Cerebellar coordination of voluntary movements relies on integrating feedback information to update motor output. With the use of a novel protocol, we identified spinal neurons constituting the ascending and descending components of the forelimb spinocerebellar system in behaving primates. The data suggest that descending information contributes to both motor preparation and execution, whereas ascending information conveys the spinal level motor command, such that internal and external feedback is relayed through parallel pathways.
Keywords: motor control, voluntary movements, primates, spinocerebellar
Abstract
Cerebellar control of voluntary movements is achieved by the integration of external and internal feedback information to adjust and correct properly ongoing actions. In the forelimb of primates, rostral-spinocerebellar tract (RSCT) neurons are thought to integrate segmental, descending, and afferent sources and relay upstream a compound signal that contains both an efference copy of the spinal-level motor command and the state of the periphery. We tested this hypothesis by implanting stimulating electrodes in the superior cerebellar peduncle and recording the activity of cervical spinal neurons in primates. To dissociate motor commands and proprioceptive signals, we used a voluntary wrist task and applied external perturbations to the movement. We identified a large group of antidromically activated RSCT neurons located in deep dorsal sites and a smaller fraction of postsynaptically activated (PSA) cells located in intermediate and ventral laminae. RSCT cells received sensory input from broad, proximally biased receptive fields (RFs) and were not affected by applied wrist perturbations. PSA cells received sensory information from distal RFs and were more strongly related to active and passive movements. The anatomical and functional properties of RSCT and PSA cells suggest that descending signals converging on PSA cells contribute to both motor preparation and motor control. In parallel, RSCT neurons relay upstream an integrated signal that encodes the state of working muscles and can contribute to distal-to-proximal coordination of action. Thus the rostral spinocerebellar system sends upstream an efference copy of the motor command but does not signal abrupt errors in the performed movement.
NEW & NOTEWORTHY Cerebellar coordination of voluntary movements relies on integrating feedback information to update motor output. With the use of a novel protocol, we identified spinal neurons constituting the ascending and descending components of the forelimb spinocerebellar system in behaving primates. The data suggest that descending information contributes to both motor preparation and execution, whereas ascending information conveys the spinal level motor command, such that internal and external feedback is relayed through parallel pathways.
skilled motor actions are carried out in a smooth and coordinated manner. To achieve this level of motor performance, the system continuously inspects the state of the periphery and accordingly updates the motor command and the gain of multiple reflex pathways. Much evidence links the cerebellum with these integrative tasks (Ebner 1998; Garwicz 2002; van Kan et al. 1993a, 1993b). To perform these tasks, the cerebellum integrates a broad spectrum of peripheral and central sources of information, including multiple spinocerebellar pathways (Bosco and Poppele 2001; Garwicz 2002). Cerebellar output is relayed to the motor cortex via the motor thalamus (Horne and Butler 1995; Thach 1987) and spinal cord via the red nucleus (Jankowska 1988; ten Donkelaar 1988).
Direct forelimb-related information ascends to the cerebellum through two anatomically segregated pathways. The cuneocerebellar tract originates in the external cuneate nucleus of the medulla (Cooke et al. 1971a, 1971b) and delivers proprioceptive information that is unaffected by either spinal processing or descending motor signals, although some cortical feedback from the somatosensory cortex targets the nucleus and is possibly involved with sensory gating (Cheema et al. 1985). The rostral-spinocerebellar tract (RSCT) originates in cells in the cervical enlargement (Matsushita and Ikeda 1987; Petras 1977; Petras and Cummings 1977) that integrates inputs from peripheral and intraspinal sources. In the cat, RSCT cells are further subjected to indirect descending control relayed via C3–C4 propriospinal neurons (Alstermark et al. 1990), although in the monkey, the impact of C3–C4 may have different organization, as well as different functional implications (Kirkwood et al. 2002). In the cerebellum, RSCT axons mostly terminate as mossy fibers in the anterior lobe with collaterals to the deep cerebellar nuclei (Matsushita and Xiong 1997). This anatomical organization of RSCT neurons may suggest that they relay upstream an integrated signal that contains an efference copy of the descending motor commands (Azim et al. 2014) and afferent information about the state of the periphery.
To test this hypothesis, we implanted two monkeys with stimulating electrodes in the superior cerebellar peduncle (SCP). We tested the activity of identified spinal neurons during voluntary wrist movements and in response to passive perturbations of the wrist applied during task performance. We found a large group of antidromically activated RSCT neurons in the dorsal horn that integrated inputs from large, proximally biased, sensory receptive fields (RFs) but were generally insensitive to positional errors imposed during task performance. A second group of neurons that were activated postsynaptically were found in deeper spinal laminae. These neurons were sensitive to distal RFs and expressed robust task-related activity. A subset of postsynaptically activated (PSA) neurons was specifically active during preparation for movement but not during actions.
These findings suggest that descending task-related activity is integrated at the spinal level by PSA cells that may contribute to both motor preparation and execution. RSCT neurons integrate segmental information together with proximal-related sensory cues to produce a task-correlated signal that is relayed upstream. The properties of the ascending information are unsuitable for online signaling of movement errors but can support cerebellar control of interjoint coordination. It thus appears that RSCT neurons relay internal feedback that signals the state of working muscles, whereas detailed and precise external sensory feedback is predominantly delivered to the cerebellum by the cuneocerebellar system.
MATERIALS AND METHODS
Two monkeys (Macaca fascicularis; monkeys V and H, 3–4 kg) performed an isometric wrist task with an instructed delay period. The sequence of events comprising a single trial is shown in Fig. 1A; the trial structure has been detailed elsewhere (Asher et al. 2010; Yanai et al. 2007). In short, during task performance, a cast held the monkey’s hand in either a pronated or supinated position. The monkey controlled the two-dimensional (2D) movements of an on-screen cursor by applying isometric wrist flexion/extension and radial/ulnar torques. A trial was initiated by the appearance of a central target (trial onset). The monkey positioned the cursor inside the target by generating zero torque for a rest period (500–600 ms). Then, eight uniformly distributed peripheral targets appeared around the center target at a fixed distance, defining the onset of a delay period. One of these peripheral targets was presented in a distinct color for 500 ms (cue onset). The disappearance of the central target served as a “Go” signal (monkey V, 1,300–1,700 ms; monkey H, 850–1,200 ms after cue onset). The monkey then had to move the cursor into the previously cued target by generating an isometric torque in the appropriate direction and magnitude and keep the cursor within the target for an active torque period (350–750 ms). Subsequently, the peripheral targets disappeared, and the central target reappeared. The monkey returned to the rest position and received a reward, after which the screen went blank for 1,000–1,500, ms and a new trial started. The timing between externally induced events (e.g., between cue onset and the Go signal) was randomly varied from trial to trial. Task-related activity of single neurons was recorded with the wrist held in the pronated and/or supinated positions.
Fig. 1.
