Abstract
Background
Left ventricular assist devices (LVADs) have been used as an effective therapeutic option in patients with advanced heart failure, either as a bridge to transplantation, as destination therapy, or in some patients, as a bridge to recovery.
Objectives
This study evaluated whether patients undergoing an LVAD bridge-to-recovery protocol can achieve cardiac and physical functional capacities equivalent to those of healthy controls.
Methods
Fifty-eight male patients—18 implanted with a continuous-flow LVAD, 16 patients with LVAD explanted (recovered patients), and 24 heart transplant candidates (HTx)—and 97 healthy controls performed a maximal graded cardiopulmonary exercise test with continuous measurements of respiratory gas exchange and noninvasive (rebreathing) hemodynamic data. Cardiac function was represented by peak exercise cardiac power output (mean arterial blood pressure × cardiac output) and functional capacity by peak exercise O2 consumption.
Results
All patients demonstrated a significant exertional effort as demonstrated with the mean peak exercise respiratory exchange ratio >1.10. Peak exercise cardiac power output was significantly higher in healthy controls and explanted LVAD patients compared with other patients (healthy 5.35 ± 0.95 W; explanted 3.45 ± 0.72 W; LVAD implanted 2.37 ± 0.68 W; and HTx 1.31 ± 0.31 W; p < 0.05), as was peak O2 consumption (healthy 36.4 ± 10.3 ml/kg/min; explanted 29.8 ± 5.9 ml/kg/min; implanted 20.5 ± 4.3 ml/kg/min; and HTx 12.0 ± 2.2 ml/kg/min; p < 0.05). In the LVAD explanted group, 38% of the patients achieved peak cardiac power output and 69% achieved peak O2 consumption within the ranges of healthy controls.
Conclusions
The authors have shown that a substantial number of patients who recovered sufficiently to allow explantation of their LVAD can even achieve cardiac and physical functional capacities nearly equivalent to those of healthy controls.
Key Words: cardiac power, exercise capacity, heart transplant, LVAD, recovery
Abbreviations and Acronyms: HTx, heart transplant candidates; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction
Central Illustration
Heart transplantation is the gold standard treatment for patients with advanced heart failure resistant to medical therapy. Because demand exceeds the availability of donor hearts, the use of mechanical circulatory support and left ventricular assist devices (LVADs) has emerged as an alternative form of therapy for such patients (1). LVADs have been used as an effective therapeutic option in patients with advanced heart failure, either as a bridge to transplantation, as destination therapy, or in some patients, as a bridge to recovery 2, 3. Improvement in myocyte histology and biochemistry, as well as in left ventricular anatomy, physiology, and hemodynamics, has been observed in patients with advanced heart failure receiving prolonged mechanical circulatory support 4, 5. It has also been demonstrated that the use of LVADs improves the rate of survival, quality of life, and functional capacity in patients with advanced heart failure 6, 7, 8, 9, 10. LVADs provide cardiac function, not only at rest, but also during exercise in patients with severe heart failure 11, 12, 13, 14.
The improvement in cardiac function of implanted LVAD patients may be such that the pump could be removed and transplantation avoided with good long-term outcomes 15, 16, 17, 18, 19. Sufficient recovery to allow device explantation has been observed in only 5% to 24% of patients 19, 20, 21, 22, 23. However, a strategy of combining mechanical and pharmacological therapy may enhance further myocardial recovery and allow the device to be safely explanted in a larger proportion of LVAD implanted patients 24, 25, 26, 27.
The explanted LVAD patients demonstrate improved quality of life, cardiac performance, and exercise performance several years following device removal 3, 12, 18, 28. However, to what extent the explanted LVAD patients can restore their cardiac and physical function remains unknown. Therefore, in the present study, we tested the hypothesis that patients whose LVAD was explanted due to myocardial recovery using such protocols can achieve cardiac and physical functional capacities equivalent to those of healthy controls.
Methods
Study design
This was a cross-sectional, observational study in which cardiac and respiratory measurements at rest and in response to maximal graded cardiopulmonary exercise testing were compared among the 4 groups: 1) heart transplant candidates; 2) LVAD implanted patients (on LVAD support); 3) LVAD explanted patients; and 4) healthy controls. The LVAD implanted and LVAD explanted groups were consecutive patients subject to our previous investigations 12, 18, 26, 27.
Study participants
The study population consisted of 58 male patients—16 LVAD explanted (recovered) patients, 18 LVAD implanted, and 24 heart transplant candidates (HTx)—and 97 healthy controls with no known cardiovascular diseases. Exclusion criteria included inability to perform treadmill exercise tests, inability to exercise beyond anaerobic threshold, symptomatic angina limiting exercise, and unwillingness to provide a consent form.
