Abstract
Fraxinus rhynchophylla Hance (Oleaceae), its stem barks are known as Cortex fraxini (秦皮 qín pí) listed in Chinese Pharmacopoeia. Phytochemical study has indicated that methanol extracts from Qinpi has protective effect on acute liver injury. The present study investigates the hepatoprotective activity of EtOH–water extract from the seeds of F. rhynchophylla Hance against carbon tetrachloride-induced liver injury in mice. The EtOH–water extract significantly alleviated liver damage as indicated by the decreased levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), the malondialdehyde (MDA) content, and increased the levels of superoxide dismutase (SOD), glutathione (GSH) and glutathione peroxidase (GSH-Px), and reduced the pathological tissue injury induced by CCl4. Quantitative analysis of seven major constituents (1–7) in EtOH–water extract (EWE) was developed by high performance liquid chromatography-diode-array detector (HPLC-DAD). The current research indicates that the EWE from the seeds of F. rhynchophylla Hance decreased liver index, inhibited the increase of serum aminotransferase induced by CCl4, and decreased hepatic MDA content, SOD and GSH-Px activities. These results suggested that the pretreatment with EWE protected mice against CCl4-induced liver injuries. Based on the results, the EtOH–water extract from the seeds of F. rhynchophylla Hance is efficacious for prevention and treatment of CCl4-induced hepatic injury in mice. Secoiridoid and tyrosol glucosides might be the active ingredients responsible for the biological and pharmacological activities of hepatoprotection.
Keywords: Fraxinus rhynchophylla Hance, Hepatoprotective, Secoiridoid glucosides, ALT and AST, CCl4-induced hepatic injury mice
Graphical abstract
1. Introduction
The liver plays an important role in human metabolism and detoxification of endogenous and exogenous chemicals.1 Liver injuries or dysfunctions have been recognized as serious health problem. Especially acute and chronic liver injuries resulted from the exposure to toxic chemicals, drugs, and virus infiltration from ingestion or infection, have gained more attention in recent years.2, 3, 4 Corticosteroids and interferon has been used for the treatment of hepatic diseases, however, these synthetic chemical drugs are not well accepted by patients due to limited therapeutic efficacy and serious complications.5 Therefore, more effective complementary and therapeutic drugs with low or no side-effects are needed for the treatment of liver diseases.6, 7, 8, 9 In recent years, some effective and safe dietary ingredients for liver-protection have been isolated from traditional medicinal plants, such as glycyrrhizin,10 curcumin,11 resveratrol,12 as well as silybin and silymarin.13, 14 Fraxinus rhynchophylla Hance (Oleaceae) is a commonly used Chinese traditional medicinal plant, mainly distributed in China and Korea.15 Its stem bark also known as Cortex fraxini (Qinpi) is Chinese herbal drug for treating diseases such as acute conjunctivitis and psoriasis; arresting discharges; curing chronic bronchitis; and bacillary dysentery, diuretic, antirheumatic, analgesic, antiperspiratory effects, and enhancing eyesight.16 Phytochemical study has indicated that methanol extracts from Qinpi has protective effect on acute liver injury.17 Many natural products such as secoiridoid glucosides, coumarins, lignans, sesquilignans, and coumarinolignans have been identified from this plant.18, 19, 20, 21, 22, 23 Recently, several pharmacological activities of phytochemical constituents isolated from the barks and leaves of F. rhynchophylla have been carried out, including anti-diabetes effects,24, 25, 26, 27, 28 anti-Toxoplasma gondii effects,29 including antioxidant enzymes,30 inhibiting amyloid-β-induced neuronal cell damage,31 and inhibiting nitric oxide synthesis activities.32 Hydrangeside B, along with other secoiridoid glucosides showed hepatoprotective activities against dl-galactosamine-induced toxicity in human hepatocyte HL-7702 (HL-7702) cells.33 Secoiridoid glycoside, oleuropein, showed anti-hepatitis B virus (HBV) activity and effectively blocked hepatitis B surface antigen (HBsAg) secretion with an IC50 of 23.2 μg/mL in HepG2.2.15 cells with no significant cytotoxicity.34 The hepatoprotective activity of oleuropein against carbon tetrachloride (CCl4)-induced liver damage in mice was achieved through the NF-E2-related factor 2-mediated induction of heme oxygenase-1.35 Recently, Peng et al.36 investigated the effect of Fraxinus rhynchophylla ethanol extract (FREtOH) on liver fibrosis induced by CCl4 in rats. However, the hepatoprotective activity of the seeds of F. rhynchophylla Hance has not been evaluated so far.
