Abstract
Drug delivery across the skin is used for several millennia to ease gastrointestinal (GI) ailments in Traditional Persian Medicine (TPM). TPM topical remedies are generally being applied on the stomach, lower abdomen, lower back and liver to alleviate GI illnesses such as dyspepsia, gastritis, GI ulcers, inflammatory bowel disease, intestinal worms and infections. The aim of the present study is to survey the topical GI remedies and plant species used as ingredients for these remedies in TPM. In addition, pharmacological activities of the mentioned plants have been discussed. For this, we searched major TPM textbooks to find plants used to cure GI problems in topical use. Additionally, scientific databases were searched to obtain pharmacological data supporting the use of TPM plants in GI diseases. Rosa × damascena, Pistacia lentiscus, Malus domestica, Olea europaea and Artemisia absinthium are among the most frequently mentioned ingredients of TPM remedies. β-asarone, amygdalin, boswellic acids, guggulsterone, crocin, crocetin, isomasticadienolic acid, and cyclotides are the most important phytochemicals present in TPM plants with GI-protective activities. Pharmacological studies demonstrated GI activities for TPM plants supporting their extensive traditional use. These plants play pivotal role in alleviating GI disorders through exhibiting numerous activities including antispasmodic, anti-ulcer, anti-secretory, anti-colitis, anti-diarrheal, antibacterial and anthelmintic properties. Several mechanisms underlie these activities including the alleviation of oxidative stress, exhibiting cytoprotective activity, down-regulation of the inflammatory cytokines, suppression of the cellular signaling pathways of inflammatory responses, improving re-epithelialization and angiogenesis, down-regulation of anti-angiogenic factors, blocking activity of acetylcholine, etc.
Keywords: Gastrointestinal, Medicinal plants, Olea europaea, Pistacia lentiscus, Rosa × damascene, Topical delivery, Traditional medicine
Introduction
The evidence of herbal medicines dates back over 5,000 years. The application of medications to the skin to cure illnesses is a practice that has been utilized by humankind for thousands of years and has included the application of poultices, oils, gels, ointments, pastes, and lotions (1). Skin which is known as the largest organ of the human body plays important role in drug delivery. Three important modes including topical, regional and transdermal are used for delivery of various dosage forms. Topical delivery is used mainly to directly affect cutaneous disorders while regional delivery requires deeper penetration than topical delivery and is used to alleviate disease symptoms in deep tissues such as muscles and vasculature joints, beneath or near the site of application (2). Regional delivery is also applied to reduce drug toxicity, as it is established that systemic delivery, can produce inadequate doses of the drug in target tissue, as well as toxicity in healthy tissue. Transdermal delivery is applied to the skin to achieve systemically active levels of the drug to cure systemic disease (2-4). Transdermal delivery has also several advantages over other routes of administration. It is used to bypass hepatic first-pass effect and other variables associated with the gastrointestinal (GI) tract such as pH and gastric emptying time that can prematurely metabolize or degrade drugs. Moreover, transdermal systems also are non-invasive and can be self-administered. They also improve patient compliance and would cause fewer systemic adverse effects (5-7). Particularly, transdermal administration of medicines has been shown to reduce GI track related side effects (8).
Drug delivery across the skin is used for several millennia to ease GI ailments in various traditional medicine systems. In Traditional Persian Medicine (TPM), which is based on quadratic elements (9), a majority of GI remedies are being applied to skin and mostly aimed at regional and/or transdermal delivery (10). These remedies are especially administrated for the treatment of gastric weakness and dyspepsia, gastritis, loss of appetite, belching, GI ulcers, colitis, intestinal worms and infections (11, 12). Several medicinal plants, animal products and minerals generally in compound formulations have been recommended to cure these conditions. The recommended formulations are in the forms of poultices, lotions, ointments, rubbing oils, bathes, etc. A number of papers have already well studied the medicinal plants used for the treatment of some GI diseases especially peptic ulcer in view of TPM (13, 14). However, there is not any scientific study to specifically survey topical remedies used to alleviate GI problems. Therefore, here we present an overview of the topical GI remedies in TPM and the plant species used as ingredients for these remedies. In addition, relevant pharmacological activities of the mentioned plants in GI tract have been discussed.
Materials and Methods
Firstly, we searched major TPM textbooks to find medicinal plants used for the treatment of GI problems in topical use. These books included Al-Hawi fi’l-Tebb (Comprehensive Book of Medicine) by Razi (865-925), Canon of Medicine by Ibn Sina (980-1037), Ferdows al-Hekmah fi’l-Tebb (Paradise of Wisdom on Medicine) by Tabari (9th century), Konnash fi’l-Tebb by Kashkari (9th-10th century), Hedayat al-Mota’allemin fi’l-Tebb (An Educational Guide for Medical Students) by Akhawayni (10th century), and Qarabadin-e-Kabir by Aqili-Khorasani (16th-17th century). The search was performed using a software namely Jamee al-Tibb containing a majority of TPM books. Afterwards, the scientific names of the retrieved plant names were authenticated using botanical textbooks, including the Dictionary of Medicinal Plants (15), Qamus al-qanun fi’l-tibb (16), Illustrated polyglottic dictionary of plant names in Latin, Arabic, Armenian, English, French, German, Italian, and Turkish languages (17), Encyclopedia of Medicinal Plants: Arabic-English-French-German-Latin (18) and Tafsir kitāb Diyusquiridis (Explanation of Dioscorides’ Book) (19).
The scientific names were then entered as key terms for the second search. ScienceDirect, PubMed, Scopus, and Google Scholar databases were searched to obtain pharmacological data supporting the use of TPM plants in GI diseases using the following keywords: Gastrointestinal diseases, peptic ulcer, anti-secretory, gastro-protective effects, anti-inflammatory effects, antibacterial, Helicobacter pylori, anti-diarrhea, colitis, etc. Different steps of the present research are illustrated schematically in Figure 1.
Figure 1.
Different steps of the present research
Topical GI dosage forms in TPM
The use of topical remedies is probably coeval with the appearance of medical knowledge. In TPM, topical medications are almost as applicable as internal formulations (20). In GI problems, topical remedies mostly in the forms of poultices or zemad, ointments or marham, bathes or notul, lotions or tali and compresses or kemad, are being applied on the stomach area, lower abdomen, lower back and liver.
Poultices are topical preparations usually containing whole fresh medicinal plants or herbal powders occasionally in mixture with herbal distillates, infusions or oils. These dosage forms are directly applied to the skin near the affected area (12).
Herbal oils are common ingredients of topical remedies. In TPM, herbal oils are mostly extracted by maceration method through which the flowers and other herbal tissues are soaked in a base oil (commonly olive, almond or sesame oils), then filtered (12). This process is repeated several times to obtain rich herbal oils containing essential oils and other lipophilic phytpchemicals. Traditional ointments are defined as mixtures of herbal or animal oil and bees wax as a base for bioactive herbal extracts and powders (21). The hydrophobic nature of ointment bases offers an improved percutaneous absorption of herbal extracts. Ointment bases influence drug bioavailability due to their occlusive properties of the stratum corneum, which increases the flux of drug across the skin. Moreover, they affect drug dissolution and drug partitioning within or from the ointment to the skin (2). Oleo-gum-resins such as mastic, olibanum, guggul, opobalsam, etc. which are rich sources of essential oils are important ingredients of TPM cutaneous GI formulations (12). A number of essential oils have been reported to exert GI protective activities (22, 23). Terpenes, the primary constituents of the essential oils obtained from many types of plants and flowers have been shown to have percutaneous permeation through the intact skin (24). Moreover, some terpene-containing essential oils such as fennel oil, peppermint oil, cardamom oil and sweet basil oil are capable of accelerating the percutaneous absorption of co-administered drugs probably due to the increased skin-vehicle partitioning by the oils (25). Various sesquiterpenes have also been found to enhance percutaneous penetration of the drugs possibly by disrupting the intercellular lipid bilayers in the stratum corneum, thus improving co-administered drugs diffusivity, and/or increasing drug partitioning. Some other phytochemicals present in TPM formulations such as fixed oils and fatty acids, aloe juice and α-tocopherol also have percutaneous penetration enhancing effects (26). Thus, these phytochemicals exert multidimensional activities in TPM topical remedies. For instance, the presence of aloe juice in a multi-herbal preparation not only offers multiple GI activities such as anti-ulcerogenic, anti-H. pylori, anti-diarrheal, anthelminthic and anti-ulcerative colitis (UC) effects (27-31), but also act as a base or carrier and penetration enhancing agent for other ingredient of the preparation (26).
TPM cutaneous GI formulations aimed at developing percutaneous absorption and deposition of bioactive phytochemicals as well as offering higher regional concentrations than systemic administration at the same total body exposure to the drug. Cutaneous application of these formulations along with oral preparations offers a multifaceted therapeutic strategy for the treatment of GI diseases.
TPM recommended medicinal plants for topical use in gastrointestinal diseases
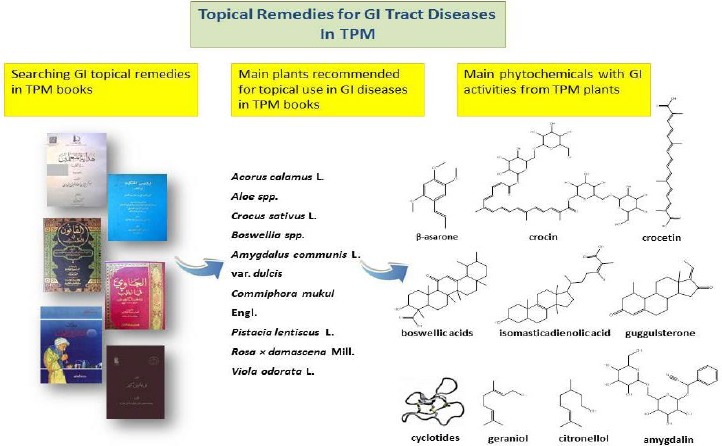
Around 60 plant species from 34 families have been frequently noted in TPM textbooks to be topically active in the treatment of GI diseases. Most of these species belong to the Apiaceae (eight species) and Rosaceae (four species) families. Rosa × damascena. Mill. flowers, Pistacia lentiscus L. oleo-gum-resin, Malus domestica Baumg. fruits, Olea europaea L. fruit oil and aerial parts of Artemisia absinthium L. are among the most frequently mentioned herbal ingredients of TPM-recommended remedies. A wide spectrum of GI diseases including GI ulcers, gastric inflammations and swellings, diarrheal illnesses caused by gastric dysfunction, bacterial infections and intestinal problems such as inflammatory bowel disease (IBD) and colitis has been traditionally treated by a combination of internal and topical medications (16, 20, 32). Medicinal plants used to alleviate or cure GI diseases and their TPM information are listed in Table 1.
Table 1.
