Abstract
Radiology provides a crucial clinical adjunct in patients with plasma cell disorders, in particular multiple myeloma, and its uses are evolving and expanding. This pictorial review illustrates the role of imaging throughout the patient’s clinical course, with specific reference to recently updated international diagnostic criteria. At presentation, imaging optimises characterisation and staging of the plasma-cell disorder, while later in the course of the disease, its roles include the monitoring of disease progression, assessment of post-treatment response and the investigation of clinical deterioration.
Keywords: Magnetic resonance imaging, multiple myeloma, plasmacytoma
Introduction
Multiple myeloma (MM) is a neoplastic proliferation of plasma cells which affects multiple organ systems. It typically evolves from monoclonal gammopathy of unknown significance (MGUS) and smouldering myeloma.1 The spectrum of neoplastic plasma cell disorders also includes solitary plasmacytoma arising from bone or soft tissue, multiple plasmacytomas (regarded as an unusual form of myeloma and treated as such) and the rare condition plasma cell leukaemia.1 Radiology provides a crucial clinical adjunct to blood tests, serum examination and bone-marrow biopsy in patients with plasma cell disorders. This pictorial review illustrates the importance of imaging throughout the patient’s clinical course. At diagnosis, radiology aids in the correct characterisation and staging of the plasma cell disorder, and identifies appropriate targets for histological confirmation. Later in the course of the disease, imaging is important for disease monitoring, assessment of post-treatment response and the investigation of clinical deterioration. It plays an important role in guiding management, for example the commencement of systemic therapy upon progression to MM, or radiotherapy or surgery to critical lesions causing neurologic compromise.
Diagnosis and disease characterisation
According to the recently updated International Myeloma Working Group (IMWG) criteria (2014), the diagnosis of MM requires that all three of the following be met: ≥10% monoclonal plasma cells in the bone marrow and/or a biopsy-proven plasmacytoma; monoclonal protein in the serum and/or urine; and at least one feature of myeloma-related organ dysfunction as per the ‘CRAB’ criteria, namely serum calcium elevation, renal insufficiency, anaemia and lytic bone lesions (≥5 mm).1 Skeletal involvement is reported as occurring in 80–90% of patients with MM,2 highlighting the importance of imaging in the diagnosis of MM. Osteoporosis was considered evidence of myeloma bone disease in the preceding version of the IMWG criteria,3 being taken to reflect diffuse bone marrow infiltration. However, this has been removed from the current criteria to avoid over-diagnosis,1 as it may be incidental to the plasma cell disorder in this typically older age group. Another important update to the diagnostic criteria is that the presence of more than one focal lesion on magnetic resonance imaging (MRI) ≥5 mm in size is also considered evidence of myeloma bone disease.1 This modification recognises the important role of MRI and hopes to identify disease progression earlier, thus allowing treatment before substantial end-organ damage has occurred.1
Radiology is also important for the diagnosis and correct characterisation of other plasma cell disorders. The diagnosis of both MGUS and smouldering myeloma requires that no ‘CRAB’ features be present. Hence, imaging is required to exclude focal bone lesions, which would up-stage the patient to MM.1. Less frequently than MM, a solitary plasmacytoma can occur in the absence of other features of MM, either a solitary bone plasmacytoma (SBP) or solitary extramedullary plasmacytoma (SEP). Both conditions have a better prognosis than MM.4 Radiotherapy can provide high rates of local control in SBP, but the majority of patients progress to MM, after a median of two to four years.4 SEP has a better prognosis than SBP, as the majority of patients can be cured with local radiotherapy, and the rates of progression to MM are substantially lower.4 In both SPB and SEP, radiology is important to exclude additional lesions in addition to characterisation of the index lesion. For example, MRI of the spine can detect evidence of more extensive disease in 26–33% of patients thought to have SBP,5,6 thus up-staging the patient to MM.
Imaging appearances
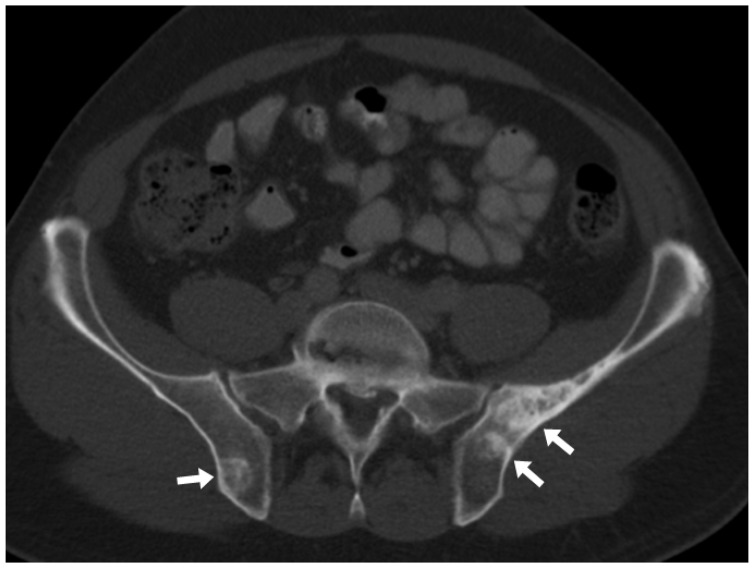
Traditionally, X-ray skeletal surveys have been the imaging of choice in the work-up of patients with suspected myeloma and for follow-up. The typical plain film appearance of a plasmacytoma is that of a well-circumscribed lytic lesion. Expansion can occur, but is often better appreciated in locations such as the ribs than in the spine. Sclerotic lesions (Figure 1) are less common and should raise the suspicion of POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein and skin changes).7 Vertebral crush fractures are common, related to osteopaenia and/or discrete lesions (Figures 2 and 3). If seen in a young patient, further imaging is warranted to look for underlying marrow pathology.1 In recent times, some centres have introduced low-dose whole-body computed tomography (CT) and/or whole-body MRI as an alternative to traditional skeletal surveys, with both techniques being shown to have greater sensitivity.8
Figure 1.
Axial CT scan on bone windows showing multiple sclerotic lesions in the bony pelvis in a patient with osteosclerotic myeloma. CT: computed tomography.