The present study aimed to evaluate the hepatoprotective activity of EtOH–water extract from the seeds of F. rhynchophylla Hance employing a widely used CCl4-induced liver damage model in mice and quantitative analysis of six secoiridoid glucosides (1–6) and one tyrosol glucoside (7) by high performance liquid chromatography-diode-array detector (HPLC-DAD) method.
2. Materials and methods
2.1. Chemicals and reagents
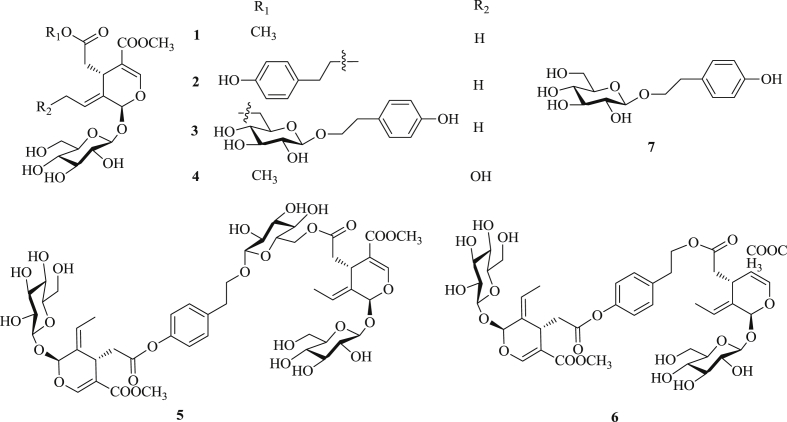
CH3OH (HPLC grade), CH3CH2OH (HPLC grade), and CH3CN (HPLC grade) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other organic solvents used in the current study, such as CH3OH, ethyl acetate (EtOAc), acetone, and chloroform (CHCl3) were of analytical grade. They are commercially available from Hengxing Chemical Reagent Co., Ltd. (Tianjin, China). Chemical standards of oleoside dimethyl ester (1), ligstroside (2), nuzhenide (3), 10-hydroxoleoside dimethyl ester (4), GI3 (5), GI5 (6), and salidroside (7) were prepared in our laboratory. The purity of each compound was >98%, determined by HPLC analysis. The chemical structures of these reference compounds are shown in Fig. 1.
Fig. 1.
Structures of compounds 1–7.
2.2. Materials
The seeds of F. rhynchophylla Hance were provided in August 2013 from Baoji City, Shaanxi province, China. The herbariums of F. rhynchophylla Hance (FRH001) were deposited in Room 612, Department of Pharmaceutical Engineering, College of Chemical Engineering, Northwest University.
2.3. HPLC analysis conditions
HPLC analysis was performed on an Agilent 1260 separation module connected to a G1315D DAD detector using a Synergi 4u Hydro-RP 80R column (250 × 4.6 mm, 4 μm, 100 Å) with a flow rate of 1.0 mL/min. Solvent system: 0 min: 95% A (1% phosphoric acid) and 5% B (acetonitrile), 2 min: 95% A (1% phosphoric acid) and 5% B (acetonitrile), 5 min: 80% A (1% phosphoric acid) and 20% B (acetonitrile), 25 min: 75% A (1% phosphoric acid) and 25% B (acetonitrile), 27 min: 95% A (1% phosphoric acid) and 5% B (acetonitrile), 30 min: 95% A (1% phosphoric acid) and 5% B (acetonitrile). At the end of the run, 100% of acetonitrile was allowed to flush the column for 10 min, and an additional 10 min of post run time was set to allow for equilibration of the column with the starting eluant. The UV detector was operating at 230 nm, and the column temperature was maintained at 30 °C.
2.4. Calibration curves
Methanol stock solutions containing the seven standard compounds 1–7 were prepared and diluted to five different final concentrations. A calibration curve was constructed for each of the compounds by plotting peak areas versus compound concentrations.
