Significance
Animals use memories of their recent environment to regulate their behavioral priorities. The basis for this cross-modal, experience-dependent plasticity is poorly understood. Caenorhabditis elegans feeds on bacteria in rotting fruit. It monitors O2 levels, and switches behavioral state when O2 approaches 21%. We show that C. elegans' memory of its recent O2 environment reconfigures how it processes sensory information. Pheromones that attract animals acclimated to 21% O2 repel animals acclimated to 7% O2. O2 memory is encoded in the activity history of a circuit that continuously signals O2 levels. This circuit is connected to neurons driving pheromone attraction and repulsion. O2 experience changes the pheromone responsiveness of these sensors and their postsynaptic targets, correlating with the switch in pheromone valence.
Keywords: neural circuit, experience-dependent plasticity, tonic circuit, oxygen sensing, acclimation
Abstract
Animals adjust their behavioral priorities according to momentary needs and prior experience. We show that Caenorhabditis elegans changes how it processes sensory information according to the oxygen environment it experienced recently. C. elegans acclimated to 7% O2 are aroused by CO2 and repelled by pheromones that attract animals acclimated to 21% O2. This behavioral plasticity arises from prolonged activity differences in a circuit that continuously signals O2 levels. A sustained change in the activity of O2-sensing neurons reprograms the properties of their postsynaptic partners, the RMG hub interneurons. RMG is gap-junctionally coupled to the ASK and ADL pheromone sensors that respectively drive pheromone attraction and repulsion. Prior O2 experience has opposite effects on the pheromone responsiveness of these neurons. These circuit changes provide a physiological correlate of altered pheromone valence. Our results suggest C. elegans stores a memory of recent O2 experience in the RMG circuit and illustrate how a circuit is flexibly sculpted to guide behavioral decisions in a context-dependent manner.
The body comprises multiple highly integrated subsystems working together to sustain life from moment to moment and over long time scales (1). Much of this coordination involves dynamically interacting neural circuits that optimize responses to current circumstances by taking into account sensory input, organismal state, and previous experience (2–8). Circuit cross-talk enables animals to adjust their behavioral priorities to changing environments, e.g., variation in temperature, humidity, day length, or oxygen (O2) levels (9–13). Whereas some behavioral adjustments can be rapid (14, 15), others develop over time, as animals adapt to changed conditions. How animals store information about their recent environment, and use this information to modify behavioral choices, is poorly understood.
The compact nervous system of Caenorhabditis elegans, which comprises only 302 uniquely identifiable neurons (wormwiring.org) (16), provides an opportunity to study the links between prior environmental experience, circuit plasticity, and behavioral change. This nematode is adapted to a life feeding on bacteria in rotting fruit (17). It has sensory receptors for odors, tastants, pheromones, and respiratory gases, as well as temperature, mechanical, and noxious cues (18–21). Despite this simplicity, the mechanisms by which its nervous system marshals information about past and present sensory experience to shape behavioral priorities remain largely mysterious. The anatomical connectome, while valuable (22), is insufficient to explain or predict neuronal network function (23, 24), partly because neuromodulators can dynamically reconfigure and specify functional circuits (25–27).
When ambient O2 approaches 21%, C. elegans wild isolates become persistently aroused and burrow to escape the surface (28–30). This state switch is driven by tonically signaling O2 receptors called URX, AQR, and PQR (31, 32) whose activity increases sharply when O2 approaches 21% (28, 30, 33, 34). The URX neurons are connected by gap junctions and reciprocal synapses to the RMG interneurons, and tonically stimulate RMG to promote escape from 21% O2 (wormwiring.org) (16, 35). URX and RMG are both peptidergic, and at 21% O2 tonically release neuropeptides (28, 35). Gap junctions connect RMG to several other sensory neurons besides URX, including pheromone sensors (16, 36). Whether information communicated from URX to RMG about the O2 environment modulates other sensory responses is unknown.
Here, we show that acclimating C. elegans to different O2 environments gradually reconfigures this animal's response to sensory cues. Animals acclimated to 7% O2 but not 21% O2 are aroused by CO2. Pheromones that attract animals acclimated at 21% O2 repel animals acclimated to 7% O2. These changes are driven by experience-dependent remodeling of URX O2 sensors, RMG interneurons, and the ASK and ADL pheromone sensors.
Results
Acclimation to Different O2 Environments Reprograms CO2 Responses.
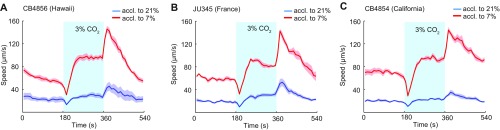
C. elegans escape 21% O2, which signals that animals are at the surface, and accumulate at lower O2 levels, which likely indicate that animals are burrowed (28, 30, 31). We speculated that C. elegans gradually change their sensory preferences when shifted between these environments. To test our hypothesis, we first examined responses to CO2. CO2 is aversive to C. elegans, and, due to respiration, its concentration will typically rise as O2 levels fall. Animals escaping 21% O2 will thus often encounter high CO2, creating conflicting drives that could be ecologically significant. Previous work showed that natural C. elegans isolates immediately suppress CO2 avoidance when O2 levels approach 21%, due to increased tonic signaling from URX O2 sensors (37–40). We speculated that not only current but also prior O2 experience changes C. elegans’s CO2 responses. To test this speculation, we kept wild isolates from California, France, and Hawaii overnight at 21% or 7% O2, and compared their responses to 3% CO2 on a thin lawn of bacteria kept at 7% O2. After halting briefly, animals acclimated to 7% O2 became persistently aroused at 3% CO2 (Fig. S1 A–C), whereas animals acclimated to 21% O2 showed little change in locomotory activity.
