Abstract
Natural Killer (NK) cells are one of the major components of innate immunity, with the ability to mediate antitumor activity. Understanding the role of NK cell mediated tumor killing in controling of solid tumor growth is still in the developmental stage. We have shown recently that bitter melon extract (BME) modulates the regulatory T cell (Treg) population in head and neck squamous cell carcinoma (HNSCC). However, the role of BME in NK cell modulation against HNSCC remains unknown. In this study, we investigated whether BME can enhance the NK cell killing activity against HNSCC cells. Our results indicated that treatment of human NK cell line (NK3.3) with BME enhances ability to kill HNSCC cells. BME increases granzyme B accumulation and translocation/accumulation of CD107a/LAMP1 in NK3.3 cells exposed to BME. Further, an increase in cell surface expression of CD16 and NKp30 in BME treated NK3.3 cells was observed when co-cultured with HNSCC cells. Collectively, our results demonstrated for the first time that BME augments NK cell mediated HNSCC killing activity, implicating an immunomodulatory role of BME.
Keywords: Bitter melon, Head and neck cancer, Natural killer cell, LAMP1
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer and one of the leading causes of cancer related mortality worldwide. Improvement has been made with respect to chemotherapeutic drugs, radiation and surgical techniques for HNSCC. However, recurrence of the disease and drug resistance still causes a significant amount of death. Thus, there is a great need for additional effective therapies to increase cure rates and reduce morbidity.
Natural killer (NK) cells are cytotoxic lymphoid cells of the innate immune system. Unlike T cells, these cells are able to respond quickly to “invaders” without a “priming” period (1). They recognize target cells with low cell surface expression of MHC class I (MHC-I). Cancer cells having characteristics of reduced MHC-I expression thus can be recognized by NK cells, which consequently release cytokines and cytolytic granules containing perforin and granzyme B to kill tumor cells. Due to the variability in the expression of MHC-I on the surface of different tumor cells, NK cell responses vary (2, 3). NK cell based therapies include different strategies such as increasing NK cell lytic function or enhancing their homing to tumor sites, cytokine mediated activation of endogenous NK cells, antibody dependent cell mediated cytotoxicity (ADCC)-promoting therapeutic antibodies, NK cell infusions, anti-KIR antibody, bispecific antibody or combined therapies (4–7). Preclinical and clinical studies suggest that NK cell mediated immunotherapy would be efficient in treating minimal residual disease (8).
Natural products play a leading role in the discovery and the development of various drugs for the treatment of human diseases including cancer. Bitter melon (Momordica charantia) is extensively cultivated in Asia, Africa, and South America, and is widely used in folk medicines to treat diabetes (9). In our previous studies, we demonstrated that BME is effective in killing various types of solid tumors including head and neck cancer (10–13). We found that BME inhibits HNSCC cell proliferation through modulation of c-Met signaling in in vitro as well as in vivo using a xenograft model (12). We recently observed that BME treatment reduces the regulatory T cell (Treg) activity in a HNSCC syngeneic mouse model (14). NK cells display rapid and potent killing of hematological cancers (15). However, the effect of BME on NK cell cytotoxicity remains unknown in solid tumors including HNSCC. In this study, we demonstrated for the first time that pretreatment of NK cells with BME enhances their killing activity against HNSCC cells. We also observed that BME mediated increase in NK cell killing activity is associated with translocation of CD107a/LAMP1, increased accumulation of granzyme B, and increase of CD16 (FcγRIIIa) and NKp30 cell surface expression.
Materials and Methods
BME preparation
BME was prepared from the Chinese variety of young bitter melons (raw and green) as discussed previously (12, 14). Briefly, BME was extracted using a household juicer and centrifuged at 560 × g at 4°C for 30 min, freeze dried at −45°C for 72 h and stored at −80°C. We next prepared BME by suspending 1 gm of freeze-dried powder in 10 ml of water, mixed overnight, and separated the aqueous portion by centrifugation for 30 minutes. BME was aliquoted and stored at −80°C. We generally prepare a big batch and tested each batch for cytotoxicity using 3–4 previously tested cancer cell lines.