2.5. Preparation of seeds of F. rhynchophylla Hance extract
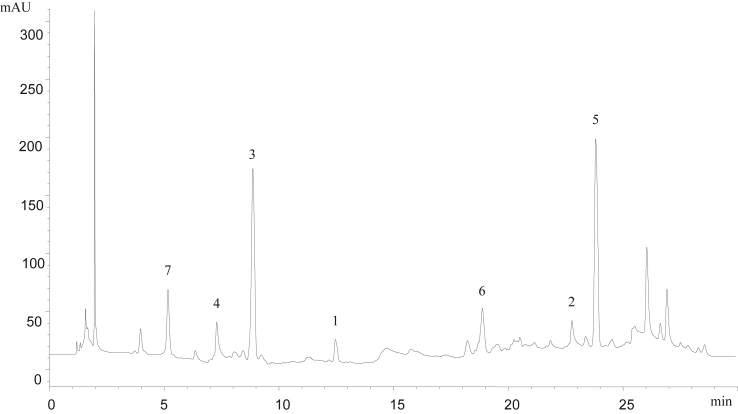
The 5 kg air-dried seeds of F. rhynchophylla Hance were percolated twice with absolute ethyl alcohol at room temperature. The ethyl alcohol was evaporated under vacuum. The herb residue was then percolated twice with water at room temperature and made the SFR-water extracts. Finally, mixed the above two extracts, and the in vivo bioactivity study of seeds of F. rhynchophylla Hance was carried on by this sample. The HPLC spectrum of the extract is shown in Fig. 2.
Fig. 2.
The HPLC chromatogram of EtOH–Water extract.
2.6. The hepatoprotective activities assays
2.6.1. Animals
Male Kunming mice (weighing 18–22 g), obtained from the Experimental Animal Center of Xi'an Jiaotong University, were used. The animal ethical approval communication number is SCXK 2012-003 (Northwest University, Xi'an, Shaanxi, China). All animals were grouped and housed in polyacrylic cages (29 × 18 × 16 cm) with no more than six animals per cage and maintained under standard laboratory conditions (temperature 25 ± 2 °C, relative humidity 50 ± 10%) with dark and light cycle (14/10 h). The mice were acclimatized to laboratory condition for 5 days before commencement of experiment. All the experiments were performed in accordance with the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of People's Republic of China.
2.6.2. CCl4-induced hepatotoxicity model
Mice were randomly divided into five groups of 12 animals each. In the control group (group I) and CCl4-intoxicated group (group II), mice were given a single daily dose of distilled water (0.2 mL/10 g, intragastrically (ig)). In the test groups, mice were given 2.0 g (group IV) and 4.0 g (group V) of EtOH–water extract (EWE) per kg body weight (BW) once daily. In group III, silymarin was served as a positive control, and mice were given 0.1 g/kg BW. All administrations were conducted for 7 weeks. On the 49th day, all mice except those in the control group were given simultaneously a CCl4/peanut oil mixture (0.1:100, intraperitoneally, 0.1 mL/10 g, ig) 2 h after the last administration, while the control group received peanut oil alone. Then all the animals were fasted for 16 h and were subsequently tested for the following analysis.
2.6.3. Liver index
Liver index was determined as percent of wet liver weight to body weight.
2.6.4. Assessment of liver function
After blood collection, serum was separated by centrifugation at 604 g at room temperature for 20 min. The serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values were measured with commercially available diagnostic kits produced by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.6.5. Determination of MDA, SOD and GSH-Px activity
After the animals were sacrificed, livers were immediately excised. With the exception of a portion of the left lobe to be used for histopathological examination, the livers were homogenized in phosphate buffer (50 mM, pH 7.4) and centrifuged at 420 g for 20 min at 4 °C. The malondialdehyde (MDA) content, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities along with protein levels in the supernatant were measured according to commercially available diagnostic kits.
2.6.6. Histopathological examinations
A left lobe portion of the liver was incubated for 24 h in 10% neutral formalin solution. Based on standard procedures, we obtained 5 μm sections for histopathological studies using hematoxylin and eosin (H&E) staining.
2.6.7. Statistical analysis
The data were analyzed in triplicate, using SAS software, version 8.1 (SAS Institute, Cary, NC, USA). Tukey's test was used to determine statistical significance. The values were considered significantly different when the P-value was lower than 0.05.
3. Results
3.1. HPLC method validation
All calibration curves made by the HPLC method exhibited excellent linear regressions with the determination coefficients (r2) ranging from 0.99 to 0.9999, and the calibration ranges adequately covered variations (Table 1).
Table 1.
