Abstract
Determining the properties of proteins prior to purification saves time and labor. Here, we demonstrate a native mass spectrometry approach for rapid characterization of overexpressed proteins directly in crude cell lysates. The method provides immediate information on the identity, solubility, oligomeric state, overall structure and stability, as well as ligand binding, without the need for purification.
Introduction
Characterizing an overexpressed protein is essential for assessing its quality1. In cases of mass production, or high throughput investigations, a preliminary characterization step reduces waste of resources on inadequate protein material. It also provides a means to ensure that different batches of the same protein have similar features. Properties such as molecular weight, folding and assembly state, and ability to bind relevant ligands including cofactors, or drugs, are critical protein characteristics. However, these characteristics are usually assayed with purified proteins, with significant cost of time and labor. Therefore, quality assessment of overproduced proteins with no prior purification offers a great advantage.
Native mass spectrometry (MS) is a powerful method for structural characterization of intact proteins and protein complexes, providing insight into the composition, stoichiometry, network of interaction and overall shape of protein assemblies2–4. Generally, a prerequisite for acquiring native MS data is prior purification of the protein complex of interest5. Additionally, as most common buffers used during purification or storage of protein complexes contain salts and solubilizing agents such as imidazole, DTT, and EDTA that are largely incompatible with MS, a buffer exchange step into a volatile buffer is necessary5. Here we show that native MS also offers a way of direct characterization of recombinant, over-expressed proteins in crude cell lysates, overcoming the need for purification and buffer exchange procedures. The method relies on the high expression levels that are typically achieved in E. coli that result in production of the recombinant protein outperforming the endogenous gene products1. Consequently, MS analysis of unpurified bacterial cultures can be performed6. Here we show that highly resolved native MS data can be generated, wherein the intact recombinant protein becomes the dominant component in the mass spectra, thus enabling in-depth characterization.
Experimental Section
Direct MS sample preparation
For direct native MS analysis, E. coli expressing cells were grown at 37 °C as described above, until OD600nm of 0.5 was reached. Protein expression was induced by the addition of 0.5 mM IPTG for 3.5 h. 25 ml of the induced cell cultures were collected and centrifuged at 5,000g for 15 min. Cell pellets were washed once with double-distilled water to remove residual growth medium. Cells were then resuspended in 2 ml of buffer containing 1 M ammonium acetate, pH 7.0 and protease inhibitors (1 mM PMSF, 1 mM benzamidine, 1.4 μg/ml pepstatin A) and lysed by sonication. The lysate was cleared by centrifugation at 11,000 g for 30 min and the supernatant was directly used for MS analyses.
For the minimal cell culture experiment, E. coli expressing DJ-1 or CBR3 were grown at 37 °C as described above, until OD600nm of 0.5 was reached. Protein expression was induced by the addition of 0.5 mM IPTG for 3.5 h and then 25, 10, 5, 2 and 1 mL cell cultures were collected and processed for direct MS analysis as described above.
Protein expression quantification
E. coli cells expressing DJ-1 or CBR3 were grown at 37 °C, in 300 ml medium as described above, until OD600nm of 0.5 was reached. Protein expression was induced by the addition of 0.5 mM IPTG. At different time points, 25 mL of bacterial cultures were collected, and processed for direct MS analysis as described above. To measure the levels of the expressed proteins, equal volumes of the cell extracts were two-fold diluted and run on 15% polyacrylamide-SDS gels. Gels were stained with InstantBlueTM (Expedeon), scanned and band intensity was measured with the ImageJ software (NIH). For the determination of the concentration of the expressed proteins at the different time points, different amounts of BSA (between 0.05 and 8 μg) were run on the same gel and the BSA band intensities were used to generate a standard curve.