The majority of LVAD explanted patients (87%) were in New York Heart Association (NYHA) functional class I, and 13% were in NYHA functional class II. Left ventricular ejection fraction (LVEF) ranged from 50% to 72%. The mean period of LVAD support was 396 days, with a range of 22 to 638 days. The explantation was considered if the following criteria were met while the LVAD was off for 15 min: a left ventricular end-diastolic diameter of <60 mm; a left ventricular end-systolic diameter of <50 mm and a LVEF of >45%; a left ventricular end-diastolic pressure (or pulmonary-capillary wedge pressure) of <12 mm Hg; a resting cardiac index of >2.8 l/min/m2 of body surface area; and a maximal oxygen consumption with exercise of >16 ml/kg/min and an increase in minute ventilation relative to the carbon dioxide production (ventilatory response) of <34 (26). Patients in the present study were tested at an average of 3.3 ± 1.1 years (range 0.3 to 5.8 years) following device explantation. All explanted LVAD patients completed 2 stages of a pharmacological regimen suggested by Birks et al. 26, 27. In the first stage (intended to enhance reverse remodeling), treatment with 4 drugs was initiated immediately after the patient had been weaned from inotropic therapy with adequate end-organ recovery. The 4 drugs and the maximum titrated doses were as follows: lisinopril 40 mg daily; carvedilol 50 mg twice daily; spironolactone 25 mg daily; and losartan 100 mg daily. The second stage of pharmacological therapy was instituted after maximal regression in the left ventricular end-diastolic diameter had been achieved while the LVAD was in place. When a constant left ventricular size had been maintained for at least 2 weeks, according to echocardiographic assessment, clenbuterol was administered at an initial dose of 40 μg twice daily, then at a dose of 40 μg 3 times daily, and finally at a dose of 700 μg 3 times daily. The dose was adjusted to maintain the resting heart rate at a level below 100 beats/min. Before clenbuterol was started, carvedilol was replaced by the selective β1-blocker bisoprolol (26). In addition patients were advised to increase their physical activity by walking >30 min per day.
All LVAD patients implanted were with a continuous blood flow LVAD. Fifteen patients had a HeartMate II (Thoratec, Pleasanton, California) and 3 patients had a HeartWare (HeartWare Limited, Sydney, Australia) device. All LVAD patients had dilated cardiomyopathy. LVEF (measured on pump) ranged from 45% to 68%. Time since device implantation averaged 219 ± 87 days, with a range of 62 to 388 days. The indication for insertion of the LVAD was the development of severe heart failure that was not responsive to intensive medical treatment, including inotropic support, with evidence of impending or actual multiorgan failure due to low cardiac output (26).
The heart transplant candidates were patients placed on the transplant waiting lists and their clinical characteristics are presented in Table 1.
Table 1.
Clinical Characteristics of Studied Population
| Heart Transplant Candidates (n = 24) | LVAD Implanted (n = 18) | LVAD Explanted (n = 16) | Healthy Controls (n = 97) | |
|---|---|---|---|---|
| Male/female | 24/0 | 18/0 | 16/0 | 97/0 |
| Age, yrs | 46.5 ± 12.5 | 39 ± 14 | 41 ± 14 | 43 ± 18 |
| Height, m | 1.76 ± 0.08 | 1.78 ± 0.06 | 1.79 ± 0.07 | 1.77 ± 0.07 |
| Body weight, kg | 78.9 ± 11.5 | 79.3 ± 14.4 | 85.4 ± 13.2 | 81.1 ± 1.06 |
| Body mass index, kg/m2 | 25.6 ± 3.8 | 25.1 ± 4.1 | 26.7 ± 4 | 26.0 ± 3.1 |
| Body surface area, m2 | 1.94 ± 0.16 | 1.96 ± 0.17 | 2.04 ± 0.17 | 1.98 ± 0.15 |
| Left ventricular ejection fraction, % | 19.8 ± 8.3∗ | 50 ± 08 | 58 ± 14 | 63 ± 12 |
| Etiology of heart failure | ||||
| Idiopathic dilated cardiomyopathy | 16 | 16 | 15 | — |
| Coronary heart disease | 8 | 2 | 1 | — |
| Medical therapy | ||||
| Diuretic agents | 24 (100) | 16 (89) | — | — |
| Aldosterone antagonists | 17 (71) | 15 (83) | 10 (63) | — |
| ACE inhibitors | 9 (38) | 13 (72) | 12 (75) | — |
| Angiotensin II antagonists | 12 (50) | 3 (17) | 6 (38) | — |
| Beta-blockers | 16 (67) | 15 (83) | 13 (81) | — |
| Digoxin | 15 (63) | 11 (61) | 13 (81) | — |
| Antiarrhythmic agents | 5 (21) | 4 (22) | 6 (38) | — |
Values are n, mean ± SD, or n (%).
ACE = angiotensin-converting enzyme; LVAD = left ventricular assist device.
p < 0.01 transplants vs. LVAD implanted, LVAD explanted, and controls.
The ethics committee of the Royal Brompton and Harefield Foundation Trust approved the study, and all procedures were in accordance to the Declaration of Helsinki. All patients who participated in the study provided written informed consent.
Resting and peak exercise measurements
Upon arrival in the transplantation exercise laboratory, each study participant’s weight and height were measured. Electrocardiographic electrodes were attached according to the standard lead configuration for exercise testing, and electrocardiographic cables were connected. The patient then sat on a chair, and after a 5-min resting period, arterial blood pressure was assessed from the brachial artery by cuff sphygmomanometry. This was followed by measurement of cardiac output in a seated position using the inert gas rebreathing method (Innocor A/S, Innovision, Odense, Denmark) as we have previously described 11, 12, 29, 30. Briefly, the Innocor device employs a rebreathing system that uses an oxygen-enriched mixture of an inert soluble gas (0.5% nitrous oxide) and an inert insoluble gas (0.1% sulfur hexafluoride) from a 4-l pre-filled anesthesia bag. Photoacoustic analyzers measure gas concentrations over a 5-breath interval. Nitrous oxide concentration decreases during the rebreathing maneuver at a rate proportional to pulmonary blood flow, allowing estimation of cardiac output. Three to 4 respiratory cycles are needed to obtain a value for nitrous oxide washout. After cardiac output measurement at rest, each patient was connected to the metabolic cart using a facemask. Oxygen consumption, carbon dioxide production, and minute ventilation were measured. After 3 min of metabolic measurements at rest, heart transplant and LVAD implanted patients performed a modified Bruce protocol, and LVAD explanted patients and healthy controls performed the Bruce protocol. Continuous breath-by-breath sampling of respiratory gases and heart rate measurements were undertaken. During the last 30 s of each exercise stage, arterial blood pressure was measured, and Borg scale recordings for dyspnea and fatigue were performed. Patients were instructed to give an approximately 1-min warning before they felt they would end the exercise so that a final cardiac output rebreathing measurement was obtained at the peak of exercise. This is because the Innocor device takes approximately 30 s to boot up followed by 15 s to make the measurement. The patient continued to exercise through the measurement, and cool down was started after it was complete. At the same time during the rebreathing maneuver, peak arterial blood pressure was measured.