Calibration curves and LOD and LOQ data of 7 compounds investigated by HPLC.
| No. | Calibration curvesa | r2 | Linear range (mg/mL) | LODb (mg/mL) | LOQb (mg/mL) |
|---|---|---|---|---|---|
| 1 | Y = −26.3302 + 15081.4372X | 0.9998 | 0.02–0.48 | 0.0005 | 0.0016 |
| 2 | Y = 40.3439 + 13692.4304X | 0.9986 | 0.01–0.24 | 0.0048 | 0.0158 |
| 3 | Y = 667.3964 + 4408.5855X | 0.9999 | 0.07–3.50 | 0.0012 | 0.0035 |
| 4 | Y = −1578.7230 + 15651.8470X | 0.9993 | 0.10–1.20 | 0.0330 | 0.0992 |
| 5 | Y = 481.0792 + 6299.1942X | 0.9984 | 0.20–3.20 | 0.0010 | 0.0034 |
| 6 | Y = −2.0423 + 1721.8740X | 0.9999 | 0.05–1.20 | 0.0038 | 0.0119 |
| 7 | Y = 1228.5054 + 2292.3870X | 0.9999 | 0.20–2.40 | 0.0250 | 0.0840 |
Y is the value of peak area, and X is the value of the reference compound's concentration (μg/mL).
LOD and LOQ were determined at S/N of about 3 and 10, respectively.
3.2. Quantification of the seven compounds
According to these results, the content of compound 3 (377.280 mg/g) was higher than other compounds, and the content of compound 5 was 330.960 mg/g in EtOH–water extract from the seed of F. rhynchophylla Hance (Table 2). The content of total analytes is 778.086 mg/g or 77.81% in EtOH–water extract from the seed of F. rhynchophylla Hance.
Table 2.
Contents of seven compounds in the EtOH–water extract of seeds of F. rhynchophylla Hance.
| No | Contents of analytesa (mg/g, n = 3) |
Extraction rate (w %)b | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| EtOH–water | 2.247 | 2.930 | 377.280 | 11.244 | 330.960 | 27.368 | 26.057 | 9.08 |
Extraction rate = solids content of the extract/sample volume.
3.3. The hepatoprotective activities assays
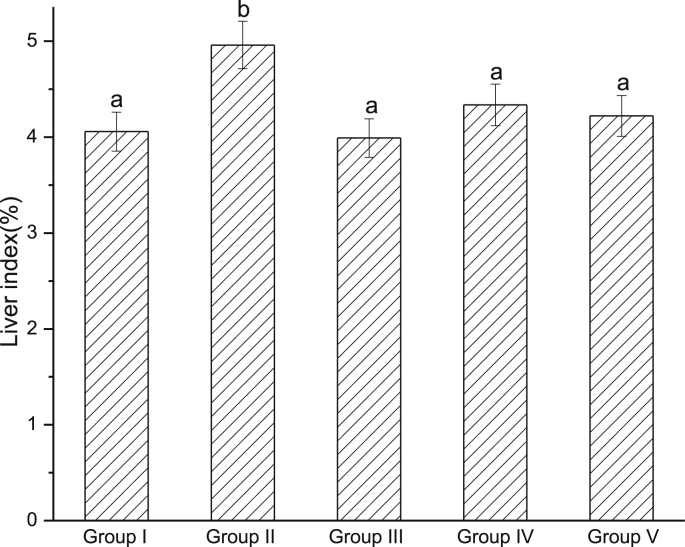
3.3.1. EtOH–water extract (EWE) decreased liver index
Liver swelling induced by CCl4 was manifested on the increase of liver index. As shown in Fig. 3, in the model group (CCl4-intoxicated group), liver index was 4.96, which was significantly higher than control group (P < 0.05). Pretreatment with EWE significantly decreased liver index compared with model group (P < 0.05).
Fig. 3.
Effects of EWE on liver index. Different lower case letters correspond to significant differences at P < 0.05. Group I was the control group. Group II was CCl4-treated group. Group III was given CCl4 + silymarin. Group IV and V were given CCl4 + 2.0 and 4.0 g/kg BW of EWE respectively.