Native mass spectrometry analysis
Experiments were performed using a Q Exactive™ Plus mass spectrometer (Thermo Fisher Scientific) modified for the transmission and detection of high m/z ions7 and a high-mass Q-TOF instrument adapted for a QSTAR Elite (MDS Sciex) platform8. All spectra were calibrated externally, using cesium iodide. Spectra acquired on the QSTAR instruments are shown with minimal smoothing; no smoothing was done for the Orbitrap data. Typically, an aliquot of 3 μL protein solution was loaded into a gold-coated nano-ESI capillary prepared in-house, as previously described9, and sprayed into the instruments. Conditions within the mass spectrometers were adjusted to preserve noncovalent interactions, with the source operating in positive mode. Lysates samples were prepared as described. Purified proteins were buffer-exchanged into 100 mM ammonium acetate before analysis. Purified PHGDH was buffer exchanged into 250 mM ethylenediammonium diacetate, supplemented with 2 mM of the cofactor nicotinamide adenine dinucleotide (NAD).
The following experimental parameters were used on the Orbitrap platform: capillary voltage 1.7 kV, inlet capillary temperature 180 °C and argon was used as the collision gas in the HCD cell. Mass spectra were recorded at a resolving power of 10,000 - 17,500. Bent flatapole DC bias was set to 1.8 - 2 V. Axial gradient was set to 10 - 30 V. To facilitate efficient desolvation of some proteins, the HCD cell bias was dynamically switched to -50 V or -100 V. Trapping gas pressure was set to values ranging between 1 and 3.5, corresponding to HV pressures of 3.8x10-5 and 2.2x10-4 mbar, respectively.
The following experimental parameters were used on the Qstar Elite platform: For DJ-1 – capillary voltage 1.2 kV, declustering potential 30 V, focusing potential 220 V, declustering potential-2 15 V, focusing rod offset 50, collision gas 2. For Hsp31 - capillary voltage 1.1 kV, declustering potential 40 V, focusing potential 200 V, declustering potential 2 15 V, focusing rod offset 60, collision gas 3. For CBR3 - capillary voltage 1.1 kV, declustering potential 30 V, focusing potential 220 V, declustering potential-2 15 V, focusing rod offset 130, collision gas 2. For PHDGH - capillary voltage 1.15 kV, declustering potential 200 V, focusing potential 220 V, declustering potential 2 18 V, focusing rod offset 60, collision gas 2.
Ion mobility–mass spectrometry measurements
IM-MS measurements were performed on the Synapt G2 instrument (Waters, Hertfordshire, UK). Instrument parameters were as follows: capillary voltage of 1.4 kV, sampling cone 20 V, extraction cone 2 V, trap collision energies 5 V, transfer collision energy 5-15 V, trap DC bias 45 V, helium cell gas flow 120 ml/min. Nitrogen was used as the IMS gas, at a flow of 60 ml/min, IM wave velocity was set to 300 m/s, and wave height was set to 20 V. Collision cross-sections were calculated for the 12+ and 14+ charge state of DJ-1 and Hsp31 dimers, respectively. In brief, five proteins or protein complexes were used for calibration: β-lactoglobulin (37.3 kDa), BSA (67.2 kDa), concanavalin A (103.1 kDa), ADH (149.3 KDA) and pyruvate kinase (227.0 KDa) (all purchased from Sigma). All calibrants were dissolved in 200 mM ammonium acetate solutions, to retain a native-like conformation. All IM-MS results presented were averaged from three independent experiments. For each experiment, measurements were conducted on the same day; the only parameters modified for the test and calibrating proteins were capillary voltages and transfer collision energy, which was set to 5 or 15 V for Hsp31 and DJ-1 analysis, respectively. The CCS values were then calculated by Driftscope CCS calculation software (V2.8, Waters Corp., Manchester, U.K.). Theoretical CCSs values for human DJ-1 (PDB 1UCF) and Hsp31 (1IZY) were calculated using the Driftscope Projection Approximation (PA) algorithm (Waters).
