3 ng/ml was found to be the IC50 value. c Effect of TA on domoic acid induced toxicity. Pre-treatment with TA (10–30 µg/ml) followed by domoic acid exposure (75 ng/ml) for 24 h. d Effect of phytocompounds present in TA against domoic acid induced cytotoxicity. Cell viability on pre-treatment of ursolic acid (UA) 250 ng/ml, kaempferol (K) 200 ng/ml, ellagic acid (EA) 300 ng/ml, arjunolic acid (AA) 150 ng/ml, arjungenin (AG) 200 ng/ml, followed by domoic acid (DA) exposure (75 ng/ml) for 24 h. Data are expressed as mean ± SEM of three independent experiments. In the TA treated groups Asterisk indicates statistically significance p < 0.05 vs control and Double asterisk indicates p < 0.05 vs domoic acid, whereas in phytocompounds treated groups Asterisk indicates significant difference p < 0.05 with respect to domoic acid; # indicates significant difference at p < 0.05 with respect to control
Effect of TA on domoic acid induced cell morphology and SEM characterisation
Caco-2 cells were observed for general morphological differences in various groups i.e. control group, domoic acid exposed group and domoic acid with TA or the phytocompounds pre-treatment groups (Fig. 3a, c). Domoic acid exposed cells showed loss of adherence to the flask. Caco-2 cells pre-treated with TA, kaempferol and arjungenin showed improvement in their adherence property. Domoic acid exposure showed considerable morphological apoptotic characteristics, however the same was decreased with pre-treatment of TA, kaempferol and arjungenin. Cells pretreated with phytocompounds viz., ellagic acid, ursolic acid and arjunolic acid did not show any significant difference in cell morphology with respect to the domoic acid treated group. SEM analysis showed that domoic acid exposure led to ultra structural changes like detached cells, shrinkage and reduction in cell size when compared to control group cells (Fig. 3b). Moreover, pre-treatment with TA (30 µg/ml) protected cells from ultra structural morphological changes induced by domoic acid (Fig. 3b).
Fig. 3.


Protective effect of TA pre-treatment on domoic acid induced structural alterations of Caco-2 cells. a Phase-contrast microscopy: (i) Control Caco-2 cells, (ii) Domoic acid (75 ng/ml) exposed cells for 24 h, (iii–v) Pre-treatment with TA (10 µg/ml, 20 µg/ml and 30 µg/ml) followed by domoic acid (75 ng/ml) exposure for 24 h. b Scanning electron microscopy: I and (i) Control Caco-2 cells showing normal morphology and normal cell size, II and (ii) Domoic acid exposed cells showing cell damage, III and (iii) Pre-treatment with TA (30 µg/ml) followed by domoic acid exposure showing protective effect. Images from I to III at 500× magnification and images from (i–iii) are at 3000× magnification. c Effect of pre-treatment with phytocompounds on domoic acid induced morphological changes in Caco-2 cells. (i): Control Caco-2 cells, (ii): Domoic acid (75 ng/ml) exposed cells for 24 h, (iii–vii): Pre-treatment with ursolic acid (UA) 250 ng/ml, kaempferol (K) 200 ng/ml, ellagic acid (EA) 300 ng/ml, arjunolic acid (AA)150 ng/ml, arjungenin (AG) 200 ng/ml respectively, followed by domoic acid (DA) exposure (75 ng/ml) for 24 h
Effect of TA on domoic acid induced apoptosis
Effect of TA on domoic acid induced loss of mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential is one of the major indicators of apoptosis detection in cell line studies. In the present study, domoic acid exposure induced apoptosis at a greater extent assessed by means of fluorescence intensity (41.2 ± 3.5%), when compared with untreated cell line (Fig. 4a). Different doses of TA pre-treatment attenuated domoic acid induced loss of mitochondrial membrane potential and improved the fluorescence intensity by 49.5 ± 4.3% (TA-10 µg/ml), 64.8 ± 3.38% (TA-20 µg/ml) and 86.2 ± 6.1% (TA-30 µg/ml).
Fig. 4.