Calculations and statistical analyses
The cardiac power output was calculated from the product of cardiac output and mean arterial pressure using the following equation (12): CPO = (QT × MAP) × K, where CPO is cardiac power output measured in watts, QT is cardiac output in liters per min, MAP is mean arterial pressure in millimeters of mercury, and K is the conversion factor (2.22 × 10−3). Mean arterial pressure was calculated as: SBP + 0.412 × (SBP − DBP), where SBP is systolic blood pressure and DBP is diastolic blood pressure (31). Systematic vascular resistance to blood flow at peak exercise was estimated as the ratio between mean arterial pressure and cardiac output and, according to convention, was multiplied by a factor of 80 to convert units to dynes per second per centimeter to the fifth power.
For the purpose of comparing patients with controls, the regression line of the controls can be assigned the value of unity (100%), and the 2 SD on either side of this unity line can therefore form the boundaries of the reference range that represents the “normal” values (32). The cardiac and functional data of patients can therefore be represented as a percentage of the corresponding average control value. Data points falling within these boundaries are considered to be within the “normal” range as previously suggested (32).
All statistical analysis was carried out using SPSS version 21.0 (SPSS, Chicago, Illinois). Before statistical analysis, data were checked for univariate and multivariate outliers using standard Z-distribution cutoffs and Mahalanobis distance tests, respectively. Normality of distribution was assessed using a Kolmogorov-Smirnov test. To test differences in measured variables between the patients’ groups, a 1-way analysis of variance was used. To identify the groups that differed significantly from one another, a post hoc Tukey test was performed. Nominal variables were evaluated using the chi-square test. Pearson coefficient of correlation was used to determine the relationships between variables. Statistical significance was indicated if p < 0.05. All data are presented as mean ± SD unless otherwise indicated.
Results
Patients’ demographic and clinical characteristics are presented in Table 1. There were no significant differences in demographic details between the patients groups. LVEF was significantly lower in HTx compared with LVAD patients and controls (p < 0.01).
Table 2 shows resting and peak exercise cardiac and physical function variables in cardiac transplant candidates, patients with implanted and explanted LVAD, and healthy controls. It is apparent from Table 2 that relative to healthy controls, the HTx and LVAD implanted and LVAD explanted patients demonstrate cardiac and physical functional capacities that were significantly diminished. Individual data for patient groups are presented in Table 3.
Table 2.
Resting and Peak Exercise Cardiac and Physical Function Variables in HTx, Implanted and Explanted LVAD Patients, and Healthy Controls
| Heart Transplant Candidates (n = 24) | LVAD Implanted (n = 18) | LVAD Explanted (n = 16) | Healthy Controls (n = 97) | |
|---|---|---|---|---|
| Sitting rest | ||||
| Heart rate, beats/min | 76.7 ± 19.7 | 72.6 ± 13.3 | 73.3 ± 6.2 | 65.7 ± 10.6 |
| Stroke volume, ml | 49.1 ± 12.3∗ | 76.7 ± 17.0 | 71.1 ± 11.3 | 73.7 ± 18.0 |
| Systolic BP, mm Hg | 94.5 ± 17.5 | 95.3 ± 21.1 | 105.2 ± 15.8 | 123.3 ± 11.7† |
| Diastolic BP, mm Hg | 61.4 ± 10.3 | 63.3 ± 17.9 | 70.9 ± 12.7 | 76.3 ± 9.1‡ |
| Mean arterial BP, mm Hg | 74.4 ± 12.1 | 72.8 ± 15.6 | 85.5 ± 12.6 | 95.7 ± 8.98‡ |