Results and Discussion
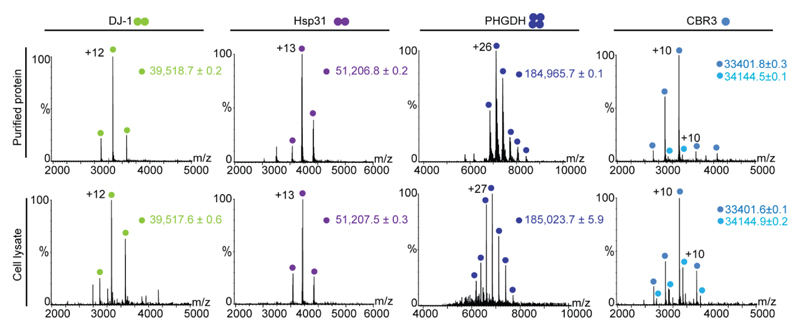
To investigate the applicability of this direct-MS approach we selected a range of proteins with different masses (from 19 to 47 kDa), oligomeric states and structural features. Initially, we examined the overexpressed human Parkinson’s-related protein DJ-1 (39.5 kDa, homodimer). Following induction by isopropyl-beta-D-thiogalactoside (IPTG), and 3.5 h subsequent growth, cells were harvested and lysed by sonication. The lysis buffer comprised 1M ammonium acetate at pH =7.0, to ensure compatibility with MS analysis, supplemented with protease inhibitors. The pellet was removed by centrifugation and native MS analysis was performed immediately after, using either an Orbitrap™ or QTOF-based platforms (Fig. 1 and S1). The acquired spectrum indicated a well-resolved charge state series corresponding to DJ-1. The measured mass, and the low number of charge states, indicated that DJ-1 is expressed in a soluble form and that the initial methionine was removed. Moreover, owing to the observation of peaks corresponding to the native, homodimeric form, the spectrum also indicated that the protein is correctly folded. In fact, the spectrum obtained from crude lysates was comparable to one recorded for purified DJ-1. Similar results were obtained for Hsp31, a homodimeric stress response protein from Saccharomyces cerevisiae.
Figure 1. Native MS analysis directly from crude cell lysates produces comparable information to that obtained from purified proteins.
Mass spectra were recorded on an Orbitrap platform for dimeric DJ-1 and Hsp31, tetrameric phosphoglycerate dehydrogenase, and monomeric CBR3 in its apo- and NADPH bound forms. Spectra recorded from purified samples are shown on the top and those generated directly from lysates are at the bottom. The oligomeric states of each of the proteins are indicated by a cartoon of circles beside the protein’s name.
We also examined two oxidoreductases: CBR3, a human, monomeric, NADPH-dependent carbonyl reductase; and, SerA, a homotetrameric, NADH-dependent phosphoglycerate dehydrogenase (PHGDH). Mass measurements of both purified and crude lysate samples of PHGDH indicated that the initial methionine is removed, and that the protein assembles into a homotetrameric structure. Further, the measured mass of PHGDH from crude lysate, indicates that it is bound to four NAD+ molecules, as expected10 (Fig. 1). Likewise, analysis of CBR3 revealed the co-existence of two populations, the protein in its apo state, and an NADPH-bound state. The purified form of CBR3 indicated a significant reduction in the relative abundance of the NADPH-bound state, probably due to its loss during purification. Overall, all spectra acquired revealed highly resolved peaks, narrow charge state distributions and the anticipated stoichiometry and cofactor associations, regardless of the high-mass mass spectrometer used. These results suggest that isolation and purification of these four proteins is not necessarily a prerequisite for their native MS characterization.