Cell lines and cytotoxicity assay
We used two HNSCC cell lines in this study. Cal27 cell line (tongue origin) was purchased from ATCC and was maintained in Dulbecco’s Modified Eagle Medium (Sigma) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Sigma). JHU-29 (tongue) cell line was procured from the Johns Hopkins University, and was maintained in RPMI-1640 medium (Sigma) supplemented with 10% FBS and 1% penicillin/streptomycin. The human NK cell line (NK3.3) was cultured in RPMI-1640 medium supplemented with 10% FBS, 1% glutamine, 1% penicillin-streptomycin, and 200 IU/ml recombinant IL-2 (rIL-2) (R & D Systems) (16). We added IL-12 overnight to the NK 3.3 cells, then removed residual IL-2 by washing, exposed with BME (1% v/v) for additional 20 h before incubating with cancer cells. HNSCC cells were co-cultured with BME treated NK3.3 cells at different Tumor Cell/Target: Effector Cell (T:E) (1:10) ratios for 24 hr. Cytotoxicity was measured by using a multiTox-fluor multiplex cytotoxicity assay kit (Promega) following the manufacturer’s protocol, and readings were taken using a Bio-Tek plate reader.
Western blot analysis
Cell lysates were analyzed by SDS-PAGE and transferred onto 0.45 μM nitrocellulose membrane (Bio-Rad). Membranes were blocked using 5% low fat dry milk and probed with the specific antibodies. Proteins were detected using ECL Western blotting substrate (Thermo Scientific) and autoradiography. The protein loading was normalized using antibody to β-actin. The following antibodies were used in this study: granzyme B, pSTAT3, STAT3 and LAMP1 (Cell Signaling Technologies) and β actin (Santa Cruz Biotech).
Flow cytometry
NK cells were treated with 2% BME or left untreated as a control for 16 hr, washed extensively and then co-cultured with adherent HNSCC cells for another 24 hr. NK3.3 cells were separated from the HNSCC cells, washed with buffer (0.5% BSA in 1X phosphate buffer pH-7.4) and stained with anti-CD45 (FITC), anti-CD56 (APCA700), anti-CD107a (PE), anti-CD16 (ECD), anti-NKp30 (PE), anti-CD314/NKG2D (APC), anti-CD161 (A750), anti-CD158e (BV421), or anti-NKp46 (PECy7) antibody for surface expression. Brilliant stain buffer (BD Biosciences) was used for the dilution of the stains. Next, cells were washed with staining buffer, fixed with 4% formaldehyde, and analyzed using an LSRII flow cytometer (BD Biosciences). Data were evaluated using FlowJo software. The antibodies were purchased from Beckman Coulter, Miltenyi Biotec or Biolegend.
Statistical analysis
Results were expressed as the mean ± standard deviation (SD), and statistical analyses were performed using two-tailed paired or unpaired Student’s t test in GraphPad Prism 6 (GraphPad, La Jolla, CA). A p value of < 0.05 was considered statistically significant.
Results
BME enhances NK cell mediated cytotoxicity
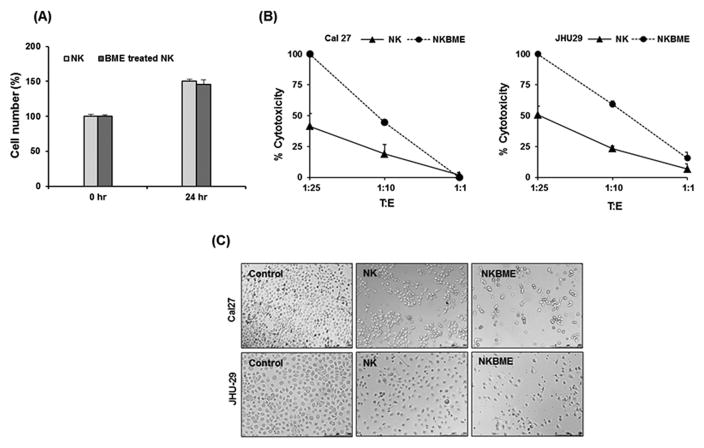
We initially examined whether BME has an effect on NK3.3 cell growth. For this, NK cells were exposed to BME for 24 h and cell viability were assessed. We did not observe an effect of BME treatment on NK cell growth or viability (Fig. 1, panel A). We next examined whether BME treatment of NK3.3 cells enhanced tumor cell killing activity. For this, control or BME treated NK3.3 cells were co-cultured with HNSCC (Cal27 or JHU-29) cells at different T:E ratios for 24 hr. Our results suggested that BME treated NK cells showed enhanced cytotoxicity as compared to untreated NK cells (Fig. 1, panels B and C). BME treatment alone, without prior IL-2 stimulation of NK cells, does not significantly induce NK cell activity.