Protective effect of TA pre-treatment on domoic acid induced apoptosis. a Effect of TA on domoic acid induced loss of mitochondrial membrane potential. Pre-treatment with TA (10 µg/ml, 20 µg/ml and 30 µg/ml, respectively) followed by domoic acid (75 ng/ml) exposure for 24 h. The value of MMP was expressed as relative fluorescence intensity compared to control. *Statistically significant differences, *p < 0.05 vs control and **p < 0.05 vs domoic acid. b Effect of TA on domoic acid induced nuclear damage using DAPI staining. (i) Control Caco-2 cells, (ii) Domoic acid (75 ng/ml) treated cells exposed for 24 h, (iii–v) Pre-treatment of TA (10, 20, and 30 µg/ml, respectively) followed by domoic acid (75 ng/ml) exposure for 24 h. Arrow marks in the image represent cells undergoing apoptosis
Effect of TA on domoic acid induced nuclear damage
Caco-2 cells were examined for nuclear damage using DAPI staining. As shown in Fig. 4b, domoic acid exposed cells were observed with condensed nuclei in greater numbers; however, TA pre-treatment reduced the number of cells with nuclear damage in a concentration dependent manner. Cells pre-treated with TA-30 µg/ml appeared similar to those of control cells.
Effect of TA on domoic acid induced oxidative stress
Potential effect of TA on domoic acid induced ROS production
Caco-2 cells treated with domoic acid showed a three-fold increase in ROS generation when compared with the control group (Fig. 5a). The value of ROS was expressed as relative fluorescence compared to group which is treated with only domoic acid, which was taken as 100%. The intensity of fluorescence in cells treated with TA, kaempferol and arjungenin showed a significant reduction (p < 0.05) of ROS production compared to the group which was treated with only domoic acid (Fig. 5a, b).
Fig. 5.
Effect of pre-treatment of TA and phytocompounds on domoic acid induced oxidative stress. a, b Reactive oxygen species (ROS). c, d Nitrite production (NO). Pre-treatment of TA (10, 20, and 30 µg/ml, respectively) and phytocompounds (ursolic acid (UA) 250 ng/ml, kaempferol (K) 200 ng/ml, ellagic acid (EA) 300 ng/ml, arjunolic acid (AA) 150 ng/ml, arjungenin (AG) 200 ng/ml,) followed by domoic acid (75 ng/ml) exposure for 24 h. The values of ROS were expressed as relative fluorescence intensity and NO was expressed as relative absorbance compared to domoic acid treatment. In TA treated groups *indicates statistically significance p < 0.05 vs control and ** indicates p < 0.05 vs domoic acid, whereas in phytocompounds treated groups * indicates significant difference p < 0.05 with respect to domoic acid; # indicates significant difference at p < 0.05 with respect to control
Potential effect of TA on domoic acid induced NO release
NO production by Caco-2 cells culture medium was determined by the measurement of total nitrite in culture medium. Domoic acid exposure triggered significant release of nitrite with respect to the control group (p < 0.05). Pre-treatment of cells with TA, kaempferol and arjungenin significantly inhibited domoic acid induced NO release (p < 0.05) (Fig. 5c, d).
Effect of TA on domoic acid induced changes in glutathione reductase and catalase
Domoic acid exposure significantly down regulated the levels of antioxidant enzymes (p < 0.05), such as, glutathione reductase and catalase when compared to control Caco-2 cells (Fig-6). Pre-treatment of TA showed protective effect in a dose depended manner on domoic acid induced oxidative stress with respect to levels of glutathione reductase and catalase (Fig. 6).
Fig. 6.