Experimental setup and neuronal types. A: illustration of the behavioral task, recorded signals, and sequence of events composing a single trial (bottom). sup, Supination; pro, pronation. B: illustration of recording configuration and an example of an antidromically activated single action potential triggered by SCP stimulation. C: example of antidromic responses occurring at a short latency and low trial-to-trial response variability (stimulation amplitude = 50 µA; response latency = 1.54 ± 0.04 ms). Traces in blue highlight cases of failed antidromic response due to collision with prestimulus spikes. Frequency-following test (bottom; train of 150 Hz and 150 µA) highlights the reliable response of the neuron (indicated by arrows) throughout the train. Shading shows the response for the first and fifth stimuli. D: example of postsynaptic response (stimulation amplitude = 150 µA; response latency = 4.3 ± 0.12 ms). Bottom: a frequency-following test for a postsynaptically activated (PSA) site (same conventions as in C). The cell failed to respond on the fourth stimulus (shading). Red arrows indicate the expected response time, based on the response latency computed for the first stimulus. E: example of a single site showing a mixed response pattern, including both antidromic and postsynaptic spikes. Stimulation amplitude 100 µA and occurrence time of early response (blue arrow) and late response (red arrow) are indicated. Blue traces highlight sweeps where the antidromic response failed due to collision with a prestimulus spike, although the postsynaptic response was unaffected. F: distribution of response latency for antidromic (blue) and postsynaptic (red) sites computed for a stimulation amplitude of 100 µA. Mean response latencies are indicated by colored arrows.
Passive Wrist Displacement
In one monkey (H), we also tested the neuronal response to passive wrist displacement (PWD), composed of an angular wrist deviation of 8° (when the wrist was midpronated), directed in either flexion or extension. Each PWD lasted 400 ms (100 ms ramp, 200 ms maintain position, and 100 ms return to center). Each set of PWDs included 60–70 displacements applied every 3 s in a randomly selected direction (either flexion or extension), during which the monkeys continued performing the target-reaching task.
Surgical Procedures
All surgeries were performed with aseptic techniques while the animals were under isoflurane, ketamine (7.5 mg/kg), and Domitor (0.1 mg/kg) anesthesia. Antibiotics and analgesics were administered postoperatively. The surgical procedures are described in detail below. All surgical and animal handling procedures were according to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, complied with Israeli law, and were approved by the Ethics Committee of The Hebrew University.
Insertion of stimulating electrodes into the SCP.
Three stimulating electrodes (parylene-coated platinum-iridium electrodes; We Sense, Nazareth, Israel) were chronically implanted in the SCP ipsilateral to the working arm in each monkey. An MRI scan was used preoperatively for trajectory planning and postoperatively to verify the electrode locations.
Implanting the spinal chamber.
In each monkey, a spinal chamber was implanted above the cervical spinal cord (segments C5–T1). During surgery, the soft tissue was retracted and laminectomy used to expose the spinal cord, while leaving the dura mater intact. Orthopedic screws and dental cement attached the recording chamber to the underlying vertebrae.
Recording Procedures
The extracellular activity of spinal interneurons (INs) was recorded using one to two electrodes. During recording, the monkey sat upright in a primate chair with its head and upper back restrained. The working arm was placed with the elbow bent at 90°. Its hand was held with the fingers straight and the wrist in the supination or pronation position. The neural recording configuration is illustrated in Fig. 1B. Activity from each electrode (band-pass filtered at 300–6,000 Hz, gain = 10,000) was continuously recorded. Online and offline spike-sorting algorithms (ASD and AlphaSort; Alpha-Omega Engineering, Nazareth, Israel) were used to extract the spiking activity of single neurons from the compound signal. Spikes assigned to each neuron were tested for stationarity by measuring the consistency of the rate in the pre-cue intervals across trials and the reproducibility of neuronal response to torque onset at each target direction.
In addition to neural data, we collected the 2D torque signals and the behavioral events that composed single trials. An ad hoc algorithm was applied offline on the torque data to detect automatically the time of torque onset (“To”). This algorithm first identified the time of the maximal torque derivative during target acquisition. With the use of this event as a marker, we then defined the earliest time at which the torque derivative was lower than a threshold level (one-tenth of the maximal derivative value). A post hoc visual inspection was used to confirm the validity of the results produced by the algorithm.
SCP Stimulation Protocol
After the monkey performed a sufficient number of trials, we delivered single-pulse stimulations via the SCP electrodes (during which the monkey kept performing the task) using a constant current stimulator (A-M Systems, Sequim, WA). The stimulation protocol consisted of 200–300 single biphasic stimulation pulses (50–200 μA), 0.2 ms each phase, delivered at 3 Hz. Subsequently, we applied a frequency-following test using a sequence of 10–20 bursts of single biphasic pulses applied at 120 or 150 Hz at an amplitude of 45–150 μA.
Data Analysis
Neural response to SCP stimulations.
We identified recording sites that were responsive to SCP stimulation by computing the stimulus-triggered averaging of neural activity. To do so, we aligned all sweeps (after full-wave rectification) around the stimulation onset and computed the bin-wise SD of the entire set of sweeps. This measure was found to be a sensitive estimator of any deviation in the signal that was locked to the aligning event (Yanai et al. 2007). Responsive sites were defined as those cases in which a response was found to exceed the prestimulation baseline (means + 2 SDs). We then defined antidromic responses using several criteria: 1) short response latency (<2.5 ms); 2) collision test with prestimulus spikes (when available); and 3) frequency following test (postsynaptic responses had a longer latency, and the neurons were unable to follow persistently the trains applied).
Task-related activity and tuning properties of single cells.
For each neuron, we measured task-related activity by comparing the single-trial mean firing rate around torque onset (−250 to +750 ms) with the pre-cue period (during the 1,000 ms before cue onset) using a paired t-test of single-trial firing rates.
Directionally tuned cells were defined further as cells with a significant target-related modulation of firing rate, as estimated for the same time window (0 to +750 ms around torque onset) using a resampling method (Crammond and Kalaska 1996; Shalit et al. 2012). For tuned cells, we defined a preferred direction (PD) as the target to which the cell had the highest mean rate compared with the other targets.
Task-related rate modulations of single cells were estimated by computing the peri-event time histogram (PETH). The PETH was computed by averaging the single-cell activity around torque onset (from −1,000 to +1,000 ms) in the PD and the immediate neighboring targets (i.e., PD ± 1 target). For untuned cells, the preferred target was taken as the target with maximal firing around torque onset. PETHs were computed using a 20-ms bin size and separately for pronation and supination trials (when available). This means that the total number of PETHs used for computing the population average could be larger than the number of cells, since some single cells contributed two PETHs (one for each hand posture). Single-cell PETHs were subsequently normalized by dividing by the average firing rate during the pre-cue period (from −1,000 ms until cue onset) and were multiplied by 100 to present the rate in percent change relative to the pre-cue period.
