Abstract
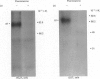
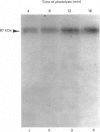
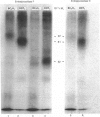
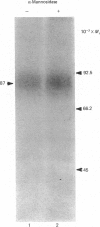
In this study, we clarify the structural aspects of the oligosaccharides associated with the alpha 1-adrenergic receptor in two muscle cell lines. Photoaffinity labelling of intact BC3H1 or DDT1 muscle cells with 2-[4-(4-azido-3-[125I]iodobenzoyl)piperazin-1-yl]-4-amino-6, 7-dimethoxyquinazoline ([125I]azidoprazosin) followed by SDS/polyacrylamide-gel electrophoresis (PAGE) and autoradiography revealed specifically labelled proteins of molecular mass = 87,000 and 81,000, respectively. Treatment of photoaffinity-labelled receptors in DDT1 cells with 33 u. of endoglycosidase F/ml for 24 h resulted in the loss of the 81 kDa receptor and the appearance of a 52.5 kDa protein. When lower concentrations of glycosidase or shorter incubation times were used, the 81 kDa receptor was converted to a 66 kDa protein. Treatment of the photoaffinity-labelled BC3H1 receptor with endoglycosidase F resulted in the appearance of a 50.5 kDa protein. Neither alpha-mannosidase nor endoglycosidase H had an effect on the photoaffinity labelling patterns of the receptor from the two cell types. alpha 1-Adrenergic receptors, solubilized from membranes prepared from BC3H1 and DDT1 cells, bound to wheat germ agglutinin-Sepharose and were displaced by N-acetylglucosamine. Taken together, these results indicate that alpha 1-adrenergic receptors in BC3H1 and DDT1 cells contain complex, but not high, mannose oligosaccharide chains; differences in the composition or number of chains partially accounts for the different molecular mass of the receptor in the two cell lines. The results further indicate that the oligosaccharide chains contribute substantially to the apparent molecular mass of alpha 1-adrenergic receptors, as detected by SDS/PAGE, and that the protein backbone of these receptors is likely to be approximately 50 kDa.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler S. K., Brown R. D., Taylor P. The relationship between phosphatidylinositol metabolism and mobilization of intracellular calcium elicited by alpha1-adrenergic receptor stimulation in BC3H-1 muscle cells. Mol Pharmacol. 1984 Nov;26(3):405–413. [PubMed] [Google Scholar]
- Bause E., Hettkamp H. Primary structural requirements for N-glycosylation of peptides in rat liver. FEBS Lett. 1979 Dec 15;108(2):341–344. doi: 10.1016/0014-5793(79)80559-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. D., Berger K. D., Taylor P. Alpha 1-adrenergic receptor activation mobilizes cellular Ca2+ in a muscle cell line. J Biol Chem. 1984 Jun 25;259(12):7554–7562. [PubMed] [Google Scholar]
- Cornett L. E., Norris J. S. Characterization of the alpha 1-adrenergic receptor subtype in a smooth muscle cell line. J Biol Chem. 1982 Jan 25;257(2):694–697. [PubMed] [Google Scholar]
- Cornett L. E., Norris J. S. Photoaffinity labeling of the DDT1 MF-2 cell alpha 1-adrenergic receptor. Mol Cell Biochem. 1985 May;67(1):47–53. doi: 10.1007/BF00220985. [DOI] [PubMed] [Google Scholar]
- Fratelli M., De Blasi A. Agonist-induced alpha 1-adrenergic receptor changes. Evidence for receptor sequestration. FEBS Lett. 1987 Feb 9;212(1):149–153. doi: 10.1016/0014-5793(87)81575-2. [DOI] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- George S. T., Ruoho A. E., Malbon C. C. N-glycosylation in expression and function of beta-adrenergic receptors. J Biol Chem. 1986 Dec 15;261(35):16559–16564. [PubMed] [Google Scholar]
- Heidenreich K. A., Brandenburg D. Oligosaccharide heterogeneity of insulin receptors. Comparison of N-linked glycosylation of insulin receptors in adipocytes and brain. Endocrinology. 1986 May;118(5):1835–1842. doi: 10.1210/endo-118-5-1835. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Hughes R. J., Boyle M. R., Brown R. D., Taylor P., Insel P. A. Characterization of coexisting alpha 1- and beta 2-adrenergic receptors on a cloned muscle cell line, BC3H-1. Mol Pharmacol. 1982 Sep;22(2):258–266. [PubMed] [Google Scholar]