Figure 1. BME treatment increases cytotoxic activity of NK3.3 cells on HNSCC cells.
(A) NK3.3 cells were treated with BME for 24 hr. Cell number and viability was measured by Trypan blue dye exclusion method. Results shown are an average of three independent experiments. Cell viability at 0 hr time point was arbitrarily set to100%. (B) Cal27 or JHU-29 cells were co-cultured with different T:E ratios of control or BME treated NK cells, and cytotoxicity was measured. Data are represented as mean ± SD.* p<0.05. (C) Representative microscopic images (10X) showing untreated HNSCC cells (control), HNSCC cells exposed to untreated NK (NK) or BME treated NK cells (NKBME) with T:E ratio 1:10. Photographs were taken after 24 hr of co-culturing BME treated NK cells with target cells.
BME treatment enhances NK cell cytotoxicity by increasing granzyme B expression
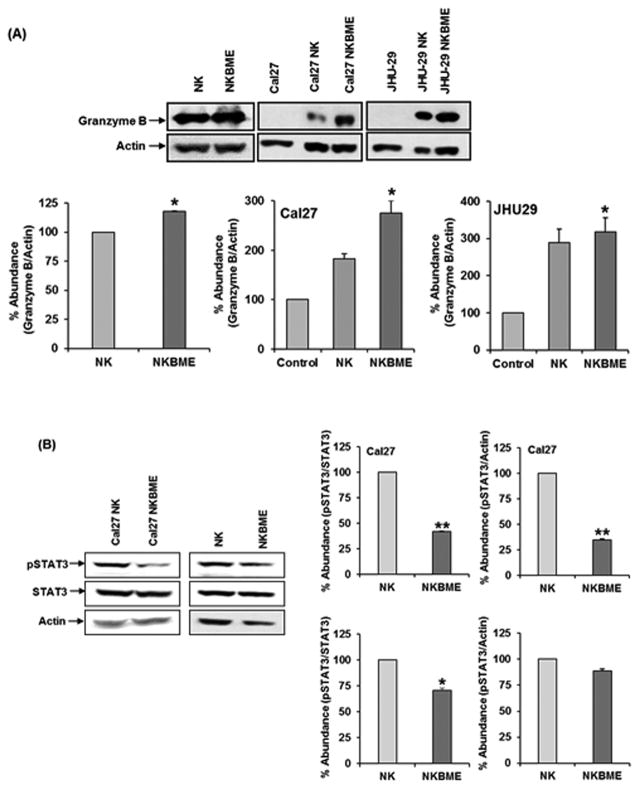
NK cells, upon encounter with potential target cells, form an immunological synapse. This event provides the activation signals to NK cells to augment granzyme B expression and release (17). One of the defining properties of NK cells is the expression and regulated secretion of granzyme B. To gain further insights into the effects of BME on NK cell-mediated cytotoxicity, we examined the effect of BME on the expression of granzyme B. We observed higher granzyme B expression in BME treated NK3.3 cells as compared to untreated cells. BME treated NK3.3 cells, when co-cultured with HNSCC tumor cells, also displayed a significantly higher expression of granzyme B as compared to untreated NK3.3 cells (Fig. 2, panel A). We did not observe a significant upregulation of perforin in BME treated NK cells when exposed to HNSCC cells.
Figure 2. BME treatment increases granzyme B expression in NK 3.3 cells.
(A) Western blot analysis of granzyme B expression in control or BME treated NK3.3 cells when co-cultured with Cal27 and JHU-29 cells. (B) PhosphoSTAT3/STAT3 expression in control or BME treated NK3.3 cells co-cultured with Cal27 was examined by Western blot using specific antibody. Similarly, lysates from NK cell were untreated or BME treated were analyzed for phosphoSTAT3/STAT3 expression. Blots were re-probed with an antibody to actin for comparison of protein loading in each lane. Densitometric analyses of was performed using Image J software. Data are represented as mean ± SD. *, p<0.05, **, p<0.01
Recent studies suggested that STAT3 activation impairs tumor immune surveillance and allows the tumor to escape immune control (18). STAT3 activation in NK cells indeed suppressed cytotoxicity in mouse model (19). We therefore examined the status of activated STAT3. Our results demonstrated that phosphoSTAT3 activation is significantly lower in BME treated NK3.3 cells as compared to control NK3.3 cells when co-cultured with Cal27 cells (Fig. 2, panel B). We also observed inhibition of phosphoSTAT3 in BME treated NK3.3 cells alone. The modulation of STAT3 has been shown to inversely correlate with expression of granzyme B (19). Together, our results suggested that tumor-NK cell interaction is prerequisite for BME treatment to maximally enhance granzyme B expression.