TPM suggested medicinal plants used to treat GI diseases in topical application
| Scientific names | Family | Traditional names | Plant part | Medicinal uses | References |
|---|---|---|---|---|---|
| Acacia arabica (Lam.) Muhl. ex Willd. | Fabaceae | Aqaqia | dried extract of the leaves and legumes | Gastritis, vomiting caused by yellow bile | (10, 21, 32) |
| Acorus calamus L. | Acoraceae | Vaj | Rhizome | Stomach weakness, loss of appetite, cholera | (10, 21, 32) |
| Aloe spp. | Asphodelaceae | Sabr | Dried sap | Stomach weakness, gastritis, stomach swelling | (10-12) |
| Althaea officinalis L. | Malvaceae | Khatmi | Flowers, seeds | Gastritis, stomach swelling, gastric abscess | (10, 11) |
| Amygdalus communis L. var. dulcis | Rosaceae | Badam talkh | Seeds | Stomach swelling and inflammation | (32) |
| Anethum graveolens L. | Apiaceae | shebet | Seeds, leaves | Gastritis, stomach swelling, Nausea and vomiting, IBD | (10, 11, 20) |
| Apium graveolens L. | Apiaceae | Karafs | Seeds | Stomach swelling | (20) |
| Aquilaria agallocha Roxb. | Thymelaeaceae | Ood | Stem wood | Loss of appetite, diarrhea, digestive aid, stomach tonic, cholera | (10, 20, 21, 32) |
| Artemisia absinthium L. | Asteraceae | Afsantin | Aerial parts | Stomach weakness, stomach swelling and pain, gastric abscess, vomiting, diarrhea, intestinal worms | (10-12, 20, 21, 32) |
| Boswellia spp. | Burseraceae | Kondor | Oleo-gum-resin | Stomach weakness, gastritis, Stomach swelling, loss of appetite, diarrhea, intestinal worms | (10-12, 20, 21, 32) |
| Brassica oleracea L. | Brassicaceae | Kalam | Leaves, seeds | Gastrointestinal swellings, colic, hemorrhoids | (10, 21, 32) |
| Carum carvi L. | Apiaceae | Zireh | Fruits | Stomach weakness, gastric swellings, flatulence | (10, 20, 21) |
| Carum copticum Benth. & Hook.f. | Apiaceae | Zenyan | Fruits | gastric swellings | (20) |
| Cissus quadrangularis L. | Vitaceae | Hamama | Berries | Stomach weakness, gastric swelling caused by phlegm | (10-12, 21) |
| Cistus ladaniferus Curtis | Cistaceae | Ladan | Sap | Stomach weakness, gastric swelling, gastric trauma, bulimia, diarrhea, diarrhea caused by stomach coldness and weakness | (10-12, 20, 21, 32) |
| Commiphora mukul Engl. | Burseraceae | Moql azraq | Oleo-gum-resin | Stomach weakness, distention and swelling, belching, intestinal ulcers, IBD, hemorrhoids | (10-12, 20, 21) |
| Commiphora opobalsamum Engl. | Burseraceae | Balsan | Oleo-gum-resin | Stomach weakness, distention and coldness, gastritis | (10, 11, 21) |
| Costus speciosus (J. Koenig) Sm. | Costaceae | Qost | Rhizome | Stomach coldness, diarrhea, colic | (11, 12, 32) |
| Crocus sativus L. | Iridaceae | Zaafaran | Stigma | Cold stomach, gastric distension and swelling, gastritis, nausea, vomiting, diarrhea | (10-12, 20, 21, 32) |
| Cucurbita pepo L. | Cucurbits | Kadu | Fruits, seeds, peel | Gastric weakness in pregnancy, hot and dry stomach, gastritis, heart burn, peptic ulcer, nausea, thirst, diarrhea | (10, 20, 21, 32) |
| Cupressus sempervirens L. | Cupressaceae | Sarv | Berries, leaves | Gastric weakness, swelling and distension, cholera, intestinal ulcers, rectal prolapse | (10, 11, 21, 32) |
| Cydonia oblonga Mill. | Rosaceae | Beh | Fruits, leaves, oil | Poor digestion, nausea, vomiting, gastritis, heartburn, diarrhea, flatulence, cholera | (10, 20, 21) |
| Cymbopogon schoenanthus (L.) Spreng. | Poaceae | Ezkher | Roots, flowers | Gastric weakness, swelling and distension, diarrhea | (20, 21, 32) |
| Cyperus rotundus L. Cyperus longus L. | Cyperacea | Soad | Rhizome | Stomach weakness, coldness and swelling, dyspepsia, gastritis, nausea, vomiting, diarrhea | (10-12, 20, 21, 32) |
| Dorema ammoniacum D. Don | Apiaceae | Oshaq | Oleo-gum-resin | Stomach weakness, coldness, swelling and hardness, gastritis, belching, gastric abscess | (10, 11, 21, 32) |
| Eugenia caryophyllata Thunb. | Myrtaceae | Mikhak | Flowers | Dyspepsia, stomach weakness, severe nausea, diarrhea, cholera | (11, 20, 21, 32) |
| Foeniculum vulgare L. | Apiaceae | Razianeh | Fruits | Hard swelling of stomach | (20) |
| Glossostemon bruguieri Desf. | Sterculiaceae | Moghat | Roots, fruits | Hard swelling of stomach | (10, 11) |
| Hordeum vulgare L. | Poaceae | Jo | Seeds flour | Stomach swelling, gastritis, peptic ulcer, nausea, thirst, chronic diarrhea, gripe, flatulence, rectal prolapse, anal fissure | (10-12, 20, 21, 32) |
| Hyoscyamus niger L. | Solanaceae | Bangdaneh | Seeds, leaves, flowers | Diarrhea, intestinal ulcers, hemorrhoids pain and inflammation, anal fissure | (10, 12, 21, 32) |
| Iris florentina L. | Iridaceae | Irsa | Rhizome | Chronic vomiting, belching, hemorrhoids | (21, 32) |
| Lawsonia inermis L. | Lythraceae | Hana | Leaves, flowers, oil | Coldness of stomach, belching, gastritis, IBD, anal fissure, colic | (10, 21) |
| Linum usitatissimum L. | Linaceae | Katan | Seeds | Gastritis, gastric hard swelling, vomiting, chronic diarrhea, flatulence, IBD, colic, ileus, hemorrhoids | (10-12, 21, 32) |
| Malus domestica Baumg. | Rosaceae | Seeb | Fruits, fruits oil | Gastric hard swellings, gastric trauma, stomach weakness, pain and inflammation, loss of appetite, intestinal worms, nausea, cholera, chronic diarrhea | (10, 11, 20, 21, 32) |
| Matricaria Chamomila L. | Asteraceae | Babuneh | Flowers | Gastric hard swelling, burning and inflammation, flatulence, belching, vomiting, colic, proctitis | (10, 11, 20, 21, 32) |
| Melilotus officinalis (L.) Lam. | Fabaceae | Eklil al-malek | Legumes | Gastric swelling and inflammation, gastric abscess, dyspepsia, hard swelling, gastric pain, flatulence, vomiting, diarrhea | (11, 20, 21, 32) |
| Myristica fragrans Houtt. | Myristicaceae | Joz Buya | Seeds, aryls | Nausea, vomiting, diarrhea, hemorrhoids | (10, 32) |
| Nymphaea alba L. Nymphaea lotus L. | Nymphaeaceae | Nilufar | Flowers | Gastritis | (21) |
| Olea europaea L. | Oleaceae | Zeytun | Fruit oil | Gastric pain and inflammation, bulimia, abdominal pain caused by flatulence, hiccups, dyspepsia, nausea, vomiting, cholera, IBD, hemorrhoids | (10-12, 21) |
| Opopanax chironium W.D.J. Koch | Apiaceae | Gavshir | Oleo-gum-resin | Gastric swelling and inflammation, belching | (11, 20, 21) |
| Phoenix dactylifera L. | Arecaceae | Khorma | Fruits | Diarrhea, cholera, | (10, 21) |
| Pimpinella anisum L. | Apiaceae | Anisun | Fruits | Intestinal ulcers | (21) |
| Pistacia atlantica Desf. Pistacia terebinthus L. | Anacardiaceae | Botm | Oleo-gum-resin | Gastric hard swelling, anal pain, gastric weakness, belching, gastric abscess, colic, hemorrhoids, anal fissure | (10, 12, 21) |
| Pistacia lentiscus L. | Anacardiaceae | Mastaki | Oleo-gum-resin | Gastric weakness, hard swelling, pain and inflammation, loss of appetite, dyspepsia, hiccups, severe nausea, intestinal ulcers, diarrhea, cholera | (21) |
| Portulaca oleracea L. | Portulacaceae | Khorfeh | Aerial parts | Gastric weakness, vomiting, excessive thirst, hemorrhagic hemorrhoids | (11, 12, 21) |
| Punica granatum L. | Punicaceae | Golnar | Flowers | Gastric weakness and inflammation, loss of appetite, excessive vomiting, diarrhea, cholera, intestinal ulcers, anal fissure, rectal prolapse | (10, 20, 21, 32) |
| Rhus coriaria L. | Anacardiaceae | Somaq | Fruits | Gastric diarrhea, nausea, intestinal ulcers, rectal prolapse, diarrhea, hemorrhoids | (10, 20, 21) |
| Rosa × damascena Mill. | Rosaceae | Gol-e-sorkh | Flowers, seeds, oil | Gastric hard swelling, pain and inflammation, chronic hiccups, dyspepsia, excessive thirst, bulimia, gastric diarrhea, nausea, intestinal ulcers, cholera, IBD, anal inflammation, anal fissure and fistula, rectal prolapse | (10-12, 20, 21, 32) |
| Santalum album L. | Santalaceae | Sandal | Wood | Gastric hard swelling and inflammation, nausea, hiccups, loss of appetite, diarrhea, cholera, colic | (10-12, 20, 21, 32) |
| Tragopogon graminifolius DC Tragopogon pratensis L. | Asteraceae | Lehyat al-tees | Aerial parts | Gastric diarrhea, intestinal ulcers | (21, 32) |
| Trigonella foenum-graecum L. | Fabaceae | Holbeh | Aerial parts | Gastric hard swelling, gastric abscess, gastritis, IBD, ileus, hemorrhoids | (10, 11, 20, 21, 32) |
| Valeriana celtica L. Nardostachys jatamansi DC. | Caprifoliaceae | Nardin | Rhizome | Gastric weakness, hard swelling and inflammation, loss of appetite, belching, colic | (10, 12, 20, 21, 32) |
| Viola odorata L. | Violaceae | Banafsheh | Aerial parts | Gastric weakness, swelling and inflammation, vomiting, thirst, colic, hemorrhoids | (10, 11, 20, 21) |
Pharmacological activities of TPM recommended GI plants
Pharmacological GI activities of TPM recommended medicinal plants have been shown by a large number of in vitro and animal investigations as well as some clinical trials.
Mastic gum (oleo-gum-resin from Pistacia lentiscus L.) as one of the most emphatic TPM recommended GI plants has been found to exert anti-Helicobacter pylori activities in vivo (33). In a randomized clinical trial (RCT) in 148 patients with functional dyspepsia, administration of 350 mg mastic gum three times daily for 3 weeks significantly improved symptoms of functional dyspepsia when compared to placebo (34). Mastic gum decreased histological damage in trinitrobenzene sulfonic acid (TNBS)-induced colitis, regulated oxidant/antioxidant balance and modulated inflammation (35). It improved the clinical features of Chron’s disease (CD) (36). Additionally, mastic gum exhibited antibacterial activity against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis (37).
Artemisia absinthium L. another important GI active TPM plant could induce a significant decrease in volume of gastric juice, acid output and peptic activity in rats. It also decreased the ulcer index significantly (38). In a 6 weeks controlled clinical trial in patients with CD, administration of A. absinthium improved symptoms of CD by increased production of pro-inflammatory cytokines such as TNF-α (39). A. absinthium also exhibited anti-inflammatory, anti-nociceptive, anthelmintic activities properties and antibacterial activities against GI pathogens (40-42).
Olive oil has traditionally been applied to relieve gastric pain and inflammation, dyspepsia, abdominal pain caused by flatulence, bulimia, hiccups, nausea and vomiting, cholera, IBD and hemorrhoids (11, 20, 32). Odabasoglu et al demonstrated that olive oil could prevent the indomethacin-induced gastric damages in rats, enhanced the efficacy of indomethacin for reducing carrageenan-induced paw edema and exerted anti-inflammatory activity against paw edema (43). In a human study, a 30-day olive oil containing diet resulted in attenuating gastric secretory function, suppression of serum gastrin and higher levels of peptide YY in patients with gallstones (44). Olive oil also exhibited strong anti-H. pylori activity, decreased acid secretion in the GI tract and reduced the size of peptic ulcers (45).
Additionally, olive oil phenols inhibited the NF-κB driven transcription in a concentration-dependent manner supporting its use in gastric inflammation (46).
Guggul gum (oleo-gum-resin from Commiphora mukul) has been widely applied in TPM to alleviate stomach distention and swelling, belching, intestinal ulcers, IBD and hemorrhoids (10, 21). In a randomized controlled trial in 99 patients with hemorrhoids, administration of 3 g/day guggul gum for 4 weeks decreased flatulence, dyspepsia, gastro-esophageal reflux, and colonoscopic grading scores significantly compared to control. The rate of constipation, and proctorrhagia were also significantly improved after 4-weak follow-up (47). Guggulsterone, a steroid found in guggul gum, exhibited anti-inflammatory activities in mouse models of colitis by targeting lamina propria T cells (48). In addition, guggulsterone significantly increased apoptosis in HT-29 cells through activating caspases-3 and -8. It decreased cIAP-1 and 2, and Bcl-2 levels and increased the levels of truncated Bid, Fas, p-c-Jun, and p-JNK. The size of HT-29 xenograft tumors in guggulsterone-treated mice was significantly smaller than control group (49).