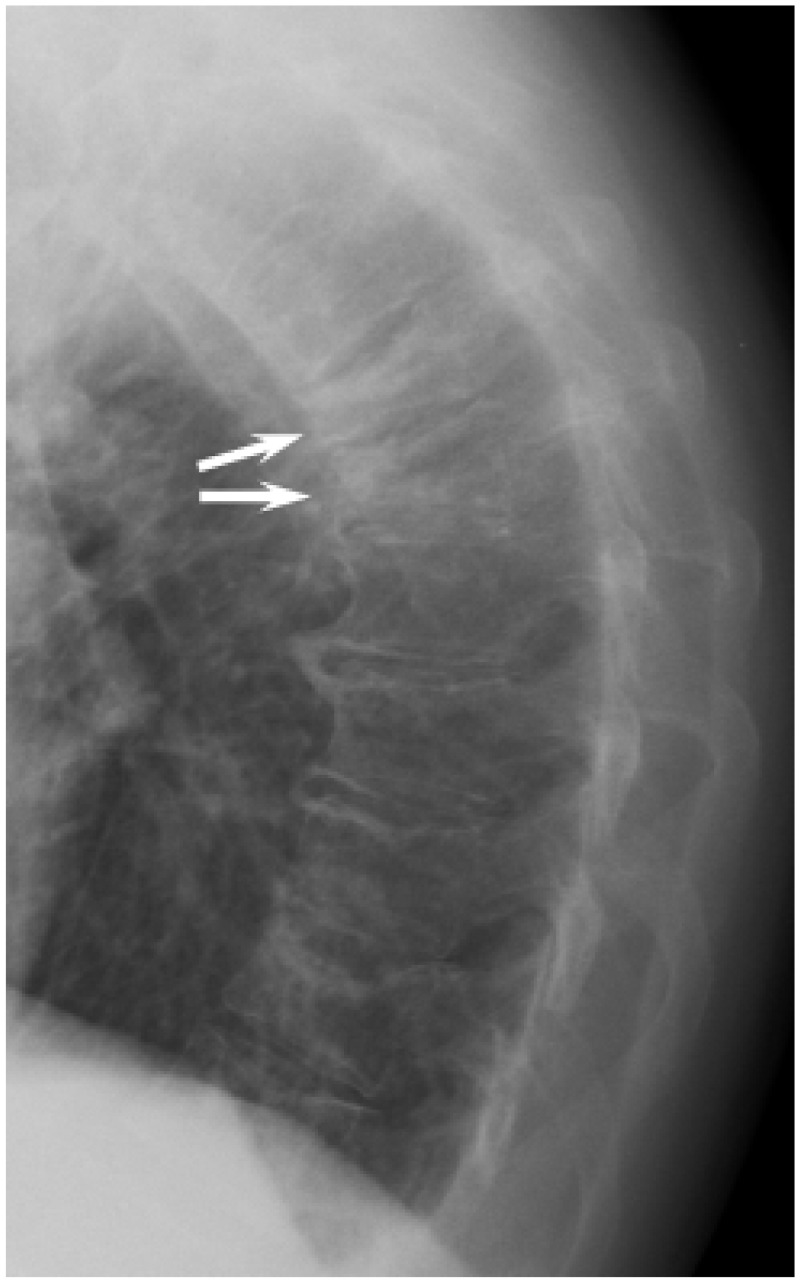
Figure 2.
Lateral chest X-ray showing two contiguous thoracic vertebral crush fractures (arrows). The visualised spine is osteopaenic, but there are no definite discrete lesions.
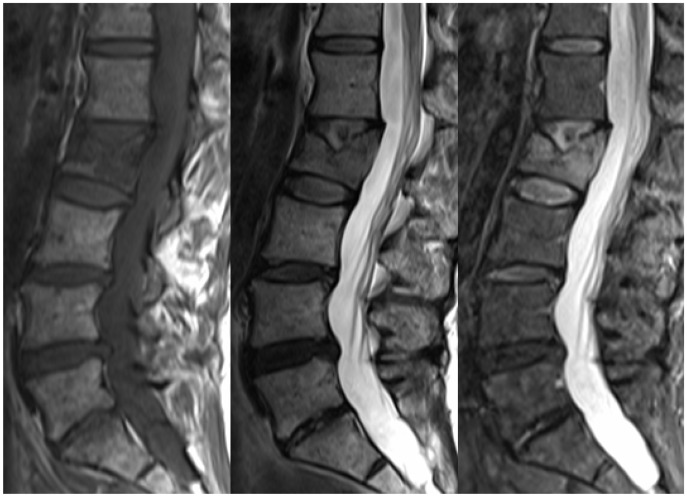
Figure 3.
Sagittal T1, T2 and T2 STIR images of the lumbar spine showing a pathological crush fracture of the L2 vertebral body. The diffuse T1 hypointensity of the vertebral body indicates an underlying lesion, consistent with a plasmacytoma given known MM. MM: multiple myeloma.
The CT appearances of MM are analogous to the plain film findings (Figure 4), though with the inherent greater sensitivity of CT. CT also better demonstrates features such as cortical breach, discrete fracture lines and extra-osseous extension. In addition, CT may visualise extramedullary plasmacytomas (Figure 5), which are most common in the soft tissues surrounding the axial skeleton, and occur in about 13% of patients with MM through the course of their disease.9
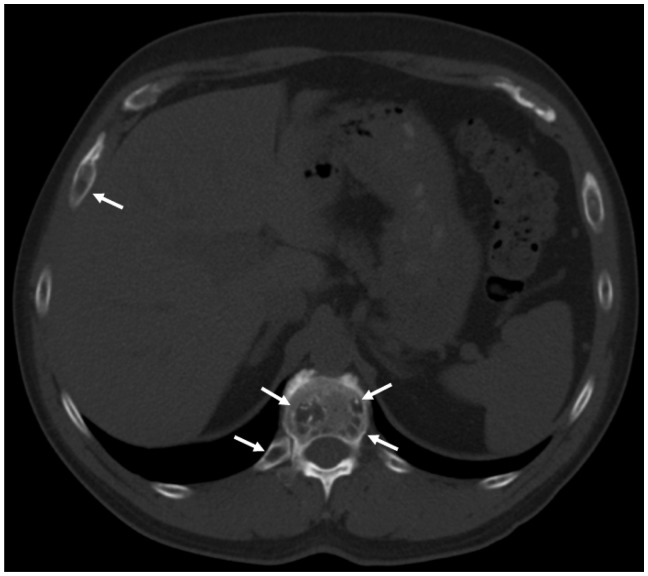
Figure 4.