Protective effect of TA on domoic acid induced modulation of glutathione reductase and catalase. 1. Control 2. Domoic acid (75 ng/ml) 3–5. TA-10, 20 and 30 µg/ml, respectively. GAPDH - Glyceraldehyde 3-phosphate dehydrogenase, GR-glutathione reductase, CAT-catalase. Relative band intensities are shown in separate bar diagrams for GR and CAT
Discussion
Exposure to domoic acid through contaminated sea food consumption, particularly shell fish causes several toxic effects to humans sometimes proving fatal. One report on such an intoxication indicated that consumption of domoic acid contaminated mussel led to three deaths and neurological abnormalities in more than hundred individuals (Lelong et al. 2012). Few other studies observed the symptoms of illness with respect to domoic acid consumption including gastrointestinal symptoms within 24 h viz., vomiting, abdominal cramps, diarrhoea and followed by neurological symptoms up to 48 h like behavioural changes, seizures and memory loss (Lefebvre and Robertson 2010; Ramsdell and Gulland 2014). The present study was conducted to investigate the role of TA against diverse toxic responses following domoic acid exposure on Caco-2 cells. The entry of toxin in the food chain can primarily exert its effect on the digestive system. Several studies concerning the role of domoic acid on primary neuronal cells have been carried out (Berman and Murray 1997; Berman et al. 2002). The present study is focused on Caco-2 cells, since intestinal cells are likely to be more sensitive to domoic acid in vivo in the first few hours of exposure.
Domoic acid exposure decreased cell viability in a concentration dependent manner (20–150 ng/ml), indicating the sensitivity of Caco-2 cell line to the toxic effects of domoic acid. The estimated IC50 (0.2 µM) of domoic acid on Caco-2 cells was in accordance with the previous findings of Carvalho et al. (2008). However, pre-treatment with TA increased cell viability up to a certain extent and prevented domoic acid induced cell death. Bright field microscopic observation and SEM analysis of the present study revealed that domoic acid (75 ng/ml) caused structural changes in Caco-2 cells which include shrunken cells, decreased cell size, irregular cell borders and formation of intra-cytoplasmic vacuoles. Pre-treatment with TA at 30 µg/ml prevented ultra-structural alterations in the colonic cell line Caco-2 subsequent to domoic acid exposure suggesting its protective role against domoic acid induced toxicity.
Mitochondria play a key role in integration and transmission of cell death signals by activating caspases or programmed cell death pathways. Effect of domoic acid on cell apoptosis has been observed by a decline in the mitochondrial membrane potential (ΔΨm). TA pre-treatment increased ΔΨm in a dose dependent manner. The increase in ΔΨm may be due to active regulation of pro-apoptotic/apoptotic/anti-apoptotic factors like cytochrome c (Cyt c), caspase-2, caspase-9 or Bcl-2 proteins (Samraj et al. 2007). Carvalho et al. (2008) demonstrated that exposure of Caco-2 cells to domoic acid (60–100 ng/ml), damaged membranes (lysosomes, and mitochondria) and induced apoptosis through up-regulation of Bax. Present findings of domoic acid induced cytotoxicity are in accordance with the results of previous studies of Carvalho et al. (2008) on the intestine cell line. Earlier studies by Ananth et al. (2001) strongly suggest that domoic acid induces apoptosis by the modulation of gene expressions such as, of Bcl-2, Bax, and caspase-3 in degenerating neurons of the hippocampus.
Present observation on in vitro free radical scavenging activities showed that T.arjuna bark extract scavenged free radical generation of DPPH·, ABTS·, NO·, H2O·2 and has high ferric reducing antioxidant power. Hydro-alcoholic extract of TA showed presence of high quantity of polyphenols and flavonoids. Our previous studies on bioactivity guided fractionation of TA showed presence of triterpenoids, tannins, polyphenols and flavonoids (Kumar et al. 2013). Further, LC–MS results of the present study identified bioactive polyphenols and terpenoids i.e. ellagic acid, ursolic acid, kempherol-3-glucuronide, trocotrienol, arjungenin, arjunolic acid, etc. Upon exposure to domoic acid, Caco-2 cell lines showed chromatin condensation as revealed by DAPI staining in the present study. Moreover, administration of TA decreased the number of condensed cell in a dose dependent manner. In our previous studies on in vitro PC12 cell line, different fractions of TA has shown a protective role on DNA damage (Kumar et al. 2013). Previous studies of Carvalho et al. (2008) also confirmed the genotoxicity of domoic acid through DNA damage, strand breaks and chromatin condensation.