The response profile of neurons around the onset of PWD was computed separately for flexion-directed and extension-direction PWDs. Here, we used a 10-ms bin size, and normalization was computed by dividing by the average firing rate in the pre-PWD period (from −1,000 to −500 ms before PWD onset).
The significance of single-cell responses to PWD was computed by paired t-testing between the single trial number of spikes during the 300 ms after perturbation onset and the number of spikes during the same time window before perturbation onset. The time window was selected to include the ramp and hold phases of the perturbations.
Torque encoding of single neurons.
The 2D torque signal was continuously recorded during task performance at 1 kHz sampling rate. Offline processing of this signal included smoothing and downsampling to 50 Hz and calculating the radial torque. Next, the torque derivative was computed and smoothed using a moving average filter spanning 11 bins (smooth function in Matlab). For each trial, we extracted two parameters: the mean radial torque during the hold period (i.e., in the last 500 ms before returning to center) and the maximal value of the derivative at a time window of 0–500 ms around torque onset. We computed for each neuron the correlation coefficients between single-trial firing rates (computed at the corresponding time windows) and torque level or torque derivative. For cells that were directionally tuned, the correlation coefficient was computed using only those trials in which movement was toward either the PD or to its neighboring targets (i.e., PD ± 1 target). For cells that were recorded during the two-hand postures, the relationships between firing rate and torque data were estimated for each hand posture separately.
Posture sensitivity of single spinal cells.
We computed the posture sensitivity of single spinal cells. This was done by measuring the tendency of spinal neurons to modulate their firing rate during pronation vs. supination trials by first calculating the single-trial firing rate for each cell (computed over a time window spanning −1,000 to +1,000 around torque onset). We defined posture-sensitive cells as those for which the single-trial rates during pronation trials were significantly different from the rates during supination trials (P < 0.05, t-test).
Classifying single-cell response patterns.
We used k-means clustering methods to categorize single-cell, task-related activity into functional subgroups. Specifically, we computed the torque-related PETH for cells in pronation and/or supination trials (treating the activity of cells that were recorded during the 2 postures separately). PETHs were computed for a time window spanning −1,000 to +1,000 ms around torque and smoothed using a Gaussian kernel spanning 5 ms and normalized by dividing by the norm of each vector. For classification purposes, we focused on a time window spanning −500 to +750 ms around torque onset. This time window captures the entire task-related modulation and thus yielded a more robust categorization of the data into subgroups. Vectors were classified into subgroups using k-means clustering analysis with a correlation-based distance measure (D = 1 − r, with r corresponding to the correlation coefficient between the 2 vectors). This distance measure quantifies the similarity between response shapes, irrespective of the response-rate level.
Mapping RFs of Spinal Sites
At the end of each recording session, we applied passive manipulation of the arm that included natural skin stimulation and passive joint rotation, while monitoring the neuronal activity. In this manner, we identified the RFs converging on the recorded spinal sites. This mapping was done manually by the experimentalist, while the hand of the monkey was released from the cast.
RESULTS
We recorded neuronal activity during task performance from 280 cervical spinal sites located between C5 and T1 in two monkeys (252 and 28 sites for monkeys H and V, respectively). Each spinal site corresponded to a specific location of a single recording electrode, from which we were able to extract (using offline sorting techniques) the activity of one or more single units. Of the 280 tested sites, 124 (44.3%) contained neurons that responded to SCP stimulation. In 64 sites of the 124, we identified SCP responsive single cells (n = 124 units) that were stable both during the stimulation test and during the prestimulation trials so that their task-related activity could be studied as well. These 64 sites were used in the subsequent analyses.
Single-pulse SCP stimulation evoked two types of responses. The most frequent response was antidromic activation (Fig. 1C), occurring at a short latency after stimulation and with little trial-to-trial variability. In cases where the baseline firing of the cell was sufficiently high (29/73 units), a collision test between prestimulation spontaneous spikes and stimulus-evoked spikes confirmed the antidromic nature of the response (highlighted traces in Fig. 1C). For cells where collision could not be detected, we used the spike waveform to match between the antidromic responsive cell and the cell recorded during task performance during prestimulation trials. Frequency following tests, routinely run for all tested neurons, further highlighted the antidromic origin of this response (Fig. 1C). These cells were identified as RSCT neurons, since the RSCT is the only pathway that ascends from the recording site (cervical enlargement) to the cerebellum through the ispsilateral SCP to the cerebellum. The second type of response comprised transmission of the SCP stimulation across at least one synapse (i.e., postsynaptic; Fig. 1D). Postsynaptic responses expressed weaker cross-stimuli consistency and could not follow high-frequency trains of stimuli (Fig. 1D). Some sites contained antidromically activated as well as PSA cells (Fig. 1E). The average response latency was measured only for cases where we applied stimulation at 100 µA to minimize the intensity-dependent impact on measured values (a total of 70 RSCT and 35 PSA neurons). We found marked differences in latencies between the antidromic and postsynaptic responses (Fig. 1F). For antidromically activated spinal sites, the latency was 1.51 ± 0.33 ms (median = 1.4 ms, n = 70), whereas postsynaptic responses expressed a broader distribution of response latencies, with a mean value of 4.71 ± 1.64 ms (median = 5.16 ms, n = 35). Table 1 summarizes the frequency of sites that contained RSCT and/or PSA neurons and the number of neurons that were found.
Table 1.
Summary of identified data set
A site corresponds to the signal recorded from a single electrode at a specific spinal location.
Each neuron corresponds to single-cell activity extracted from the compound signal at a given site.
Unless stated otherwise, pronation and supination trials were tested separately. Some neurons were recorded in both hand postures; thus these neurons contributed 2 sets of trials, and the total number of tested postures was larger than the number of neurons.
Of the 73 neurons, 29 (39.7%) were tested for collision with prestimulus spikes.
Mixed sites correspond to sites in which both rostral-spinocerebellar tract (RSCT) and postsynaptically activated (PSA) cells were recorded by a single electrode in a given spinal site.
Spatial and Functional Segregation of SCP Responsive Sites
In the rostrocaudal axis (Fig. 2A), RSCT cells tended to cluster in the lower cervical enlargement (segments C7–T1), whereas PSA sites were distributed more evenly in the cervical enlargement (from C6 to T1). The tendency of RSCT neurons to accumulate at lower rostrocaudal levels (i.e., rostrocaudal < 0) was significantly different than the tendency of PSA cells (P < 0.0264, χ2 test). In addition, the distribution of counts found for RSCT but not PSA cells was significantly different than the expected counts based on uniform distribution (P < 0.029 and P < 0.39, respectively). In the dorsoventral axis (Fig. 2, B and C), RSCT sites were found in more dorsal laminae (mean depth 0.43 mm, n = 30) compared with PSA sites, which were found significantly more ventrally (mean depth 1.4 mm, n = 12, P < 0.01, ANOVA test). Sites with mixed responses (i.e., where both PSA and RSCT cells were found) were situated at intermediate positions between these two groups (mean depth 0.97 mm, n = 22). The abundance of sites with mixed response patterns highlights the substantial spatial overlap between PSA and RSCT neurons.