- Hughes R. J., Insel P. A. Agonist-mediated regulation of alpha 1- and beta 2-adrenergic receptor metabolism in a muscle cell line, BC3H-1. Mol Pharmacol. 1986 Jun;29(6):521–530. [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Segal M., Krieger M. Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid-linked carbohydrate chains. J Cell Biol. 1986 May;102(5):1576–1585. doi: 10.1083/jcb.102.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., DeBlasi A., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. I. Agonist-promoted desensitization and phosphorylation of alpha 1-adrenergic receptors coupled to inositol phospholipid metabolism in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1987 Mar 5;262(7):3098–3105. [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Dickinson K. E., Heald S. L., Wikberg J. E., Hagen P. O., DeBernardis J. F., Winn M., Arendsen D. L., Lefkowitz R. J., Caron M. G. Photoaffinity labeling of mammalian alpha 1-adrenergic receptors. Identification of the ligand binding subunit with a high affinity radioiodinated probe. J Biol Chem. 1984 Feb 25;259(4):2579–2587. [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Maisel A. S., Motulsky H. J., Insel P. A. Life cycles of cardiac alpha 1- and beta-adrenergic receptors. Biochem Pharmacol. 1987 Jan 1;36(1):1–6. doi: 10.1016/0006-2952(87)90375-3. [DOI] [PubMed] [Google Scholar]
- Meier K. E., Sternfeld D. R., Insel P. A. Alpha 1- and beta 2-adrenergic receptors co-expressed on cloned MDCK cells are distinct glycoproteins. Biochem Biophys Res Commun. 1984 Jan 13;118(1):73–81. doi: 10.1016/0006-291x(84)91069-6. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Tzartos S., Lindstrom J. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J Biol Chem. 1982 Mar 10;257(5):2694–2701. [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Reed B. C., Ronnett G. V., Lane M. D. Role of glycosylation and protein synthesis in insulin receptor metabolism by 3T3-L1 mouse adipocytes. Proc Natl Acad Sci U S A. 1981 May;78(5):2908–2912. doi: 10.1073/pnas.78.5.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. E., Dubyak G. R. Agonist-induced calcium transients in cultured smooth muscle cells: measurements with fura-2 loaded monolayers. Biochem Biophys Res Commun. 1986 May 14;136(3):927–934. doi: 10.1016/0006-291x(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Knutson V. P., Kohanski R. A., Simpson T. L., Lane M. D. Role of glycosylation in the processing of newly translated insulin proreceptor in 3T3-L1 adipocytes. J Biol Chem. 1984 Apr 10;259(7):4566–4575. [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman C. E., Hess H. J., Homcy C. J., Graham R. M. Photoaffinity labeling of the alpha 1-adrenergic receptor using an 125I-labeled aryl azide analogue of prazosin. Biochemistry. 1984 Jul 31;23(16):3765–3770. doi: 10.1021/bi00311a031. [DOI] [PubMed] [Google Scholar]
- Slieker L. J., Lane M. D. Post-translational processing of the epidermal growth factor receptor. Glycosylation-dependent acquisition of ligand-binding capacity. J Biol Chem. 1985 Jan 25;260(2):687–690. [PubMed] [Google Scholar]
- Stiles G. L., Benovic J. L., Caron M. G., Lefkowitz R. J. Mammalian beta-adrenergic receptors. Distinct glycoprotein populations containing high mannose or complex type carbohydrate chains. J Biol Chem. 1984 Jul 10;259(13):8655–8663. [PubMed] [Google Scholar]
- Stiles G. L. Deglycosylated mammalian beta 2-adrenergic receptors: effect on radioligand binding and peptide mapping. Arch Biochem Biophys. 1985 Feb 15;237(1):65–71. doi: 10.1016/0003-9861(85)90254-1. [DOI] [PubMed] [Google Scholar]
- Swiedler S. J., Freed J. H., Tarentino A. L., Plummer T. H., Jr, Hart G. W. Oligosaccharide microheterogeneity of the murine major histocompatibility antigens. Reproducible site-specific patterns of sialylation and branching in asparagine-linked oligosaccharides. J Biol Chem. 1985 Apr 10;260(7):4046–4054. [PubMed] [Google Scholar]
- Terman B. I., Insel P. A. Photoaffinity labeling of alpha 1-adrenergic receptors of rat heart. J Biol Chem. 1986 Apr 25;261(12):5603–5609. [PubMed] [Google Scholar]
- Terman B. I., Slivka S. R., Hughes R. J., Insel P. A. Alpha 1-adrenergic receptor-linked guanine nucleotide-binding protein in muscle and kidney epithelial cells. Mol Pharmacol. 1987 Jan;31(1):12–20. [PubMed] [Google Scholar]
- Toews M. L. Comparison of agonist-induced changes in beta- and alpha 1-adrenergic receptors of DDT1 MF-2 cells. Mol Pharmacol. 1987 Jan;31(1):58–68. [PubMed] [Google Scholar]
- Yarden Y., Rodriguez H., Wong S. K., Brandt D. R., May D. C., Burnier J., Harkins R. N., Chen E. Y., Ramachandran J., Ullrich A. The avian beta-adrenergic receptor: primary structure and membrane topology. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6795–6799. doi: 10.1073/pnas.83.18.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]