BME treatment of NK cells enhances CD107a/LAMP1 surface expression and accumulation
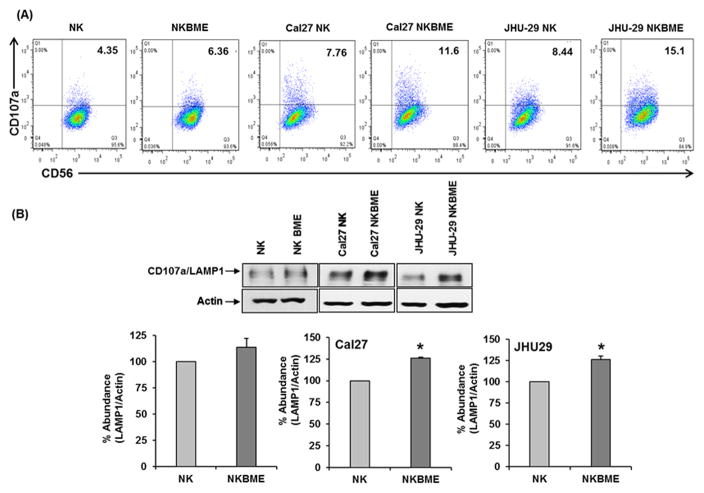
NK cell cytotoxicity is a multistep complex process that involves adhesion to target cells, synapse formation and signal transduction leading to granule polarization and exocytosis. CD107a/LAMP1 participates in the degranulation of NK cell lytic granules, mobilizing perforin and granzyme B towards the immunological synapse. CD107a/LAMP1 also plays a crucial role in the protection from degranulation-associated suicide of NK cells (20). Therefore, it is likely that BME might affect the degranulation process by modulating the surface expression of CD107a on NK cells. Our results demonstrated that BME treated NK3.3 cells, when co-cultured with HNSCC cells, display higher surface CD107a expression than control NK3.3 cells (Fig. 3, panel A). Additionally, we examined the expression of total CD107a/LAMP1 by Western blot analysis. Our data indicates an accumulation of CD107a/LAMP1 in BME treated NK3.3 cells co-cultured with Cal27 or JHU-29 cells (Fig. 3, panel B). Interestingly, NK3.3 cells treated only with BME displayed a similar level of CD107a/LAMP1 expression to that of untreated NK3.3 cells.
Figure 3. BME treatment increases CD107a/LAMP1 expression on NK 3.3 cells.
(A) FACS analysis of the cell surface expression of CD107a/LAMP-1 on NK cells. Dot plot analysis of the surface expression of CD107a on CD56 positive NK3.3 cells and BME exposed NK3.3 cells with or without co-culture with HNSCC cells. (B) Western blot data showing CD107a/LAMP1 expression in NK3.3 cells. Blots were re-probed with an antibody to actin for comparison of protein loading in each lane. Densitometric analyses were performed using Image J software. Data are represented as mean ± SD. * p<0.05
BME treatment of NK cells enhances CD16 and NKp30 expression
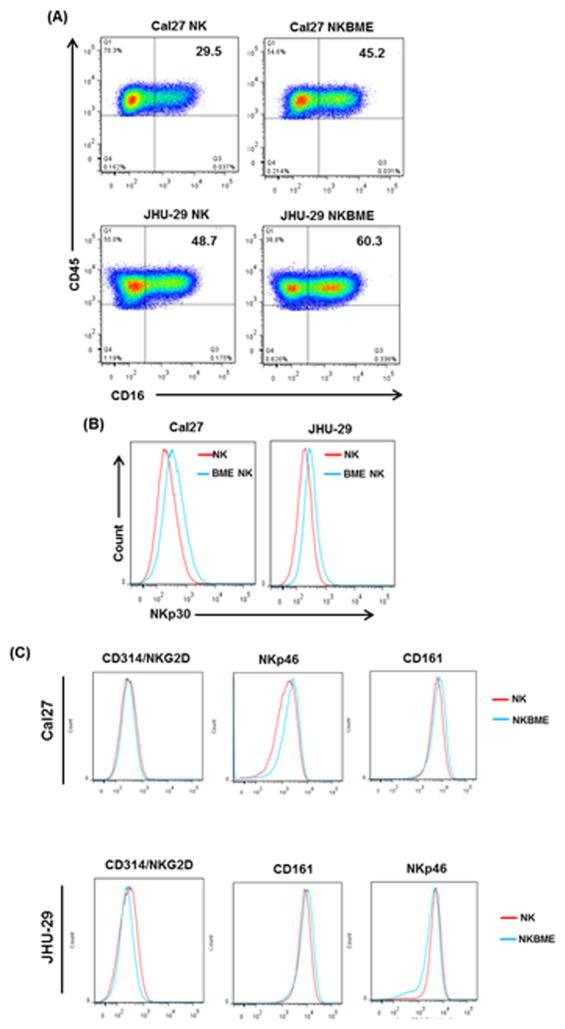
CD16, a member of the IgG superfamily, is expressed on a subset of NK cells. It is involved in ADCC and in mediating direct natural killer cell cytotoxicity (21). Engagement of CD16 by its ligand, the Fc region of IgG, triggers degranulation of lytic granules from NK cells (22). We observed an increase in CD16 surface expression in BME treated NK3.3 cells co-cultured with HNSCC cells (Fig. 4, panel A). CD16 signaling is mediated by transmembrane adaptor proteins that possess immunoreceptor tyrosine-based activation motifs (ITAM). The natural cytotoxicity receptor (NCR) NKp30 is also associated with ITAM containing adaptor proteins (22). We observed an increase in NKp30 surface expression in BME treated NK3.3 cells co-cultured with tumor cells as compared with untreated NK cells (Fig. 4, panel B). We did not find modulation of other NK receptors (NKG2D, CD161, or NKp46) on BME treated NK3.3 cells when co-cultured with HNSCC cells (Fig. 4, panel C).
Figure 4. BME treatment increases CD16 and NKp30 surface expression on NK3.3 cells.
(A) FACS analysis showing the populations of CD16+ NK3.3 cells exposed with BME or left untreated when co-cultured with Cal27 or JHU-29 cells. The right panel showed the quantitation of the CD16 as % of NK cell population (B) FACS analysis showing expression of NKp30 on NK3.3 and BME treated NK3.3 cells co-cultured with Cal27 and JHU-29 cells. Data are represented as mean ± SD. * p<0.05 (C) Overlay of histograms showing expression of CD314/NKG2D, NKp46, and CD161 by FACS analysis on NK3.3 cells exposed with BME as described above.
Discussion
BME displayed anticancer activities in various cancer models (10–13). In addition to its direct inhibitory effect on tumor growth and survival, BME also has demonstrated immunomodulatory activity in a syngeneic mouse model of HNSCC by inhibiting Treg activity (14). However, the effect of BME on NK cell activation and subsequently killing of HNSCC cells was unknown. The tumor microenvironment is not only a passive recipient of immune cells but an active contributor to the establishment of immunosuppressive conditions (23). In the tumor microenvironment, NK cells display a modified phenotype with reduced cytotoxic activity as well as reduced immune surveillance. A direct correlation between high intra-tumoral levels of NK cells and increased survival has been shown in several types of cancers (24). Studies suggested that various chemotherapeutic drugs augment IL-2-activated NK cell lysis of tumor cells (25).
In this study, we demonstrated that BME treatment augments NK cell mediated tumor cell toxicity to induce tumor cell death via activation of NK cell receptor dependent pathways. The mechanistic study suggested that BME treated NK cells, when co-cultured with HNSCC cells, accumulate and translocate CD107a/LAMP1 to the cell surface. We further observed upregulation of CD16 and NKp30 receptors on BME treated NK cells when co-cultured with HNSCC cells.