Fig. 2.
Segmental organization of identified sites. A: rostrocaudal distribution of responsive sites. Minimal noise (up to 0.25 mm) was added to the x-axis values to reduce overlap between points. Nonresponsive sites are indicated by gray symbols. Left: the counts of the different responsive sites in each segmental level are shown; right: an illustration of the laminectomy and spinal chamber location with respect to the recording area. B: dorsoventral (“depth”) position of identified spinal sites. Right: an illustration of the laminar organization [adapted from (Dum and Strick 1996)] depicts the presumed laminar location; left: the counts of sites per lamina are plotted. As in A, some noise was added to the mediolateral values to minimize overlap between points. C: statistical analysis of recording depth found for the 3 groups of cells (P < 2 × 10−5, one-way ANOVA). The dashed line indicates the average depth of spinal sites that responded to motor cortical stimulations (Zinger et al. 2013); the red cross symbol illustrates a single outlier case of the RSCT neuron. D: cumulative distribution of receptive fields (RFs) counted from distal to proximal sites. Distal fields (d), fingers and wrist; intermediate fields (i), forearm and elbow; proximal fields (p), upper arm, shoulder, and upper trunk. Sites that responded to RFs spanning the entire limb were omitted.
We identified the peripheral RFs of 61 of the 64 SCP responsive sites using passive manipulation of the ipsilateral upper limb (see materials and methods). We found RSCT sites responsive to proximal (upper arm, shoulder, and upper trunk), distal (fingers and wrist), and intermediate (elbow and forearm) regions with a clear proximal bias (62% with a proximal RF component vs. 38% with intermediate and/or distal RF, n = 29). In contrast, PSA sites tended to respond more to distally located RFs (25% with proximal RF vs. 66.6% with distal RF, n = 12), although a larger sample size of PSA sites will be required either to confirm or reject this difference in RF properties. PSA and RSCT sites differed significantly in their RF distributions (P < 0.0491, χ2 test). Sites with mixed responses to SCP stimulation tended to have either an RF that spanned the entire limb (15%, n = 20) or proximally biased RFs (75% proximal vs. 10% distal RF). The proximal bias of the antidromically activated sites was further highlighted in the cumulative RF distribution for the different groups computed from distal to proximal parts of the upper limb (Fig. 2D). Whereas 70% of the PSA sites responded from distal to intermediate RFs, only roughly 30% of the RSCT sites responded when activating these regions.
Task-Related Activity of Identified Neurons
PSA neurons were more sensitive to the task parameters than RSCT neurons. Figure 3 illustrates four examples of identified RSCT (Fig. 3, A and B) and PSA (Fig. 3, C and D) neurons recorded during task performance. All four neurons were task related (i.e., significantly changed their rate around torque onset relative to baseline level), although they exhibited variable relationships to behavioral events and motor actions. Only the two PSA cells (shown in Fig. 3, C and D) were directionally tuned during the active-hold period (i.e., their torque-related firing rate was significantly modulated by target direction, resampling method, P < 0.01).
Fig. 3.
Activity of identified single cells. Examples of raster plots and peri-event time histograms (PETHs) computed for RSCT (A and B) and PSA (C and D) cells. For each cell, the left plot presents trial segments aligned on cue onset, and the right plot presents segments from the same trials but aligned on torque onset. Trials were sorted according to target directions, with green horizontal lines distinguishing trials where different directional targets were cued. Total number of trials (Ntr) is indicated in the top left corner of each raster. PETHs were computed using a 25-ms bin size. sp/s, spikes per second.
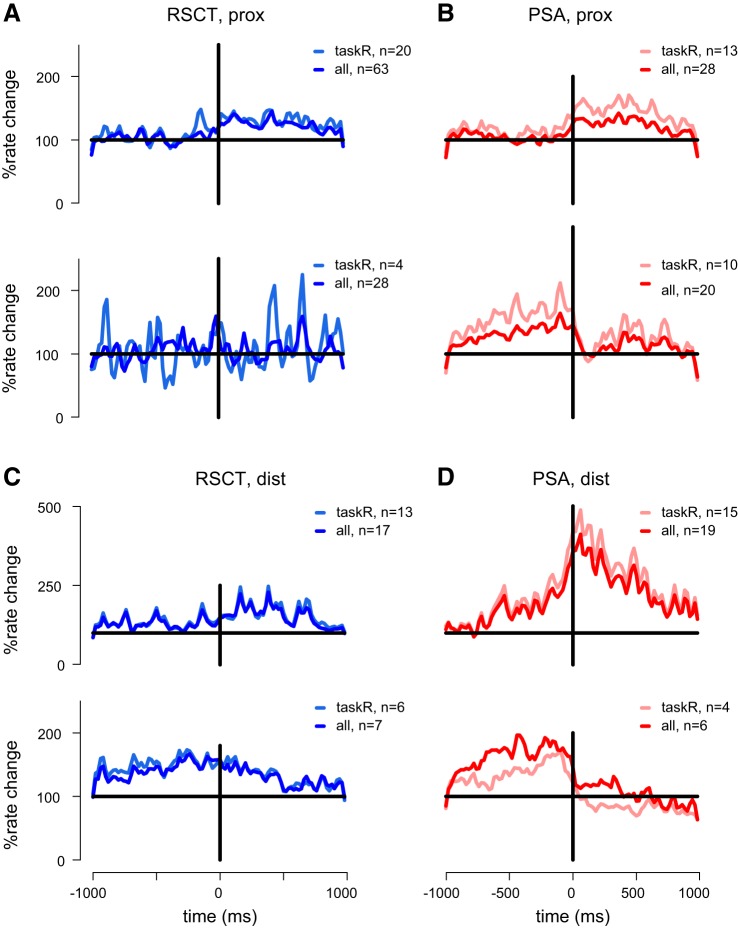
The relationships between single-cell activity and the task were quantified, first by computing the PETH around torque onset (Fig. 4) averaged across cell groups. Cells were divided into subgroups based on their response pattern (increase or decrease in rate relative to the baseline level computed during the pre-cue period) and RF properties (cells recorded from sites receiving sensory input from distal or proximal arm regions). Generally, we found that cells that were located in sites with proximal RF (Fig. 4, A and B) had weaker correlations with the task compared with cells that were recorded in sites with distal RF (Fig. 4, C and D). However, among cells with distal RFs, PSA cells (Fig. 4D) had stronger relationships to the task than RSCT cells (Fig. 4C). This was true for both excitatory and inhibitory responses and when computing PETH averaged across all cells or only task-related cells.
Fig. 4.