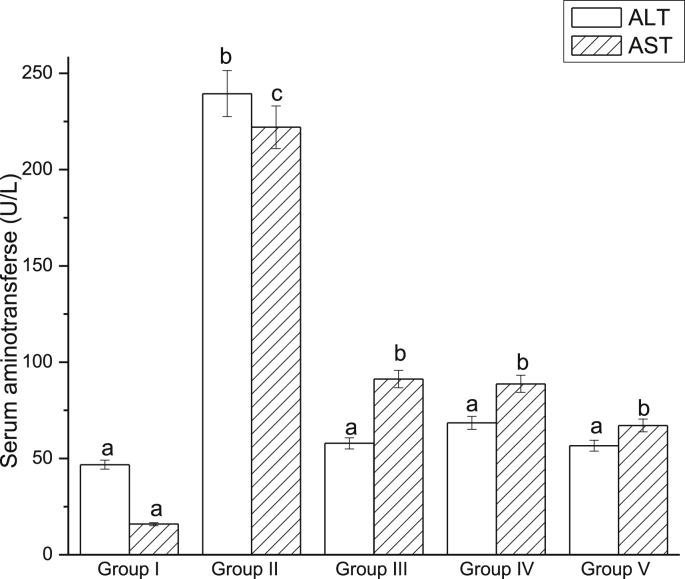
3.3.2. EWE inhibited the increase of serum aminotransferase induced by CCl4
The results of hepatoprotective effect of EWE on the serum ALT and AST activities are shown in Fig. 4. In CCl4-intoxicated group, serum ALT and AST activities were 239.46 and 11.97 U/L, respectively, whereas the values of control group were only 46.79 and 2.34 U/L, respectively. Therefore, a significant increase in the activities of serum ALT and AST was observed when liver was exposured to CCl4 (P < 0.05). Administration with different doses of EWE for 49 days significantly reduced the activities of serum ALT and AST as compared to the control group (P < 0.05) in a dose-dependent manner. Silymarin, which has been used as medicine for liver disease, had superior effects on decreasing serum ALT and AST activities.
Fig. 4.
Effects of EWE on serum ALT and AST. Different lower case letters correspond to significant differences at P < 0.05. Group I was the control group. Group II was CCl4-treated group. Group III was given CCl4 + silymarin. Group IV and V were given CCl4 + 2.0 and 4.0 g/kg BW of EWE respectively.
3.3.3. EWE decreased hepatic MDA content, SOD and GSH-Px activities
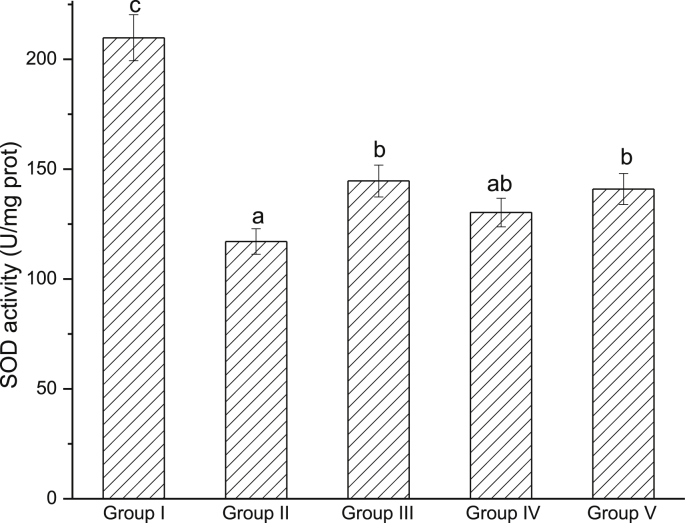
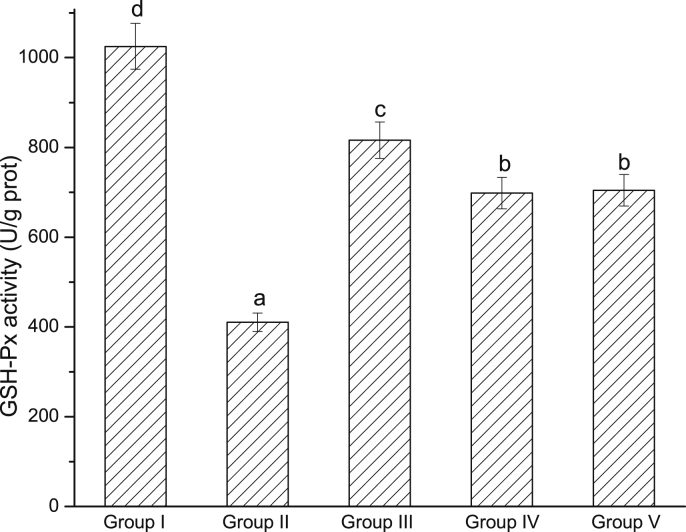
The levels of MDA, SOD and GSH-Px are indicators of oxidative stress. A 30.77% increase of MDA content was observed in model group compared with control group (Fig. 5). Pretreatment with EWE and silymarin could completely inhibit CCl4-induced increase of liver MDA content. As shown in Fig. 6, Fig. 7, hepatic SOD and GSH-Px activities were decreased by 44.21% and 59.96%, respectively, after CCl4 treatment. However, pretreatment with EWE significantly increased SOD and GSH-Px activities (P < 0.05).
Fig. 5.
Effects of EWE on hepatic MDA. Different lower case letters correspond to significant differences at P < 0.05. Group I was the control group. Group II was CCl4-treated group. Group III was given CCl4 + silymarin. Group IV and V were given CCl4 + 2.0 and 4.0 g/kg BW of EWE respectively.