Fig. S1.
Acclimation to different O2 environments reprograms CO2 responses in natural C. elegans isolates. (A–C) Wild strains modulate their CO2 response according to recent O2 experience. These strains encode NPR-1 215F, the natural low activity isoform of NPR-1. n = 116–171.
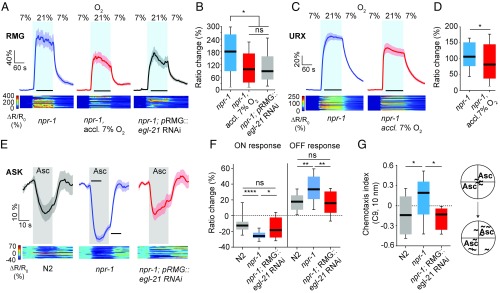
To probe this plasticity, we studied the N2 laboratory strain. Unlike natural isolates, N2 is aroused by 3% CO2 regardless of prior O2 experience (Fig. 1A and Fig. S2A). N2 animals have altered responses to 21% O2, because output from the RMG interneurons, a major relay of the circuit signaling 21% O2, is blocked by a hyperactive neuropeptide receptor, NPR-1 215V (35, 36). Natural C. elegans isolates have a less active receptor, NPR-1 215F, which does not block RMG output (41, 42). Does this account for altered CO2 responses? Disrupting npr-1 caused N2 animals to behave like natural isolates: They were not aroused by CO2 if previously acclimated to 21% O2 (Fig. 1A). The effects of acclimating npr-1 mutants to 7% O2 developed over 16 h and were reversed within 3 h if animals were transferred to 21% O2 (Fig. S2 B and C). Selectively expressing NPR-1 215V in RMG interneurons prevented npr-1 animals from acclimating to 21% O2—restoring N2-like behavior (Fig. 1B). Disrupting the GCY-35 soluble guanylate cyclase, a molecular O2 sensor in URX required for the URX—RMG circuit to signal 21% O2 (28, 30, 31, 33, 43) had the same effect (Fig. 1C).
Fig. 1.
Recent O2 experience regulates CO2-evoked arousal. (A) N2 animals and npr-1 animals acclimated to 7% O2 persistently increase their speed when CO2 rises to 3%; npr-1 animals acclimated to 21% O2 do not. n = 247–302 animals. ****P < 0.0001; ns, not significant; Wilcoxon signed-rank test. Animals were assayed on food at 7% O2. In this and subsequent figures, animals were acclimated to 21% O2 unless noted otherwise; solid lines indicate the mean and shaded areas, the SEM. Black bars here and throughout indicate intervals used for statistical comparisons; boxplots show the median and the 25th–75th percentiles; whiskers represent 10th–90th percentiles. (B) Selectively expressing NPR-1 215V in RMG, (C) disrupting gcy-35, or (D) RNAi knockdown of EGL-21 in RMG prevent npr-1 animals acclimated to 21% O2 from suppressing CO2-evoked arousal. n = 104–235.
Fig. S2.
Time line and reversibility of acclimation to different O2 levels. (A) N2 are strongly aroused by a 3% CO2 stimulus, irrespective of whether they have been acclimated at 21% or 7% O2. n = 462–518 animals. ****P < 0.0001; Wilcoxon signed-rank test. (B and B′) Mean speed of npr-1 animals at 3% CO2, plotted against the time animals were previously exposed to 7% or atmospheric (∼21%) O2. (B) Acclimation to 7% happens gradually and animals continue to increase their speed over many hours. n = 151–171. (C) Acclimation is reversed rapidly, and after ≤3 h, animals behave like siblings grown at 21% O2. n = 138–188. Error bars (B and C) and shaded regions (B′ and C′) represent SEM. ****P < 0.0001; ***P < 0.001; ns, not significant; Kruskal–Wallis ANOVA with Dunn’s multiple comparisons test.
The NPR-1 215V receptor isoform inhibits RMG peptidergic transmission (35). This finding led us to speculate that circuitry changes caused by prior O2 experience might reflect prolonged differences in RMG peptidergic release. To test this possibility, we selectively knocked down the carboxypeptidase E ortholog egl-21 in RMG using RNAi. Processing of most C. elegans neuropeptides depends on EGL-21 (44). RMG knockdown of egl-21 prevented npr-1 animals from acclimating to 21% O2 in the CO2 assay (Fig. 1D). These data suggest that neuropeptide release from RMG is required for C. elegans acclimated to 21% O2 to suppress CO2-evoked arousal.
Pheromone Valence Changes with Prior O2 Experience.
We studied how prior O2 experience alters CO2 responses because the special relationship between these gases has ecological implications. However, C. elegans has many CO2-responsive neurons, complicating analysis of how persistent differences in RMG activity alter the CO2 circuits (38, 45, 46). Several studies have reported differences in the sensory responses of N2 and npr-1 mutants that are associated with altered RMG function (35, 36, 47, 48). We speculated that at least some of these behavioral differences could reflect a diminished capacity of N2 animals to acclimate to different O2 environments due to reduced neurosecretion from RMG.