Task-related activity of identified neuronal groups. A: mean response modulation (computed as the percent of rate change relative to the firing rate during the pre-cue period) around torque onset computed for RSCT cells that were recorded from sites with proximal (prox) RFs. Top: mean modulation computed for RSCT neurons with positive torque-related response; bottom: mean modulation computed for RSCT neurons with negative torque-related response. Each panel contains the mean activity computed for all (dark hue) and significant task-related (taskR; light hue) neurons. B: same as A but for PSA neurons recorded from proximal sites. C and D: activity of RSCT and PSA neurons that were recorded from sites with distal (dist) RFs.
We further quantified the relationships between cell firing and task performance for RSCT and PSA cells (Fig. 5). First, we computed the mean firing rate during the pre-cue period (i.e., baseline-rate level) and the direction-dependent rate modulation around torque onset (Fig. 5A). Firing rates were computed separately for pronation and supination trials. Note that since some cells were recorded during the two-hand postures, the total number of cases used to construct the histogram was larger than the number of cells. We found that the rate of PSA cells was generally higher than the firing rate of RSCT cells. These differences were significant (Wilcoxon rank sum test) around torque onset for both distal and proximal sites and during the pre-cue period for distal sites alone.
Fig. 5.
Response properties of identified neurons. A: mean firing rates computed for RSCT (blue bars) and PSA (red bars) cells during the pre-cue period (PC) and around torque onset (dPD). PC rate was computed for 1 s before cue onset. dPD rate modulation was computed as the difference in rate levels between the activity in the preferred target (±1 target) around torque onset (−250 to +750 ms around onset time) and the anti-preferred target. Darker bars correspond to mean activity computed for cells recorded from sites with proximal RFs, and lighter bars correspond to mean activity computed for cells recorded from sites with distal RFs. Significant differences between RSCT and PSA activity were tested for each site and each epoch (*P < 0.05, **P < 0.01, ***P < 0.001, Wilcoxon rank sum test). B: fraction of neurons that expressed significant sensitivity to torque onset [task-related (taskR)] torque direction (tuned), rate of torque change at movement onset (dTrq/dt), and torque level at hold period (Trq level). Fractions were computed for RSCT (blue bars) and PSA (red bars) separately and for cells recorded from sites with proximal (dark bars) and distal (light bars) RFs. For tuned cells, torque sensitivity was computed for the preferred target ± 1 target around it. C: time-resolved modulation of directionally tuned cells computed for RSCT and PSA cells. Time 0 corresponds to torque onset. Tuning was computed separately for each hand posture and sensory RFs. Color scheme is the same as in B.
We then measured the fraction of cells that were task related and/or coded other task-related parameters (torque direction, torque magnitude, and torque-change rate). Table 2 summarizes the data set and the results of this analysis. Significant task-related modulations of firing rate were often found among RSCT and PSA cells recorded from sites with distal RF and to a lesser extent, from sites with proximal RF (Fig. 5B). However, the encoding of torque-related parameters was more frequent in PSA cells. These cells were sensitive to torque direction (i.e., expressed directional tuning) and torque rate change, especially when recorded in sites with distal RFs. Finally, we computed the time-resolved directional tuning of the different cell groups (Fig. 5C). Here again, cells recorded from distal sites were more often directionally tuned than cells recorded from proximal sites. Nonetheless, PSA cells in both distal and proximal sites expressed stronger tuning than RSCT neurons. Specifically, the tuning of PSA cells occurred earlier along the trial (~300 ms before torque onset) and was maintained throughout the hold period. Interestingly, despite the general insensitivity of RSCT neurons to torque direction, a large fraction of these cells (22 cells of 49 tested, 45%) exhibited significant posture-dependent modulation in firing rate (P < 0.01, rank sum test between single-trial firing rates in pronation vs. supination trials). For PSA cells, we found a similar fraction of significantly posture-sensitive cells (8 of 22 cells, 36%).
Table 2.
Parametric encoding for different receptive fields during task performance
| RSCT |
PSA |
|||||
|---|---|---|---|---|---|---|
| n | Task Related | Tuned | n | Task Related | Tuned | |
| Proximal RF | 91 | 24 | 8 | 48 | 23 | 12 |
| Distal RF | 24 | 19 | 2 | 25 | 19 | 13 |
| Total | 115 | 43 | 10 | 73 | 42 | 25 |
RF, receptive field.
Functional Subgroups among Identified Neurons
We further explored the relationships between the activity of identified spinal cells and motor behavior by searching for frequent response prototypes in each group of neurons. Response profiles were classified using k-means clustering analysis (k = 3, the number of centers used in the analysis), and several underlying response prototypes were identified for RSCT and PSA neurons, reflecting differential involvement in the behavioral task. Figure 6 presents the normalized single-cell responses found for RSCT (Fig. 6A) and PSA neurons (Fig. 6B). Each set of neurons was sorted by the cluster allocation and the timing of the response peak (within each cluster). To compensate for the different noise levels in the clusters, we selected those neurons that were closer to the cluster center [distance measure based on correlation (1 − r, as defined above) < 0.5]. The variability in fractions of neurons in each group that met this criterion (38.5% for RSCT neurons and 63.3% for PSA cells) indicates that the PSA neurons were less diverse than the RSCT neurons in terms of their task-related activity.
Fig. 6.
Classification of cell activity into subgroups. Activity of RSCT (A) and PSA (B) cells sorted by cluster allocation (separated by thick horizontal lines). Data in each cluster were further sorted by response peak time. Each line corresponds to the normalized PETH (see materials and methods) of a single cell around torque onset (time = 0). Only cells that were close to the cluster center (distance, D < 0.5) are shown. The average response profile for each cluster is shown for RSCT (C) and PSA (D) cells. E: the overlaying of the 2 prototypes that showed phasic-tonic response patterns from RSCT (blue) and PSA (red) neurons. Selected profiles are indicated with arrows in C and D. F: averaged depth (ordinate) and medial-lateral location (abscissa) computed separately for each group of identified neurons. Color coding corresponds to the 3 different clusters in C and D. Horizontal and vertical lines reflect the SE of the depth and medial-lateral location, respectively.
Despite the differences in response magnitude between RSCT and PSA cells, we found a general resemblance in the response profiles. Specifically, for RSCT cells (Fig. 6C), we found a group of neurons with a phasic-tonic response profile locked to torque onset, a second group of neurons with a late “ramp” response profile following torque onset, and a third group of neurons with pre-movement (delay-period) activity. The number of cells in each group was roughly even, and the expressed change in discharge rate was moderate. For PSA cells (Fig. 6D), we found a large group of neurons with a phasic-tonic response profile, a smaller group of neurons with preparatory ramp activity that was truncated at torque onset, and a third group of neurons that were inhibited around torque onset. Unlike RSCT neurons, the discharge rate expressed by PSA cells was large relative to baseline levels. The overlaying of the subgroups of RSCT and PSA cells expressing the phasic response pattern (Fig. 6, C and D) revealed that although the onset times of both groups occurred before torque onset, PSA activity slightly preceded RSCT activity (Fig. 6E). Finally, we found no significant spatial organization among the different subgroups (Fig. 6F).