The amount of free radical production is determined by the balance of many factors, and ROS are produced both endogenously and exogenously. The endogenous sources of ROS include mitochondria, cytochrome P450 metabolism, peroxisomes, and inflammatory cell activation. (Rahman 2007). The accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) can cause decreased oxidative phosphorylation and damage to mitochondrial DNA resulting in mitochondrial dysfunction. In the present study, DCFDA fluorescence and Griess reagent were used to determine the accumulation of ROS and NO, respectively, in Caco-2 cells. Our present results demonstrated that ROS and NO generation were significantly triggered (p < 0.05) by domoic acid exposure, and the same were decreased by administration of TA in a dose dependent manner. This domoic acid induced oxidative stress was also confirmed by others in in vitro and in vivo studies (Giordano et al. 2008; Vranyac-Tramoundanas et al. 2011; Pulido 2008). A previous study on California sea lions suggested that oxidative stress may occur during excitotoxicity induced by domoic acid (Giordano et al. 2008; Silvagni et al. 2005). Lu et al. (2013) demonstrated that domoic acid induces inflammatory markers, mitochondrial damage and generates oxidative species, which results in amnesia in domoic acid treated mice through PKC-z–dependent K-ras/Raf/MEK/ERK1/2 pathway.
Catalase (CAT) is one of the antioxidant enzymes that catalyzes the conversion of H2O2 into H2O and O2 and is important during elimination of ROS. Glutathione Reductase (GR) is a flavo-protein oxidoreductase, GR catalyzes the reduction of GSH and is involved in defense against ROS. Present protein expression studies revealed that both enzymes i.e. CAT and GR were down regulated by the domoic acid exposure. However, theses enzyme activities were up regulated two fold by TA-20 and TA-30 pre-treatments even after the domoic acid exposure. Previous studies on experimental animal models also suggested that the T. arjuna alcoholic extract may help in altering the levels of cytochrome P-450 and other antioxidant enzymes like SOD, CAT, GST and GSH in different organs. The exogenous antioxidants from T. arjuna may act directly or indirectly with the internal antioxidant system for synergistic effects to protect against several diseases linked to oxidative stress (Das et al. 2010; Kumar et al. 2013).
Attempt was also made to identify the most promising phytocompounds in TA extract against domoic acid toxicity. Morphological studies using bright field microscopy, MTT assay, ROS and nitrite estimation were carried out using arjungenin, arjunolic acid, ellagic acid, ursolic acid and kaempferol. The study showed that kaempferol and arjungenin at the concentration of 200 ng/ml showed protective effect against domoic acid induced toxicity in terms of increase in cell viability, decrease in cell damage with respect to cell morphology, reduced ROS and nitric oxide production in Caco-2 cell line. Arjungenin and kaempferol are known to have antioxidant potential to combat against a wide range of oxidative stress related diseases (Ghosh et al. 2010; Rajendran et al. 2014). Kaempferol belongs to the flavonol group which has attracted great interest owing to its apparent beneficial effects on human health attributed to its antioxidant activities (Wattel et al. 2003). A previous study by Choi (2011) strongly evidenced that kaempferol can help in reducing or preventing osteoblasts degeneration in degenerative disorders by decreasing reactive oxygen species generation and mitochondrial membrane dissipation. Further Hong et al. (2009) demonstrated modulatory effect of kaempferol on cellular antioxidant defense capacity and cell apoptosis by regulating HO-1 expression and MAPK signal transduction. Since oxidative stress is one of the major outcomes of amnesic shell fish poisoning, arjungenin and kaempferol can act as promising therapeutic compounds to combat the toxic insult caused by domoic acid.
To date, however, only a few studies have examined drugs/phytochemicals/herbal extracts to ameliorate domoic acid induced toxicity. In the present study TA prevented domoic acid induced toxic effects in Caco-2 cells to a greater extent. The present study inferred that the presence of kaempferol and arjungenin in the TA extract might have individually or synergistically affected the observed protective activity against domoic acid induced toxicity in Caco-2 cell line. Moreover, our study offers the possibility of potential therapeutic use of T. arjuna bark for the prevention domoic acid induced damages.
Acknowledgements
Authors are thankful to Director, DFRL, Mysore for providing constant guidance, encouragement and necessary facilities during this investigation.
