Abstract
Importance
The rabbit is the primary animal model used to investigate aspects of nasal surgery. Although several studies have used this model, none has provided a comprehensive analysis of the surgical anatomy and techniques used to gain access to the rabbit nasal fossae and septum.
Objective
To describe and optimize the surgical anatomy and approach to the rabbit nasal vault and septal cartilage.
Design, Setting, and Participants
In an ex vivo animal study conducted at an academic medical center, preliminary cadaveric dissections were performed on rabbit head specimens to establish familiarity with relevant anatomy and rehearse various approaches. Live Pasteurella-free New Zealand white rabbits (3.5-4.0 kg) were used to further develop this surgical technique developed here. Access of the nasal vault was gained through a midline nasal dorsum incision and creation of an osteoplastic flap with a drill. Submucosal resection was performed with preservation of the mucoperichondrium. All rabbits were monitored daily for 4 weeks in the postoperative period for signs of infection, pain, and complications. The study was conducted from June 1, 2014, to December 1, 2014.
Main Outcomes and Measures
Surgical anatomy and techniques used to gain access to the rabbit nasal vault and harvest septal cartilage.
Results
Four Pasteurella-free New Zealand white rabbits (Western Organ Rabbit Co), ranging in age from 9 to 12 months and weighing between 3.5 and 4.0 kg, were used in this study. Initial dissections demonstrated the feasibility of harvesting septal cartilage while preserving the mucoperichondrial envelope. Access to the nasal vault through this 3-osteotomy approach allowed for maximal exposure to the nasal cavity bilaterally while maintaining the integrity of the mucoperichondrium following septal cartilage harvest. The maximum amount of bulk, en bloc, cartilage harvested was 1.0 × 2.5 cm. Following surgical dissection, all animals maintained adequate airway patency and support to midface structures. Furthermore, all specimens preserved the integrity of the mucoperichondrium, septum, vascular anatomy, and airway dynamics. No operative complications, postoperative airway compromise, or infections were observed.
Conclusions and Relevance
Access to the rabbit nasal vault and septal cartilage is feasible through a variety of surgical approaches and techniques. To date, this is the first study to meticulously document and review the surgical approaches to the rabbit nasal cavity. This approach describes a novel, 3-osteotomy method of accessing the nasal cavity bilaterally and successfully harvesting rabbit septal cartilage in a submucoperichondrial plane. The ability to preserve native anatomy and function allows for improved outcomes in translational and animal guided clinical research.
Level of Evidence
NA.
Key Points
Question
What is the surgical anatomy and approach to the New Zealand white rabbit nasal vault and septal cartilage?
Findings
Although the rabbit septum and nasal vault exhibits gross similarities to its human counterpart, the smaller cranium, and bony snoutlike nose prohibits conventional approaches that would otherwise be used in humans. Most publications provide vague details generally involving osteotomies without any information on instrument selection or surgical landmarks. Our study is the first to meticulously document and review the surgical approaches to the rabbit nasal cavity. We describe a novel three-osteotomy method of accessing the nasal cavity bilaterally and successfully harvest rabbit septal cartilage in a sub-mucoperichondrial plane.
Meaning
A novel and reproducible three-osteotomy dorsal approach to the nasal cavity bilaterally allows for the meticulous dissection of septal cartilage with preserved septal integrity, vascular anatomy, and airway dynamics.
This ex vivo study examines the anatomy and approaches to rabbit septal surgery.
Introduction
The rabbit nasal septum has similar morphologic characteristics to the human septum and maintains the same anatomic relationships between the skull base, vomer, and perpendicular plate of the ethmoid bone. Many investigators relied on rabbit models in early attempts to investigate the effect of removing the nasal septum (eg, submucosal resection) on the development of the human facial skeleton. The rabbit nasal fossae and paranasal sinuses have also been used to study sinus disease and endoscopic surgery. More recently, the rabbit septal cartilage model has been used to study advances in cartilage reshaping.
Despite numerous publications involving rabbit septal surgery, descriptions of the relevant surgical anatomy, operative considerations, and techniques used to gain access to the nasal fossae are lacking. Although the rabbit septum exhibits gross similarities to its human counterpart, the smaller cranium and bony snoutlike nose prohibit conventional approaches that would otherwise be used in humans. Most publications provide vague details generally involving osteotomies, without any information on instrument selection or surgical landmarks. Even among the more detailed studies, sufficient technical details are lacking, making reproduction of the experiment a challenge. Accordingly, we set out to review the relevant lagomorph anatomy in the New Zealand White rabbit, describe our surgical approaches, and detail our experience and operative technique.