Differential Sensitivity of RSCT and PSA Neurons to Imposed Motor Perturbations
In a subset of trials, we applied PWDs while the monkey performed the task. These perturbations displaced the position of the working hand and required the monkey to correct for this interference to complete the trial successfully. An example of such displacements and their effect on the torque signal are shown in Fig. 7A. Previously, we showed that these displacements recruit a broad spectrum of peripheral afferents and spinal neurons (Prut and Perlmutter 2003). Figure 7 depicts examples of a single RSCT cell (Fig. 7B) and a PSA cell (Fig. 7C) that responded to the applied passive perturbation. We computed the mean response to perturbations expressed by RSCT and PSA neurons after dividing the neurons into subgroups based on the RF of the recording sites (distal vs. proximal). We found that RSCT neurons that were recorded from proximal sites expressed very low sensitivity to the applied perturbations (Fig. 7D), with only a small fraction of the examined cases (3/52 cases = 6%) showing a significant change in discharge rate relative to the pre-perturbation level. Moreover, even the few responsive RSCT cells expressed a low amplitude and temporally variable response to the passive displacement, which therefore canceled out at the population level. In contrast, PSA cells recorded from proximal sites were more often responsive to these perturbations compared with RSCT cells in matching sites (6/28 = 21%, P < 0.0345, χ2 test), and the observed response was strong and sharp (Fig. 7E). For PSA neurons recorded from sites with distal RFs (Fig. 7F), the fraction of responsive cells was higher (4/8 = 50%) but not significantly higher than the fraction of responsive PSA cells recorded from sites with proximal RFs (P > 0.11, χ2 test). In addition, the rate modulation expressed by PSA cells recorded in distal sites was comparable with the response profile of the PSA cells recorded from proximal sites. This result is consistent with our previous findings showing that PWD is an efficient trigger that activates extensive regions in the spinal cord, but apparently not all cell types are affected to the same extent.
Fig. 7.
Neural response to passive wrist displacements (PWDs). A: three examples of radial torque (black traces) and position signal (gray traces) measured during 3 different trials in which PWDs were applied. The positional displacements occurred at different points along the trial and were directed toward either flexion (up deflection in the position signal) or extension (down deflection) direction. Each displacement lasted 400 ms. Vertical colored lines show the timing of the different events along the trial. B: example of an RSCT neuron that responded to the applied displacements. Raster plots are aligned to the onset of left- and right-directed displacements (left and right, respectively). PETHs were computed for each raster (computed at a 1-ms bin size and convolved with a Gaussian kernel spanning 30 bins) and are shown on top of each raster, and the position signal is shown at the bottom. C: same as B but for a PSA cell. D: averaged perturbation-related PETHs of all RSCT cells that were recorded from sites with proximal RFs (dark blue) and the response of the subset of neurons that expressed a significant response to the perturbations (light blue). E: same as in D but for PSA cells recorded in sites with proximal RFs (all cells: dark red; responsive cells: light red). F: same as in E but for PSA cells recorded in sites with distal RFs.
DISCUSSION
The main goal of this study was to document information relayed by the forelimb-related spinocerebellar system in behaving primates and to examine its relationship to sensory feedback and motor commands. We found that SCP stimulation activates two neighboring but functionally and anatomically distinct groups of neurons. The first group is composed of RSCT neurons that were antidromically activated and located in the deep dorsal horn. The second group of neurons is composed of PSA cells located at more ventral layers of the spinal gray matter. The input/output properties of these neurons and their task-related activity shed new light on the operation of the spinocerebellar circuitry during preparation and execution of voluntary movements in nonhuman primates.
Spatial Organization of the RSCT System
RSCT neurons were encountered at high frequency, especially in the dorsal horn, indicating that these cells form an extensive network of neurons. The spatial distribution of RSCT neurons was broad, but they were found more frequently in the dorsal laminae and lower cervical segments (C7–T1), which is the location of the nucleus centrobasalis, previously identified in the cervical cord of primates (Petras 1977; Petras and Cummings 1977). Several RSCT neurons were found in dorsal layers that were more superficial than expected by anatomical studies. This inconsistency probably reflects the technical difficulty of identifying the exact entry point to the gray matter during chronic spinal recordings in primates where the dura is intact and its elasticity and thickness change over the course of experiments.
Task-Related Information Carried by RSCT Neurons
The activity patterns of single RSCT neurons were diverse, indicating that although these neurons targeted the same supraspinal site, their input was sparsely sampled from a broad and heterogeneous set of sources, such that each neuron integrated a different set of inputs and thus expresses a unique pattern of response. RSCT neurons often expressed task-related rate modulations, especially when recorded from sites that integrated sensory input from distal RFs. Nonetheless, the task-dependent rate modulations expressed by these cells were modest, and the neurons were generally insensitive to the motor parameters of the task (i.e., torque direction, level, or change rate). The activity pattern of RSCT neurons was coarsely separable into several subgroups, with some cells that were active around torque onset and others that were more active during the pre-torque delay period. The source for the task-related activity of RSCT neurons is not fully understood, but it is unlikely that this activity is solely generated by sensory feedback. This assumption is supported by the fact that for a large group of RSCT neurons, the onset time of the task-related response was reported to be earlier than expected from an exclusive feedback mechanism subsequent to the onset of muscle activity (Asher et al. 2010; Shalit et al. 2012). This means that task-related activity of RSCT neurons is, at least in part, triggered by descending and/or intraspinal inputs.
Previous studies (Lundberg 1971; Oscarsson 1973) have argued that the convergence pattern on ventral spinocerebellar tract cells (the lumbar equivalent of the RSCT system) may generate a signal related to the state of IN systems (e.g., the spinal flexor reflex afferent system). A more recent study (Jankowska and Hammar 2013) suggested that these IN systems include premotor INs, and thus ventral spinocerebellar tract neurons may signal the excitability level of segmental motoneurons. Our findings for RSCT neurons are in line with this hypothesis. The tonic increase in the firing rate of RSCT neurons around torque onset and their sensitivity to hand posture appear to inform upstream elements of task-related changes that are strongly correlated with the motoneuronal excitability level. This suggests that the RSCT system transmits spinal-based internal feedback (Azim et al. 2014).