Pharmacological activities of other TPM GI recommended plants are shown in Table 2. Most of the mentioned plants exhibited various GI activities which support their extended application in TPM. Nonetheless, the majority of studies have investigated the effects of internal administration of the plants and there is scarcity in studies dealing with their topical application as it is recommended in TPM. Therefore, future studies are needed to elucidate GI effects of TPM plants in topical use. Interestingly, some of the mentioned plants like saffron are traditionally used in depression, tension, anxiety and insomnia even in topical use (21, 50, 51). These effects can exert additional relieving effects on stress-related GI diseases such as peptic ulcers, IBD, etc.
Table 2.
Gastrointestinal activities of TPM-recommended plants for topical use and their main phytochemicals
| Scientific name | Common name | Extract/phytochemical/plant part | Pharmacological activities | Model | Reference |
|---|---|---|---|---|---|
| Acacia arabica (Lam.) Muhl. ex Willd. | Gum arabic tree | Gum arabic-supplemented oral rehydration solution | Anti-diarrhea | in vivo | (62) |
| Acorus calamus L. | Sweet flag | Crude extract/ n-hexane fraction | Spasmolytic activity by inhibition of spontaneous and high K+-induced contractions through Ca2+ channel blockade in the isolated rabbit jejunum preparation | ex vivo | (63) |
| Methanol extract | Anti-diarrhoeal effect | in vivo | (64) | ||
| Ethanol extract of rhizome containing β-asarone | Potent anthelmintic activity, anti-amoebic and antibacterial activity | in vitro | (65) | ||
| Ethanol extract of rhizome | Anti-secretory, anti-ulcer, cytoprotective | in vivo | (66) | ||
| Aloe spp. | Aloes | Aqueous extract of the leaves of A. ferox Mill | Improving intestinal motility, increasing fecal volume in loperamide-induced constipation | in vivo | (27) |
| A. vera gel | Inhibitory effects on colorectal prostaglandin E2 and interleukin-8 production | in vitro | (28) | ||
| Aqueous extract of A. vera leaves | Inhibition of gastric acid secretion | in vivo | (30) | ||
| A. vera extract | Strong anti-H. pylori activity, ulcer healing properties | in vitro, in vivo | (29) | ||
| Aqueous extract of leaves of A. ferox | Anthelminthic activity | in vitro | (67) | ||
| Ethanolic extract of A. barbadensis | Antimicrobial activity | in vitro | (68) | ||
| Althaea officinalis L. | Marsh mallow | Hydro-ethanolic extract of aerial parts | Antibacterial against Escherichia coli | in vitro | (69) |
| Aqueous extract of aerial parts | Antiulcer activity: reduction of the ulcer number, ulcer index and peptic activity after pyloric ligation, reduction of oxidative stress and histamine release | in vivo | (70) | ||
| Amygdalus communis L. var. dulcis | Bitter almond | Amygdalin | Protection against gastric ulcer | in vivo | (71) |
| Ethanol extract of seeds | Laxative effect | in vivo | (72) | ||
| Anethum graveolens L. | Dill | Seed ethanolic extract | Inhibiting acid secretion and the occurrence of lesions in stomach | in vivo | (73) |
| Aqueous and ethanolic extracts of seeds Seeds powder | Protection against gastric ulcer, attenuation in the changes in gastric juice volume, pH, acid-output and ulcer index, acid buffering activities pepsin binding activity | in vitro in vivo | (74) | ||
| Hydroalcoholic extract | Potent spasmolytic activity in ileum | ex vivo | (75) | ||
| Hot water and acetone extracts of seed | Antibacterial activity | in vitro | (76) | ||
| Apium graveolens L. | Celery | Methanolic and aqueous extracts of aerial part and seeds | Inhibition of gastric ulcers | in vivo | (77) |
| Methanolic and aqueous extracts of leaves | Antimicrobial activity against enteric pathogens | in vitro | (78) | ||
| Ethanolic and aqueous extracts of leaves | Inhibition of spontaneous rat ileum contractions | ex vivo | (79) | ||
| Aquilaria agallocha Roxb. | Agarwood | Ethyl acetate extract | Analgesic, anti-inflammatory | in vivo | (80) |
| Artemisia absinthium L. | Absinthe | Essential oil containing trans-sabinyl acetate, myrcene, β-thujone | Anti-fungal, antibacterial activity | in vitro | (81) |
| Ethanol extract of aerial parts | Anti-gastric ulcer effects, decrease in volume of gastric juice and acid output | in vivo | (38) | ||
| Powder | TNF-α suppression, remission of symptoms of CD | RCT | (39) | ||
| Methanol extract | Anti-inflammatory | in vivo | (82) | ||
| Methanol extracts | Antibacterial (GI pathogens) | in vitro | (40) | ||
| Essential oil, aqueous extract | Anti-inflammatory, anti-nociceptive | in vivo | (41) | ||
| Aqueous extracts ethanolic extracts | Anthelmintic | in vitro in vivo | (42) | ||
| A multiherbal preparation containing ethanolic-aqueous extracts | Cure upper abdominal complaints | RCT | (83) | ||
| Boswellia spp. | Olibanum | B. serrate oleo-gum-resin | Complete resolution of ulcers in chronic colitis, loss of friability of mucosa, and granulation, loss of hypercellularity of lamina propria without distorted crypt architecture in rectal mucosa, healing of ulcers and loss of fibrous tissue and chronic inflammatory cells | clinical trial | (84) |
| B. serrata gum-resin hydroalcoholic extract | Antidiarrheal activity, inhibition of acetylcholine- and electrical field stimulation-induced contractions in the isolated guinea-pig ileum | in vivo, ex vivo | (85) | ||
| Boswellic acids | Gastric ulcer protective effect | in vivo | (86) | ||
| B. serrata gum-resin extract, acetyl-11-keto-β-boswellic acid | Attenuating leu-kocyte–endothelial cell adhesive interactions, ameliorating inflammation- associated tissue injury in a rat model of experimental IBD | in vivo | (87) | ||
| Boswellic acids | Attenuating the recruitment of both leukocytes and platelets, blunting P-selectin expression, protecting the colonic mucosa against tissue injury, and reducing colitis activity | in vivo | (88) | ||
| β-boswellic acid derivatives | H. pylori urease inhibitory activities | in vitro | (89) | ||
| Brassica oleracea L. | Cabbage | Hydroalcoholic extract of leaves | Protection against gastric ulcer | in vivo | (90) |
| Carum carvi L. | Persian cumin | Methanol extract of seeds | Anti-H. pylori | in vitro | (91) |
| Essential oil | Treatment of intestinal dysbiosis | in vitro | (92) | ||
| Ethanol extract of the seeds | Inhibiting the response of intestinal smooth muscle cells to acetylcholine | ex vivo | (93) | ||
| Powdered seeds | Modulatory role on tissue lipid peroxidation, antioxidant profile and preventing 1,2-dimethylhydrazine-induced histopathological lesions in colon cancer rats | in vivo | (94) | ||
| Alcoholic extract | anti-ulcerogenic activity: reducing acid output, increasing mucin secretion, increasing prostaglandin E2 release, decrease in leukotrienes, protection against gastric ulceration | in vivo | (95) | ||
| Carum copticum Benth. & Hook.f. | Ajwain | Ethanol and aqueous extract of fruits | Antidiarrhoeal activity | in vivo | (96) |
| Aqueous extract of fruits | Inhibitory effect on ACh-induced contraction in rat’s ileum | ex vivo | (97) | ||
| Aqueous extract | Treatment of peptic ulcer | in vivo | (98) | ||
| An equal mixture of methanol, diethyl ether and petroleum benzene extract | Anti-H. pylori | in vitro | (99) | ||
| Cissus quadrangularis L. | Veldt grape | Methanol extract of stem | Attenuation in levels of TNF-α, IL-1β), microvascular permeability, activity of nitric oxide synthase-2, mitochondrial antioxidants, lipid peroxidation, DNA damage, Decrease in tissue damage glutathione, superoxide dismutase and catalase, reducing size of NSAID induced ulcer crater, restoration of mucosal epithelium | in vivo | (100, 101) |
| Stem extract | Attenuation in aspirin-induced gastric lesions, an increase in uric acid, antioxidative enzymes, SH groups, decrease in lipid peroxidase, TNF-α, xanthine oxidase, myeloperoxidase activities | in vivo | (102) | ||
| Methanolic extract | Increase in the mucosal defensive factors like mucin secretion, mucosal cell proliferation, glycoproteins, and life span of cells in experimentally induced gastric ulcer | in vivo | (103) | ||
| Chloroform extract | Potent anti-H. Pylori | in vitro | (104) | ||
| Cistus ladaniferus Curtis | Labdanum | Aqueous extract of aerial parts | Effective against reserpine- and serotonin-induced mucosal congestion and haemorrhagic ulcers | in vivo | (105) |
| Aqueous extract of leaves and stems | Antispasmodic action in the rabbit jejunum through calcium channel blockade | ex vivo | (106) | ||
| aerial parts aqueous extract | Anti-diarrhoeal activity in castor oil-induced diarrhoea | in vivo | (107) | ||
| Commiphora mukul Engl. | Guggul | Guggulsterone | Anti-inflammatory activities in mouse models of colitis by targeting lamina propria T cells | in vivo | (48) |
| Guggulsterone | Activation of the mitochondria-dependent pathway and the extrinsic pathway of apoptosis in colon cancer cells, inhibition of the growth of HT-29 xenografts | in vitro | (49) | ||
| Guggulsterone | Inducing apoptosis, inhibition of angiogenesis and metastasis in colon cancer cells through blocking STAT3 and VEGF expression | in vitro | (108) | ||
| Oleo-gum-resin powder | Reduction in symptoms of uncomplicated hemorrhoids grade 1 and 2. | RCT | (47) | ||
| Commiphora opobalsamum Engl. | Arabian balsam tree | Oleo-gum-resin ethanol extract | Protecting against gastric ulcers, anti-secretion | in vivo | (109) |
| Methalonic extract of aerial parts | analgesic and anti-inflammator y activity | in vivo | (110) | ||
| Essential oil | Antimicrobial activity | in vitro | (111) | ||
| Costus speciosus (J. Koenig) Sm. | Crêpe ginger | - | - | - | - |
| Crocus sativus L. | Saffron | Extract of stigma, crocin | Inhibiting the growth of colorectal cancer cells | in vitro | (112) |
| Methanol and aqueous extracts, crocin and safranal | Anti-H. pylori effects | in vitro | (113) | ||
| Crocetin | Ameliorating UC by down-regulation of NFkB | in vivo | (114) | ||
| Aqueous extract | Inhibition of gastric cancer progression | in vivo | (115) | ||
| Hydro-ethanol extract | Strong inhibitor of IL-8 secretion from H. pylori-infected epithelial cells | in vitro | (116) | ||
| Cucurbita pepo L. | Pumpkin, squash | Aqueous extract of pulp | Anti-ulcer activity by enhancement of gastric adherent mucus in aspirin-induced gastric and duodenal ulcer | in vivo | (117) |
| Cupressus sempervirens L. | Mediterranean cypress | Essential oil | Inhibition of the growth of H. pylori | in vitro | (118) |
| Ethanolic extract of leaves, cupressuflavone | Anti-ulcerogenic activity through enhancement of endogenous antioxidant enzymes, disposal of free radicals and anti-apoptotic activity | in vivo | (119) | ||
| Essential oil | Antimicrobial | in vitro | (120) | ||
| Cydonia oblonga Mill. | Quince | Juice | Diminishing inflammation and ulcer indices in TNBS-induced ulcerative colitis | in vivo | (121) |
| Polyphenol extract of peel | Potent anti-inflammatory effect | in vitro | (122) | ||
| Ethanolic extract of seeds aqueous extract | Anti-E.coli, anti- Enterobacter aerogenes | in vitro, in vivo | (123) | ||
| A fruit preparation | Inhibiting the gastrointestinal content advance, reducing castor oil-induced diarrhea | in vivo | (124) | ||
| Cymbopogon schoenanthus (L.) Spreng. | Camel grass | - | - | - | - |
| Cyperus rotundus L. | Java grass | Decoction of rhizome | Gastric ulcer inhibitory effect | in vivo | (125) |
| Hydro-methanol extract of whole plant | Antinociceptive effect | in vivo | (126) | ||
| Dorema ammoniacum D. Don | Gum ammoniac tree | - | - | - | - |
| Eugenia caryophyllata Thunb. | Clove | Hydro-ethanolic extract of flowers | Anti-H.pylori | In vitro | (127) |