One such behavior is pheromone preference (36). Select pheromone blends attract npr-1 hermaphrodites but repel N2 hermaphrodites (36, 49). We replicated these observations using an equimolar 10 nM mix of asc-ωC3 (also called ascaroside C3, ascr#5 and daumone 5), asc-C6-MK (also called ascaroside C6, ascr#2 and daumone 2), and asc-ΔC9 (also called ascaroside C9, ascr#3 and daumone 3) pheromones (36, 50–52), (Fig. 2A). We then asked whether acclimating N2 and npr-1 hermaphrodites overnight in different O2 environments altered their pheromone response. We assayed animals at 21% O2. Whereas npr-1 animals acclimated to 21% O2 were attracted to the pheromone mix, npr-1 animals acclimated to 7% O2 avoided it (Fig. 2B). Acclimating N2 animals at different O2 levels did not alter pheromone avoidance (Fig. 2B), recapitulating our observations with CO2 (Fig. S2C). Disrupting gcy-35 switched the pheromone attraction exhibited by npr-1 animals acclimated at 21% O2 into repulsion (Fig. 2B). In summary, reducing the activity of O2 sensing circuitry for prolonged periods of time—either via environmental or genetic manipulation—transforms pheromone attraction to pheromone avoidance.
Fig. 2.
Pheromone valence changes with prior O2 experience. (A) Quadrant assay for pheromone preference (as in ref. 36). (B) Behavioral responses to an equimolar 10 nM mix of C3, C6, and C9 ascaroside pheromones. npr-1 animals acclimated to 21% O2 are attracted to the pheromone, whereas siblings acclimated to 7% O2 avoid it. N2 avoid pheromones irrespective of whether they have been acclimated to 7% or 21% O2. The soluble guanylate cyclase GCY-35 is required for normal O2 responses and pheromone attraction in npr-1 animals acclimated at 21% O2. **P < 0.01; ns, not significant; one-way ANOVA with Tukey’s multiple comparisons test. n = 8 assays each. (C) Previous O2 experience sculpts pheromone responses in ASK sensory neurons. Acclimation to 7% O2 reduces pheromone-evoked Ca2+ responses in ASK, consistent with altered behavioral preference. The gray shading in this and subsequent figures indicates addition of pheromone. (D) Quantification of data shown in C. Heat maps in this and all subsequent figures show individual Ca2+ responses. n = 35–36 animals. ****P < 0.0001; **P < 0.01; *P < 0.05; ns, not significant; Mann–Whitney U test.
O2 Experience Changes Pheromone Responses in ASK Neurons.
How does prior O2 experience switch pheromone valence? The altered behavior must reflect some lasting change in the circuitry that couples sensory detection to motor output. The principal neurons driving pheromone attraction are the ASK ciliated head neurons. Pheromone evokes a decrease in Ca2+ levels in ASK (the “ON” response) that quickly returns to above baseline when pheromone is removed (the “OFF” response). The pheromone-evoked Ca2+ response in ASK is bigger in npr-1 animals compared with N2 animals, a difference thought to contribute to the opposite pheromone preference of these strains (36). Does prior O2 experience change the responsiveness of ASK neurons to pheromones? To test this possibility, we measured pheromone-evoked Ca2+ responses in ASK using the ratiometric Ca2+ indicator YC3.60. Overnight acclimation at 7% O2 attenuated the ASK pheromone response in npr-1 animals to levels found in N2 (Fig. 2 C and D). Thus, prior O2 experience alters ASK pheromone responses, commensurate with a change in behavioral preference.
Peptidergic Feedback Heightens RMG Responsiveness to 21% O2 After Sustained Exposure to 21% O2.
RMG interneurons are connected to both the URX O2 receptors and the ASK pheromone sensors via gap junctions (wormwiring.org) (16, 36). A simple prediction made by our data is that the response properties of RMG change when npr-1 animals are acclimated to different O2 levels, and this alters the properties of ASK. To explore this prediction, we compared RMG Ca2+ responses to a 7–21% O2 shift in npr-1 animals previously acclimated to either 21% or 7% O2. Animals acclimated to 7% O2 showed significantly smaller RMG responses than those acclimated to 21% O2 (Fig. 3 A and B). Thus, prolonged exposure to 21% O2 augments RMG responses to this stimulus.
Fig. 3.
Peptidergic feedback regulates RMG properties and pheromone preference. (A and B) Acclimation to 7% O2, or knockdown of egl-21, similarly reduces the RMG Ca2+ responses evoked by a 7–21% O2 stimulus. (B) Quantification of data in A. n = 20–21 animals. *P < 0.05; ns, not significant; Mann–Whitney U test. (C and D) Acclimation to 7% O2 reduces URX Ca2+ responses evoked by a 7–21% O2 stimulus. (D) Quantification of data in C. n = 38–39 animals. *P < 0.05; Mann–Whitney U test. (E and F) Knockdown of egl-21 in RMG diminishes pheromone-evoked Ca2+ responses in ASK. (F) Quantification of data in E. n = 20–21 animals. *P < 0.05; **P < 0.01; ****P < 0.0001; ns, not significant; Mann–Whitney U test. (G) RNAi knockdown of egl-21 in RMG prevents npr-1 animals acclimated to 21% O2 from being attracted to pheromone. n = 12 assays. *P < 0.05; one-way ANOVA followed by Dunnett’s multiple comparisons test.
RMG responses to 21% O2 are driven by URX neurons (35). Do the altered RMG properties in animals acclimated to 7% O2 ultimately reflect reduced input from URX? URX responses were smaller in animals acclimated to 7% O2 (Fig. 3 C and D), suggesting that changes in RMG properties at least partly reflect plasticity in URX. However, a causal relationship remains to be proven, because RMG could feed back to alter URX properties following acclimation to 7% O2.
If we selectively knocked down peptidergic transmission from RMG, by RNAi of egl-21 CPE, npr-1 animals acclimated to 21% O2 failed to increase RMG responsiveness to a 7–21% O2 stimulus (Fig. 3 A and B). These data suggest there is a positive feedback loop by which tonic peptidergic signaling from RMG in npr-1 animals previously kept at 21% O2 increases RMG responsiveness to 21% O2. Thus, experience-dependent plasticity in RMG and URX represents a neural correlate of acclimation to different O2 environments.