On the other hand, the moderate and sluggish rate change exhibited by RSCT neurons at movement onset and during passive movements indicates that these neurons do not carry external sensory feedback and rules out their possible role in the online error correction of executed voluntary actions (Cooke et al. 1971a; Lundberg 1971). This is because an error-correcting system is expected to fire vigorously in response to PWDs that introduce acute motor errors and thus impede task performance. In contrast, the RSCT signal was, by and large, insensitive to the detailed and specific sensory consequences of the performed action, unlike PSA neurons in matching sites (based on their RFs) and intermediate zone spinal INs studied previously (Prut and Perlmutter 2003). Functionally, this finding implies that internal feedback (relayed via the RSCT) reaches the cerebellum in parallel and independently of external feedback that is carried via the cuneocerebellar system. This parallel processing differs from the integrated flow of information shown in the dorsal spinocerebellar system (Hantman and Jessell 2010) and the spinocerebellar component of the C3–C4 systems (Alstermark et al. 1981; Azim et al. 2014; Kinoshita et al. 2012). Our findings may thus reflect organizational changes required by the unique motor tasks that the forelimb of primates needs to perform compared with rodents.
Identity of the PSA Neurons
PSA responses were also evoked by SCP stimulations, although less frequently than antidromic activation. These responses could have been mediated locally via segmental collaterals of RSCT and/or by activating cerebellar output fibers that travel through the SCP to the red nucleus (Walberg et al. 1986) and then to the spinal cord via the rubrospinal tract (Belhaj-Saïf et al. 1998; Gibson et al. 1985; Houk et al. 1988). Note that the latency of PSA responses was too short to be mediated by transcortical circuitry (Zinger et al. 2013). Several lines of evidence suggest that postsynaptic cells are activated by descending rubrospinal input. Postsynaptic responses were found in a consistent (sweep-by-sweep) manner. Such a faithful activation of a polysynaptic pathway is less likely to reflect intraspinal transmission, since these synapses are generally weak (Prut and Perlmutter 2003) and sparse (Harrison and Jankowska 1985). Furthermore, in cases where RSCT and PSA cells were recorded by the same electrode, the postsynaptic spikes occurred independently of the antidromic response (which was sometimes abolished due to collision with earlier spikes). This uncorrelated firing between nearby RSCT and PSA cells (which are more likely to be connected than remotely located cells) appears to be inconsistent with a model where PSA cells receive sufficiently strong input from RSCT cells to be serially activated in response to SCP stimulation. Finally, PSA cells were related to distal RFs, both during active and passive movements, which is consistent with available information on the rubrospinal tract (Belhaj-Saïf et al. 1998; Miller et al. 1993; Van Kan and McCurdy 2002).
The response pattern of PSA cells was more frequently related to the task and the applied perturbation, which is consistent with a system that actively contributes to motor output. Note, however, that the behavioral paradigm used here cannot dissociate well activation of proximal and distal muscles. We further found a unique group of PSA neurons with enhanced preparatory activity that was rapidly suppressed at torque onset. We previously reported spinal preparatory activity that was inversely correlated with movement-related activity (Prut and Fetz 1999). The activity pattern of PSA cells could substantially modify the excitability of segmental motoneurons in a specific manner during motor preparation (Cohen et al. 2010). The fact that this activity pattern was uniquely found for PSA cells indicates that at least part of the cerebellar control of motor timing (Ivry 1997) is mediated by changing the excitability of the segmental networks in an epoch-dependent manner.
The Role of RSCT Neurons in Controlling Voluntary Movements
RSCT neurons were not strongly affected by sensory input from distal RFs or during PWD. On the other hand, RSCT cells are likely to integrate multiple central sources of task-related information, such as indirect cortical input (Alstermark et al. 1990), premotor INs (Jankowska et al. 2010), and INs that receive corticospinal commands (Dum and Strick 1996; He et al. 1993; Zinger et al. 2013). All of these inputs can act to shape the task-related activity of RSCT neurons. Interestingly, it is possible that some of the intraspinal task-related input driving RSCT neurons originate, in fact, from nearby PSA cells. This reasoning is supported by the considerable spatial overlap found between PSA and RSCT neurons and the fact that the task-related activity of RSCT neurons was a scaled, yet delayed version of PSA activity. The response properties of RSCT neurons may suggest that the spinocerebellar system of the upper limb constitutes a functional loop in which the cerebellum affects spinal circuitry (indirectly via midbrain-descending pathways) and in turn, receives a report of the net (spinal-level) motor result via the ascending RSCT system.
The fact that RSCT neurons were activated by proximally biased RFs, in addition to the input they receive from segmental INs that relay motor commands to distal muscles, may suggest that the ascending RSCT signal contains integrated cross-joint information. According to this hypothesis, the termination of distal and proximal afferents has some degree of overlapping termination onto RSCT and PSA neurons. This integration of distal and proximal information can act to update and coordinate spinal pathways that control the distal musculature with pathways that control more proximal effectors (e.g., maintaining posture and balance). This spinal-level integration may act in parallel to equivalent supraspinal systems (Crammond and Kalaska 1996; Kurtzer et al. 2005; Pruszynski et al. 2011; Scott et al. 2015) and be necessary for low-level cross-linking between the two systems.
GRANTS
Support for this work was provided, in part, by the Israel Science Foundation (ISF-1787/13) and the German-Israeli Science Foundation (GIF-I-1224-396.13/2012) and through the generous support of the Baruch Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.H., T.D.A., and Y.P. conceived and designed research; O.C., R.H., and Z.I. performed experiments; O.C. analyzed data; O.C. interpreted results of experiments; O.C. prepared figures; Y.P. drafted manuscript; T.D.A. and Y.P. edited and revised manuscript; O.C., R.H., T.D.A., Z.I., and Y.P. approved final version of manuscript.