| Essential oil/ eugenol | Protection against gastric ulcer | in vivo | (128) | ||
| Essential oil/ eugenol | Anti-Giardia activity | in vitro | (129) | ||
| Foeniculum vulgare L. | Fennel | Aqueous-ethanol extract of seeds | Suppressing ROS generation in H. pylori-infected gastric epithelial cells | in vitro | (116) |
| aqueous extract of seeds | Anti-ulcerogenic and antioxidant effects | in vivo | (77) | ||
| Glossostemon bruguieri Desf. | Dombeya arabica | - | - | - | - |
| Hordeum vulgare L. | Barley | Seeds | Antiinflammtory | in-vitro, in-vivo | (130, 131) |
| Hyoscyamus niger L. | Henbane | Crude extract of seeds/ β-sitosterol | GI antispasmodic effect through a combination of anticholinergic and Ca2+ antagonist mechanisms. | in-vivo | (132) |
| Iris florentina L. | Iris | - | - | - | - |
| Lawsonia inermis L. | Henna | Aqueous, ethanol and chloroform extract of leaves | Decrease in the volume of gastric acid secretions, free acidity and total acidity and ulcer index in gastric ulcers induced rats. | in vivo | (133) |
| Aqueous extract of leaves | Antibacterial activity | in vitro | (134) | ||
| Linum usitatissimum L. | Linseed | Crude extract of lignans of seeds | Protection and recovery against gastric ulcers | in vivo | (135) |
| Seeds oil and mucilage | Protection against gastric ulcers | in vivo | (136) | ||
| Aqueous-methanol extract of seeds | Antidiarrheal and antispasmodic activities through inhibition of Ca2+ channels | in vivo, Ex vivo | (137) | ||
| Malus domestica Baumg. | Apple | Methanol extract of fruit flesh containing polyphenols | Preventing aspirin-induced gastric injury, counteracting aspirin-induced up-regulation of HB-EGF and COX-2 expression | in vivo | (138) |
| Fruit juice | Antiulcerative activity | in vivo | (139) | ||
| Fruit sauce | Antidiarrheal activity | in vivo | (140) | ||
| Matricaria Chamomila L. | Chamomile | Hydroalcoholic extract of aerial parts | Protective effect against ethanol-induced gastric mucosal lesions by reducing gastric lesions and malondialdehyde and increasing glutathione levels in gastric tissue or whole blood | in vivo | (141) |
| aqueous-methanolic extract of aerial parts | Antidiarrhoeal, antisecretory and antispasmodic activities through K+-channels activation and weak Ca2+ antagonist effect | in vivo | (142) | ||
| aqueous extract of aerial parts | Spasmolytic activity by cAMP- cGMP-phosphodiesterases inhibition | in vitro | (143) | ||
| decoction of aerial parts | Potent antidiarrheal and antioxidant: protection against castor oil-induced diarrhea and intestinal fluid accumulation | in vivo | (144) | ||
| Melilotus officinalis (L.) Lam. | Common melilot | Gel and aqueous extract containing catechin and cinnamic acid | Attenuating acetic acid induced UC antioxidant and anti-inflammatory effects | in vivo | (145) |
| Myristica fragrans Houtt. | Nutmeg | Crude suspension and petroleum ether extract of seeds | Antidiarrheal effect | in vivo | (146) |
| Hydro-ethanolic extract | Anti-H. pylori activity | in vitro | (147) | ||
| Nardostachys jatamansi DC. | Spikenard | - | - | - | - |
| Nymphaea lotus L. | White lotus | Aqueous extract | Protection against gastric ulcer | in vivo | (148) |
| Nymphaea alba L. | White water rose | Ethanol extract of rhizome | Antioxidant and analgesic | in vivo, in vitro | (149) |
| Olea europaea L. | Olive | Olive oil | Preventing the indomethacin-induced gastric damages in rats, enhancing efficacy of indomethacin for reducing carrageenan-induced paw edema, anti-inflammatory effect against paw edema | in vivo | (43) |
| A 30-day period of diets containing olive oil | Attenuating gastric secretory function, suppression of serum gastrin and higher levels of peptide YY. | Patients with gallstones | (44) | ||
| Polar fraction of extra-virgin olive oil | Inhibition of NF-κB driven transcription and nuclear translocation in AGS cells (a model for gastric inflammation) | in vitro | (46) | ||
| Virgin olive oil extracts rich in phenolic compounds especially dialdehydic form of decarboxymethyl ligstroside (Ty-EDA) | Strong anti-H. pylori activity, decrease acid secretion in the GI tract, reduction in the size of peptic ulcers | in vitro | (45) | ||
| Leaves extract | Attenuation of the ethanol-induced gastric lesions, prevention of an increase in gastric lipid peroxidation, prevention of a decrease in antioxidative enzyme activity | in vivo | (150) | ||
| Opopanax chironium W.D.J. Koch | Sweet myrrh | - | - | - | - |
| Phoenix dactylifera L. | Date | Aqueous and ethanolic extracts of fruits | Ameliorative effect on ethanol-induced gastric ulcer | in vivo | (151) |
| Ethanol and water extracts of the flesh and pits | Enhancing the GI transit | in vivo | (152) | ||
| Pimpinella anisum L. | Anise | Aqueous suspension of fruits | Cytoprotective and anti-ulcer activities against experimentally-induced gastric lesions | in vivo | (153) |
| Aqueous and ethanol extracts of fruits | Antioxidant and antimicrobial activities | in vitro | (154) | ||
| Pistacia atlantica Desf. | Persian turpentine tree | Essential oil of oleo-gum-resin | Antimicrobial activity | in vitro | (155) |
| Pistacia atlantica subsp. kurdica | Baneh tree | Oleo-gum-resin, essential oil | Anti-colitis activity | in vivo | (156) |
| Pistacia lentiscus var. Chia | Mastic | Oleo-gum-resin | Improving symptoms in patients with functional dyspepsia | RCT | (34) |
| Oleo-gum-resin | Antibacterial activity against H. pylori | in vivo | (157) | ||
| Oleo-gum-resin total extract/ isomasticadienolic acid | Reducing H. pylori colonization | in vivo | (33) | ||
| Oleo-gum-resin powder | Decreasing histological damage in TNBS-induced colitis, regulating oxidant/ antioxidant balance and modulating inflammation | in vivo | (35) | ||
| Oleo-gum-resin powder | Improving the clinical features of CD and regulating inflammation and antioxidant status | RCT | (36) | ||
| Oleo-gum-resin essential oil | Antibacterial activity against E. coli, Staphylococcus aureus, and Bacillus subtilis | In vitro | (37) | ||
| Portulaca oleracea L. | Purslane | Aqueous and ethanolic extracts | Gastric anti-ulcerogenic effects | in vivo | (158) |
| Punica granatum L. | Pomegranate | Methanol extract of peel | Potent anti-H. pylori | in vitro | (159) |
| Aqueous-methanolic extract of flowers | Gastric anti-ulcerogenic effects | in vivo | (160) | ||
| Ethanolic extract of pericarp: ethyl acetate and n-butanol fractions | Anti-enterohemorrhagic E. coli | in vitro | (161) | ||
| Aqueous extract of peels | Antidiarrheal effects | in vivo | (162) | ||
| Methanol-water extract of flowers and its ellagic acid rich fraction | Attenuation of colonic inflammation in UC, attenuation of histamine, myeloperoxidase and oxidative stress | in vivo | (163) | ||
| Rosa × damascena Mill. | Damask rose | Hydroalcoholic extract of flowers | Inhibition of ileum contraction at mg concentrations, stimulatory effect on ileum at μg concentrations | ex vivo | (164) |
| Flowers essential oil containing geraniol and citronellol | Inhibitory effect on ileum contraction | ex vivo | (165) | ||
| Hydroalcoholic extract of flowers | Improving macroscopic and histopathological parameters of acetic acid-induced colitis | in vivo | (166) | ||
| Rhus coriaria L. | Sumac | Crude methanolic extract | Anti-secretory, antidiarrheal and antispasmodic properties through Ca2+ blockade | in vivo, in vitro | (167) |
| Ethanol extract | Anti-H. pylori activity | in vitro | (168) | ||
| Hydroalcoholic extract of leaves | Analgesic effect | in vivo | (169) | ||
| Santalum album L. | Indian sandalwood | Methanol extract of wood | Anti-diarrhoeal activity | in vivo | (170) |
| Hydro-alcoholic extract | Protection against gastric ulcer | in vivo | (171) | ||
| Methanolic extract of wood | Analgesic and anti-inflammatory activities | in vivo | (172) | ||
| Tanacetum balsamita L. subsp. Balsamitades (Schultz Bip.) Grierson | Costmary | Essential oil | Antimicrobial activity | in vitro | (173) |
| Tragopogon pratensis L. | Meadow salsify | Ethanol extract of aerial part | Antibacterial properties | in vitro | (174) |
| Tragopogon graminifolius | Goatsbeard | Ethanol extract of aerial part | Alleviating colitis via anti-inflammatory effects | in vivo | (175) |
| Hydroalcoholic extract of aerial part | Protection against gastric ulcer | in vivo | (176) | ||
| Trigonella foenum-graecum L. | Fenugreek | Aqueous extract and a gel fraction of seeds | Gastric ulcer protective effects | in vivo | (177) |
| Valeriana celtica L. | Alpine valerian | - | - | - | - |
| Viola odorata L. | Sweet violet | Aqueous extract of aerial parts | Antibacterial effects | in vitro | (178) |
| Cyclotides | Anti-gastrointestinal nematodes | in vitro | (179) | ||
| Hydro-ethanol extract | Strong inhibitor of IL-8 secretion from H. pylori-infected epithelial cells | in vitro | (116) | ||
| Ziziphus spina-christi (L.) Willd. | Christ’s Thorn Jujube | Methanol extract of stem bark | Anti-diarrhoeal effects | in vivo | (180) |
Essential oils from aromatic plants have com-ponents with antibacterial activities. Cinnamaldehyde, thymol analogues, geraniol, menthol and carvacrol are examples of these components which mostly derive from terpenes and terpenoids (52, 53). Topical use of plants containing antibacterial essential oils may reduce bacterial pathogens in GI track especially in the intestines. Interestingly, phenolic monoterpenes and phenylpropanoids (typically showing strong anti-microbial activities) in combination with other components were found to increase the bioactivities of these mixtures which support the application of the combination of herbal oils in TPM (12, 54). It is well-established that the combination of phenolics such as thymol and carvacrol, with monoterpenes alcohols like eugenol produced synergistic effects on several microorganisms. There are some generally accepted mechanisms of antimicrobial interaction that produce synergistic effects. These mechanisms include the sequential inhibition of a common biochemical pathway, inhibition of protective enzymes of microorganisms; and the use of cell wall active agents to enhance the uptake of other antimicrobials (54). Polyphenols have been found to exhibit numerous beneficial activities in the gastrointestinal tract, including antispasmodic, anti-ulcer, anti-secretory, anti-colitis, anti-diarrheal, and anti-oxidative stress properties (55). For instance, flavonoids and other phenolic compounds such as flavone, quercetin and naringenin which are present in many TPM plants have been found to be effective in inhibiting the growth of the microorganisms (56). In addition, a number of polyphenolic compounds including oleuropein, cinnamic acid, baicalein, rutin, quercetin, and tephrosin have been reported to exhibit anti-ulcerogenic activity with a good level of gastric protection (57). Generally, polyphenols possess anti-ulcer activities through improving cytoprotection, re-epithelialization, angiogenesis, and neovascularization which are mediated by the up-regulation of tissue growth factors, PGs, and vWF/ factor VIII complex, together with the down-regulation of anti-angiogenic factors. Moreover, polyphenols have been shown to suppress vascular permeability and leukocyte-endothelium interaction mediated by the down-regulation of cellular and intercellular adhesion agents. Polyphenols can palliate inflammatory responses and down-regulate pro-inflammatory cytokines within mucosal ulcers by inhibiting intracellular signaling pathways of the inflammatory process (ERK, JNK, and MAPK), as well as modulating intracellular transcriptional factors (55). Besides their action as gastroprotectives, flavonoids also can be alternative agents for alleviating peptic ulcers associated with H. pylori (58).