Next, we examined what is the minimal induction time that allows direct native MS analysis. To this end, a time course for DJ-1 expression was performed by collecting aliquots every 30 minutes following induction. The amount of overproduced protein in each sample was quantified by SDS-PAGE and the ability to acquire MS data with adequate signal-to-noise was examined. The results indicate that 30 min after induction, 0.39 mg/ml of DJ-1 was measured in crude lysate (Fig. 2a), a quantity that is already sufficient for obtaining a well resolved charge state series of DJ-1 (Fig. 2b and S2). Similar results were obtained for CBR3 (Fig. 2 and S2). In this case, 30 minutes after induction, the concentration of CBR3 in the lysate was 0.46 mg/ml, yielding enough protein for recording a highly resolved spectrum that discriminates between apo- and NADPH-bound forms. In both cases, MS spectra of samples with longer induction times benefited from improved signal-to-noise ratio. Notably, even before IPTG induction charge states of DJ-1 and CBR3 could be identified, due to leaky expression of the proteins (Fig. S3). However, without induction, the charge state series of both proteins were not easily resolved since the relative abundance of DJ-1 and CBR3 is comparable to the levels of certain endogenous bacterial proteins (as the ~43 kDa band obtained in Fig. 2a). Hence, the direct MS approach could be applied once the overexpressed target becomes the dominant cellular protein, which in the case of DJ-1 and CBR3 was already 30 min post-induction.
Figure 2. Well resolved direct-MS spectra of DJ-1 and CBR3 can be generated 30 minutes post-induction.
(a) Time-course analysis monitoring the overexpression rate of DJ-1 (top) and CBR3 (bottom) post-induction. The recombinant proteins’ concentrations were calculated based on relative band intensity, by densitometry, using known amounts of BSA as a standard. Arrows point to the band of the overexpressed protein. (b) Mass spectra of DJ-1 and CBR3 collected 0.5, 1 and 1.5 h post-induction.
Monitoring the time course of expression, while being able to detect natively folded and functional proteins is valuable, as in many cases, longer growth periods result in misfolding and/or proteolysis. This aspect is demonstrated in Figure S4, which shows that 48 h post-induction, CBR3 and Hsp31, both appear in the non-soluble fraction of the cell lysate, eliminating their detection by native MS. Thus, direct MS allows easy and informative time course measurements that can be used for optimization of expression conditions.
To determine the minimal bacterial culture volume that is sufficient for the direct-MS approach, decreasing amounts of culture volumes, ranging from 25 to 1 ml, were used. Following centrifugation cells were resuspended in 2 ml lysis buffer and lysed by sonication. As can be seen in Figure 3, even at 1 ml of cell culture, well resolved charge series of CBR3 and Hsp31 were obtained. Both apo- and NADH bound forms of CBR3 were obtained in all tested cell culture volumes, although a decrease in signal-to-noise ratio was obtained in the 1 ml culture. In the case of Hsp31, high signal-to-noise ratio was maintained throughout the decrease in culture volume, however, it was accompanied with gradual dissociation of the homodimeric protein into monomers. Such protein quaternary structure disruption is likely to be due to heat buildup during the sonication process, a scenario that is significantly reduced upon increasing the density of the bacterial suspension.
Figure 3. One ml of bacterial culture is sufficient for native MS analysis directly from crude cell lysates.
Different volumes of CBR3 and Hsp31 cell cultures, ranging from 25 to 1 ml, were lysed in 2 ml buffer and analyzed directly by native MS. Clear charge state series of apo CBR3 and its NADPH-bound form were detected in all cell culture volumes. The homodimeric Hsp31 protein was detected in all tested culture volumes, however, dissociation into monomers occurred in correlation with the reduction in sample volume, probably due to denaturing during the sonication lysis procedure.
In order to determine whether spectra of denatured proteins can be recorded directly from the crude cell lysate, denaturing solvents, as methanol and acetic acid were added to the crude cell lysate samples. The results indicate that under mild denaturing conditions charge state series corresponding to CBR3 and Hsp31 are identified, wherein dissociation of the NADPH cofactor from CBR3 and disassembly of Hsp31 into monomers could be monitored (Fig. S5). However, harsh denaturation conditions prevented the detection of both CBR3 and Hsp31. This is probably due to the fact that a fully denatured protein gives rise to a larger distribution of charge states with lower m/z values in comparison to the folded protein11. As a result peak intensity is reduced and the peaks of the recombinant protein become indistinguishable from background signals. On the whole, direct-MS measurements under mild denaturation conditions enable validation of the assembly state and ligand binding.