Axial CT of the upper abdomen on bone windows demonstrating multiple lytic vertebral and rib lesions (arrows). CT: computed tomography.
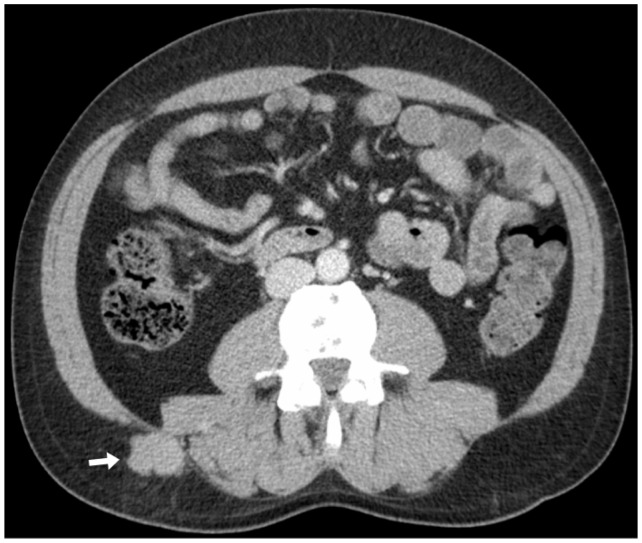
Figure 5.
Axial CT on soft-tissue windows shows a subcutaneous mass in the right flank adjacent to the paravertebral musculature (arrowhead), proven to represent an extramedullary plasmacytoma on biopsy. CT: computed tomography.
MRI has a number of advantages over other imaging modalities and has become part of the routine assessment of patients with plasma cell disorders. The typical appearances of a plasmacytoma are that of a T1- and T2-hypointense mass, which shows high signal on T2 STIR or T2 fat-saturated images. Contrast administration is not necessary routinely, but will show enhancement of the lesion(s) (Figure 6). The appearances described above are the same as for differentials, including metastatic disease, lymphoma and some primary bone tumours, but the clinical context will generally favour MM over these alternatives. A single bone lesion on MRI is not sufficient evidence of myeloma bone disease, but may warrant further investigation with CT (as one lytic lesion on CT is sufficient for the diagnosis) and/or biopsy.1
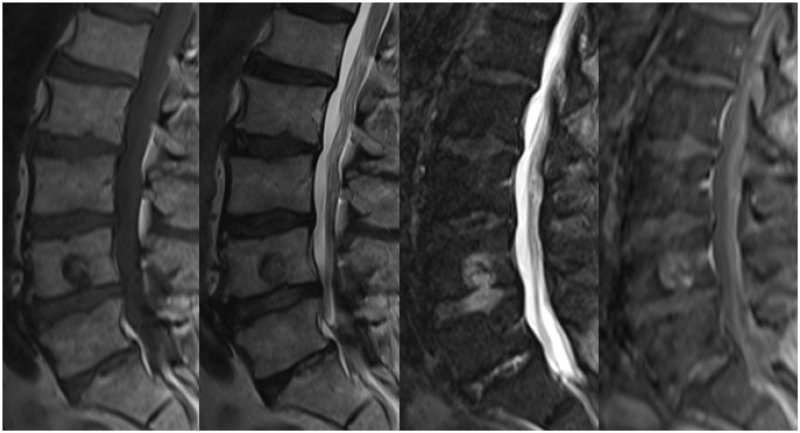
Figure 6.
Sagittal T1, T2, T2 STIR and T1 post-contrast with fat saturation images demonstrating a plasmacytoma in the L4 vertebral body. The mass is hypointense on the T1 and T2 images, hyperintense on the T2 STIR sequence and demonstrates enhancement. The background marrow appearances are normal.
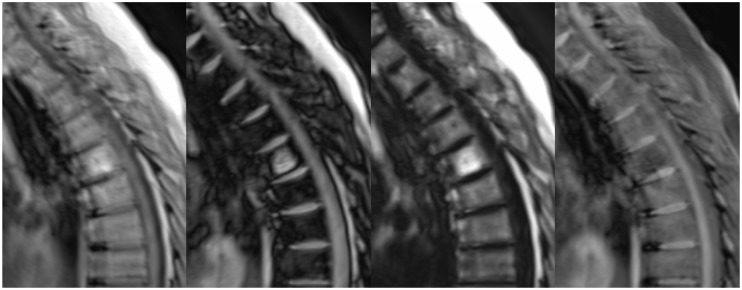
MRI will often show evidence of diffuse bone marrow infiltration, manifest as T1- and T2-hypointensity, though this is not sufficient to up-stage MGUS, SBP or SEP to MM.1 Stäbler et al. described five appearances of the vertebral bone marrow in the setting of MM, namely normal, diffuse infiltration, focal lesion(s), combined focal and diffuse infiltration and a salt-and-pepper appearance (Figure 7).10 In their study, normal and salt-and-pepper appearances both correlated with stage I disease clinically, while the other appearances were associated with more advanced disease.10 Marrow infiltration may mask the presence of discrete lesions unless T2 STIR, T2 fat-saturated and/or contrast-enhanced images are utilised (Figure 8).
Figure 7.
Sagittal T1 images showing possible appearances of the vertebral marrow: normal, diffuse infiltration, discrete lesions and a salt-and-pepper appearance.
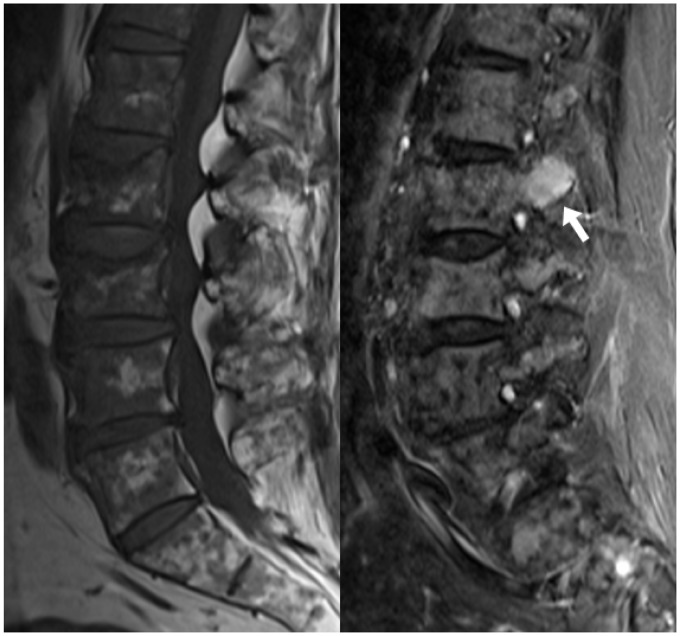
Figure 8.