Importantly, RNAi knockdown of egl-21 in RMG also altered pheromone-evoked Ca2+ responses in the ASK neurons of npr-1 animals acclimated to 21% O2, reducing them to N2-like levels (Fig. 3 E and F). The physiological effects on ASK and RMG were accompanied by a behavioral switch to pheromone avoidance (Fig. 3G). Thus, peptidergic signaling from RMG appears to mediate multiple effects of acclimation to 21% O2: an increased tonic Ca2+ response to 21% O2 in RMG, a bigger ASK response to pheromone cues, and decreased C. elegans avoidance of pheromone.
Communication Between Neurons in the RMG Circuit.
The wiring diagram suggests RMG is electrically connected to multiple sensory neurons through gap junctions, including the ASK neurons, the ADL and ASH nociceptors, the AWB olfactory neurons, and the IL2 chemo/mechanoreceptors (Fig. 4A) (wormwiring.org) (16). Altered RMG properties may therefore directly influence each of these electrically coupled neurons, and vice versa. Previous studies suggest that the O2-sensing URX neurons cooperate with the nociceptive ADL and ASH neurons and the ASK pheromone sensors, to promote C. elegans aggregation and escape from 21% O2 (32, 35, 36, 53). However, in the absence of physiological data, it is unclear what information RMG neurons receive or transmit, apart from tonic O2 input from URX (28, 35). We first asked whether O2-evoked responses in RMG propagated to ADL and ASK. The wiring diagram suggests ASK and ADL are connected to RMG exclusively via gap junctions. ASK and ADL each showed O2-evoked Ca2+ responses in npr-1 animals (Fig. S3 A–D). Conversely, RMG responded to stimulation with the pheromone mix we used to stimulate ASK (Fig. 4B); the dynamics of the response are remarkably similar to those observed in ASK, and a robust ON response was typically followed by an increase—or rebound—beyond baseline levels after stimulus removal (OFF response) (Fig. 4B). These results lend direct support to a hub-and-spoke model in which multiple sensory inputs are integrated through gap junctions with the RMG hub (36).
Fig. 4.
RMG hub neurons respond to pheromones and alter their response according to recent O2 experience. (A) Circuit showing connections between RMG interneurons and O2-sensing, nociceptive, and pheromone-sensing neurons. (B) An equimolar (100 nM) mix of C3, C6, and C9 ascarosides evokes a decrease in RMG Ca2+ in npr-1 animals acclimated to 21% O2. n = 57 animals. (C and D) RMG shows robust pheromone-evoked Ca2+ responses in both N2 animals and npr-1 animals acclimated to 21% O2. Acclimating npr-1 animals to 7% O2 alters RMG properties and diminishes both ON and OFF responses to pheromone addition and removal. (D) Quantification of data shown in C. n = 36 animals each. ***P < 0.001; ****P < 0.0001; Mann–Whitney U test. (E–G) Acclimation to 7% O2 enhances ADL pheromone responses and acute pheromone repulsion. npr-1 animals show decreased avoidance of the C9 ascaroside compared with N2 when grown under standard conditions (∼21% O2), but not when acclimated to 7% O2. Plotted are the avoidance index (E) and fraction of animals reversing (E′), in response to a drop of diluted C9 (10 nM) applied to the nose. n = 260–280 animals each. *P < 0.05; ***P < 0.001; ****P < 0.0001; ns, not significant; one-way ANOVA followed by Tukey’s multiple comparisons test. (F and G) The Ca2+ responses evoked in ADL by 10 nM C9 pheromone are larger in npr-1 animals acclimated to 7% O2 compared with siblings acclimated at 21% O2. (G) Quantification of data plotted in F. n = 23–24 animals. **P < 0.01; Mann–Whitney U test. (H) Model showing acclimation to different O2 levels has opposite effects on antagonistic circuit elements promoting attractive and repulsive pheromone responses. This experience-dependent plasticity arises from prolonged changes in the activity state of RMG hub neurons, which receive tonic input from URX O2-sensing neurons. Sustained peptide release from RMG at 21% O2 feeds back to alter RMG properties. In animals kept at 7% O2 peptide release from RMG is low, disrupting the feedback. The time required for animals to acclimate to 7% or 21% O2 corresponds to hysteresis in the onset or decay of peptide signaling. See also Figs. S3 and S4.
Fig. S3.
ASK and ADL sensory neuron spokes respond to O2. (A) O2-evoked Ca2+ responses in ASK do not differ between N2 and npr-1 animals. (B) Quantification of data plotted in A. n = 21–24 animals; Mann–Whitney U test. Blue shading indicates a shift from 7% to 21% O2. (C) ADL sensory neurons show robust responses to a 21% O2 stimulus in npr-1 but not in N2 animals. (D) Quantification of data shown in C. n = 30 animals each. ****P < 0.0001; Mann–Whitney U test. Note different Ca2+ sensors were used to image ASK (YC3.60) and ADL (GCaMP3 var500).