Abbreviations
- TA
Terminalia arjuna hydro-alcoholic extract
- MMP
Mitochondrial membrane potential
- DAPI
2-(4-amidinophenyl)-1H -indole-6-carboxamidine
- ROS
Reactive oxygen species
- NO
Nitric Oxide
- CAT
Catalase
- GR
Glutathione reductase
- ABTS
2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
Complaince with ethical standards
Conflicts of interest
The authors declare that there is no conflict of interest.
References
- Amira OC, Okubadejo NU. Frequency of complementary and alternative medicine utilization in hypertensive patients attending an urban tertiary care centre in Nigeria. BMC Complement Altern Med. 2007;7(1):30. doi: 10.1186/1472-6882-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth C, Thameem Dheen S, Gopalakrishnakone P, Kaur C. Domoic acid-induced neuronal damage in the rat hippocampus: changes in apoptosis related genes (Bcl-2, Bax, caspase-3) and microglial response. J Neurosci Res. 2001;66:177–190. doi: 10.1002/jnr.1210. [DOI] [PubMed] [Google Scholar]
- Berman FW, Murray TF. Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J Neurochem. 1997;69:693–703. doi: 10.1046/j.1471-4159.1997.69020693.x. [DOI] [PubMed] [Google Scholar]
- Berman FW, LePage KT, Murray TF. Domoic acid neurotoxicity in cultured cerebellar granule neurons is controlled preferentially by the NMDA receptor Ca 2 + influx pathway. Brain Res. 2002;924:20–29. doi: 10.1016/S0006-8993(01)03221-8. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. A necessary role for cell shrinkage in apoptosis. Biochem Pharmacol. 1998;56:1549–1559. doi: 10.1016/S0006-2952(98)00225-1. [DOI] [PubMed] [Google Scholar]
- Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1992;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Carvalho Pinto-Silva CR, Moukha S, Matias WG, Creppy EE. Domoic acid induces direct DNA damage and apoptosis in Caco-2 cells: recent advances. Environ Toxicol. 2008;23:657–663. doi: 10.1002/tox.20361. [DOI] [PubMed] [Google Scholar]
- Choi EM. Kaempferol protects MC3T3-E1 cells through antioxidant effect and regulation of mitochondrial function. Food Chem Toxicol. 2011;49:1800–1805. doi: 10.1016/j.fct.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Das K, Chakraborty PP, Ghosh D, Nandi DK. Protective effect of aqueous extract of Terminalia arjuna against dehydrating induced oxidative stress and uremia in male rat. Iran J Pharm Res. 2010;9:153–161. doi: 10.18579/jpcrkc/2010/9/4/79508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi S. Terminalia arjuna Wight Arn.—a useful drug for cardiovascular disorders. J Ethnopharmacol. 2007;114:114–129. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160:171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Das J, Manna P, Sil PC. Protective effect of the fruits of Terminalia arjuna against cadmium-induced oxidant stress and hepatic cell injury via MAPK activation and mitochondria dependent pathway. Food Chem. 2010;123:1062–1075. doi: 10.1016/j.foodchem.2010.05.062. [DOI] [Google Scholar]
- Giordano G, Klintworth HM, Kavanagh TJ, Costa LG. Apoptosis induced by domoic acid in mouse cerebellar granule neurons involves activation of p38 and JNK MAP kinases. Neurochem Int. 2008;52:1100–1105. doi: 10.1016/j.neuint.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DR, Manalo JL. The activation of glutamate receptors by kainic acid and domoic acid. Nat Toxins. 1998;6:153–158. doi: 10.1002/(SICI)1522-7189(199805/08)6:3/4<153::AID-NT16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Hasan MdK, Kabir AKL, Mistry S. Chemical and biological investigation of leaves of polygonum plebeju. SJ Pharm Sci. 2009;2:71. [Google Scholar]