The sagittal T1 image of the lumbar spine shows a salt-and-pepper appearance of the vertebral marrow in a patient with MM, obscuring discrete lesions. The T2 STIR sequence, however, reveals a discrete hyperintense mass within the left posterior elements of L2 (arrow), consistent with a plasmacytoma. MM: multiple myeloma.
The Dixon technique, which separates fat and water based on their chemical shift, aids characterisation of focal lesions, in particular the differentiation between plasmacytomas and incidental haemangiomas. After acquiring in-phase and out-of-phase images, these are added and subtracted to receive fat- and water-selective images, respectively. Haemangiomas typically contain fat, and will thus have high signal on fat-selective images and low signal on water-selective images (Figure 9). Plasmacytomas will show the opposite signal characteristics, being hyperintense on the water-selective images and hypointense on fat-selective images (Figure 10). This technique also increases conspicuity of plasmacytomas in the setting of bone marrow infiltration, with the plasmacytomas shown as hyperintense on out-of-phase images.11
Figure 9.
Sagittal in-phase, out-of-phase, fat-selective and water-selective images of a haemangioma within the T6 vertebral body. Most notably, the lesion shows high signal on the fat-selective image but low signal on the water-selective image.
Figure 10.
Sagittal in-phase, out-of-phase, fat-selective and water-selective images of a plasmacytoma within the L4 vertebral body. In contrast to Figure 9, the lesion shows low signal on the fat-selective image and high signal on the water-selective image. Note the fatty marrow signal (high signal on the fat-selective image) in the L5 vertebral body and sacrum due to radiotherapy for management of a previous sacral plasmacytoma.
Monitoring of precursor conditions
MGUS and smouldering myeloma do not typically require treatment outside the context of a clinical trial. Similarly, radiotherapy provides high rates of local control for patients with a solitary plasmacytoma,4 and they do not generally require systemic treatment at diagnosis. The prognosis of patients with these precursor conditions is largely related to progression of their disease to MM, however, rather than the initial diagnosis itself. Modern treatment of MM is both safer and more effective than previously. Thus, it is important to detect progression to MM early, and imaging follow-up plays a crucial role in this. Early detection of progression allows the prompt institution of systemic therapies in an effort to minimise end-organ damage.
Occasionally, imaging features of patients with these precursor conditions do not meet the criteria for MM, but suggest a greater risk of progression to MM. Such high-risk imaging findings include evidence of diffuse bone marrow infiltration on MRI; a single bone lesion on MRI; multiple MRI lesions <5 mm in size that remain indeterminate after CT and/or biopsy; and equivocal or tiny lucencies seen on CT (including computed tomography–positron emission tomography).1 These features warrant a shorter imaging follow-up interval (three to six months).1
Post-treatment follow-up
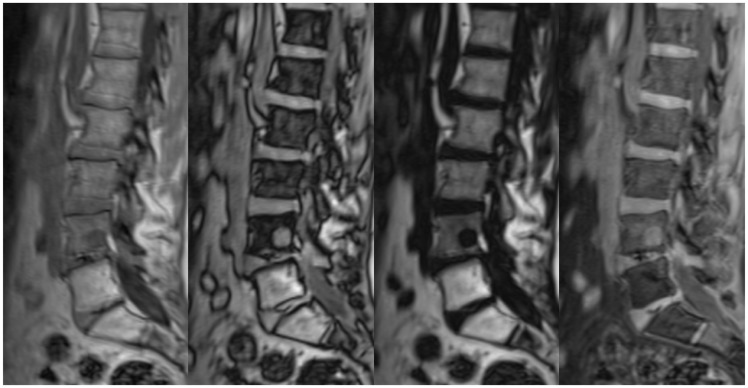
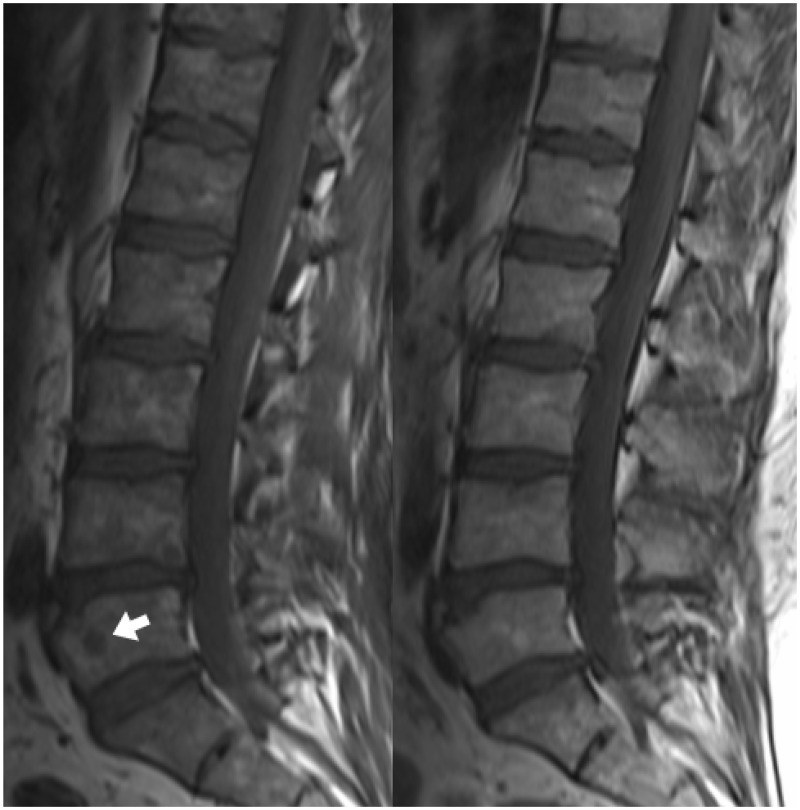
Once a diagnosis of MM has been made, treatment is warranted, and imaging provides information on response. In patients with bone marrow involvement on MRI, successful treatment typically results in an increase (or normalisation) of the T1 signal, reflecting a greater proportion of fatty marrow (Figure 11). This may also manifest as a change in the MRI pattern of marrow infiltration, for example from diffuse infiltration to a salt-and-pepper appearance or focal lesions (Figure 12). The marrow signal can return to normal, though the persistence of signal abnormality does not exclude a complete response to treatment.12 Imaging also assesses the response to treatment of discrete plasmacytomas. This is particularly important for lesions that are symptomatic or produce neurological compromise. There may be a significant delay between systemic therapy and an appreciable structural change, however, and discrete lesions may remain stable in size, despite a satisfactory response.13 PET is useful in this setting, helping to distinguish between active disease and fibrotic lesions.14
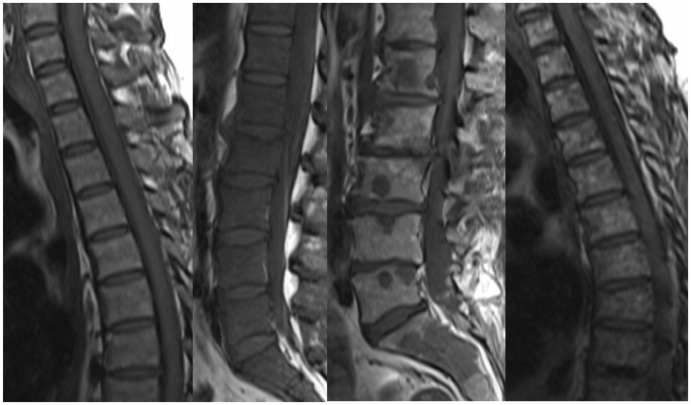
Figure 11.