NPR-1 215V signaling has been proposed to silence the hub-and-spoke circuit (23, 26). One attractive model is that signaling from the neuropeptide receptor closes RMG gap junctions (36, 49). To investigate this model, we first compared pheromone-evoked Ca2+ responses in RMG in N2 and npr-1 animals, but did not observe any significant differences (Fig. 4 C and D). We then compared O2-evoked responses in ASK and also did not observe differences between the two genotypes (Fig. S3 A and B). By contrast, npr-1 but not N2 animals displayed a strong O2-evoked response in ADL neurons (Fig. S3 C and D). Disrupting GCY-35, the molecular O2 sensor in URX, abolished the ADL O2 response in npr-1 animals (Fig. S4 A and B). Expressing gcy-35 cDNA selectively in URX neurons restored the ADL O2 response. These data suggest the URX O2 response drives the ADL O2 response. Sensory responses in ADL require the TRPV1 ortholog OCR-2 (Fig. S4 C and D) (49). By contrast, the ADL response to O2 did not require OCR-2, consistent with URX neurons driving the ADL O2 response and ADL retaining an ability to function as an interneuron in ocr-2 mutants (Fig. S4 E and F). Although other interpretations are possible, a simple model to explain our data is that NPR-1 215V signaling in RMG affects different gap junctions differently, inhibiting RMG–ADL communication but having smaller or no effects on the RMG–ASK connection.
Fig. S4.
ADL responses to 21% O2 require GCY-35 signaling, but not the TRPV channel OCR-2. (A) The soluble guanylate cyclase GCY-35 is required for ADL responses to 21% O2. The defect of gcy-35(ok769) mutants is fully rescued by expressing gcy-35 cDNA in URX using the flp-8 promoter. (B) Quantification of data shown in A. n = 25–27 animals each. ****P < 0.0001; ***P < 0.001; ns, not significant; Mann–Whitney U test. (C) OCR-2 is required cell autonomously for ADL response to the C9 ascaroside. (D) Quantification of data shown in C. n = 11–12 animals each. ****P < 0.0001; Mann–Whitney U test. Gray shading indicates stimulation with 10 nM C9. (E) OCR-2 is not required for ADL responses to a 21% O2 stimulus, although Ca2+ rises less sharply in mutants. (F) Quantification of data plotted in E. n = 21–22 animals each; Mann–Whitney U test. Blue shading indicates a shift from 7% to 21% O2.
O2 Experience Sculpts RMG and ADL Pheromone Responses.
The pheromone attraction mediated by ASK neurons and promoted by RMG signaling is proposed to antagonize pheromone avoidance driven by the ADL neurons in a push–pull mechanism (49). The relative strength of these arms determines the animal’s response. We found that acclimating npr-1 animals to 7% O2 greatly reduced pheromone-evoked responses in RMG compared with animals kept at 21% O2 (Fig. 4 C and D). Thus, acclimation to 7% O2 weakens both the ASK (Fig. 2 C and D) and RMG circuit elements that drive attraction to pheromone. Does acclimation to different O2 levels also alter pheromone responses in ADL? ADL neurons are activated by the asc-ΔC9 (previously known as ascaroside C9), and a drop of this pheromone increases the probability of animals reversing (49). In this behavioral paradigm, the fraction of animals reversing in response to a drop of C9 provides a measure of the pheromone’s repulsiveness; this fraction is significantly higher in N2 than npr-1 animals at low pheromone concentrations (10 nM) (49). We confirmed that N2 animals were more strongly repelled by 10 nM C9 than npr-1 animals (Fig. 4E). We then showed that acclimating npr-1 animals overnight at 7% O2 enhanced their avoidance of C9, so that they behaved indistinguishably from N2 (Fig. 4E). The avoidance index (A.I.) used in this assay (49, 54) is calculated as [(fraction reversing to pheromone) − (fraction reversing to buffer alone)], and any change in the A.I. could reflect an altered response to the buffer rather than to C9. Consistent with enhanced pheromone avoidance, npr-1 animals reversed more in response to C9 if they were acclimated to 7% O2 (Fig. 4E′).
Pheromone-evoked Ca2+ responses in ADL neurons were previously characterized using 100 nM C9, a concentration that elicits strong and comparable repulsion in N2 and npr-1 animals (49). By using GCaMP3 var500 we could record ADL responses to 10 nM C9 and assess the impact of previous O2 experience under conditions similar to those used in behavioral assays. npr-1 animals acclimated to 7% O2 showed significantly bigger ADL Ca2+ responses compared with siblings acclimated to 21% O2 (Fig. 4 F and G). Together our data suggest that acclimation to 7% O2 simultaneously weakens the ASK and RMG circuit elements that drive attraction to pheromone and strengthens the ADL pheromone response driving repulsion, thereby switching the animal’s behavioral choice.
Discussion
Unfavorable environments can evoke slow, sustained changes in behavioral priorities that reflect an altered internal state. The neural mechanisms mediating such integrative, experience-dependent plasticity are poorly understood. C. elegans persistently attempts to escape 21% O2 (28), presumably because surface exposure is unfavorable (30, 31). We find that the O2 environment experienced recently by C. elegans changes the way it processes sensory information. Pheromones that attract C. elegans acclimated to 21% O2 repel animals acclimated to 7% O2; 3% CO2 triggers sustained arousal in animals acclimated to 7% O2 but has comparatively little effect on the speed of animals acclimated to 21% O2.
A memory of previous O2 experience arises from prolonged differences in the activity of a tonically active circuit. Exposure to 21% O2 tonically stimulates the URX O2 receptor neurons and their synaptic partners, the RMG interneurons. Sustained stimulation of URX and RMG at 21% O2 increases their signaling at 21% O2. The reprogramming of RMG requires peptidergic signaling competence in this interneuron. Our data suggest a simple model in which over time, sustained peptide release from RMG at 21% O2 feeds back to alter RMG properties. In animals kept at 7% O2 peptide release from RMG is low, disrupting the feedback. In this neural integrator model the time required for animals to acclimate to 7% or 21% O2 corresponds to hysteresis in the onset or decay of peptide signaling. We previously showed that neuropeptide expression in RMG is positively coupled to neurosecretion from RMG (36), further evidence of a positive feedback loop in this interneuron. Tonically signaling circuits are common in brains. It will be interesting to explore how often such circuits store information about their activity history, and potentially about the animal’s experience, by incorporating peptidergic positive feedback loops.