| Cardiac output, l/min | 3.53 ± 0.66∗ | 5.5 ± 1.4 | 5.2 ± 0.8 | 4.87 ± 1.20 |
| Cardiac power output, W | 0.59 ± 0.16∗ | 0.89 ± 0.23 | 0.94 ± 0.20 | 1.02 ± 0.22 |
| Vascular resistance, dyn/s/cm−5 | 1,735 ± 357∗ | 1,143 ± 433 | 1,351 ± 293 | 14,85 ± 509 |
| Oxygen consumption, ml/min | 314.4 ± 54.2 | 371 ± 47 | 407 ± 35 | 374 ± 43 |
| Oxygen consumption, ml/kg/min | 4.32 ± 0.92 | 4.74 ± 0.56 | 4.88 ± 0.09 | 3.63 ± 0.66 |
| Peak exercise | ||||
| Heart rate, beats/min | 101.9 ± 21.6∗ | 142.3 ± 26.0§ | 162.8 ± 12.9 | 173.9 ± 16.7 |
| Stroke volume, ml | 82.9 ± 22.2 | 88.9 ± 16.8 | 90.6 ± 15.2‖ | 116.9 ± 18.5† |
| Systolic BP, mm Hg | 94.9 ± 16.3∗ | 115.6 ± 25.0§ | 140.1 ± 21.9‖ | 199.1 ± 18.4† |
| Diastolic BP, mm Hg | 59.4 ± 10.1 | 72.0 ± 14.5 | 78.1 ± 12.6‖ | 66.0 ± 14.3 |
| Mean arterial BP, mm Hg | 74.0 ± 11.8∗ | 86.9 ± 17.9§ | 103.5 ± 18.2‖ | 120.8 ± 11.0† |
| Cardiac output, l/min | 8.12 ± 1.52∗ | 12.4 ± 1.9§ | 14.7 ± 2.37‖ | 20.3 ± 3.9† |
| Cardiac power output, W | 1.31 ± 0.31∗ | 2.37 ± 0.67§ | 3.45 ± 0.72‖ | 5.36 ± 0.94† |
| Vascular resistance, dyn/s/cm−5 | 758 ± 195∗ | 569 ± 118 | 583 ± 159‖ | 495 ± 116 |
| Oxygen consumption, ml/min | 916 ± 227∗ | 1,814 ± 422§ | 2,511 ± 420‖ | 3,090 ± 740† |
| Oxygen consumption, ml/kg/min | 12.0 ± 2.2∗ | 20.5 ± 4.3§ | 29.8 ± 5.9‖ | 36.4 ± 10.3 |
| Arterial-venous oxygen difference, ml O2/100 ml of blood | 11.3 ± 2.5∗ | 15.1 ± 4.1§ | 17.2 ± 2.3 | 18.2 ± 3.7 |
| Respiratory exchange ratio | 1.10 ± 0.15 | 1.13 ± 0.07 | 1.11 ± 0.07 | 1.11 ± 0.08 |
Values are mean ± SD.
BP = blood pressure; HTx = heart transplant candidate; LVAD = left ventricular assist device.
p < 0.05 transplants vs. LVAD implanted, LVAD explanted, and controls.
p < 0.05 controls vs. transplants, LVAD explanted, LVAD implanted.
p < 0.05 controls vs. transplants and LVAD implanted.
p < 0.05 LVAD implanted vs. LVAD explanted, and controls.
p < 0.05 LVAD explanted vs. controls.
Table 3.
Individual Patient Data for Peak Exercise Cardiac Power Output and Oxygen Consumption
| Patient # | Heart Transplant Candidates |
LVAD Implanted |
LVAD Explanted |
|||
|---|---|---|---|---|---|---|
| Peak Cardiac Power (W) | Peak Oxygen Consumption (ml/kg/min) | Peak Cardiac Power (W) | Peak Oxygen Consumption (ml/kg/min) | Peak Cardiac Power (W) | Peak Oxygen Consumption (ml/kg/min) | |
| 1 | 1.69 | 11.56 | 3.71 | 20.32 | 2.81 | 26.13 |
| 2 | 1.07 | 7.96 | 2.55 | 18.71 | 2.83 | 27.86 |
| 3 | 1.51 | 7.67 | 1.75 | 23.43 | 3.67 | 45.91 |
| 4 | 1.44 | 11.38 | 1.33 | 25.13 | 3.98 | 25.56 |
| 5 | 1.45 | 17.03 | 3.34 | 17.26 | 4.08 | 30.01 |
| 6 | 1.64 | 13.06 | 2.06 | 18.18 | 3.36 | 21.52 |
| 7 | 1.66 | 13.21 | 1.98 | 19.42 | 2.83 | 25.30 |
| 8 | 1.52 | 15.47 | 2.14 | 25.68 | 2.90 | 33.85 |
| 9 | 0.91 | 9.48 | 1.63 | 10.89 | 3.70 | 28.05 |
| 10 | 1.65 | 11.60 | 2.57 | 16.94 | 3.29 | 36.16 |
| 11 | 1.50 | 12.88 | 2.13 | 15.58 | 4.10 | 23.41 |
| 12 | 1.84 | 12.70 | 2.63 | 18.82 | 2.20 | 27.16 |
| 13 | 0.98 | 13.68 | 2.10 | 25.22 | 2.39 | 27.79 |
| 14 | 1.23 | 13.68 | 1.36 | 17.03 | 4.12 | 32.47 |
| 15 | 1.08 | 10.56 | 2.64 | 22.20 | 4.50 | 32.76 |
| 16 | 1.38 | 11.13 | 2.89 | 22.02 | 4.36 | 33.20 |
| 17 | 1.37 | 9.44 | 2.41 | 24.08 | — | — |
| 18 | 1.38 | 13.22 | 3.45 | 28.12 | — | — |
| 19 | 1.41 | 14.70 | — | — | — | — |
| 20 | 0.55 | 10.26 | — | — | — | — |
| 21 | 0.90 | 11.07 | — | — | — | — |
| 22 | 0.90 | 11.07 | — | — | — | — |
| 23 | 1.21 | 12.30 | — | — | — | — |
| 24 | 1.26 | 12.33 | — | — | — | — |
| Mean | 1.31 | 11.98 | 2.37 | 20.5 | 3.45 | 29.82 |
| SD | 0.31 | 2.21 | 0.68 | 4.34 | 0.72 | 5.90 |
LVAD = left ventricular assist device.