- Hong JT, Yen JH, Wang L, Lo YH, Chen ZT, Wu MJ. Regulation of heme oxygenase-1 expression and MAPK pathways in response to kaempferol and rhamnocitrin in PC12 cells. Toxicol Appl Pharmacol. 2009;237:59–68. doi: 10.1016/j.taap.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Jain S, Yadav PP, Gill V, Vasudeva N, Singla N. Terminalia arjuna a sacred medicinal plant: phytochemical and pharmacological profile. Phytochem Rev. 2009;8:491–502. doi: 10.1007/s11101-009-9134-8. [DOI] [Google Scholar]
- Jayaraj R, Anand T, Rao PL. Activity and gene expression profile of certain antioxidant enzymes to microcystin-LR induced oxidative stress in mice. Toxicology. 2006;220:136–146. doi: 10.1016/j.tox.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Kumar GP, Navya K, Ramya EM, Venkataramana M, Anand T, Anilakumar KR. DNA damage protecting and free radical scavenging properties of Terminalia arjuna bark in PC-12 cells and plasmid DNA. Free Radic Antioxid. 2013;3:35–39. doi: 10.1016/j.fra.2013.04.001. [DOI] [Google Scholar]
- Lefebvre KA, Robertson A. Domoic acid and human exposure risks: a review. Toxicon. 2010;56:218–230. doi: 10.1016/j.toxicon.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Lelong A, Hégare H, Soudant P, Bates SS. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: revisiting previous paradigms. Phycologia. 2012;51:168–216. doi: 10.2216/11-37.1. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Li MQ. Troxerutin counteracts domoic acid–induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein β–mediated inflammatory response and oxidative stress. J Immunol. 2013;190:3466–3479. doi: 10.4049/jimmunol.1202862. [DOI] [PubMed] [Google Scholar]
- Ordonez AAL, Gomez JD, Vattuone MA. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006;97:452–458. doi: 10.1016/j.foodchem.2005.05.024. [DOI] [Google Scholar]
- Pulido OM. Domoic acid toxicologic pathology: a review. Mar Drugs. 2008;6:180–219. doi: 10.3390/md6020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219. [PMC free article] [PubMed] [Google Scholar]
- Rajendran P, Rengarajan T, Nandakumar N, Palaniswami R, Nishigaki Y, Nishigaki I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur J Med Chem. 2014;86:103–112. doi: 10.1016/j.ejmech.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Ramsdell JS, Gulland FM. Domoic acid epileptic disease. Mar Drugs. 2014;12:1185–1207. doi: 10.3390/md12031185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10:1003–1008 [DOI] [PubMed]
- Samraj AK, Sohn D, Schulze-Osthoff K, Schmitz I. Loss of caspase-9 reveals its essential role for caspase-2 activation and mitochondrial membrane depolarization. Mol Biol Cell. 2007;18:84–93. doi: 10.1091/mbc.E06-04-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvagni PA, Lowenstine LJ, Spraker T, Lipscomb TP, Gulland FMD. Pathology of domoic acid toxicity in California sea lions (Zalophus californianus) Vet Pathol. 2005;42:184–191. doi: 10.1354/vp.42-2-184. [DOI] [PubMed] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49e55. [Google Scholar]
- Sutherland RJ, Hoesing JM, Whishaw IQ. Domoic acid, an environmental toxin, produces hippocampal damage and severe memory impairment. Neurosci Lett. 1990;120:221–223. doi: 10.1016/0304-3940(90)90043-9. [DOI] [PubMed] [Google Scholar]
- Szende B, Kéri G, Szegedi Z, Benedeczky I, Csikos A, Örfi L, Gazit A. Tyrphostin induces non-apoptotic programmed cell death in colon tumor cells. Cell Biol Int. 1995;19:903–912. doi: 10.1006/cbir.1995.1028. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranyac-Tramoundanas A, Harrison JC, Sawant PM, Kerr DS, Sammut IA. Ischemic cardiomyopathy following seizure induction by domoic acid. Am J Pathol. 2011;179:141–154. doi: 10.1016/j.ajpath.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattel A, Kamel S, Mentaverri R, Lorget F, Prouillet C, Petit JP, Brazier M. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem Pharmacol. 2003;65:35–42. doi: 10.1016/S0006-2952(02)01445-4. [DOI] [PubMed] [Google Scholar]