Sagittal T1 images of the lumbar spine in a patient with MM, pre and post treatment. Before treatment, the T1 signal is heterogeneous and generally decreased. There is also a small T1-hypointense lesion in the L5 vertebral body (arrow). After treatment, the T1 signal has normalised, and the L5 lesion is no longer visible. MM: multiple myeloma.
Figure 12.
Sagittal T1 images pre and post treatment in another patient. Before treatment, the T1 signal is diffusely decreased, consistent with diffuse bone-marrow infiltration. After treatment, the appearances have altered to a ‘salt-and-pepper’ pattern. Crush fractures of the T11 and L2 vertebral bodies are also present.
More recently, advanced MRI techniques such as whole-body diffusion-weighted imaging and whole-body dynamic contrast-enhanced imaging have been shown to be valuable in differentiating between good and poor responders to treatment.15,16 As bone marrow involvement is heterogeneous, bone marrow biopsy is liable to sampling error, and imaging has the potential to assess overall disease burden better. MRI is likely to be particularly useful in patients with oligo- or non-secretory MM,16 in whom the value of bone marrow biopsy is lower. Further research in such techniques may decrease the reliance on bone marrow biopsy and its associated morbidity.
Disease complications
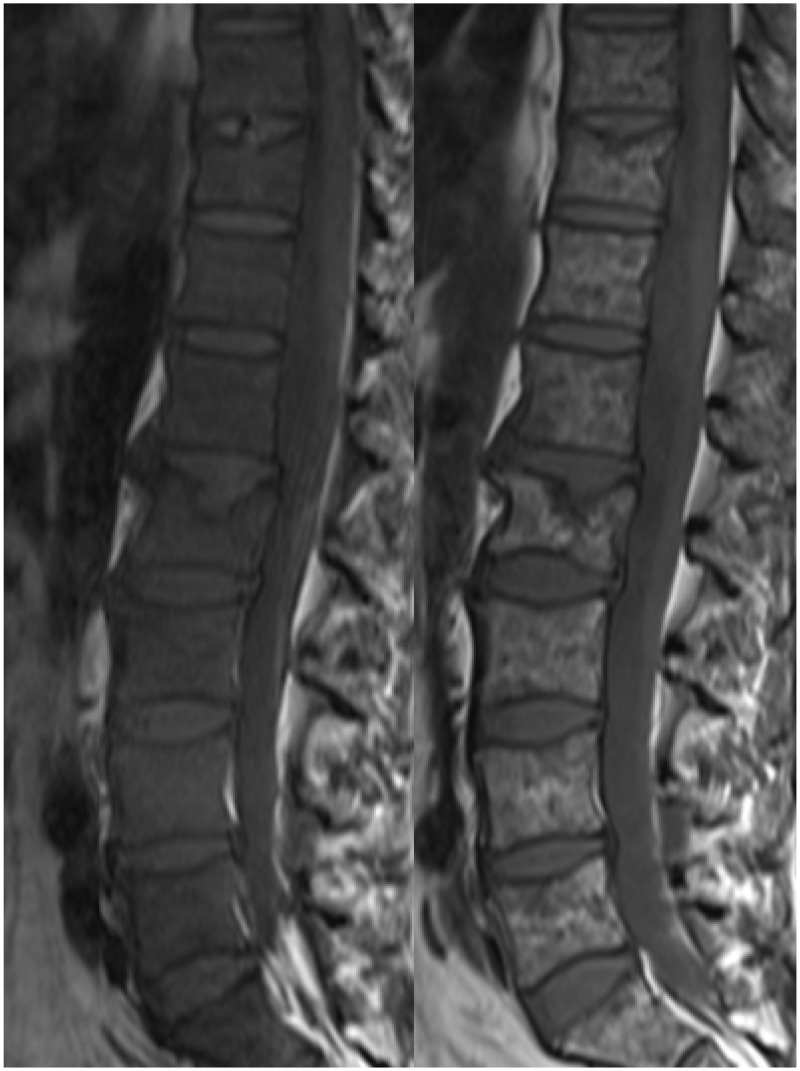
Imaging is also important for evaluating patients when they deteriorate clinically. Back pain is a common complaint in patients with MM, and possible causes include the development of a new lesion and/or a pathological fracture (Figure 13). Identification of a symptomatic lesion allows radiotherapy for pain relief, and radiotherapy may also promote healing in the setting of a pathological fracture.2 The possibility of vertebral infection should also be considered as a possible cause of back pain.17 Patients with MM are at increased risk of infection due to both the disease and the treatment, and infection is a major cause of morbidity and mortality in MM patients.18 Chemotherapy with dexamethasone may be a predisposing factor for vertebral infection.17 The appearances are as for vertebral osteomyelitis and discitis occurring outside of the context of MM.