To obtain a perspective of the spans involved, the highest recorded cardiac and functional capacities (i.e., cardiac power output and oxygen consumption obtained at peak exercise were 7.7 W and 4.75 l/min, respectively), and the corresponding mean values were 5.4 ± 0.9 W and 3.09 ± 0.74 l/min. At the other extreme, the lowest recorded peak cardiac power and oxygen consumption values were in the HTx group (i.e., 0.55 W and 0.45 l/min, respectively), and the corresponding mean values were 1.4 ± 0.2 W and 0.94 ± 0.21 l/min, respectively. The mean values of peak exercise cardiac power and oxygen consumption of LVAD implanted patients were 2.4 ± 0.4 W and 1.81 ± 0.42 l/min, and of explanted patients, 3.4 ± 0.7 W and 2.51 ± 0.42 l/min, respectively (all p < 0.05 compared with healthy controls) (Table 2).
To provide further insight into the cardiac and functional benefits associated with LVAD bridge-to-recovery protocol, the data confirmed that the ratio between peak oxygen consumption and cardiac power output was the lowest in healthy controls (6.79 ± 1.68 ml O2/W), followed by LVAD explanted (8.64 ± 2.27 ml O2/W), then LVAD implanted patients (8.65 ± 3.41 ml O2/W), and then heart transplant candidates (9.16 ± 2.88 ml O2/W). Additionally, LVAD explanted patients who achieved a peak exercise cardiac power similar to that of healthy controls demonstrated a 22% lower ratio between peak oxygen consumption and cardiac power output than those LVAD explanted patients who had not achieved the level of healthy controls (7.2 vs. 9.2 ml O2/W; p = 0.03).
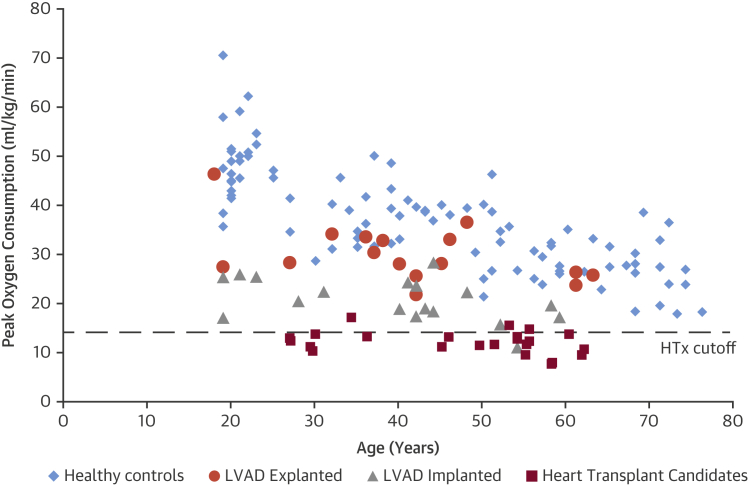
When expressed relative to the reference values of healthy subjects, as percentages of the average control values of cardiopulmonary exercise variables, it is possible to compare directly the relative cardiac versus physical functional capabilities of the patient groups. As shown in Figure 1, the diminution in cardiac functional capacity (peak cardiac power output) was most marked in the HTx group from 100% (the normalized average in healthy controls) to 25.6 ± 4.6% (p < 0.01), whereas the corresponding diminution in physical functional capacity (peak oxygen consumption) was to 33.7 ± 8.5% (p < 0.01). Of patients who recovered sufficiently to enable LVAD explantation, 6 patients (37.5%) achieved peak exercise cardiac power output that fell within the normal range of age- and sex-matched healthy controls (Figure 2), whereas 10 patients (62.5%) achieved peak exercise oxygen consumption levels within the range of healthy controls (Figure 3). By placing the relative values of cardiac versus physical functional capacities of the patients groups side by side as shown in Figure 1, it is apparent that in these groups of patient cardiac functional capacity (peak cardiac power) was more severely impaired than physical impairments at peak exertion (peak oxygen consumption). Figures 2 and 3 also show a well-known normal phenomenon of age-related decline in cardiac and physical function performance. Each of the HF cohorts also showed similar decline with age, but also stepwise decreases with increasing severity of HF.
Figure 1.
Cardiac and Functional Capacities in LVAD Implanted, LVAD Explanted, and HTx Patients Expressed as % of Healthy Controls
Peak exercise cardiac power output (orange bars) and oxygen consumption (blue bars) in patients (LVAD explanted, n = 16; LVAD implanted, n = 18; heart transplant candidates, n = 24) expressed as percentage achieved of healthy controls. HTx = heart transplant candidates; LVAD = left ventricular assist device.
Figure 2.
Individual Data of Cardiac Pumping Capability in HTx, LVAD Implanted and LVAD Explanted Patients, and Healthy Controls in Relation to Age
Peak exercise cardiac power output in healthy controls (blue diamonds), LVAD explanted (orange circles), LVAD implanted (gray triangles), and heart transplant candidates (red squares) individual data in relation to age. Six of 16 (38%) LVAD explanted patients achieved a level of peak exercise cardiac power output equivalent to that of healthy controls. Dashed line (HTx cutoff) represents clinical threshold value for heart transplantation referral. Abbreviations as in Figure 1.
Figure 3.