NK cells contain various proteins including granzyme and perforin, which are secreted from NK cells upon contact with target cells, and ultimately cause death of target cells. CD107a/LAMP-1 is present in the membranes of cytolytic granules. This protein is also expressed on the surface of NK cells upon degranulation and is considered a discrete marker for the NK cell-mediated killing of target cells (26). CD107a plays a vital role in the survival of NK cells against degranulation mediated self-destruction (20). Silencing of CD107a/LAMP1 inhibits cytotoxic activity of NK cells (27). We observed the enhanced expression of CD107a on the surface of NK cells when they were exposed to BME and co-cultured with HNSCC cells. This phenomenon is closely related to the effect of BME on degranulation and the expression of granzyme B on BME treated NK cells. Further, STAT3 activation was inversely correlated with granzyme B expression. It is likely reflecting the ability of BME to induce the killing of HNSCC cells through the enhancement of NK cytolytic activity. The function of NK cells is dependent on the balance between engagement of both activation and inhibitory receptors with the target cell. CD16 is a member of IgG superfamily, involved in ADCC and also mediating direct natural killer cell cytotoxicity (21). We observed that BME treatment increases CD16 expression on NK cells when co-cultured with HNSCC cells. Additionally, we observed that treatment with BME also increases the expression of NKp30, a natural cytotoxic receptor. NKp30 plays an important role in mediating tumor immunosurveillance in several clinical settings. One of its ligands, B7H6, is expressed on many types of tumor cells but absent on normal tissues. Interaction of NKp30 with B7H6 has been shown to enhance degranulation of NK cells (28). Other activation receptors on NK cells, including NKG2D and NKp46 did not indicate a modulation after BME treatment.
Based on our in vitro data, we examined granzyme B and CD107a/LAMP1 expression in tumor tissues from HNSCC syngeneic mouse (14). We performed Western blot analysis and qRT-PCR to examine the status of granzyme B in tumor following BME treatment. Additionally we examined LAMP1 expression by Western blots. Interestingly, we did not observe a significant modulation of granzyme B or CD107a/LAMP1 expression in tumors isolated from BME treated mice as compared with the control group in the HNSCC syngeneic mouse model (data not shown). The role of NK cells in anti-tumor responses in vivo may be difficult to interpret due to the difference between human and mouse NK cells (29). Human NK cells can be subclassified based upon the level of expression of CD56. CD56 dim positive subsets of NK cells present in the circulation have high level of cytotoxicity while CD56 bright positive cells have less cytotoxicity. These subsets of NK cells are not present in mice. BME treatment in a HNSCC syngeneic mouse model does not appear to alter NK cell activity. One possible reason for these results might be the difference in expression of NKp30 by mouse and human NK cells. NKp30 expression is increased in human NK3.3 cells after BME treatment. NKp30 (NCR3 or 1C7), a natural killer (NK) cell activation receptor is a functional gene in human (30) but a pseudogene in mouse (31). Further work is indeed necessary to tease out the in-depth mechanism for BME mediated NK cell killing against HNSCC. Together our data demonstrated that BME modulates human NK cell cytotoxic activity against HNSCC by modulating LAMP1, Granzyme BCD16, and NKP30 expression. Our findings also suggested that besides direct anti-tumor action, enhancement of effector functions of the immune system may also contribute to the therapeutic effects of BME in HNSCC.