Figure 13.
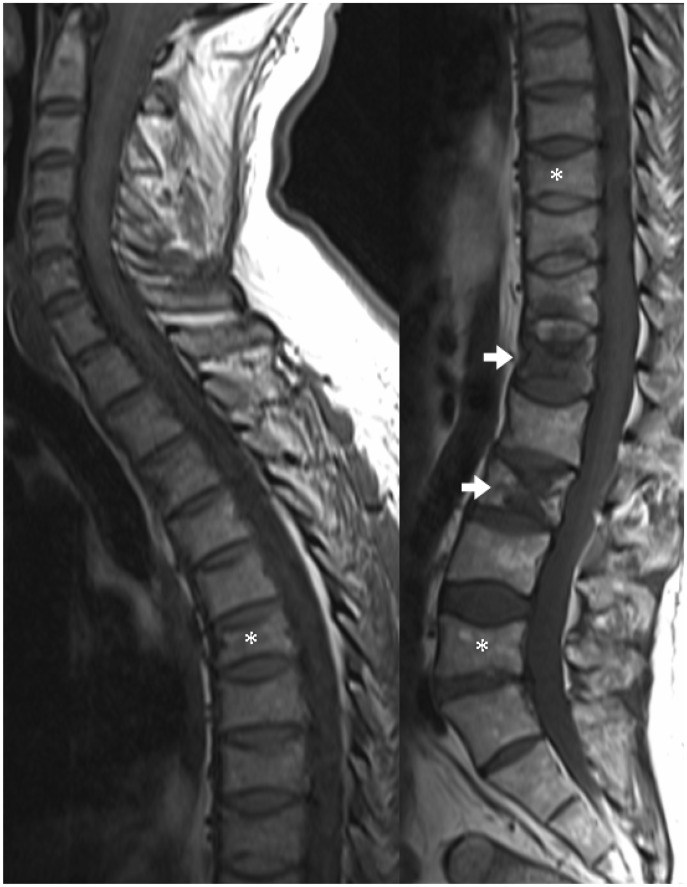
Sagittal T1 images of the upper and lower spine in a patient with multiple vertebral plasmacytomas. Multiple vertebral crush fractures are present, some due to underlying bone lesions (arrows) and others without discrete lesions (asterisks).
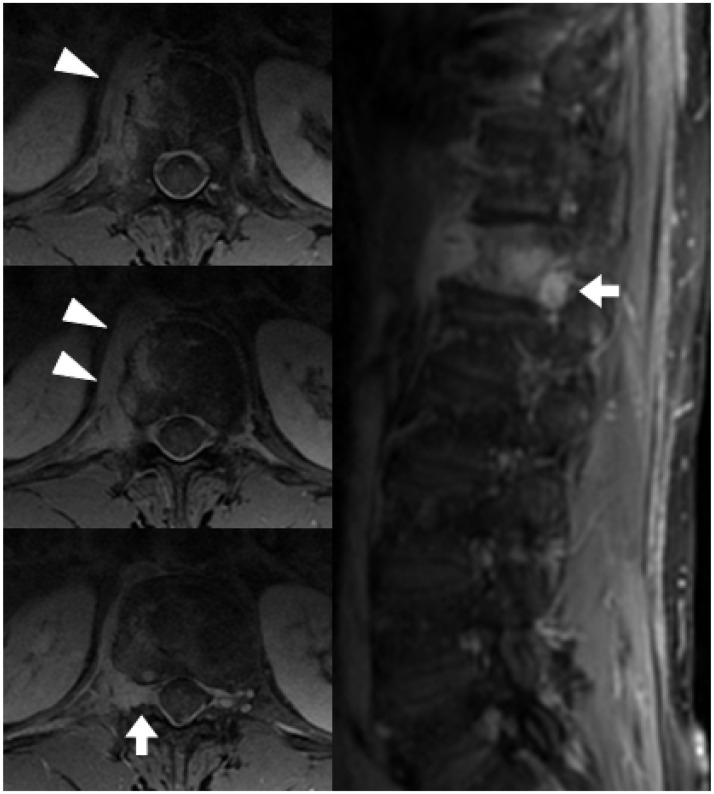
Spinal cord compression is cited as occurring in up to 20% of MM patients during the course of their disease, and MRI is the investigation of choice.2,18 It is a medical emergency, as a delay in diagnosis and treatment may leave a patient with significant ongoing neurological impairment. Spinal cord compression can either be due to extra-osseous extension of a plasmacytoma (Figure 14) or, less frequently, a pathological crush fracture.2,18 Differentiation between these two causes is important to determine the most appropriate management. Radiotherapy is the treatment of choice if the cord compression is due to extra-osseous extension of tumour, as plasmacytomas are highly radio-sensitive, and should be performed within 24 hours.2 If due to a pathological fracture, however, surgical decompression and/or stabilisation is warranted, often consolidated by postoperative radiotherapy.2 Radiology subsequently provides important information on the adequacy of treatment. Extra-osseous tumour can also involve the neural exit foramina (Figure 15), with the potential for nerve root compression or infiltration.
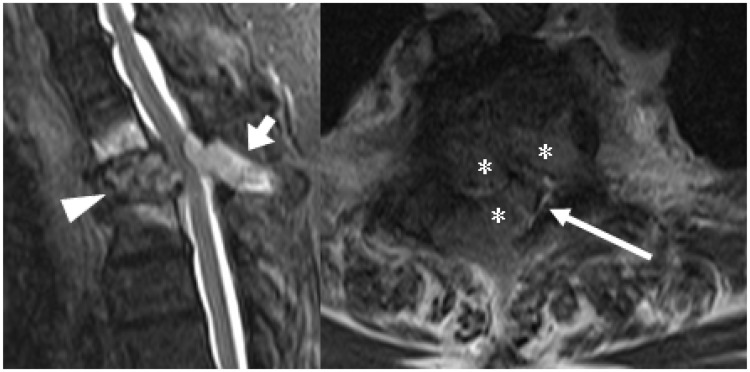
Figure 14.
The sagittal STIR image (left) in a patient with leg symptoms shows a plasmacytoma involving both the anterior (arrowhead) and posterior elements (short arrow) of the T2 vertebra. The axial T2 image (right) confirms the presence of tumour extending into the central vertebral canal (asterisks), compressing and distorting the spinal cord (long arrow), but without cord signal change.