Individual Data of Functional Capacity in HTx, LVAD Implanted and LVAD Explanted Patients, and Healthy Controls in Relation to Age
Peak oxygen consumption in healthy controls (blue diamonds), LVAD explanted (orange circles), LVAD implanted (gray triangles), and heart transplant candidates (red squares) individual data in relation to age. Eleven of 16 (69%) LVAD explanted patients achieved a level of peak exercise oxygen consumption equivalent to that of healthy controls. Dashed line (HTx cutoff) represents clinical threshold value for heart transplantation referral. Abbreviations as in Figure 1.
An important aspect in LVAD explanted (recovered) patients to be considered is the time since the device was explanted (3.3 ± 1.1 years, range 0.3 to 5.8 years). To address this point, we assessed the relationship between time since LVAD explantation and the ratio between oxygen consumption and cardiac power at peak exercise. There was a nonsignificant relationship between time since LVAD explantation and the ratio between oxygen consumption and cardiac power at peak exercise (r = −0.10; p = 0.68) (Central Illustration), suggesting that cardiac and functional recovery, once achieved with the LVAD bridge-to-recovery program, is sustainable for several years following LVAD explantation.
Central Illustration.
Cardiac Recovery Following LVAD Therapy In Advanced Heart Failure
Relationship between time since LVAD explantation and the cardiac and physical functional recovery. Peak cardiac power output relative to peak oxygen consumption individual data in relation to the time since LVAD was explanted. Figure suggests that cardiac and functional recovery, once achieved with LVAD bridge-to-recovery protocol, are sustainable for several years following LVAD explantation. LVAD = left ventricular assist device.
Discussion
The major finding from the present study suggests that a significant percentage of LVAD explanted patients can achieve cardiac and physical functional capacities that are within the normal range of healthy controls. Our data further confirm previous findings suggesting that LVAD therapy improves cardiac and functional capacity in patients with confirmed cardiac end-stage failure and provide direct comparison with HTx. A limited number of heart transplantation centers have demonstrated cardiac recovery that is sufficient to permit LVAD explantation 22, 33. The findings of the present study support the hypothesis that use of a LVAD can lead to improvement in cardiac and functional capacities to allow for the LVAD to be efficiently explanted (i.e., bridge to recovery).
On average, the cardiac functional gain by LVAD compared with HTx patients was 1.1 W of peak cardiac power output (Δ = 56%), and the physical functional gain was 8 ml/kg/min of peak oxygen consumption (Δ = 39%). If a perfect cardiac transplantation were realizable, such as when an average HTx candidate could be transformed into an average healthy control, the ideal cardiac functional gain would be 4.0 W of peak cardiac power (Δ = 75%) and the ideal physical functional gain would be ∼20 ml/kg/min of peak oxygen consumption (Δ = 70%). For a comparison with real-life clinical cases, corrective operations on appropriate valve disease patients can confer an average cardiac functional gain of 0.79 W (Δ = 23%) and an average physical functional gain of 11%, as shown in a longitudinal follow-up investigation (34). Similarly, the functional benefits of cardiac resynchronization therapy confer an average cardiac functional gain of 0.72 W (Δ = 27%) and an average physical functional gain of 16% (35). Data from the present cross-sectional study of LVAD implanted patients suggest that this therapeutic modality in suitable patients can confer cardiac and physical functional gains equivalent or superior to currently established cardiac interventions.
LVAD explanted patients demonstrated significantly better cardiac and functional capacities than LVAD implanted patients and HTx. It is a remarkable finding that 38% of LVAD explanted patients achieved the level of peak exercise cardiac function equivalent to that of healthy controls. It is interesting that 69% of LVAD explanted patients demonstrated peak oxygen consumption equivalent to that of healthy controls. Taken together, these findings suggest that a significant number of LVAD explanted patients can achieve cardiac and functional capacity similar to healthy controls confirming benefits of LVAD therapy and directing future investigations towards strategies to enhance myocardial recovery to allow for the device to be explanted. Our data also suggest that these functional improvements can be sustained.
The data from the present study can also throw some light on a long-standing debate about whether the cardiac dysfunction per se or the peripheral factors (including vasodilatory incapacity, pulmonary or neurohumoral derangements, and skeletal muscle contractile and metabolic abnormalities) are primarily responsible for the exercise limitation seen in heart failure patients (36). Hitherto, there has been no methodology to determine whether the heart or the periphery is the weaker link. The problem is further complicated by the fact that any abnormality in either factor (heart or periphery) can influence the performance measures of the other (37). One way of resolving this uncertainty is by investigating whether cardiac functional limitation is more (or less) severely compromised than the physical functional impairment in heart failure patients relative to a reference cohort of healthy controls. Whichever one is more severely impaired can be postulated as the likely primary cause, and moreover, a reversal of the impairment would show a greater improvement in the primary limiting factor than the secondary. This investigation referenced the functional capacities of healthy controls. In relative terms, in each heart failure group in this study, the cardiac functional reserve was on average more severely impaired than the aerobic exercise capacity (Figure 1). This confirmed previously reported nonlinear relationship between cardiac and functional capacity in LVAD and heart failure patients (38).
Insight into what constitutes abnormal response to stress observed in LVAD patients and heart transplant candidates can be gained by unscrambling how their peak exercise hemodynamic responses deviate from the normal physiological responses observed in healthy controls. In the absence of any valid biomedical model of how each central hemodynamic variable varies with peak oxygen uptake in normal subjects, it is reasonable to assume that central hemodynamic variables are linearly related to peak oxygen uptake (39). A significant deviation from the linear relation would constitute a departure from physiology into the realm of pathology. Moreover, it can be hypothesized that the greater the extent of departure from physiological response would imply a more severe pathology.