RMG has gap junctions not only with URX, but also with the ASK and ADL pheromone sensors (16, 36). This arrangement suggests that information can be integrated across the circuit (36). We find that ASK and ADL show O2-evoked Ca2+ responses, and moreover, that acclimating animals to different O2 levels alters O2 and/or pheromone-evoked responses in each of the URX, RMG, ASK, and ADL neurons. Inhibiting peptidergic transmission from RMG prevents RMG and ASK neurons from changing their pheromone responsive properties in animals acclimated to 21% O2; it also prevents the experience-dependent switch in pheromone valence. Changes in the pheromone-evoked responses of ASK and ADL neurons are consistent with RMG regulating communication across the network. For example, we speculate that in animals acclimated to 21% O2 pheromone-evoked responses in ASK may be able to inhibit ADL pheromone responses via RMG, whereas in animals acclimated at 7% O2 this communication may be less potent. Although this conjecture is plausible, we cannot exclude that the intrinsic properties of several neurons in the circuit are altered by O2 experience.
We see parallels between our observations and a Drosophila study showing that repeated presentation of an aversive shadow cue leads to a persistent change in behavioral state that scales with the number and frequency of the presentations (55, 56). Our findings are also reminiscent of “latent modulation” in the feeding network of Aplysia, where the history of activation in some circuit elements has a lasting effect on subsequent responses, most likely by changing neuronal excitability through peptidergic modulation (57, 58).
Why should C. elegans reconfigure its sensory responses according to prior O2 experience? It is tempting to speculate about a behavioral hierarchy (59) that gives priority to escape from 21% O2 and that dominates over sensory drives that could hinder escape from the surface. Animals at the surface may gradually suppress their aversion to CO2 to facilitate escape to low O2/high CO2 environments. Once the threat of exposure at the surface recedes, escape from CO2 again becomes adaptive. In a boom-and-bust species like C. elegans (17), pheromones may be repulsive because they predict an unsustainable population density. However, if escaping the surface is more important than avoiding a crowded environment, attraction toward pheromones may be transiently adaptive because crowded environments predict reduced O2. Irrespective of the precise selective advantage(s), if any, our data reveal a form of cross-sensory and experience-dependent plasticity in C. elegans, guiding behavioral choice according to prior O2 experience.
Materials and Methods
Strains were grown under standard conditions with Escherichia coli OP50 food (60). Defined O2 environments were created using a glove box (Coy Laboratory Products). For detailed information on behavioral assays, Ca2+ imaging, molecular biology, and statistics, SI Materials and Methods.
SI Materials and Methods
Locomotion Assays.
Locomotion assays were performed as described (45, 46). A total of 20–25 adult hermaphrodites were picked to nematode growth media plates seeded 16–20 h earlier with 20 μL of E. coli OP50 grown in 2xTY. To create a behavioral arena with a defined atmosphere, we lowered a 1 cm × 1 cm × 200 μm deep polydimethylsiloxane (PDMS) chamber on top of the worms, with inlets connected to a PHD 2000 syringe pump (Harvard apparatus). We pumped in defined gas mixtures (BOC) humidified using a sintered gas bubbler (SciLabware) at a flow rate of 3.0 mL/min. Movies were recorded at two frames per second using a Point Gray Grasshopper camera mounted on a Leica M165FC dissecting microscope and analyzed using custom Matlab software (35).
Chemotaxis to Pheromones.
Chemotaxis to pheromones used either four-quadrant Petri plates without food (Falcon X plate, Becton Dickinson Labware) (36, 61) or, for acute C9 avoidance, the drop test on food (49, 54).
Quadrant assay.
For each assay, 200 worms were picked to a fresh seeded plate for 2–3 h, washed three times with chemotaxis buffer, and placed at the center of a 10-cm quadrant assay plate with pheromones in alternating quadrants. Animals were scored after ∼15 min and a chemotaxis index was calculated as (number of animals on pheromone quadrants − number of animals on buffer quadrants)/(total number of animals). We used an equimolar (10 nm) mix of the ascarosides C3, C6, and C9. Assays were repeated on at least 4 different days.
Drop test.
Responses were scored as reversals if animals initiated a backward movement <4 s after stimulation that was equal to or longer than half their body length. The fraction reversing is given by (number of animals that make a long reversal)/(number of total animals tested); the effect size or avoidance index was calculated as (fraction reversing to pheromone) − (fraction reversing to buffer alone).
Ca2+ Imaging.
Ca2+ imaging was performed as described (36, 49, 62), using microfluidic devices (MicroKosmos) to immobilize animals. For O2 and CO2 experiments, worms were glued to agarose pads (2% in M9 buffer, 1 mM CaCl2) using Dermabond tissue adhesive, with the nose and tail immersed in M9 buffer (45, 46). Imaging was on an inverted microscope (Axiovert; Zeiss) with a 40× C-Apochromat lens (water immersion, N.A. 1.0), and MetaMorph acquisition software (Molecular Devices). Recordings were at two frames per second with a 100-ms exposure time. Photobleaching was minimized using optical density filter 2.0 or 1.5. Animals were preexposed to excitation light for ∼1 min in all experiments. A custom Matlab script was used to analyze image stacks (35).