REFERENCES
- Alstermark B, Isa T, Kümmel H, Tantisira B. Projection from excitatory C3-C4 propriospinal neurones to lamina VII and VIII neurones in the C6-Th1 segments of the cat. Neurosci Res 8: 131–137, 1990. doi: 10.1016/0168-0102(90)90065-M. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lindström S, Lundberg A, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 8. Ascending projection to the lateral reticular nucleus from C3-C4 propriospinal also projecting to forelimb motoneurones. Exp Brain Res 42: 282–298, 1981. doi: 10.1007/BF00237495. [DOI] [PubMed] [Google Scholar]
- Asher I, Zinger N, Yanai Y, Israel Z, Prut Y. Population-based corticospinal interactions in macaques are correlated with visuomotor processing. Cereb Cortex 20: 241–252, 2010. doi: 10.1093/cercor/bhp095. [DOI] [PubMed] [Google Scholar]
- Azim E, Fink AJ, Jessell TM. Internal and external feedback circuits for skilled forelimb movement. Cold Spring Harb Symp Quant Biol 79: 81–92, 2014. doi: 10.1101/sqb.2014.79.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhaj-Saïf A, Karrer JH, Cheney PD. Distribution and characteristics of poststimulus effects in proximal and distal forelimb muscles from red nucleus in the monkey. J Neurophysiol 79: 1777–1789, 1998. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev 81: 539–568, 2001. [DOI] [PubMed] [Google Scholar]
- Cheema S, Rustioni A, Whitsel BL. Sensorimotor cortical projections to the primate cuneate nucleus. J Comp Neurol 240: 196–211, 1985. doi: 10.1002/cne.902400209. [DOI] [PubMed] [Google Scholar]
- Cohen O, Sherman E, Zinger N, Perlmutter S, Prut Y. Getting ready to move: transmitted information in the corticospinal pathway during preparation for movement. Curr Opin Neurobiol 20: 696–703, 2010. doi: 10.1016/j.conb.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JD, Larson B, Oscarsson O, Sjölund B. Organization of afferent connections to cuneocerebellar tract. Exp Brain Res 13: 359–377, 1971a. doi: 10.1007/BF00234337. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Larson B, Oscarsson O, Sjölund B. Origin and termination of cuneocerebellar tract. Exp Brain Res 13: 339–358, 1971b. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Differential relation of discharge in primary motor cortex and premotor cortex to movements versus actively maintained postures during a reaching task. Exp Brain Res 108: 45–61, 1996. doi: 10.1007/BF00242903. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci 16: 6513–6525, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner TJ. A role for the cerebellum in the control of limb movement velocity. Curr Opin Neurobiol 8: 762–769, 1998. doi: 10.1016/S0959-4388(98)80119-0. [DOI] [PubMed] [Google Scholar]
- Garwicz M. Spinal reflexes provide motor error signals to cerebellar modules—relevance for motor coordination. Brain Res Brain Res Rev 40: 152–165, 2002. doi: 10.1016/S0165-0173(02)00198-4. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Houk JC, Kohlerman NJ. Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. J Physiol 358: 527–549, 1985. doi: 10.1113/jphysiol.1985.sp015565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 13: 1233–1239, 2010. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol 361: 403–418, 1985. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 13: 952–980, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne MK, Butler EG. The role of the cerebello-thalamo-cortical pathway in skilled movement. Prog Neurobiol 46: 199–213, 1995. doi: 10.1016/0301-0082(95)80011-V. [DOI] [PubMed] [Google Scholar]
- Houk JC, Gibson AR, Harvey CF, Kennedy PR, van Kan PL. Activity of primate magnocellular red nucleus related to hand and finger movements. Behav Brain Res 28: 201–206, 1988. doi: 10.1016/0166-4328(88)90097-6. [DOI] [PubMed] [Google Scholar]
- Ivry R. Cerebellar timing systems. Int Rev Neurobiol 41: 555–573, 1997. doi: 10.1016/S0074-7742(08)60370-0. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Target cells of rubrospinal tract fibres within the lumbar spinal cord. Behav Brain Res 28: 91–96, 1988. doi: 10.1016/0166-4328(88)90083-6. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Interactions between spinal interneurons and ventral spinocerebellar tract neurons. J Physiol 591: 5445–5451, 2013. doi: 10.1113/jphysiol.2012.248740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Hammar I. Collateral actions of premotor interneurons on ventral spinocerebellar tract neurons in the cat. J Neurophysiol 104: 1872–1883, 2010. doi: 10.1152/jn.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature 487: 235–238, 2012. doi: 10.1038/nature11206. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Maier MA, Lemon RN. Interspecies comparisons for the C3-C4 propriospinal system: unresolved issues. Adv Exp Med Biol 508: 299–308, 2002. doi: 10.1007/978-1-4615-0713-0_35. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci 8: 498–504, 2005. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res 12: 317–330, 1971. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Spinocerebellar projections from the cervical enlargement in the cat, as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol 263: 223–240, 1987. doi: 10.1002/cne.902630206. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Xiong G. Projections from the cervical enlargement to the cerebellar nuclei in the rat, studied by anterograde axonal tracing. J Comp Neurol 377: 251–261, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Miller LE, van Kan PL, Sinkjaer T, Andersen T, Harris GD, Houk JC. Correlation of primate red nucleus discharge with muscle activity during free-form arm movements. J Physiol 469: 213–243, 1993. doi: 10.1113/jphysiol.1993.sp019812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O, Iggo A. Functional organization of spinocerebellar paths. In: Handbook of Sensory Physiology. New York: Springer, 1973, vol. II, p. 339–380. [Google Scholar]
- Petras JM. Spinocerebellar neurons in the rhesus monkey. Brain Res 130: 146–151, 1977. doi: 10.1016/0006-8993(77)90850-2. [DOI] [PubMed] [Google Scholar]
- Petras JM, Cummings JF. The origin of spinocerebellar pathways. II. The nucleus centrobasalis of the cervical enlargement and the nucleus dorsalis of the thoracolumbar spinal cord. J Comp Neurol 173: 693–716, 1977. doi: 10.1002/cne.901730405. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011. doi: 10.1038/nature10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature 401: 590–594, 1999. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- Prut Y, Perlmutter SI. Firing properties of spinal interneurons during voluntary movement. I. State-dependent regularity of firing. J Neurosci 23: 9600–9610, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH, Cluff T, Lowrey CR, Takei T. Feedback control during voluntary motor actions. Curr Opin Neurobiol 33: 85–94, 2015. doi: 10.1016/j.conb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Shalit U, Zinger N, Joshua M, Prut Y. Descending systems translate transient cortical commands into a sustained muscle activation signal. Cereb Cortex 22: 1904–1914, 2012. doi: 10.1093/cercor/bhr267. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar HJ. Evolution of the red nucleus and rubrospinal tract. Behav Brain Res 28: 9–20, 1988. doi: 10.1016/0166-4328(88)90072-1. [DOI] [PubMed] [Google Scholar]
- Thach WT. Cerebellar inputs to motor cortex. Ciba Found Symp 132: 201–220, 1987. [DOI] [PubMed] [Google Scholar]
- van Kan PL, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol 69: 74–94, 1993a. [DOI] [PubMed] [Google Scholar]
- van Kan PL, Houk JC, Gibson AR. Output organization of intermediate cerebellum of the monkey. J Neurophysiol 69: 57–73, 1993b. [DOI] [PubMed] [Google Scholar]
- Van Kan PL, McCurdy ML. Contribution of primate magnocellular red nucleus to timing of hand preshaping during reaching to grasp. J Neurophysiol 87: 1473–1487, 2002. [DOI] [PubMed] [Google Scholar]
- Walberg F, Dietrichs E, Nordby T. The origin and termination of the dentatorubral fibres in the cat as studied with retrograde and anterograde transport of peroxidase labelled lectin. Exp Brain Res 63: 294–300, 1986. doi: 10.1007/BF00236846. [DOI] [PubMed] [Google Scholar]
- Yanai Y, Adamit N, Harel R, Israel Z, Prut Y. Connected corticospinal sites show enhanced tuning similarity at the onset of voluntary action. J Neurosci 27: 12349–12357, 2007. doi: 10.1523/JNEUROSCI.3127-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger N, Harel R, Gabler S, Israel Z, Prut Y. Functional organization of information flow in the corticospinal pathway. J Neurosci 33: 1190–1197, 2013. doi: 10.1523/JNEUROSCI.2403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]