Study limitations
The cross-sectional study design and limited number of patients in each heart failure group may prevent generalization of the data. Also, patients enrolled in the study were men. A longitudinal, multicenter study is therefore needed to allow assessments of cardiac and functional capacities at different time points in both men and women. This will aim to identify strategies to enhance bridge-to-recovery partway and define early markers of myocardial recovery to allow for LVADs to be explanted.
Conclusions
It is remarkable that dilated cardiomyopathy patients with refractory end-stage heart failure, following the judicious use of combined LVAD and medical therapies, were able to recover cardiac and physical functional reserve capacities. The extent of this recovery is that a significant number of them can achieve the levels equivalent to those of healthy controls. More aggressive strategies to enhance cardiac remodeling and reconditioning during LVAD support should be encouraged with ultimate goal of LVAD explantation and return to a pharmacological management. Assessment of central hemodynamic measurements under cardiopulmonary exercise stress testing can lead to early identification of myocardial recovery in LVAD implanted patients.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with advanced heart failure awaiting transplantation, there was a significantly better cardiac performance and functional capacity among those in whom LVADs were explanted, a substantial proportion of whom attained peak cardiac power output and peak oxygen consumption in the range of healthy individuals.
TRANSLATIONAL OUTLOOK: Prospective clinical trials are needed to define optimal pharmacological and physiological strategies to enhance myocardial recovery and allow for use of LVADs as a bridge to recovery of cardiac function.
Acknowledgments
The authors thank Drs. R. Bougard, R.S. George, D. Nunan, and G. Donovan for their invaluable help in data collection.
Footnotes
Dr. Jakovljevic is currently supported by Research Councils’ UK Centre for Ageing and Vitality at Newcastle University. Dr. Yacoub has received institutional research funding from St. Jude Thoratec. Dr. Schueler has received research funding from HeartWare; and has served as an advisor and proctor for HeartWare. Dr. MacGowan has research funding from HeartWare. Dr. Birks has received institutional research funding from St. Jude Thoratec. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Burkhoff D., Sayer G., Doshi D., Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015;66:2663–2674. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 2.MacGowan G.A., Crossland D.S., Hasan A., Schueler S. Considerations for patients awaiting heart transplantation: insights from the UK experience. J Thorac Dis. 2015;7:527–531. doi: 10.3978/j.issn.2072-1439.2015.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenneman A.J., Birks E.J. Treatment strategies for myocardial recovery in heart failure. Curr Treat Options Cardiovasc Med. 2014;16:287. doi: 10.1007/s11936-013-0287-9. [DOI] [PubMed] [Google Scholar]
- 4.Hall J.L., Fermin D.R., Birks E.J. Clinical, molecular, and genomic changes in response to a left ventricular assist device. J Am Coll Cardiol. 2011;57:641–652. doi: 10.1016/j.jacc.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birks E.J. Molecular changes after left ventricular assist device support for heart failure. Circ Res. 2013;113:777–791. doi: 10.1161/CIRCRESAHA.113.301413. [DOI] [PubMed] [Google Scholar]
- 6.Kiernan M.S., Sundareswaran K.S., Pham D.T. Preoperative determinants of quality of life and functional capacity response to left ventricular assist device therapy. J Card Fail. 2016;22:797–805. doi: 10.1016/j.cardfail.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 7.John R., Kamdar F., Liao K., Colvin-Adams M., Boyle A., Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227–1234. doi: 10.1016/j.athoracsur.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Lahpor J., Khaghani A., Hetzer R. European results with a continuous-flow ventricular assist device for advanced heart-failure patients. Eur J Cardiothorac Surg. 2010;37:357–361. doi: 10.1016/j.ejcts.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Pagani F.D., Miller L.W., Russell S.D. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 10.Jakovljevic D.G., McDiarmid A., Hallsworth K. Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am J Cardiol. 2014;114:88–93. doi: 10.1016/j.amjcard.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakovljevic D.G., George R.S., Nunan D. The impact of acute reduction of continuous-flow left ventricular assist device support on cardiac and exercise performance. Heart. 2010;96:1390–1395. doi: 10.1136/hrt.2010.193698. [DOI] [PubMed] [Google Scholar]
- 12.Jakovljevic D.G., George R.S., Donovan G. Comparison of cardiac power output and exercise performance in patients with left ventricular assist devices, explanted (recovered) patients, and those with moderate to severe heart failure. Am J Cardiol. 2010;105:1780–1785. doi: 10.1016/j.amjcard.2010.01.362. [DOI] [PubMed] [Google Scholar]
- 13.Noor M.R., Bowles C., Banner N.R. Relationship between pump speed and exercise capacity during HeartMate II left ventricular assist device support: influence of residual left ventricular function. Eur J Heart Fail. 2012;14:613–620. doi: 10.1093/eurjhf/hfs042. [DOI] [PubMed] [Google Scholar]