Molecular Biology and Generation of Transgenic Lines.
Expression constructs were made using MultiSite Gateway (Life Technologies). Promoters used include: sra-9 (3 kb; ASK), sre-1 (4 kb; ADL), and flp-21 and ncs-1 (RMG). Constructs were injected at 30–55 ng/μL, with a coinjection marker (unc-122::RFP or unc-122::GFP) at 50–60 ng/μL.
Statistical Methods.
Statistical analyses used Prism 6 (GraphPad) and MATLAB (MathWorks). No statistical method was used to predetermine sample size, but our sample sizes are similar to those generally used in the field. Exact tests used are indicated in figure legends.
Strain List.
The following strains were used: N2 (Bristol), CB4856 (Hawaii), CB4854 (California), JU345 (France), AX204 npr-1(ad609), AX2918 gcy-35(ok769); npr-1(ad609), AX3931 N2; dbEx651[Psra-9::YC3.60; unc-122::RFP], AX4018 npr-1(ad609); dbEx651[Psra-9::YC3.60; unc-122::RFP], AX6478 npr-1(ad609); dbEx941[Psre-1p::GCaMP3 var500; unc-122::RFP], AX6479 N2; dbEx941[Psre-1::GCaMP3 var500; unc-122::RFP], AX6897 npr-1(ad609); gcy-35(ok769); dbEx941[Psre-1::GCaMP3 var500; unc-122::RFP]; dbEx1001[Pflp-8::gcy-35 cDNA::SL2::mcherry; unc-122::GFP], AX3516 npr-1(ad609); dbEx614[Pgcy -37::YC2.60; unc-122::RFP], AX3436 npr-1(ad609); dbEx637[Pncs-1::Cre;Pflp-21::LoxP-STOP-LoxP-YC2.60;unc-122::RFP], AX6078 N2; dbEx637[Pncs-1::Cre;Pflp-21::LoxP-STOP-LoxP-YC2.60; unc-122::RFP], BOL171 npr-1(ad609) lin-15(n765); dbEx[Pncs-1::Cre; Pflp-21::LoxP-STOP-LoxP-NPR-1(215V)-SL2-GFP; lin- 15(+)], AX5895 npr-1(ad609); dbEx[Pncs-1::Cre; Pflp-21::LoxP-STOP-LoxP-EGL-21RNAiSense-SL2-RFP;Pflp-21::LoxP-STOP-LoxP-EGL-21RNAiAntisense-SL2-RFP; unc122::RFP], AX5872 ocr-2(ak47); npr-1(ad609); dbEx[Psrh-220::genomic ocr-2; unc-122::GFP], AX6898 npr-1(ad609); dbEx651[Psra-9::YC3.60; unc-122::RFP]; dbEx[Pncs-1::Cre;Pflp-21::LoxP-STOP-LoxP-EGL-21RNAiSense-SL2-RFP;Pflp-21::LoxP-STOP-LoxP-EGL-21RNAiAntisense-SL2-RFP; unc-122::RFP], and AX6899 npr-1(ad609); dbEx637[Pncs-1::Cre;Pflp-21::LoxP-STOP-LoxP-YC2.60;unc-122::RFP];dbEx[Pncs-1::Cre;Pflp-21::LoxP-STOP-LoxP-EGL-21RNAiSense-SL2-RFP;Pflp-21::LoxP-STOP-LoxP-EGL-21RNAiAntisense-SL2-RFP;unc-122::RFP].
Acknowledgments
We thank Rebecca Butcher for ascarosides, members of the M.d.B. laboratory and the W. Schafer laboratory for advice and comments, and the Caenorhabditis Genetics Center for strains. This work was supported by the European Research Council (AdG 269058) and the Medical Research Council (United Kingdom).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618934114/-/DCSupplemental.
References
- 1.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol. 2001;204:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- 3.Kindt KS, et al. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;55:662–676. doi: 10.1016/j.neuron.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Su CY, Wang JW. Modulation of neural circuits: How stimulus context shapes innate behavior in Drosophila. Curr Opin Neurobiol. 2014;29:9–16. doi: 10.1016/j.conb.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoopfer ED. Neural control of aggression in Drosophila. Curr Opin Neurobiol. 2016;38:109–118. doi: 10.1016/j.conb.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Cohn R, Morantte I, Ruta V. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell. 2015;163:1742–1755. doi: 10.1016/j.cell.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owald D, Waddell S. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr Opin Neurobiol. 2015;35:178–184. doi: 10.1016/j.conb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stowers L, Liberles SD. State-dependent responses to sex pheromones in mouse. Curr Opin Neurobiol. 2016;38:74–79. doi: 10.1016/j.conb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaffee RR, Roberts JC. Temperature acclimation in birds and mammals. Annu Rev Physiol. 1971;33:155–202. doi: 10.1146/annurev.ph.33.030171.001103. [DOI] [PubMed] [Google Scholar]
- 10.Lewis L, Ayers J. Temperature preference and acclimation in the Jonah Crab, Cancer borealis. J Exp Mar Biol Ecol. 2014;455:7–13. [Google Scholar]
- 11.Chown SL, Sørensen JG, Terblanche JS. Water loss in insects: An environmental change perspective. J Insect Physiol. 2011;57:1070–1084. doi: 10.1016/j.jinsphys.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Tauber E, Kyriacou BP. Insect photoperiodism and circadian clocks: Models and mechanisms. J Biol Rhythms. 2001;16:381–390. doi: 10.1177/074873001129002088. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakar NR, et al. Long-term regulation of carotid body function: Acclimatization and adaptation--invited article. Adv Exp Med Biol. 2009;648:307–317. doi: 10.1007/978-90-481-2259-2_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 15.Grosjean Y, et al. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 16.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 17.Frézal L, Félix MA. C. elegans outside the Petri dish. eLife. 2015;4:4. doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rengarajan S, Hallem EA. Olfactory circuits and behaviors of nematodes. Curr Opin Neurobiol. 2016;41:136–148. doi: 10.1016/j.conb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman MB. 2006. Mechanosensation. WormBook 1–14.