Alkaloids have been also isolated from a number of TPM recommended plants. Isocorydine alkaloid found in some Aquilaria spp. which are used in TPM GI remedies exhibited spasmolytic effects and weak gastric H+/K+-ATPase activity (59). Tropane alkaloids such as atropine and scopolamine which are found in Solanaceae family are used to block the muscarinic activity of acetylcholine showing anti-secretory and antispasmodic effects in the treatment of peptic ulcer, gastroenteritis, and spastic colitis (60). Anthocyanins also possess beneficial activities in the management of many GI disorders such as IBD by alleviating oxidative stress, exhibiting cytoprotective activity, down-regulating the inflammatory cytokines and suppressing cellular signaling pathways of inflammatory responses (61). Gastrointestinal activities of a number of phytochemicals present in TPM plants have been shown in Table 2. As seen in Table 2, several phytochemicals from TPM plants have been found to be effective in GI ailments. β-asarone from Acorus calamus L. (potent anthelmintic, anti-amoebic and antibacterial activities), amygdalin from Amygdalus communis L. var. dulcis (anti-gastric ulcer activity), boswellic acids from Boswellia serrata (gastric ulcer protective effect, protecting the colonic mucosa against tissue injury, and reducing colitis activity), guggulsterone from C. mukul (anti-inflammatory, apoptogenic properties in colon cancer cells), crocin from Crocus sativus L. (inhibiting the growth of colorectal cancer cells), crocetin (ameliorating UC and anti-H. pylori effects), isomasticadienolic acid from P. lentiscus (Reducing H. pylori colonization), and cyclotides from Viola odorata L. (anti-gastrointestinal nematodes) are among the most GI bioactive phytochemicals. Accordingly, above-mentioned compounds are potential active principles with GI tract actions as well as good candidates for future pharmacological and clinical studies and developing new GI protective medicines.
The most emphatic TPM topical GI formulations
Numerous multi-herbal topical formulations are used in TPM for the treatment of GI diseases. Some of these formulations have been frequently mentioned in many TPM textbooks indicating their extensive effectiveness and safety in traditional medicine observations. The following formulations are examples of the most frequently applied topical TPM formulations for the treatment of GI ailments.
A topical preparation containing Valeriana celtica L., mastic oil, aloe sap and verjuice is recommended to apply on stomach area to relieve gastritis and gastric burning and discomfort. As seen in Table 2, some of the ingredients of this remedy have been found to be strongly GI-protective supporting their use in TPM. A poultice consist of barley flour in combination with diverse gastroprotective anti-ulcer plants such as pureed quince, squash, purslane, mastic, sandalwood powder, etc. has also been frequently used to alleviate gastric inflammation, pain and burning (10, 21). An ointment containing Commiphora opobalsamum Engl. oleo-gum-resin, aloe and bees wax is used to relieve symptoms of gastritis (10). Another well-experienced topical prescription for gastric discomfort, nausea and vomiting is a mixture of crushed squash, purslane, barley flour and vinegar (10).
Rubbing a mixture of rose oil and mastic oil on stomach has been frequently recommended for terminating prolonged episodes of hiccups (21). A poultice containing olibanum, mastic gum, agarwood, sweet flag, pomegranate flowers, quince juice and wine is noted in many TPM books for the treatment of poor appetite (10, 21).
An ointment containing guggul gum in mixture with dill and fenugreek seeds, henna leaves, olive oil and rose oil has been used as a potent remedy to alleviate IBD symptoms (10).
The above-mentioned prescriptions along with many other TPM remedies as invaluable sources of experienced traditional knowledge offer new horizons for future studies to find bioactive phytochemicals and develop new phytopharmaceuticals and therapeutic strategies for the treatment of GI diseases.
Conclusion
With around 60 different plant species from 34 families frequently used in hundreds of recipes of TPM for topical application to cure a wide variety of GI ailments, we can conclude that these plants (in simple use or in combination recipes) can be potential alternatives for GI medications. These medications are generally applied in forms of poultices, ointments, bathes and lotions on the stomach area, lower abdomen, lower back and liver to achieve regional and/or systemic delivery of the plant’s biologically active compounds. β-asarone from A. calamus, amygdalin from A. communis L. var. dulcis, boswellic acids from B. serrate, guggulsterone from C. mukul, crocin and crocetin from C. sativus, isomasticadienolic acid from P. lentiscus, and cyclotides from V. odorata are among the most important phytochemicals present in TPM plants with GI protective activities. These phytochemicals along with many other bioactive compounds play pivotal role in alleviating GI disorders through exhibiting numerous activities including anti-spasmodic, anti-ulcer, anti-secretory, anti-colitis, anti-diarrheal, antibacterial, anthelmintic, anti-inflammatory and anti-oxidative stress properties. Several mechanisms underlie these activities including the alleviation of oxidative stress, exhibiting cytoprotective activity, down-regulation of the inflammatory cytokines, suppression of the cellular signaling pathways of inflammatory responses, improving re-epithelialization, angiogene-sis, and neovascularization mediated by the up-regulation of tissue growth factors, PGs, and vWF/ factor VIII complex, together with the down-regulation of anti-angiogenic factors, blocking muscarinic activity of acetylcholine (resulting in antisecretory effects), etc. TPM topical GI remedies commonly contain a combination of herbal powders, oils, oleo-gum-resins and extracts which may have synergistic effects with different mechanisms. Mastic gum, aloe, absinthe and olive oil are the most frequent herbal ingredients of TPM GI recipes. Although pharmacological investigations well support the use of TPM plants, data on topical application of these plants are scarce. Accordingly, there is a need to investigate pharmacological activities, clinical efficacy, pharmacokinetic aspects as well as possible skin reactions and other adverse effects of recommended plants in topical use. In conclusion, TPM topical GI remedies, the mentioned medicinal plants and their active compounds are useful pharmacological tools to discover new active principles with GI tract actions.
References
- 1.Margetts L, Sawyer R. Transdermal drug delivery: principles and opioid therapy. BJA: CEACCP. 2007;7:171–176. [Google Scholar]
- 2.Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. 2015;22:969–987. doi: 10.3109/10717544.2013.879355. [DOI] [PubMed] [Google Scholar]
- 3.Touitou E. Drug delivery across the skin. Expert Opin Biol Ther. 2002;2:723–733. doi: 10.1517/14712598.2.7.723. [DOI] [PubMed] [Google Scholar]
- 4.Weiser JR, Saltzman WM. Controlled release for local delivery of drugs: barriers and models. J Control Release. 2014;190:664–673. doi: 10.1016/j.jconrel.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan Y, Li SM, Mo FK, Zhong DF. Investigation of microemulsion system for transdermal delivery of meloxicam. Int J Pharm. 2006;321:117–123. doi: 10.1016/j.ijpharm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13:175–187. doi: 10.1080/10717540500455975. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan AS, Sridevi S, Chalasani KB, Jain AK, Jain SK, Jain NK, et al. Dendrimer-mediated transdermal delivery: enhanced bioavailability of indomethacin. J Control Release. 2003;90:335–343. doi: 10.1016/s0168-3659(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 9.Emami SA, Sahebkar A, Javadi B. Paresthesia: A review of its definition, etiology and treatments in view of the Traditional Medicine. Curr Pharm Des. 2016;22:321–327. doi: 10.2174/1381612822666151112145348. [DOI] [PubMed] [Google Scholar]
- 10.Ibn Sina A. Al-Qanun fi’l-tibb (Canon of medicine) New Delhi: I.H.M.M.R. Printing Press; 1987. [Google Scholar]
- 11.Al-Kashkari Y. Konnash fi al-Tebb. Tehran: Research Institute for Islamic and Complementary Medicine (RICM); 2007. [Google Scholar]
- 12.Aqili Khorasani MH. Qarabadin-e-Kabir. Tehran: Research Institute for Islamic and Complementary Medicine (RICM); 2007. [Google Scholar]
- 13.Farzaei MH, Rahimi R, Abbasabadi Z, Abdollahi M. An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. Int J Pharm. 2013;9:108–124. [Google Scholar]
- 14.Farzaei MH, Shams-Ardekani MR, Abbasabadi Z, Rahimi R. Scientific evaluation of edible fruits and spices used for the treatment of peptic ulcer in traditional Iranian medicine. ISRN gastroenterol 2013. 2013 doi: 10.1155/2013/136932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soltani A. Dictionary of medicinal plants. Tehran: Arjmand Press; 2004. [Google Scholar]
- 16.Abdol-hamid . Qamus al-qanun fi’l tibb. New Delhi: Jamia Hamdard; 1997. [Google Scholar]
- 17.Bedevian AK. Illustrated polyglottic dictionary of plant names in Latin, Arabic, Armenian, English, French, German, Italian, and Turkish languages. Cairo: Argus & Papazian Presses; 1936. [Google Scholar]
- 18.Hayek M. Encyclopedia of medicinal plants: Arabic-English-French-German-Latin. Bairuūt: Librairie du Liban Publishers; 1992. [Google Scholar]
- 19.Ibn-Beythar . Tafsir kitāb Diyusquiridis (Explanation of Dioscorides’book) Beirut: Dar al-gharb al-islami; 1989. [Google Scholar]
- 20.Tabari A. Ferdows al-hekmah fi al-tibb (Paradise of wisdom on medicine) Berlin: Aftab Press; 1928. [Google Scholar]
- 21.Razi MZ. Al-Hawi fi’l-tibb (Comprehensive book of medicine) Hyderabad: Osmania Oriental Publications Bureau; 1968. [Google Scholar]
- 22.Moraes TM, Kushima H, Moleiro FC, Santos RC, Rocha LR, Marques MO, et al. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: role of prostaglandins and gastric mucus secretion. Chem Biol Interact. 2009;180:499–505. doi: 10.1016/j.cbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Esteves I, Souza IR, Rodrigues M, Cardoso LG, Santos LS, Sertie JA, et al. Gastric antiulcer and anti-inflammatory activities of the essential oil from Casearia sylvestris Sw. J Ethnopharmacol. 2005;101:191–196. doi: 10.1016/j.jep.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Med. 2006;72:311–316. doi: 10.1055/s-2005-916230. [DOI] [PubMed] [Google Scholar]
- 25.Monti D, Chetoni P, Burgalassi S, Najarro M, Saettone MF, Boldrini E. Effect of different terpene-containing essential oils on permeation of estradiol through hairless mouse skin. Int J Pharm. 2002;237:209–214. doi: 10.1016/s0378-5173(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 26.Fox LT, Gerber M, Plessis JD, Hamman JH. Transdermal drug delivery enhancement by compounds of natural origin. Molecules. 2011;16:10507–10540. [Google Scholar]
- 27.Wintola OA, Sunmonu TO, Afolayan AJ. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010;10:95. doi: 10.1186/1471-230X-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langmead L, Makins RJ, Rampton DS. Anti-inflammatory effects of aloe vera gel in human colorectal mucosa in vitro. Aliment Pharmacol Ther. 2004;19:521–527. doi: 10.1111/j.1365-2036.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- 29.Kumari C, Prasad C, Ramulu JS. Determination of in-vitro and in-vivo activities of Aloe vera L. against H. pylori. Int J Pharma Bio Scis. 2010:1. [Google Scholar]