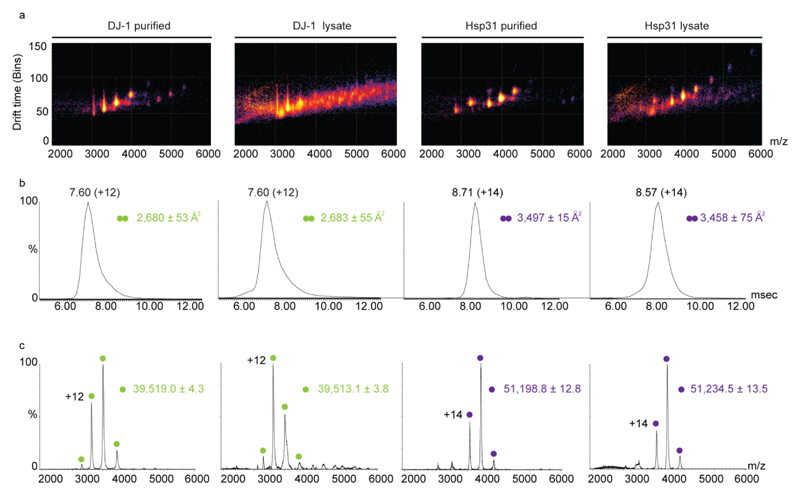
We then wished to examine whether the direct MS analysis can be expanded also to ion-mobility (IM) MS measurements, which provide information on the quaternary structure of the protein12. In this method, the time it takes a protein to transverse a weak electrical gradient in a gas-filled chamber is measured. Drift times are then converted to collision cross-sections (CCS)13, reflecting the overall shape of the protein. Using this approach, we first collected IM-MS data for DJ-1. IM–MS spectra of the protein either after cell lysis or following affinity-purification were measured. The results indicate that despite the presence of contaminant proteins within the cell extract sample, a CCS value of 2,683 ± 55 Å2 for DJ-1 could clearly be determined (Fig. 4a). A similar value of 2,680 ± 53 Å2, was measured for the purified form of DJ-1. Theoretical CCS for human DJ-1 gave a value of 2,388 Å2, which fits well with the measured CCS value, given that the projection approximation algorithm used to calculate this value is known to underestimate CCS values by ~ 13%14. Likewise, we measured CCS values for Hsp31 before and after purification. Both IM-MS spectra yielded similar CCS values of 3,458 ± 75 Å2 and 3,497 ± 15 Å2, respectively (Fig. 3). These measured CCS values were in agreement with the theoretical CCS of Hsp31, which yielded a value of 3,279 Å2. Direct-MS measurements can therefore reflect the quaternary shape of unpurified proteins.
Figure 4. Analyzing the quaternary topology of proteins by IM-MS measurements in crude lysates.
(a) Three dimensional IM-MS spectra that separate the ions based on their size and shape were measured for DJ-1 and CBR3 in crude cell lysates and in purified samples. Data was collected on a Synapt G2 mass spectrometer. (b) Representative ion mobility arrival time distributions for the 12+ and 14+ charge states of the homodimeric proteins DJ-1 and Hsp31, respectively. Calculated collision cross sections values, from at least three spectra, are indicated. (c) Representative two-dimensional plots of m/z versus intensity of DJ-1 and CBR3 from cell lysate and purified samples. Overall, comparable data were obtained for both samples, regardless if cell lysates or purified proteins were analyzed.
To assess the applicability of the direct MS approach for instant, throughput characterization of overexpressed proteins, we examined DJ-1 missense mutants associated with early-onset of familial Parkinson’s disease (A104T and D149A)15–17, as well as a mutational variant that abolishes the cytoprotective action of the protein (C106A)16. As shown in Figure 4a, the MS spectra acquired from lysed E. coli cells were well resolved for all 3 mutants. As expected, the masses of DJ-1A104T, DJ-1C106A and DJ-1D149A, indicated a mass shift of +60, -65 and -89, respectively, compared to the wild-type (WT) DJ-1. These measurements validate that all generated clones express a soluble protein, and that the homodimeric state of DJ-1 was maintained despite the mutations. In addition, it was possible to perform simultaneous measurements of WT DJ-1 and the missense variants by mixing their cell lysates prior to MS recording (Fig. 4b). Such simultaneous measurement enables to speed up the analysis in providing a direct internal reference of the WT protein18.