- 20.Bargmann CI. Chemosensation in C. elegans. WormBook. 2006:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki I, Mori I. Molecular biology of thermosensory transduction in C. elegans. Curr Opin Neurobiol. 2015;34:117–124. doi: 10.1016/j.conb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Denk W, Briggman KL, Helmstaedter M. Structural neurobiology: Missing link to a mechanistic understanding of neural computation. Nat Rev Neurosci. 2012;13:351–358. doi: 10.1038/nrn3169. [DOI] [PubMed] [Google Scholar]
- 23.Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 24.Koch C, Laurent G. Complexity and the nervous system. Science. 1999;284:96–98. doi: 10.1126/science.284.5411.96. [DOI] [PubMed] [Google Scholar]
- 25.Marder E. Neuromodulation of neuronal circuits: Back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bargmann CI. Beyond the connectome: How neuromodulators shape neural circuits. BioEssays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 27.Graebner AK, Iyer M, Carter ME. Understanding how discrete populations of hypothalamic neurons orchestrate complicated behavioral states. Front Syst Neurosci. 2015;9:111. doi: 10.3389/fnsys.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch KE, et al. Tonic signaling from O2 sensors sets neural circuit activity and behavioral state. Nat Neurosci. 2012;15:581–591. doi: 10.1038/nn.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr Biol. 2006;16:649–659. doi: 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Persson A, et al. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458:1030–1033. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- 31.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 32.Coates JC, de Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–929. doi: 10.1038/nature01170. [DOI] [PubMed] [Google Scholar]
- 33.Zimmer M, et al. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGrath PT, et al. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurent P, et al. Decoding a neural circuit controlling global animal state in C. elegans. eLife. 2015;4:4. doi: 10.7554/eLife.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macosko EZ, et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kodama-Namba E, et al. Cross-modulation of homeostatic responses to temperature, oxygen and carbon dioxide in C. elegans. PLoS Genet. 2013;9:e1004011. doi: 10.1371/journal.pgen.1004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrillo MA, Guillermin ML, Rengarajan S, Okubo RP, Hallem EA. O2-sensing neurons control CO2 response in C. elegans. J Neurosci. 2013;33:9675–9683. doi: 10.1523/JNEUROSCI.4541-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 42.Chen C, et al. IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature. 2017;542:43–48. doi: 10.1038/nature20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung BH, Arellano-Carbajal F, Rybicki I, de Bono M. Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr Biol. 2004;14:1105–1111. doi: 10.1016/j.cub.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Husson SJ, et al. Impaired processing of FLP and NLP peptides in carboxypeptidase E (EGL-21)-deficient Caenorhabditis elegans as analyzed by mass spectrometry. J Neurochem. 2007;102:246–260. doi: 10.1111/j.1471-4159.2007.04474.x. [DOI] [PubMed] [Google Scholar]
- 45.Fenk LA, de Bono M. Environmental CO2 inhibits Caenorhabditis elegans egg-laying by modulating olfactory neurons and evokes widespread changes in neural activity. Proc Natl Acad Sci USA. 2015;112:E3525–E3534. doi: 10.1073/pnas.1423808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bretscher AJ, et al. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69:1099–1113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glauser DA, et al. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics. 2011;188:91–103. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi S, Chatzigeorgiou M, Taylor KP, Schafer WR, Kaplan JM. Analysis of NPR-1 reveals a circuit mechanism for behavioral quiescence in C. elegans. Neuron. 2013;78:869–880. doi: 10.1016/j.neuron.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang H, et al. Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron. 2012;75:585–592. doi: 10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 51.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci USA. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Noguez JH, Zhou Y, Butcher RA. Analysis of ascarosides from Caenorhabditis elegans using mass spectrometry and NMR spectroscopy. Methods Mol Biol. 2013;1068:71–92. doi: 10.1007/978-1-62703-619-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang H, Bargmann CI. Acute behavioral responses to pheromones in C. elegans (adult behaviors: attraction, repulsion) Methods Mol Biol. 2013;1068:285–292. doi: 10.1007/978-1-62703-619-1_21. [DOI] [PubMed] [Google Scholar]
- 55.Gibson WT, et al. Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Curr Biol. 2015;25:1401–1415. doi: 10.1016/j.cub.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson DJ. Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci. 2016;17:692–704. doi: 10.1038/nrn.2016.125. [DOI] [PubMed] [Google Scholar]
- 57.Dacks AM, Weiss KR. Latent modulation: A basis for non-disruptive promotion of two incompatible behaviors by a single network state. J Neurosci. 2013;33:3786–3798. doi: 10.1523/JNEUROSCI.5371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marder E, O’Leary T, Shruti S. Neuromodulation of circuits with variable parameters: Single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci. 2014;37:329–346. doi: 10.1146/annurev-neuro-071013-013958. [DOI] [PubMed] [Google Scholar]
- 59.Tyrell T. The use of hierarchies for action selection. Adapt Behav. 1993;1:387–420. [Google Scholar]
- 60.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hart AC. 2006. Behavior. WormBook 1–87.
- 62.Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–731. doi: 10.1038/nmeth1075. [DOI] [PubMed] [Google Scholar]