Fig. 6.
Effects of EWE on hepatic SOD activity. Different lower case letters correspond to significant differences at P < 0.05. Group I was the control group. Group II was CCl4-treated group. Group III was given CCl4 + silymarin. Group IV and V were given CCl4 + 2.0 and 4.0 g/kg BW of EWE respectively.
Fig. 7.
Effects of EWE on hepatic GSH-Px activity. Different lower case letters correspond to significant differences at P < 0.05. Group I was the control group. Group II was CCl4-treated group. Group III was given CCl4 + silymarin. Group IV and V were given CCl4 + 2.0 and 4.0 g/kg BW of EWE respectively.
3.4. Histopatholgy
The histological observations of the hepatoprotective effects of EWE on CCl4-induced liver damage are illustrated in Fig. 8. Liver sections from control mice displayed regular cellular morphology (Fig. 8A). Compared with the normal liver tissues of control mice, liver tissue in CCl4-intoxicated mice revealed liver injury characterized by dilated sinusoidal spaces, and inflammatory cell infiltration (Fig. 8B). However, the hepatic lesions induced by treatment with CCl4 were remarkably ameliorated in hypertrophy of hepatocytes, dilated sinusoidal spaces and inflammatory cell infiltration by treatment with silymarin and different dose of EWE (Fig. 8C–E).
Fig. 8.
Effects of EWE on hepatic morphological analysis (×200 H&E): control mice (A), CCl4-treated mice (B), mice pretreated with silymarin prior to CCl4 (C), mice pretreated with 2.0 (D) and 4.0 g/kg (E) BW of EWE respectively prior to CCl4. (➝ dilated sinusoidal spaces, ⇨ inflammatory cell infiltration).
4. Discussion
The therapeutic benefits of Chinese herbal medicine have been recognized for centuries on the basis of clinical experience and practice. Qinpi, the stem barks of F. rhynchophylla Hance, is a traditional Chinese herbal drug for treating various types of chronic diseases.
ALT and AST are aminotransferases and associated with liver parenchymal cell. The enzymes normally present in the cytosol and are leaked out into the blood stream, when the hepatocellular plasma membrane is damaged. Thus, ALT and AST serum levels are very sensitive markers employed in the diagnosis of liver diseases. Rats administered once with CCl4 exhibited liver injury, as indicated by significant elevation in serum markers for hepatic cell damage (ALT and AST).37 At present study, in EWE-treated groups (2.0 and 4.0 g/kg) of rats, the studies showed that treatment with EWE significantly and dose-dependently decreased the elevations of serum levels of ALT and AST. Furthermore, the improvement of the hepatic impairment was achieved and could be observed by EWE intervention in CCl4-induced liver injury. Thus, the results showed the protective effects of EWE on hepatic pathology and orally administered EWE exerted therapeutic effects on CCl4-induced liver injury in rats.
Oxidative stress associated with lipid peroxidation is involved in liver development. The extracts and the identified compounds from F. rhynchophylla Hance had been reported anti-oxidative activity.30, 31, 32 MDA is an aldehyde, which is a product of polyunsaturated fatty acid peroxidation, and is a highly toxic molecule considered as more than just a marker of lipid peroxidation. In this study, the elevated hepatic MDA level was decreased by pretreatment of EWE, so it indicated that EWE could protect the liver against CCl4-induced lipid peroxidation. GSH and SOD are a host of antioxidant systems to protect the hepatocyte from oxidative stress. GSH, or GSH-Px, and SOD work in concert to control the cascades of uncontrolled lipid peroxidation and protect cell from oxidative damage by scavenging of reactive oxygen species (ROS) or the toxic free radicals.38, 39, 40 In this study, the CCl4-model rats present lower GSH-Px and SOD level than the control group. Thus, it was supposed that EWE possessed the properties to enhance GSH-Px and SOD, and could prevent hepatice impairment via inhibiting oxygen-free radicals and increasing anti-oxidation in CCl4-induced liver injury in rats.
5. Conclusion
In conclusions, the current research indicates that the EtOH–water extract (EWE) from the seeds of F. rhynchophylla Hance decreased liver index, inhibited the increase of serum aminotransferase induced by CCl4, and decreased hepatic MDA content, SOD and GSH-Px activities. These results suggested that the pretreatment with EWE protected mice against CCl4-induced liver injuries. Further hepatoprotective effect and the possible mechanism of active constituents of the seeds of F. rhynchophylla Hance will be studied.