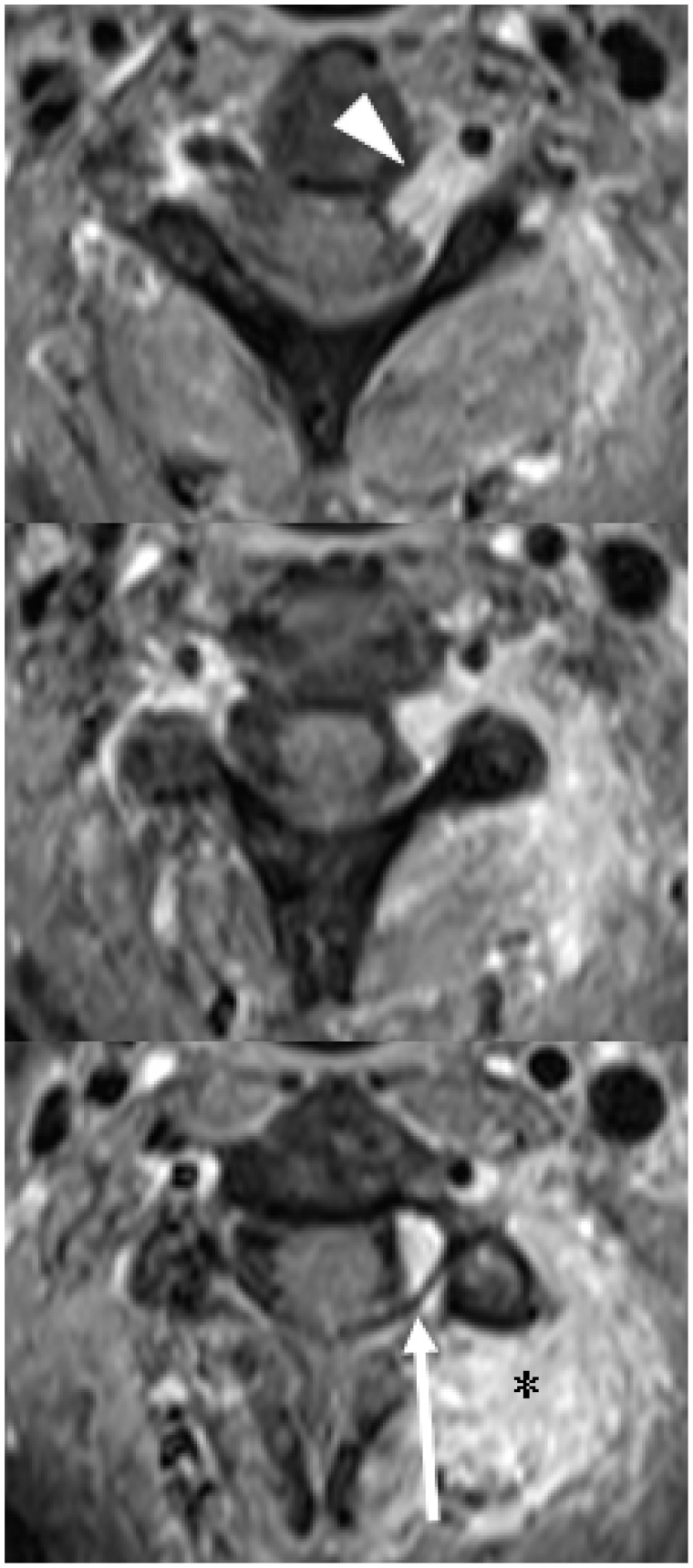
Figure 15.
Contiguous axial post-contrast T1 images (superior to inferior) showing extensive extra-osseous tumour (asterisk), including extension into the left C2/3 neural exit foramen (arrowhead). There is also some tumour within the central vertebral canal (long arrow), but without distortion of the spinal cord or cord compression.
Neurological symptoms may also occur due to meningeal involvement. This can be secondary to focal bone lesions or haematogenous dissemination, as in the brain.19 MRI typically shows enhancement on the surface of the spinal cord and/or cauda equina (Figure 16). Lumbar puncture will provide the diagnosis of meningeal myelomatosis (malignant plasma cells in the cerebrospinal fluid), but if performed prior to MRI, it may cloud the MRI appearances due to post-lumbar puncture enhancement. In this case, individual lesions should be scrutinised to determine if direct meningeal extension can be supported (Figure 17). Meningeal myelomatosis is rare, occurring in only about 1% of patients with MM.20 In the past, it was more commonly seen early in the course of the disease. More recently, however, it occurs more frequently as a late complication. The incidence of meningeal myelomatosis appears to be increasing, possibly related to modern therapies altering the tumour microenvironment.21
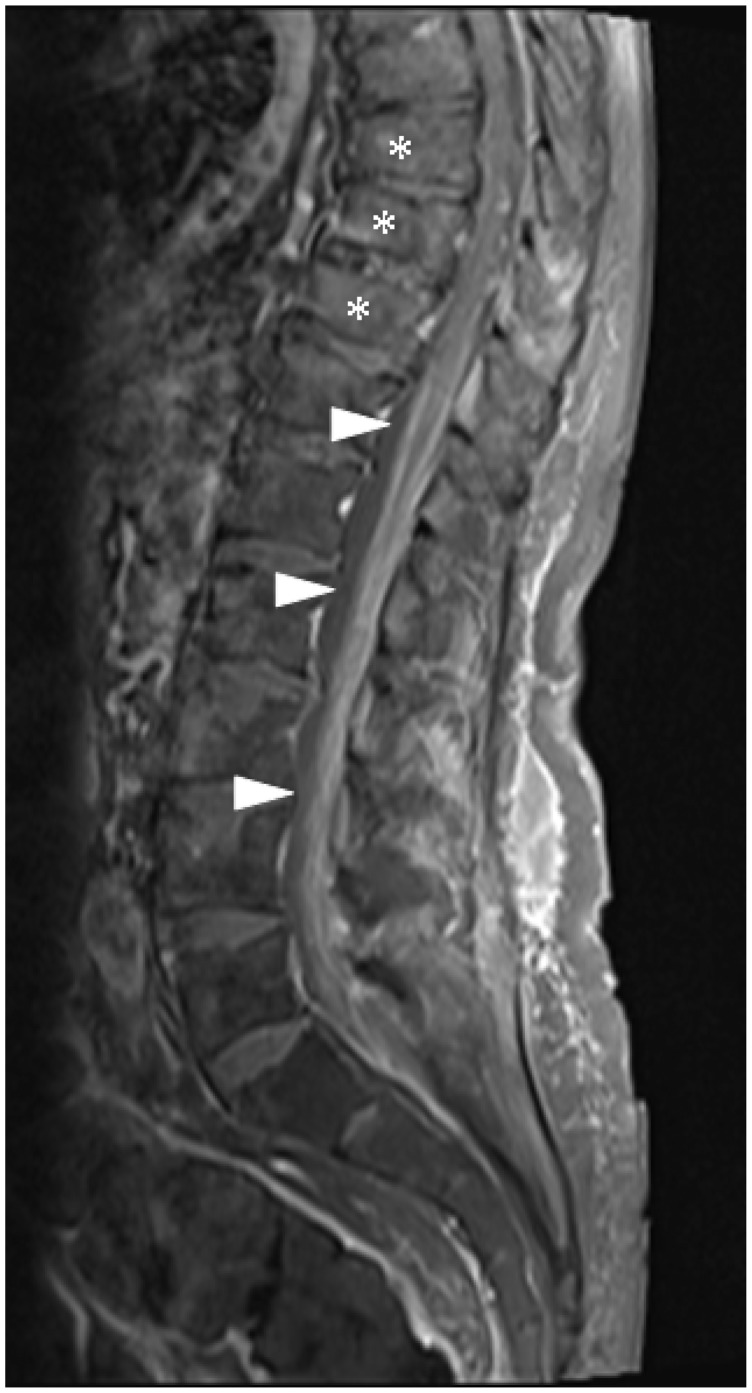
Figure 16.