We also examined variants of the carbon fixation enzyme RuBisCO from Rhodospirillum rubrum (RrRBC) that were designed to improve enzyme stability. Specifically, based on the automated structure and sequence-based design algorithm (PROSS)19, two variants were examined that include 17 (RBC17) or 31 mutations (RBC31), compared to the WT protein. Native MS analysis of crude E. coli lysates indicated that all RuBisCO variants were expressed and soluble. However, only the WT protein, RrRBC, maintained the native homodimeric state (Fig. 4c). RBC17 and RBC31 adopted a monomeric composition despite the fact that positions in the dimer interface were excluded in the design process. In addition, the spectra of the designed variants encompassed a broad charge state distribution, with high-charge states, indicating the presence of partially unfolded populations20. In accordance with the fact that RuBisCO’s active-site resides in the dimeric interface21 both RBC17 and RBC31 were enzymatically inactive (Fig. 4d). Thus, as demonstrated here for RBC17 and RBC31, the direct-MS results enabled to identify impaired protein mutants, saving the burden associated with purification.
Conclusions
In conclusion, we describe a rapid MS-based approach for characterizing overexpressed proteins directly in E. coli cell lysates. The mass accuracy afforded by this approach enables to assess sequence variations, assembly state, folding condition and association of ligands of the generated proteins. Moreover, this method considerably shortens the time gap between protein production and characterization, and is particularly suitable for optimizing the yield and quality of protein over-expression. The simplicity and generality of the approach also makes it suitable for throughput screening procedures using chip based nanoelectrospray infusion techniques22. Overall, we anticipate further application of this direct-MS method, also considering the widely-spread application of protein engineering and recombinant protein technologies in biochemical investigations and in industrial and therapeutic applications23–24.
Supporting Information
Supporting Information avilable: Computational enzyme design and cloning of RuBisCO variants, plasmids, protein expression and purification, RuBisCO activity assay and Supporting Figures. This material is available free of charge via the Internet at http://pubs.acs.org
Figure 5. Analysis of protein mutants by direct-MS indicates the solubility, fold and stoichiometry of the overproduced proteins.
(a) Direct-MS spectra recorded for DJ-1WT and its mutants, DJ-1A104T, DJ-1C106A and DJ-1D149A, indicating that all mutants are expressed and soluble and maintain the homodimeric structure. (b) Simultaneous MS measurement of crude cell lysate mix of the three DJ-1 variants: DJ-1WT DJ-1A104T and DJ-1D149A. (c) Characterization of computationally designed RuBisCO variants, RBC17 and RBC31, and WT RuBisCO. The measured mass and charge state distribution reveal that RBC17 and RBC31 are partially unfolded monomers, unlike the WT protein that is a folded homodimer. (d) Activity assays monitoring the 3-phosphoglycerate product of RuBisCO using a coupled-assay with phosphoglycerate kinase and glyceraldehyde 3-phosphate dehydrogenase. The latter’s conversion of NADH to NAD+ was spectroscopically monitored. The assay indicated that RBC17 and RBC31 are completely inactive proteins in comparison to the WT protein, as expected from the results obtained in (c). Error bars represent standard error of three experimental replicates.