Conflict of interests
The authors declare that they have no competing interest.
Acknowledgment
This study was supported by a grant from the National Natural Science Foundation of China (No. 31570348) and Shaanxi Science and Technology Foundation (No. 2016JM2001).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Alter M.J. Epidemiology of viral hepatitis and HIV coinfection. J Hepatol. 2006;44:S6. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Lee C.P., Shih P.H., Hsu C.L. Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol. 2007;45:888–895. doi: 10.1016/j.fct.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Cemek M., Aymelek F., Buyukokuroglu M.E. Protective potential of Royal Jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food Chem Toxicol. 2010;48:2827–2832. doi: 10.1016/j.fct.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Jia X.Y., Zhang Q.A., Zhang Z.Q. Hepatoprotective effects of almond oil against carbon tetrachloride induced liver injury in rats. Food Chem. 2011;125:673–678. [Google Scholar]
- 5.Stickel F., Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh N., Ghosh R., Mandal V. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol. 2011;49:970–988. doi: 10.3109/13880209.2011.558515. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A., Shivanandappa T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2010;118:411–417. [Google Scholar]
- 8.Bishayee A., Darvesh A.S., Politis T. Resveratrol and liver disease: from bench to bedside and community. Liver Int. 2010;30:1103–1114. doi: 10.1111/j.1478-3231.2010.02295.x. [DOI] [PubMed] [Google Scholar]
- 9.Lal A.A., Murthy P.B., Pillai K.S. Screening of hepatoprotective effect of a herb mixture against CCl4 induced hepatotoxicity in Swiss albino mice. J Environ Biol. 2007;28:201–207. [PubMed] [Google Scholar]
- 10.Yoshikawa M., Matsui Y., Kawamoto H. Effects of glycyrrhizin on immune-mediated cytotoxicity. J Gastroenterol Hepatol. 1997;12:243–248. doi: 10.1111/j.1440-1746.1997.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 11.Ravindran P.N., Nirmal Babu K., Sivaraman K. CRC Press; Boca Raton, BR: 2007. Turmeric: The Genus Curcuma. [Google Scholar]
- 12.Sener G., Toklu H.Z., Sehirli A.Q. Protective effects of resveratrol against acetaminophen-induced toxicity in mice. Hepatol Res. 2006;35:62–68. doi: 10.1016/j.hepres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Sturm S., Stuppner H. Analysis of iridoid glycosides from Picrorhiza kurroa, by capillary electrophoresis and high performance liquid chromatography-mass spectrometry. Chromatographia. 2001;53:612–618. [Google Scholar]
- 14.Li C.C., Hsiang C.Y., Wu S.L. Identification of novel mechanisms of silymarin on the carbon tetrachloride-induced liver fibrosis in mice by nuclear factor-kB bioluminescent imaging-guided transcriptomic analysis. Food Chem Toxocol. 2012;50:1568–1575. doi: 10.1016/j.fct.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Dou Y., Tian J. Research on medical specialty of traditional Chinese medicines using dot-immunoblotting method based on polyclonal antibody prepared from traditional Chinese medicines with hot/cold nature. Zhongguo Zhongyao Zazhi. 2009;34:438–442. [PubMed] [Google Scholar]
- 16.National Pharmacopoeia Committee . 2010. Pharmacopoeia of People's Republic of China. I. Appendix 271. [Google Scholar]
- 17.Yin M.H., Lu H.Z., Jiang L.J. Protective effect of ash bark extract against acute liver injury in mice. Lishizhen Med Materia. 2007;18:590–591. [Google Scholar]
- 18.Kostova I., Iossifova T. Chemical components of Fraxinus species. Fitoterapia. 2007;78:85–106. doi: 10.1016/j.fitote.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Kwon Y.S., Kim C.M. A study on the chemical constituents from leaves of. Fraxinus rhynchophylla, Canis Et Felis. 1996;8:52–63. [Google Scholar]
- 20.Si C.L., Zhang Y., Zhu Z.Y. Isolation and structure elucidation of secoiridoid glucosides from Fraxinus rhynchophylla leaves. Chem Nat Compd. 2009;45:814–816. [Google Scholar]