- 14.Jung M.H., Hansen P.B., Sander K. Effect of increasing pump speed during exercise on peak oxygen uptake in heart failure patients supported with a continuous-flow left ventricular assist device. A double-blind randomized study. Eur J Heart Fail. 2014;16:403–408. doi: 10.1002/ejhf.52. [DOI] [PubMed] [Google Scholar]
- 15.Hetzer R., Muller J.H., Weng Y., Meyer R., Dandel M. Bridging-to-recovery. Ann Thorac Surg. 2001;71(Suppl):S109–S113. doi: 10.1016/s0003-4975(00)02638-2. [DOI] [PubMed] [Google Scholar]
- 16.Farrar D.J., Holman W.R., McBride L.R. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002;21:516–521. doi: 10.1016/s1053-2498(01)00408-9. [DOI] [PubMed] [Google Scholar]
- 17.Birks E.J. Myocardial recovery in patients with chronic heart failure: is it real? J Card Surg. 2010;25:472–477. doi: 10.1111/j.1540-8191.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 18.Birks E.J., George R.S., Firouzi A. Long-term outcomes of patients bridged to recovery versus patients bridged to transplantation. J Thorac Cardiovasc Surg. 2012;144:190–196. doi: 10.1016/j.jtcvs.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Frazier O.H., Baldwin A.C., Demirozu Z.T. Ventricular reconditioning and pump explantation in patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015;34:766–772. doi: 10.1016/j.healun.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maybaum S., Mancini D., Xydas S. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 21.Simon M.A., Kormos R.L., Murali S. Myocardial recovery using ventricular assist devices: prevalence, clinical characteristics, and outcomes. Circulation. 2005;112:I32–I36. doi: 10.1161/CIRCULATIONAHA.104.524124. [DOI] [PubMed] [Google Scholar]
- 22.Dandel M., Weng Y., Siniawski H. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118(Suppl):S94–S105. doi: 10.1161/CIRCULATIONAHA.107.755983. [DOI] [PubMed] [Google Scholar]
- 23.Dandel M., Weng Y., Siniawski H., Potapov E., Lehmkuhl H.B., Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112:I37–I45. doi: 10.1161/CIRCULATIONAHA.104.525352. [DOI] [PubMed] [Google Scholar]
- 24.Yacoub M.H. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J. 2001;22:534–540. doi: 10.1053/euhj.2001.2613. [DOI] [PubMed] [Google Scholar]
- 25.Terracciano C.M., Miller L.W., Yacoub M.H. Contemporary use of ventricular assist devices. Annu Rev Med. 2010;61:255–270. doi: 10.1146/annurev.med.032309.063018. [DOI] [PubMed] [Google Scholar]
- 26.Birks E.J., Tansley P.D., Hardy J. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 27.Birks E.J., George R.S., Hedger M. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123:381–390. doi: 10.1161/CIRCULATIONAHA.109.933960. [DOI] [PubMed] [Google Scholar]
- 28.George R.S., Yacoub M.H., Bowles C.T. Quality of life after removal of left ventricular assist device for myocardial recovery. J Heart Lung Transplant. 2008;27:165–171. doi: 10.1016/j.healun.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Jakovljevic D.G., Nunan D., Donovan G., Hodges L.D., Sandercock G.R., Brodie D.A. Comparison of cardiac output determined by different rebreathing methods at rest and at peak exercise. Eur J Appl Physiol. 2008;102:593–599. doi: 10.1007/s00421-007-0631-4. [DOI] [PubMed] [Google Scholar]
- 30.Jakovljevic D.G., Seferovic P.M., Nunan D. Reproducibility of cardiac power output and other cardiopulmonary exercise indices in patients with chronic heart failure. Clin Sci (Lond) 2012;122:175–181. doi: 10.1042/CS20110355. [DOI] [PubMed] [Google Scholar]
- 31.Meaney E., Alva F., Moguel R., Meaney A., Alva J., Webel R. Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart. 2000;84:64. doi: 10.1136/heart.84.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bland J.M. Third edition. Oxford Medical Publications; Oxford, UK: 2000. An Introduction to Medical Statistics. [Google Scholar]
- 33.Liden H., Karason K., Bergh C.H., Nilsson F., Koul B., Wiklund L. The feasibility of left ventricular mechanical support as a bridge to cardiac recovery. Eur J Heart Fail. 2007;9:525–530. doi: 10.1016/j.ejheart.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Tan D.K., Hothi S.S., Macdonald W., Schlosshan D., Tan L.B. Impacts of valve intervention on the Functional REServe of the Heart: the FRESH-valve pilot study. Int J Cardiol. 2015;187:491–501. doi: 10.1016/j.ijcard.2015.03.177. [DOI] [PubMed] [Google Scholar]
- 35.Schlosshan D., Barker D., Pepper C., Williams G., Morley C., Tan L.B. CRT improves the exercise capacity and functional reserve of the failing heart through enhancing the cardiac flow- and pressure-generating capacity. Eur J Heart Fail. 2006;8:515–521. doi: 10.1016/j.ejheart.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Clark A.L., Poole-Wilson P.A., Coats A.J. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 37.Esposito F., Mathieu-Costello O., Shabetai R., Wagner P.D., Richardson R.S. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakovljevic D.G., Birks E.J., George R.S. Relationship between peak cardiac pumping capability and selected exercise-derived prognostic indicators in patients treated with left ventricular assist devices. Eur J Heart Fail. 2011;13:992–999. doi: 10.1093/eurjhf/hfr069. [DOI] [PubMed] [Google Scholar]
- 39.Jakovljevic D.G., Popadic-Gacesa J.Z., Barak O.F. Relationship between peak cardiac pumping capability and indices of cardio-respiratory fitness in healthy individuals. Clin Physiol Funct Imag. 2012;32:388–393. doi: 10.1111/j.1475-097X.2012.01143.x. [DOI] [PubMed] [Google Scholar]