- 30.Keshavarzi Z, Rezapour TM, Vatanchian M, Zare Hesari M, Nabizade Haghighi H, Izanlu M, et al. The effects of aqueous extract of Aloe vera leaves on the gastric acid secretion and brain and intestinal water content following acetic acid- induced gastric ulcer in male rats. Avicenna J Phytomed. 2014;4:137–143. [PMC free article] [PubMed] [Google Scholar]
- 31.Akaberi M, Sobhani Z, Javadi B, Sahebkar A, Emami SA. Therapeutic effects of Aloe spp. in traditional and modern medicine: A review. Biomed Pharmacother. 2016;84:759–772. doi: 10.1016/j.biopha.2016.09.096. [DOI] [PubMed] [Google Scholar]
- 32.Akhawayni A. Hedayat al-mota’allemin fi al-tibb (An educational guide for medical students) Mashhad: Ferdowsi University of Mashhad Publication; 1992. [Google Scholar]
- 33.Paraschos S, Magiatis P, Mitakou S, Petraki K, Kalliaropoulos A, Maragkoudakis P, et al. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob Agents Chemother. 2007;51:551–559. doi: 10.1128/AAC.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabos KJ, Sfika E, Vlatta LJ, Frantzi D, Amygdalos GI, Giannikopoulos G. Is Chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double-blind placebo controlled trial. J Ethnopharmacol. 2010;127:205–209. doi: 10.1016/j.jep.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Gioxari A, Kaliora AC, Papalois A, Agrogiannis G, Triantafillidis JK, Andrikopoulos NK. Pistacia lentiscus resin regulates intestinal damage and inflammation in trinitrobenzene sulfonic acid-induced colitis. J Med Food. 2011;14:1403–1411. doi: 10.1089/jmf.2010.0240. [DOI] [PubMed] [Google Scholar]
- 36.Kaliora AC, Stathopoulou MG, Triantafillidis JK, Dedoussis GV, Andrikopoulos NK. Chios mastic treatment of patients with active Crohn’s disease. World J Gastroenterol. 2007;13:748–753. doi: 10.3748/wjg.v13.i5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koutsoudaki C, Krsek M, Rodger A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus Var. chia. J Agric Food Chem. 2005;53:7681–7685. doi: 10.1021/jf050639s. [DOI] [PubMed] [Google Scholar]
- 38.Shafi N, Khan GA, Ghauri EG. Antiulcer effect of Artemisia absinthium L. in rats. Pak J Sci Ind Res. 2004;47:130–134. [Google Scholar]
- 39.Krebs S, Omer TN, Omer B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease - A controlled clinical trial. Phytomedicine. 2010;17:305–309. doi: 10.1016/j.phymed.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Alanis AD, Calzada F, Cervantes JA, Torres J, Ceballos GM. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2005;100:153–157. doi: 10.1016/j.jep.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Hadi A, Hossein N, Shirin P, Najmeh N, Abolfazl M. Anti-inflammatory and analgesic activities of Artemisia absinthium and chemical composition of its essential oil. Int J Pharm Sci Rev Res. 2014;38:237–244. [Google Scholar]
- 42.Tariq KA, Chishti MZ, Ahmad F, Shawl AS. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet Parasitol. 2009;160:83–88. doi: 10.1016/j.vetpar.2008.10.084. [DOI] [PubMed] [Google Scholar]
- 43.Odabasoglu F, Halici Z, Cakir A, Halici M, Aygun H, Suleyman H, et al. Beneficial effects of vegetable oils (corn, olive and sunflower oils) and alpha-tocopherol on anti-inflammatory and gastrointestinal profiles of indomethacin in rats. Eur J Pharmacol. 2008;591:300–306. doi: 10.1016/j.ejphar.2008.06.075. [DOI] [PubMed] [Google Scholar]
- 44.Serrano P, Yago MD, Manas M, Calpena R, Mataix J, Martinez-Victoria E. Influence of type of dietary fat (olive and sunflower oil) upon gastric acid secretion and release of gastrin, somatostatin, and peptide YY in man. Dig Dis Sci. 1997;42:626–633. doi: 10.1023/a:1018819714756. [DOI] [PubMed] [Google Scholar]
- 45.Romero C, Medina E, Vargas J, Brenes M, De Castro A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J Agric Food Chem. 2007;55:680–686. doi: 10.1021/jf0630217. [DOI] [PubMed] [Google Scholar]
- 46.Sangiovanni E, Colombo E, Fumagalli M, Abbiati F, Caruso D, Dell’Agli M. Inhibition of NF-kappaB activity by minor polar components of extra-virgin olive oil at gastric level. Phytother Res. 2012;26:1569–1571. doi: 10.1002/ptr.4600. [DOI] [PubMed] [Google Scholar]
- 47.Yousefi M, Mahdavi MR, Hosseini SM, Bahrami A, Davati A, Kamalinejad M, et al. Clinical evaluation of Commiphora Mukul a botanical resin, in the management of hemorrhoids: A randomized controlled trial. Pharmacogn Mag. 2013;9:350–356. doi: 10.4103/0973-1296.117832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mencarelli A, Renga B, Palladino G, Distrutti E, Fiorucci S. The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem Pharmacol. 2009;78:1214–1223. doi: 10.1016/j.bcp.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 49.An MJ, Cheon JH, Kim SW, Kim ES, Kim TI, Kim WH. Guggulsterone induces apoptosis in colon cancer cells and inhibits tumor growth in murine colorectal cancer xenografts. Cancer Lett. 2009;279:93–100. doi: 10.1016/j.canlet.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Javadi B, Emami SA. Avicenna’s contribution to mechanisms of cardiovascular drugs. Iran J Basic Med Sci. 2015;18:721–722. [PMC free article] [PubMed] [Google Scholar]
- 51.Javadi B, Sahebkar A, Emami SA. A survey on saffron in major Islamic Traditional Medicine books. Iran J Basic Med Sci. 2013;16:1–11. [PMC free article] [PubMed] [Google Scholar]
- 52.Solorzano-Santos F, Miranda-Novales MG. Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol. 2012;23:136–141. doi: 10.1016/j.copbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Shakeri A, Sahebkar A, Javadi B. Melissa officinalis L. - A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2016;188:204–228. doi: 10.1016/j.jep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Bassole IH, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farzaei MH, Abdollahi M, Rahimi R. Role of dietary polyphenols in the management of peptic ulcer. World J Gastroenterol. 2015;21:6499–6517. doi: 10.3748/wjg.v21.i21.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rauha JP, Remes S, Heinonen M, Hopia A, Kahkonen M, Kujala T, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000;56:3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 57.Sumbul S, Ahmad MA, Mohd A, Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3:361–367. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mota KS, Dias GE, Pinto ME, Luiz-Ferreira A, Souza-Brito AR, Hiruma-Lima CA, et al. Flavonoids with gastroprotective activity. Molecules. 2009;14:979–1012. doi: 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Do Nascimento RF, de Sales IR, de Oliveira Formiga R, Barbosa-Filho JM, Sobral MV, Tavares JF, et al. Activity of alkaloids on peptic ulcer: what’s new? Molecules. 2015;20:929–950. doi: 10.3390/molecules20010929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Sousa Falcao H, Leite JA, Barbosa-Filho JM, de Athayde-Filho PF, de Oliveira Chaves MC, Moura MD, et al. Gastric and duodenal antiulcer activity of alkaloids: a review. Molecules. 2008;13:3198–3223. doi: 10.3390/molecules13123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sodagari HR, Farzaei MH, Bahramsoltani R, Abdolghaffari AH, Mahmoudi M, Rezaei N. Dietary anthocyanins as a complementary medicinal approach for management of inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2015;9:807–820. doi: 10.1586/17474124.2015.1002086. [DOI] [PubMed] [Google Scholar]
- 62.Teichberg S, Wingertzahn MA, Moyse J, Wapnir RA. Effect of gum arabic in an oral rehydration solution on recovery from diarrhea in rats. J Pediatr Gastroenterol Nutr. 1999;29:411–417. doi: 10.1097/00005176-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Gilani AU, Shah AJ, Ahmad M, Shaheen F. Antispasmodic effect of Acorus calamus Linn. is mediated through calcium channel blockade. Phytother Res. 2006;20:1080–1084. doi: 10.1002/ptr.2000. [DOI] [PubMed] [Google Scholar]
- 64.Shoba FG, Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol. 2001;76:73–76. doi: 10.1016/s0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 65.McGaw LJ, Jager AK, van Staden J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J Ethnopharmacol. 2000;72:247–263. doi: 10.1016/s0378-8741(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 66.Rafatullah S, Tariq M, Mossa J, Al-Yahya M, Al-Said M, Ageel A. Anti-secretagogue, anti-ulcer and cytoprotective properties of Acorus calamus in rats. Fitoteraoia. 1994;65:19–19. [Google Scholar]
- 67.Maphosa V, Masika PJ, Bizimenyera ES, Eloff JN. In-vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, Leonotis leonurus and Elephantorrhiza elephantina against Haemonchus contortus. Trop Anim Health Prod. 2010;42:301–307. doi: 10.1007/s11250-009-9421-9. [DOI] [PubMed] [Google Scholar]
- 68.Pandey R, Mishra A. Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens. Appl Biochem Biotechnol. 2010;160:1356–1361. doi: 10.1007/s12010-009-8577-0. [DOI] [PubMed] [Google Scholar]
- 69.Watt K, Christofi N, Young R. The detection of antibacterial actions of whole herb tinctures using luminescent Escherichia coli. Phytother Res. 2007;21:1193–1199. doi: 10.1002/ptr.2238. [DOI] [PubMed] [Google Scholar]
- 70.Zaghlool SS, Shehata BA, Abo-Seif AA, El-Latif HAA. Assessment of protective effects of extracts of Zingiber officinale and Althaea officinalis on pyloric ligation-induced gastric ulcer in experimental animals. UK J Pharm Biosci. 2015:3. [Google Scholar]
- 71.Nabavizadeh F, Alizadeh AM, Sadroleslami Z, Adeli S. Gastroprotective effects of amygdalin on experimental gastric ulcer: Role of NO and TNF. J Med Plants Res. 2011;5:3122–3127. [Google Scholar]
- 72.Kazrani HR, Jalali S. Laxative effect of bitter almond (Amygdalus communis var. amara) Iran J Vet Sci Technol. 2014;6:37–47. [Google Scholar]
- 73.Hosseinzadeh H, Karimi GR, Ameri M. Effects of Anethum graveolens L. seed extracts on experimental gastric irritation models in mice. BMC Pharmacol. 2002;2:21. doi: 10.1186/1471-2210-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rifat-uz-Zaman M, Akhtar M, Khan M. Preliminary eva-luation of Anethum graveolens fruit in indomethacin-ulcer induced rats. J Biol Sci. 2004;4:151–156. [Google Scholar]
- 75.Naseri MG, Heidari A. Antispasmodic effect of Anethum graveolens fruit extract on rat ileum. Int J Pharmacol. 2007;3:260–264. [Google Scholar]
- 76.Kaur GJ, Arora DS. Antibacterial and phytochemical screening of Anethum graveolens Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med. 2009;9:30. doi: 10.1186/1472-6882-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Birdane FM, Cemek M, Birdane YO, Gulcin I, Buyukokuroglu ME. Beneficial effects of Foeniculum vulgare on ethanol-induced acute gastric mucosal injury in rats. World J Gastroenterol. 2007;13:607–611. doi: 10.3748/wjg.v13.i4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rani P, Khullar N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi . Phytother Res. 2004;18:670–673. doi: 10.1002/ptr.1522. [DOI] [PubMed] [Google Scholar]
- 79.Branković S, Gočmanac-Ignjatović M, Kostić M, Veljković M, Miladinović B, Milutinović M, et al. Spasmolytic activity of the aqueous and ethanol celery leaves (Apium graveolens L.) extracts on the contraction of isolated rat ileum. Acta medica Medianae. 2015;54:11–16. [Google Scholar]
- 80.Chitre T, Bhutada P, Nandakumar K, Somani R, Miniyar P, Mundhada Y, et al. Analgesic and anti-inflammatory activity of heartwood of Aquilaria agallocha in laboratory animals. Pharmacol Online. 2007;1:288–298. [Google Scholar]