- 21.Si C.L., Liu Z., Su Y.F. Coumarins and secoiridoid glucosides from bark of Fraxinus rhynchophylla Hance. Holzforschung. 2008;62:553–555. [Google Scholar]
- 22.Ahn J.H., Hwang B.Y., Lee M.K. Simultaneous quantitation of nine constituents of Fraxinus rhynchophylla using high performance liquid chromatography-diode array detector. Nat Prod Sci. 2013;19:236–241. [Google Scholar]
- 23.Wu Z.B., Liu Y., Tian S.S. Chemical constituents of the stem bark of Fraxinus rhynchophylla. Chem Nat Compd. 2014;49:1162–1163. [Google Scholar]
- 24.Shin E., Choi K.M., Yoo H.S. Inhibitory effects of coumarins from the stem barks of Fraxinus rhynchophylla on adipocyte differentiation in 3T3-L1 cells. Biol Pharm Bull. 2010;33:1610–1614. doi: 10.1248/bpb.33.1610. [DOI] [PubMed] [Google Scholar]
- 25.Choi K.M., Shin E., Liu Q. Hydroxyframoside B, a secoiridoid of Fraxinus rhynchophylla, inhibits adipocyte differentiation in 3T3-L1 cells. Planta Med. 2011;77:1020–1023. doi: 10.1055/s-0030-1270712. [DOI] [PubMed] [Google Scholar]
- 26.Ahn J.H., Shin E., Liu Q. Lignan derivatives from Fraxinus rhynchophylla and inhibitory activity on pancreatic lipase. Nat Prod Sci. 2012;18:116–120. [Google Scholar]
- 27.Ahn J.H., Shin E., Liu Q. Secoiridoids from the stem barks of Fraxinus rhynchophylla with pancreatic lipase inhibitory activity. Nat Prod Res. 2013;27:1132–1135. doi: 10.1080/14786419.2012.711328. [DOI] [PubMed] [Google Scholar]
- 28.Xiao K., Song Q.H., Zhang S.W. Water-soluble constituents of the root barks of Fraxinus rhynchophylla (Chinese drug Qinpi) J Asian Nat Prod Res. 2008;10:205–210. doi: 10.1080/10286020701394514. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J.H., Jin C.M., Kim Y.C. Anti-toxoplasmosis effects of oleuropein isolated from Fraxinus rhynchophylla. Biol Pharm Bull. 2008;31:2273–2276. doi: 10.1248/bpb.31.2273. [DOI] [PubMed] [Google Scholar]
- 30.Thuong P.T., Pokharel Y.R., Lee M.Y. Dual anti-oxidative effects of Fraxetin isolated from Fraxinus rhynchophylla. Biol Pharm Bull. 2009;32:1527–1532. doi: 10.1248/bpb.32.1527. [DOI] [PubMed] [Google Scholar]
- 31.Yang E.J., Kim S.I., Ku H.Y. Syringin from stem bark of Fraxinus rhynchophylla protects Aβ(25–35)-induced toxicity in neuronal cells. Arch Pharm Res. 2010;33:531–538. doi: 10.1007/s12272-010-0406-z. [DOI] [PubMed] [Google Scholar]
- 32.Kim N.Y., Pae H.O., Ko Y.S. In vitro inducible nitric oxide synthesis inhibitory active constituents from Fraxinus rhynchophylla. Planta Med. 1999;65:656–658. doi: 10.1055/s-2006-960840. [DOI] [PubMed] [Google Scholar]
- 33.Shi J., Li C.J., Yang J.Z. Hepatoprotective coumarins and secoiridoids from Hydrangea paniculata. Fitoterapia. 2014;96:138–145. doi: 10.1016/j.fitote.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Zhao G., Yin Z., Dong J. Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L. var.grandiflorum. J Ethnopharmacol. 2009;125:265–268. doi: 10.1016/j.jep.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Domitrović R., Jakovac H., Marchesi V.V. Preventive and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacol Res. 2012;65:451–464. doi: 10.1016/j.phrs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Peng W.H., Tien Y.C., Huang C.Y. Fraxinus rhynchophylla ethanol extract attenuates carbon tetrachloride-induced liver fibrosis in rats via down-regulating the expressions of uPA, MMP-2, MMP-9 and TIMP-1. J Ethnopharmacol. 2010;127:606–613. doi: 10.1016/j.jep.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Carobene A., Braga F., Roraas T. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and y-glutamyl transferase. Clin Chem Lab Med. 2013;51:1997–2007. doi: 10.1515/cclm-2013-0096. [DOI] [PubMed] [Google Scholar]
- 38.Valko M., Rhodes C.J., Moncol J. Free radicals, metals and antioxidants in oxidative stress-include cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Owen J.B., Butterfield D.A. Measurement of oxidized/reduced glutathione ratio. Methods Mol Biol. 2010;648:269–277. doi: 10.1007/978-1-60761-756-3_18. [DOI] [PubMed] [Google Scholar]
- 40.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]