Acknowledgments
Grant Support: This work was supported by research grant R01 DE024942 from the National Institutes of Health (R. B. Ray), and Saint Louis University Cancer Center Seed Grant (R. B. Ray).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Festenstein H, Schmidt W. Variation in MHC antigenic profiles of tumor cells and its biological effects. Immunol Rev. 1981;60:85–127. doi: 10.1111/j.1600-065x.1981.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 3.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 4.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012;91:299–309. doi: 10.1189/jlb.0611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahl J, Reusch U, Gantke T, Kerber A, Koch J, Treder M, Cerwenka A. AFM13 Is the Most Advanced Bispecific NK-Cell Engaging Antibody in Clinical Development Substantially Enhancing NK-Cell Effector Function and Proliferation. Blood. 2016;128:1764. [Google Scholar]
- 8.Fregni G, Perier A, Avril MF, Caignard A. NK cells sense tumors, course of disease and treatments: Consequences for NK-based therapies. Oncoimmunology. 2012;1:38–47. doi: 10.4161/onci.1.1.18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nerurkar P, Ray RB. Bitter melon: antagonist to cancer. Pharm Res. 2010;27:1049–53. doi: 10.1007/s11095-010-0057-2. [DOI] [PubMed] [Google Scholar]
- 10.Ray RB, Raychoudhuri A, Steele R, Nerurkar P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res. 2010;70:1925–1931. doi: 10.1158/0008-5472.CAN-09-3438. [DOI] [PubMed] [Google Scholar]
- 11.Ru P, Steele R, Nerurkar PV, Phillips N, Ray RB. Bitter melon extract impairs prostate cancer cell-cycle progression and delays prostatic intraepithelial neoplasia in TRAMP model. Cancer Prev Res (Phila) 2011;4:2122–2130. doi: 10.1158/1940-6207.CAPR-11-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajamoorthi A, Shrivastava S, Steele R, Nerurkar P, Gonzalez JG, Crawford S, Varvares M, Ray RB. Bitter melon reduces head and neck squamous cell carcinoma growth by targeting c-Met signaling. PLoS One. 2013;8:e78006–e78013. doi: 10.1371/journal.pone.0078006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raina K, Kumar D, Agarwal R. Promise of bitter melon (Momordica charantia) bioactives in cancer prevention and therapy. Semin Cancer Biol. 2016;40–41:116–129. doi: 10.1016/j.semcancer.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya S, Muhammad N, Steele R, Peng G, Ray RB. Immunomodulatory role of bitter melon extract in inhibition of head and neck squamous cell carcinoma growth. Oncotarget. 2016;7:33202–33209. doi: 10.18632/oncotarget.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy. 2017;19:235–249. doi: 10.1016/j.jcyt.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Kornbluth J, Flomenberg N, Dupont B. Cell surface phenotype of a cloned line of human natural killer cells. J Immunol. 1982;129:2831–7. [PubMed] [Google Scholar]
- 17.Sabry M, Lowdell MW. Tumor-Primed NK Cells: Waiting for the Green Light. Front Immunol. 2013;4:408–415. doi: 10.3389/fimmu.2013.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacalano NA. Regulation of Natural Killer Cell Function by STAT3. Front Immunol. 2016;7:128. doi: 10.3389/fimmu.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotthardt Dagmar, Sexl Veronika. STATs in NK-Cells: The Good, the Bad, and the Ugly. Front Immunol. 2016;7:694. doi: 10.3389/fimmu.2016.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohnen A, Chiang SC, Stojanovic A, Schmidt H, Claus M, Saftig P, et al. Surface CD107a/LAMP-1 protects natural killer cells from degranulation associated damage. Blood. 2013;122:1411–1418. doi: 10.1182/blood-2012-07-441832. [DOI] [PubMed] [Google Scholar]
- 21.Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci U S A. 1999;96:5640–5644. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baginska J, Viry E, Paggetti J, Medves S, Berchem G, Moussay E, et al. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol. 2013;4:490–503. doi: 10.3389/fimmu.2013.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen SK, Gao Y, Basse PH. NK Cells in the Tumor Microenvironment. Crit Rev Oncog. 2014;19:91–105. doi: 10.1615/critrevoncog.2014011142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markasz L, Stuber G, Vanherberghen B, Flaberg E, Olah E, Carbone E, et al. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Mol Cancer Ther. 2007;6:644–54. doi: 10.1158/1535-7163.MCT-06-0358. [DOI] [PubMed] [Google Scholar]
- 26.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Krzewski K, Gil-Krzewska A, Nyuyen V, Peruzzi G, Coligan JE. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood. 2013;121:4672–4683. doi: 10.1182/blood-2012-08-453738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Zheng X, Wei H, Tian Z, Sun R. Important role for NKp30 in synapse formation and activation of NK cells. Immunol Invest. 2012;41:367–81. doi: 10.3109/08820139.2011.632799. [DOI] [PubMed] [Google Scholar]
- 29.Sungur CM, Murphy WJ. Utilization of mouse models to decipher natural killer cell biology and potential clinical applications. Hematology Am Soc Hematol Educ Program. 2013;2013:227–33. doi: 10.1182/asheducation-2013.1.227. [DOI] [PubMed] [Google Scholar]
- 30.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie T, Rowen L, Aguado B, Ahearn ME, Madan A, Qin S, et al. Analysis of the gene-dense major histocompatibility complex class III region and its comparison to mouse. Genome Res. 2003;13:2621–2636. doi: 10.1101/gr.1736803. [DOI] [PMC free article] [PubMed] [Google Scholar]