- 81.Abad MJ, Bedoya LM, Apaza L, Bermejo P. The artemisia L. Genus: a review of bioactive essential oils. Molecules. 2012;17:2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad F, Khan RA, Rasheed S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium . J Islamic Acad Sci. 1992;5:111–114. [Google Scholar]
- 83.Westphal J, Horning M, Leonhardt K. Phytotherapy in functional upper abdominal complaints Results of a clinical study with a preparation of several plants. Phytomedicine. 1996;2:285–291. doi: 10.1016/S0944-7113(96)80070-5. [DOI] [PubMed] [Google Scholar]
- 84.Gupta I, Parihar A, Malhotra P, Gupta S, Ludtke R, Safayhi H, et al. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001;67:391–395. doi: 10.1055/s-2001-15802. [DOI] [PubMed] [Google Scholar]
- 85.Borrelli F, Capasso F, Capasso R, Ascione V, Aviello G, Longo R, et al. Effect of Boswellia serrata on intestinal motility in rodents: inhibition of diarrhoea without constipation. Br J Pharmacol. 2006;148:553–560. doi: 10.1038/sj.bjp.0706740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Johri RK, et al. The gastric ulcer protective effect of boswellic acids, a leukotriene inhibitor from Boswellia serrata in rats. Phytomedicine. 2008;15:408–415. doi: 10.1016/j.phymed.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 87.Krieglstein CF, Anthoni C, Rijcken EJ, Laukotter M, Spiegel HU, Boden SE, et al. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int J Colorectal Dis. 2001;16:88–95. doi: 10.1007/s003840100292. [DOI] [PubMed] [Google Scholar]
- 88.Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Muller S, et al. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1131–1137. doi: 10.1152/ajpgi.00562.2005. [DOI] [PubMed] [Google Scholar]
- 89.Golbabaei S, Bazl R, Golestanian S, Nabati F, Omrany ZB, Yousefi B, et al. Urease inhibitory activities of beta-boswellic acid derivatives. Daru. 2013;21:2. doi: 10.1186/2008-2231-21-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lemos M, Santin JR, Junior LC, Niero R, Andrade SF. Gastroprotective activity of hydroalcoholic extract obtained from the leaves of Brassica oleracea var. acephala DC in different animal models. J Ethnopharmacol. 2011;138:503–507. doi: 10.1016/j.jep.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 91.Mahady GB, Pendland SL, Stoia A, Hamill FA, Fabricant D, Dietz BM, et al. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 92.Hawrelak JA, Cattley T, Myers SP. Essential oils in the treatment of intestinal dysbiosis: A preliminary in vitro study. Altern Med Rev. 2009;14:380–384. [PubMed] [Google Scholar]
- 93.Al-Essa MK, Shafagoj YA, Mohammed FI, Afifi FU. Relaxant effect of ethanol extract of Carum carvi on dispersed intestinal smooth muscle cells of the guinea pig. Pharm Biol. 2010;48:76–80. doi: 10.3109/13880200903046161. [DOI] [PubMed] [Google Scholar]
- 94.Kamaleeswari M, Nalini N. Dose-response efficacy of caraway (Carum carvi L.) on tissue lipid peroxidation and antioxidant profile in rat colon carcinogenesis. J Pharm Pharmacol. 2006;58:1121–1130. doi: 10.1211/jpp.58.8.0014. [DOI] [PubMed] [Google Scholar]
- 95.Khayyal MT, el-Ghazaly MA, Kenawy SA, Seif-el-Nasr M, Mahran LG, Kafafi YA, et al. Antiulcerogenic effect of some gastrointestinally acting plant extracts and their combination. Arzneimittelforschung. 2001;51:545–553. doi: 10.1055/s-0031-1300078. [DOI] [PubMed] [Google Scholar]
- 96.Balaji G, Chalamaiah M, Ramesh B, Reddy YA. Antidiarrhoeal activity of ethanol and aqueous extracts of Carum copticum seeds in experimental rats. Asian Pac J Trop Biomed. 2012;2:S1151–S1155. [Google Scholar]
- 97.Hejazian YS, Dashti RM, Mahdavi SM, Qureshi MA. The effect of Carum Copticum extract on acetylcholine induced contraction in isolated rat’s ileum. J Acupunct Meridian Stud. 2009;2:75–78. doi: 10.1016/S2005-2901(09)60019-4. [DOI] [PubMed] [Google Scholar]
- 98.Komeili G, Solouki S, Maleki S, Saeidi Neek F. Effect of hydroalcholic extract of Carum copticum seed on the treatment of peptic ulcer induced by ibuprofen in rats. Horiz Med Sci. 2012;18:12–16. [Google Scholar]
- 99.Nariman F, Eftekhar F, Habibi Z, Massarrat S, Malekzadeh R. Antibacterial activity of twenty Iranian plant extracts against clinical isolates of Helicobacter pylori. Iran J Basic Med Sci. 2009;12:105–111. [Google Scholar]
- 100.Jainu M, Devi CS. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chem Biol Interact. 2006;161:262–270. doi: 10.1016/j.cbi.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Jainu M, Vijai Mohan K, Shyamala Devi CS. Gastroprotective effect of Cissus quadrangularis extract in rats with experimentally induced ulcer. Indian J Med Res. 2006;123:799–806. [PubMed] [Google Scholar]
- 102.Jainu M, Shyamala Devi CS. Attenuation of neutrophil infiltration and proinflammatory cytokines by Cissus quadrangularis: a possible prevention against gastric ulcerogenesis. J Herb Pharmacother. 2005;5:33–42. [PubMed] [Google Scholar]
- 103.Jainu M, Devi CS. Effect of Cissus quadrangularis on gastric mucosal defensive factors in experimentally induced gastric ulcer-a comparative study with sucralfate. J Med Food. 2004;7:372–376. doi: 10.1089/jmf.2004.7.372. [DOI] [PubMed] [Google Scholar]
- 104.Austin A, Jegadeesan M, Gowrishankar R. In-vitro screening of cissus quadrangularis L. Variant ii against Helicobacter pylori. Anc Sci Life. 2003;23:55–60. [PMC free article] [PubMed] [Google Scholar]
- 105.Attaguile G, Caruso A, Pennisi G, Savoca F. Gastroprotective effect of aqueous extract of Cistus incanus L. in rats. Pharmacol Res. 1995;31:29–32. doi: 10.1016/1043-6618(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 106.Aziz M, Tab N, Karim A, Mekhfi H, Bnouham M, Ziyyat A, et al. Relaxant effect of aqueous extract of Cistus ladaniferus on rodent intestinal contractions. Fitoterapia. 2006;77:425–428. doi: 10.1016/j.fitote.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 107.Aziz M, Karim A, Tab N, Mekhfi H, Bnouham M, Ziyyat A, et al. Antidiarrhoeal activity of Cistus ladaniferus aqueous extract. Spatula DD. 2011;1:175–179. [Google Scholar]
- 108.Kim ES, Hong SY, Lee HK, Kim SW, An MJ, Kim TI, et al. Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol Rep. 2008;20:1321–1327. [PubMed] [Google Scholar]
- 109.Al-Howiriny T, Al-Sohaibani M, Al-Said M, Al-Yahya M, El-Tahir K, Rafatullah S. Effect of Commiphora opobalsamum (L.) Engl. (Balessan) on experimental gastric ulcers and secretion in rats. J Ethnopharmacol. 2005;98:287–294. doi: 10.1016/j.jep.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 110.Al-Salmi AA, Sattar MA, Khan LM, Al-Harthi SE. Comparative study of analgesic and anti-inflammatory effects of Commiphora opobalsamum with diclofenac in rodents. Afr J Pharm Pharmacol. 2015;9:806–817. [Google Scholar]
- 111.Al-Massarany SM, Abbas FA, Demirci B, Baser KH, Khan SI, Al-Rehaily AJ, et al. Chemical composition and biological evaluation of the essential oil of Commiphora opobalsamum L. J Herbs Spices Med Plants. 2008;13:111–121. [Google Scholar]
- 112.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29:175–180. [PMC free article] [PubMed] [Google Scholar]
- 113.Nakhaei M, Khaje-Karamoddin M, Ramezani M. Inhibition of Helicobacter pylori growth in vitro by saffron (Crocus sativus L.) Iran J Basic Med Sci. 2008;11:91–96. [Google Scholar]
- 114.Kazi HA, Qian Z. Crocetin reduces TNBS-induced experimental colitis in mice by downregulation of NFkB. Saudi J Gastroenterol. 2009;15:181–187. doi: 10.4103/1319-3767.54750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bathaie SZ, Miri H, Mohagheghi MA, Mokhtari-Dizaji M, Shahbazfar AA, Hasanzadeh H. Saffron aqueous extract inhibits the chemically-induced gastric cancer progression in the Wistar Albino rat. Iran J Basic Med Sci. 2013;16:27–38. [PMC free article] [PubMed] [Google Scholar]
- 116.Zaidi SF, Muhammad JS, Shahryar S, Usmanghani K, Gilani AH, Jafri W, et al. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J Ethnopharmacol. 2012;141:403–410. doi: 10.1016/j.jep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 117.Sarkar S, Buha D. Effect of ripe fruit pulp extract of Cucurbita pepo Linn. in aspirin induced gastric and duodenal ulcer in rats. Indian J Exp Biol. 2008;46:639–645. [PubMed] [Google Scholar]
- 118.Ohno T, Kita M, Yamaoka Y, Imamura S, Yamamoto T, Mitsufuji S, et al. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8:207–215. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 119.Koriem KM, Gad IB, Nasiry ZK. Protective effect of Cupressus sempervirens extract against indomethacin-induced gastric ulcer in rats. Interdiscip Toxicol. 2015;8:25–34. doi: 10.1515/intox-2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mazari K, Bendimerad N, Bekhechi C, Fernandez X. Chemical composition and antimicrobial activity of essential oils isolated from Algerian Juniperus phoenicea L. and Cupressus sempervirens L. J Med Plants Res. 2010;4:959–964. [Google Scholar]
- 121.Minaiyan M, Ghannadi A, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2012;7:103–110. [PMC free article] [PubMed] [Google Scholar]
- 122.Essafi-Benkhadir K, Refai A, Riahi I, Fattouch S, Karoui H, Essafi M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-kappaB, p38MAPK and Akt inhibition. Biochem Biophys Res Commun. 2012;418:180–185. doi: 10.1016/j.bbrc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 123.Alizadeh H, Rahnema M, Semnani SN, Hajizadeh N. Detection of compounds and antibacterial effect of quince (Cydonia oblonga Miller) extracts in vitro and in vivo. J Biolog Active Prod Nat. 2013;3:303–309. [Google Scholar]
- 124.Romero M, Dávalos H, Astudillo-Vázquez A. Gastrointestinal activity of the fruit of Cydonia oblonga Miller. Rev Latinoam Quím. 2009;37:115–121. [Google Scholar]
- 125.Zhu M, Luk H, Fung H, Luk C. Cytoprotective effects of Cyperus rotundus against ethanol induced gastric ulceration in rats. Phytother res. 1997;11:392–394. [Google Scholar]
- 126.Imam MZ, Sumi CD. Evaluation of antinociceptive activity of hydromethanol extract of Cyperus rotundus in mice. BMC Complement Altern Med. 2014;14:83. doi: 10.1186/1472-6882-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y, Xu C, Zhang Q, Liu JY, Tan RX. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol. 2005;98:329–333. doi: 10.1016/j.jep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 128.Santin JR, Lemos M, Klein-Junior LC, Machado ID, Costa P, de Oliveira AP, et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:149–158. doi: 10.1007/s00210-010-0582-x. [DOI] [PubMed] [Google Scholar]
- 129.Machado M, Dinis AM, Salgueiro L, Custodio JB, Cavaleiro C, Sousa MC. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Exp Parasitol. 2011;127:732–739. doi: 10.1016/j.exppara.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 130.Choi KC, Hwang JM, Bang SJ, Son YO, Kim BT, Kim DH, et al. Methanol extract of the aerial parts of barley (Hordeum vulgare) suppresses lipopolysaccharide-induced inflammatory responses in vitro and in vivo. Pharm Biol. 2013;51:1066–1076. doi: 10.3109/13880209.2013.768274. [DOI] [PubMed] [Google Scholar]