Sagittal post-contrast T1 with fat saturation image of the lumbar spine demonstrating leptomeningeal infiltration (arrowheads) involving the lower spinal cord, conus medullaris and cauda equina. Pathological vertebral body crush fractures are also present (asterisks).
Figure 17.
Axial and coronal post-contrast T1 with fat saturation images show a vertebral plasmacytoma with extra-osseous extension (arrowheads), suspected to be producing dural involvement within the neural exit foramen (short arrows). This patient had proven meningeal myelomatosis on lumbar puncture.
Conclusion
Radiology, in particular MRI, plays a crucial and expanding role in the management of patients with plasma cell disorders. At initial presentation, imaging optimises disease characterisation and staging. Later in the course of the disease, imaging is important for disease monitoring, post-treatment follow-up and the investigation of clinical deterioration.
Acknowledgements
The authors would like to thank Dr Shane Gangatharan, Dr Anup George and Dr Kah-Lok Chan for assistance with identification of cases.
Funding
This work received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538–e548. [DOI] [PubMed] [Google Scholar]
- 2.Bird JM, Owen RG, D’Sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol 2011; 154: 32–75. [DOI] [PubMed] [Google Scholar]
- 3.Durie BGM, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J 2003; 4: 379–398. [PubMed] [Google Scholar]
- 4.Soutar R, Lucraft H, Jackson G, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol 2004; 124: 717–726. [DOI] [PubMed] [Google Scholar]
- 5.Moulopoulos LA, Dimopoulos MA, Weber D, et al. Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol 1993; 11: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 6.Wilder RB, Ha CS, Cox JD, et al. Persistence of myeloma protein for more than one year after radiotherapy is an adverse prognostic factor in solitary plasmacytoma of bone. Cancer 2002; 94: 1532–1537. [DOI] [PubMed] [Google Scholar]
- 7.Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101: 2496–2506. [DOI] [PubMed] [Google Scholar]
- 8.Regelink JC, Minnema MC, Terpos E, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol 2013; 162: 50–61. [DOI] [PubMed] [Google Scholar]
- 9.Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol 2010; 21: 325–330. [DOI] [PubMed] [Google Scholar]
- 10.Stabler A, Baur A, Bartl R, et al. Contrast enhancement and quantitative signal analysis in MR imaging of multiple myeloma: assessment of focal and diffuse growth patterns in marrow correlated with biopsies and survival rates. AJR Am J Roentgenol 1996; 167: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 11.Takasu M, Kaichi Y, Tani C, et al. Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation (IDEAL) magnetic resonance imaging as a biomarker for symptomatic multiple myeloma. PLoS ONE 2015; 10: e0116842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moulopoulos LA, Dimopoulos MA, Alexanian R, et al. Multiple myeloma: MR patterns of response to treatment. Radiology 1994; 193: 441–446. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos M, Terpos E, Comenzo RL, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia 2009; 23: 1545–1556. [DOI] [PubMed] [Google Scholar]
- 14.Zamagni E, Cavo M. The role of imaging techniques in the management of multiple myeloma. Br J Haematol 2012; 159: 499–513. [DOI] [PubMed] [Google Scholar]
- 15.Giles SL, Messiou C, Collins DJ, et al. Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 2014; 271: 785–794. [DOI] [PubMed] [Google Scholar]
- 16.Lin C, Luciani A, Belhadj K, et al. Multiple myeloma treatment response assessment with whole-body dynamic contrast-enhanced MR imaging. Radiology 2010; 254: 521–531. [DOI] [PubMed] [Google Scholar]
- 17.Desikan R, Barlogie B, Sethi R, et al. Infection – an underappreciated cause of bone pain in multiple myeloma. Br J Haematol 2003; 120: 1047–1050. [DOI] [PubMed] [Google Scholar]
- 18.Blade J, Rosinol L. Complications of multiple myeloma. Hematol Oncol Clin North Am 2007; 21: 1231–1246, xi. [DOI] [PubMed] [Google Scholar]
- 19.Lasocki A, Gangatharan S, Gaillard F, et al. Intracranial involvement by multiple myeloma. Clin Radiol 2015; 70: 890–897. [DOI] [PubMed] [Google Scholar]
- 20.Fassas ABT, Muwalla F, Berryman T, et al. Myeloma of the central nervous system: association with high-risk chromosomal abnormalities, plasmablastic morphology and extramedullary manifestations. Br J Haematol 2002; 117: 103–108. [DOI] [PubMed] [Google Scholar]
- 21.Gangatharan SA, Carney DA, Prince HM, et al. Emergence of central nervous system myeloma in the era of novel agents. Hematol Oncol 2012; 30: 170–174. [DOI] [PubMed] [Google Scholar]