Acknowledgments
We are grateful for the support of a Starting Grant from the European Research Council (ERC) (Horizon 2020)/ERC Grant Agreement no. 636752. We kindly acknowledge the collaborative support from the Thermo Fisher Scientific, Bremen team. MS is an incumbent of the Aharon and Ephraim Katzir Memorial Professorial Chair.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Structural Genomics C. China Structural Genomics C. Northeast Structural Genomics C. Graslund S, Nordlund P, Weigelt J, Hallberg BM, Bray J, Gileadi O, Knapp S, Oppermann U, et al. Nat Methods. 2008;5:135–146. [Google Scholar]
- 2.Sharon M. Science. 2013;340:1059–1060. doi: 10.1126/science.1236303. [DOI] [PubMed] [Google Scholar]
- 3.Liko I, Allison TM, Hopper JT, Robinson CV. Curr Opin Struct Biol. 2016;40:136–144. doi: 10.1016/j.sbi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Lossl P, van de Waterbeemd M, Heck AJ. EMBO J. 2016;35:2634–2657. doi: 10.15252/embj.201694818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez H, Robinson CV. Nat Protoc. 2007;2:715–726. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- 6.Easterling ML, Colangelo CM, Scott RA, Amster IJ. Anal Chem. 1998;70:2704–2709. doi: 10.1021/ac971344j. [DOI] [PubMed] [Google Scholar]
- 7.Belov ME, Damoc E, Denisov E, Compton PD, Horning S, Makarov AA, Kelleher NL. Anal Chem. 2013;85:11163–11173. doi: 10.1021/ac4029328. [DOI] [PubMed] [Google Scholar]
- 8.Chernushevich IV, Thomson BA. Anal Chem. 2004;76:1754–1760. doi: 10.1021/ac035406j. [DOI] [PubMed] [Google Scholar]
- 9.Kirshenbaum N, Michaelevski I, Sharon M. J Vis Exp. 2010 doi: 10.3791/1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuller DJ, Grant GA, Banaszak LJ. Nat Struct Biol. 1995;2:69–76. doi: 10.1038/nsb0195-69. [DOI] [PubMed] [Google Scholar]
- 11.Kaltashov IA, Mohimen A. Anal Chem. 2005;77:5370–5379. doi: 10.1021/ac050511+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanucara F, Holman SW, Gray CJ, Eyers CE. Nat Chem. 2014;6:281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- 13.Michaelevski I, Kirshenbaum N, Sharon M. J Vis Exp. 2010 doi: 10.3791/1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Atri V, Porrini M, Rosu F, Gabelica V. J Mass Spectrom. 2015;50:711–726. doi: 10.1002/jms.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle PJ, Waak J, Gasser T. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MA. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cookson MR. Cold Spring Harb Perspect Med. 2012;2:a009415. doi: 10.1101/cshperspect.a009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Nissan G, Chotiner A, Tarnavsky M, Sharon M. J Am Soc Mass Spectrom. 2016;27:1062–1070. doi: 10.1007/s13361-016-1379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenzweig A, Goldsmith M, Hill SE, Gertman O, Laurino P, Ashani Y, Dym O, Unger T, Albeck S, Prilusky J, Lieberman RL, et al. Mol Cell. 2016;63:337–346. doi: 10.1016/j.molcel.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscovitz O, Tsvetkov P, Hazan N, Michaelevski I, Keisar H, Ben-Nissan G, Shaul Y, Sharon M. Mol Cell. 2012;47:76–86. doi: 10.1016/j.molcel.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 21.Hartman FC, Harpel MR. Annu Rev Biochem. 1994;63:197–234. doi: 10.1146/annurev.bi.63.070194.001213. [DOI] [PubMed] [Google Scholar]
- 22.Maple HJ, Scheibner O, Baumert M, Allen M, Taylor RJ, Garlish RA, Bromirski M, Burnley RJ. Rapid Commun Mass Spectrom. 2014;28:1561–1568. doi: 10.1002/rcm.6925. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer-Miralles N, Domingo-Espin J, Corchero JL, Vazquez E, Villaverde A. Microb Cell Fact. 2009;8:17. doi: 10.1186/1475-2859-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Garcia L, Martin L, Mangues R, Ferrer-Miralles N, Vazquez E, Villaverde A. Microb Cell Fact. 2016;15:33. doi: 10.1186/s12934-016-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.