- 131.Gul S, Ahmed S, Kifli N, Uddin QT, Batool Tahir N, Hussain A, et al. Multiple pathways are responsible for anti-inflammatory and cardiovascular activities of Hordeum vulgare L. J Transl Med. 2014;12:316. doi: 10.1186/s12967-014-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gilani AH, Khan AU, Raoof M, Ghayur MN, Siddiqui BS, Vohra W, et al. Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca2+channels. Fundam Clin Pharmacol. 2008;22:87–99. doi: 10.1111/j.1472-8206.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 133.Goswami M, Kulshreshtha M, Rao CV, Yadav S, Yadav S. Anti-ulcer potential of Lawsonia inermis L. Leaves against gastric ulcers in rats. J appl pharm sci. 2011;1:69. [Google Scholar]
- 134.Sudharameshwari K, Radhika J. Antibacterial screening of Aegle marmelos Lawsonia inermis and Albizzia libbeck. Afr J Tradit Complement Altern Med. 2006;4:199–204. [PMC free article] [PubMed] [Google Scholar]
- 135.Joshi S, Mandawgade S, Mehta V, Sathaye S. Antiulcer effect of mammalian lignan precursors from flaxseed. Pharm Biol. 2008;46:329–332. [Google Scholar]
- 136.Dugani A, Auzzi A, Naas F, Megwez S. Effects of the oil and mucilage from flaxseed (linum usitatissimum) on gastric lesions induced by ethanol in rats. Libyan J Med. 2008;3:166–169. doi: 10.4176/080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Palla AH, Khan NA, Bashir S, Ur-Rehman N, Iqbal J, Gilani AH. Pharmacological basis for the medicinal use of Linum usitatissimum (Flaxseed) in infectious and non-infectious diarrhea. J Ethnopharmacol. 2015;160:61–68. doi: 10.1016/j.jep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 138.D’Argenio G, Mazzone G, Tuccillo C, Grandone I, Gravina AG, Graziani G, et al. Apple polyphenol extracts prevent aspirin-induced damage to the rat gastric mucosa. Br J Nutr. 2008;100:1228–1236. doi: 10.1017/S0007114508988747. [DOI] [PubMed] [Google Scholar]
- 139.Hamauzu Y, Irie M, Kondo M, Fujita T. Antiulcerative properties of crude polyphenols and juice of apple, and Chinese quince extracts. Food Chem. 2008;108:488–495. doi: 10.1016/j.foodchem.2007.10.084. [DOI] [PubMed] [Google Scholar]
- 140.Kertesz Z, Walker MS, McCay C. The effect of feeding apple sauce on induced diarrhea in rats. Am J Dig Dis. 1941;8:124–128. [Google Scholar]
- 141.Cemek M, Yilmaz E, Buyukokuroglu ME. Protective effect of Matricaria chamomilla on ethanol-induced acute gastric mucosal injury in rats. Pharm Biol. 2010;48:757–763. doi: 10.3109/13880200903296147. [DOI] [PubMed] [Google Scholar]
- 142.Mehmood MH, Munir S, Khalid UA, Asrar M, Gilani AH. Antidiarrhoeal, antisecretory and antispasmodic activities of Matricaria chamomilla are mediated predominantly through K(+)-channels activation. BMC Complement Altern Med. 2015;15:75. doi: 10.1186/s12906-015-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maschi O, Cero ED, Galli GV, Caruso D, Bosisio E, Dell’Agli M. Inhibition of human cAMP-phosphodiesterase as a mechanism of the spasmolytic effect of Matricaria recutita L. J Agric Food Chem. 2008;56:5015–5020. doi: 10.1021/jf800051n. [DOI] [PubMed] [Google Scholar]
- 144.Sebai H, Jabri MA, Souli A, Rtibi K, Selmi S, Tebourbi O, et al. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J Ethnopharmacol. 2014;152:327–332. doi: 10.1016/j.jep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 145.Safarpour AR, Kaviyani F, Sepehrimanesh M, Ahmadi N, Hosseinabadi OK, Tanideh N, et al. Antioxidant and anti-inflammatory effects of gel and aqueous extract of Melilotus officinalis L. in induced ulcerative colitis: A Rattus norvegicus model. Ann Colorectal Res. 2015:3. [Google Scholar]
- 146.Grover JK, Khandkar S, Vats V, Dhunnoo Y, Das D. Pharmacological studies on Myristica fragrans--antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24:675–680. doi: 10.1358/mf.2002.24.10.802317. [DOI] [PubMed] [Google Scholar]
- 147.Zaidi SF, Yamada K, Kadowaki M, Usmanghani K, Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori . J Ethnopharmacol. 2009;121:286–291. doi: 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 148.John-Africa L, Idris-Usman MS, Adzu B, Gamaniel KS. Protective effects of the aqueous extract of Nymphaea lotus L. (Nymphaeaceae) against ethanol-induced gastric ulcers. Int J Biol Chem Scs. 2013;6:1917–1925. [Google Scholar]
- 149.Bose A, Ray SD, Sahoo M. Evaluation of analgesic and antioxidant potential of ethanolic extract of Nymphaea alba rhizome. Oxid Antioxid Med Sci. 2012;1:217–223. [Google Scholar]
- 150.Dekanski D, Ristić S, Mitrović D. Antioxidant effect of dry olive (Olea europaea L.) leaf extract on ethanol-induced gastric lesions in rats. Med J Nutrition Metab. 2009;2:205–211. [Google Scholar]
- 151.Al-Qarawi AA, Abdel-Rahman H, Ali BH, Mousa HM, El-Mougy SA. The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. J Ethnopharmacol. 2005;98:313–317. doi: 10.1016/j.jep.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 152.Al-Qarawi A, Ali B, Al-Mougy S, Mousa H. Gastrointestinal transit in mice treated with various extracts of date (Phoenix dactylifera L.) Food Chem Toxicol. 2003;41:37–39. doi: 10.1016/s0278-6915(02)00203-x. [DOI] [PubMed] [Google Scholar]
- 153.Al Mofleh IA, Alhaider AA, Mossa JS, Al-Soohaibani MO, Rafatullah S. Aqueous suspension of anise “Pimpinella anisum” protects rats against chemically induced gastric ulcers. World J Gastroenterol. 2007;13:1112–1118. doi: 10.3748/wjg.v13.i7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gülçın İ, Oktay M, Kıreçcı E, Küfrevıoǧlu Öİ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food chem. 2003;83:371–382. [Google Scholar]
- 155.Ghalem B, Mohamed B. Essential oil from gum of Pistacia atlantica Desf.: screening of antimicrobial activity. Afr J Pharm Pharmacol. 2009;3:13–15. [Google Scholar]
- 156.Minaiyan M, Karimi F, Ghannadi A. Anti-inflammatory effect of Pistacia atlantica subsp. kurdica volatile oil and gum on acetic acid-induced acute colitis in rat. Res J Pharmacogn. 2015;2:1–12. [Google Scholar]
- 157.Dabos KJ, Sfika E, Vlatta LJ, Giannikopoulos G. The effect of mastic gum on Helicobacter pylori: a randomized pilot study. Phytomedicine. 2010;17:296–299. doi: 10.1016/j.phymed.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 158.Karimi G, Hosseinzadeh H, Ettehad N. Evaluation of the gastric antiulcerogenic effects of Portulaca oleracea L. extracts in mice. Phytother Res. 2004;18:484–487. doi: 10.1002/ptr.1463. [DOI] [PubMed] [Google Scholar]
- 159.Hajimahmoodi M, Shams-Ardakani M, Saniee P, Siavoshi F, Mehrabani M, Hosseinzadeh H, et al. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat Prod Res. 2011;25:1059–1066. doi: 10.1080/14786419.2010.501763. [DOI] [PubMed] [Google Scholar]
- 160.Alam MS, Alam MA, Ahmad S, Najmi AK, Asif M, Jahangir T. Protective effects of Punica granatum in experimentally-induced gastric ulcers. Toxicol Mech Methods. 2010;20:572–578. doi: 10.3109/15376516.2010.508079. [DOI] [PubMed] [Google Scholar]
- 161.Voravuthikunchai SP, Sririrak T, Limsuwan S, Supawita T, Iida T, Honda T. Inhibitory effects of active compounds from Punica granatum pericarp on verocytotoxin production by enterohemorrhagic Escherichia coli O157: H7. J Health Sci. 2005;51:590–596. [Google Scholar]
- 162.Qnais E, Elokda A, Abu Ghalyun Y, Abdulla F. Antidiarrheal Activity of the Aqueous extract of Punica granatum.(Pomegranate) peels. Pharm Biol. 2007;45:715–720. [Google Scholar]
- 163.Singh K, Jaggi AS, Singh N. Exploring the ameliorative potential of Punica granatum in dextran sulfate sodium induced ulcerative colitis in mice. Phytother Res. 2009;23:1565–1574. doi: 10.1002/ptr.2822. [DOI] [PubMed] [Google Scholar]
- 164.Sadraei H, Asghari G, Emami S. Effect of Rosa damascena Mill. flower extract on rat ileum. Res Pharm Sci. 2013;8:277–284. [PMC free article] [PubMed] [Google Scholar]
- 165.Sadraei H, Asghari G, Emami S. Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Res Pharm Sci. 2013;8:17–23. [PMC free article] [PubMed] [Google Scholar]
- 166.Latifi G, Ghannadi A, Minaiyan M. Anti-inflammatory effect of volatile oil and hydroalcoholic extract of Rosa damascena Mill. on acetic acid-induced colitis in rats. Res Pharm Sci. 2015;10:514–522. [PMC free article] [PubMed] [Google Scholar]
- 167.Janbaz KH, Shabbir A, Mehmood MH, Gilani AH. Pharmacological basis for the medicinal use of Rhus coriaria in hyperactive gut disorders. Bangladesh J. Pharmacol. 2014;9:636–644. [Google Scholar]
- 168.Motaharinia Y, Hazhir MS, Rezaee MA, Vahedi S, Rashidi A, Hosseini W, et al. Comparison of in vitro antimicrobial effect of ethanol extracts of Satureja khuzestanica Rhus coriaria and Ocimum basilicum L. on Helicobacter pylori. J Med Plants Res. 2012;6:3749–3753. [Google Scholar]
- 169.Mohammadi S, Zarei M, Zarei MM, Salehi I. Effect of hydroalcoholic leaves extract of Rhus Coriaria on pain in male rats. Anesth Pain Med. 2016;6:e32128. doi: 10.5812/aapm.32128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guo H, Zhang J, Gao W, Qu Z, Liu C. Anti-diarrhoeal activity of methanol extract of Santalum album L. in mice and gastrointestinal effect on the contraction of isolated jejunum in rats. J Ethnopharmacol. 2014;154:704–710. doi: 10.1016/j.jep.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 171.Ahmed N, Khan M, Jais AM, Mohtarrudin N, Ranjbar M, Amjad MS, et al. Antiulcer activity of sandalwood (Santalum album L.) stem hydroalcoholic extract in three gastric-ulceration models of wistar rats. Bol Latinoam Caribe Plant Med. 2013;12:81–91. [Google Scholar]
- 172.Saneja A, Kaushik P, Kaushik D, Kumar S, Kumar D. Antioxidant, analgesic and anti-inflammatory activities of Santalum album Linn. Planta Med. 2009;75:102. [Google Scholar]
- 173.Yousefzadi M, Ebrahimi SN, Sonboli A, Miraghasi F, Ghiasi S, Arman M, et al. Cytotoxicity, antimicrobial activity and composition of essential oil from Tanacetum balsamita L. subsp balsamita. Nat Prod Commun. 2009;4:119–122. [PubMed] [Google Scholar]
- 174.Jahani S, Saeidi S, Javadian F, Akbarizadeh Z, Sobhanizade A. Investigating the antibacterial effects of plant extracts on Pseudomonas aeruginosa and Escherichia coli. Int J Infect. 2016:3. [Google Scholar]
- 175.Farzaei MH, Ghasemi-Niri SF, Abdolghafari AH, Baeeri M, Khanavi M, Navaei-Nigjeh M, et al. Biochemical and histopathological evidence on the beneficial effects of Tragopogon graminifolius in TNBS-induced colitis. Pharm Biol. 2015;53:429–436. doi: 10.3109/13880209.2014.923004. [DOI] [PubMed] [Google Scholar]
- 176.Farzaei MH, Khazaei M, Abbasabadei Z, Feyzmahdavi M, Mohseni GR. Protective effect of Tragopogon Graminifolius DC against ethanol induced gastric ulcer. Iran Red Crescent Med J. 2013;15:813–816. doi: 10.5812/ircmj.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pandian RS, Anuradha CV, Viswanathan P. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Ethnopharmacol. 2002;81:393–397. doi: 10.1016/s0378-8741(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 178.Arora DS, Kaur GJ. Antibacterial activity of some Indian medicinal plants. J Nat Med. 2007;61:313–317. [Google Scholar]
- 179.Colgrave ML, Kotze AC, Kopp S, McCarthy JS, Coleman GT, Craik DJ. Anthelmintic activity of cyclotides: In vitro studies with canine and human hookworms. Acta Trop. 2009;109:163–166. doi: 10.1016/j.actatropica.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 180.Adzu B, Amos S, Amizan MB, Gamaniel K. Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Trop. 2003;87:245–250. doi: 10.1016/s0001-706x(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 181.Adzu B, Amos S, Wambebe C, Gamaniel K. Antinociceptive activity of Zizyphus spina-christi root bark extract. Fitoterapia. 2001;72:344–350. doi: 10.1016/s0367-326x(00)00289-6. [DOI] [PubMed] [Google Scholar]