Rabbit Anatomy
In humans, the nose functions as part of the upper respiratory tract airway, olfaction, humidification and warming of inspired air, filtration of airborne particulates, immune defense of airborne pathogens, vocal resonance, and nasal reflex functions. In comparison, the rabbit nose has a keener sense of olfaction and plays an integral part in systemic thermoregulation. The rabbit nasal mucosa provides critical thermoregulation because they lack sweat glands, which dissipate heat. Grossly, the rabbit nasal anatomy has more similarities to than differences from human anatomy and thus serves as a reasonable and economical model for studying human nasal function.
The anatomic descriptions of the nasal vault and vasculature summarized in this study were obtained from the works of Mills and Christmas, Forsgren et al,Kumlien and Schiratzki, and Godynicki. Anteriorly, the paired nasal bones, premaxilla, and incisive bone enclose the piriform aperture. The septal cartilage in its most anterior extent protrudes through the piriform aperture and terminates just superior to the incisors. The long and flat nasal bones extend posteriorly and articulate with the frontal bone at the frontonasal suture, thus forming the nasal dorsum (Figure 1A). This suture is a growth center in young rabbits. Posteriorly, the choanae communicates with the nasopharynx and is divided into 2 halves by the nasal septum. Posterosuperiorly, the septum articulates with the bony perpendicular plate of the ethmoid and posteroinferiorly with the presphenoid bone. Ventrally, the cartilaginous septum communicates with the vomer via the septovomeral joint. Anteriorly, the vomeronasal organ sits along the ventral midline margin supported by the dorsal surface of the palatine processes of the maxilla. The floor of the nasal fossae is formed primarily from the palatine process of the maxilla, which also forms the roof of the hard palate, with a central bony deficit in the midline anterior to the molars. This area is known as the palatine fissure, which provides a soft-tissue window to enter the nasal fossae from the oral cavity
Figure 1. Bony and Mucosal Depictions of the Snout Nasal Cavity.
Dorsal (A) and coronal (B) views demonstrate the humanlike qualities of the snout and paranasal structures. Adapted with permission from Evans and de Lahunta.
Internally, the nasal fossae are contained within a complex array of turbinates attached to the lateral wall (Figure 1B). The maxilloturbinate is located anteriorly, near the piriform aperture, and more ventrally. It is also known as the ventral nasal concha owing to its location. Air is humidified here as it flows toward the ethmoidoturbinate (also termed dorsal nasal concha), which is located more posteriorly. Four endoturbinates make up the ethmoidoturbinate complex and fill the remaining space in the nasal fossae. Rabbit turbinates differ from their human counterparts in that they have complex branching patterns that maximize surface area to functionally dissipate heat and regulate temperature.
Vasculature
Since thermoregulation is a primary function of the nasal mucosa, blood flow to this area is extensive. The maxillary artery and its branches, primarily the sphenopalatine artery, supply the majority of the nasal fossae. Smaller contributions from the external ethmoidal artery, malar artery, and major palatine artery also supply this region. As in human anatomy, the sphenopalatine artery enters the nasal fossae via the sphenopalatine foramen and travels a short distance before it branches into a larger caudal nasal artery and smaller nasal septal artery. The caudal nasal artery travels anteriorly to supply the mucosa of the maxilloturbinate or ventral concha. The lateral nasal artery branches from the proximal caudal nasal artery and supplies the mucosa of lateral nasal fossae and the ethmoidoturbinates or dorsal concha. These concha are also supplied in part by contributions from branches of the external ethmoidal arteries via the cribriform foramina to the nasal cavity. Arteriovenous anastomoses are present throughout the nasal concha and are tributaries of the dorsal nasal vein. The nasal septal artery, which is the smaller of the 2 branches off the sphenopalatine artery, runs medially to supply the mucosa of the septum. One of the ventral branches off the nasal septal artery supplies the vomeronasal organ. From below the nasal fossae, the major palatine artery gives rise to the small palatonasal artery, providing a minimal contribution to the nasal septum.
The nasal mucosa also contains a rich network of venous vessels. The nasal septum is covered with a venous plexus, and the ventral and dorsal concha contain cavernous plexuses. The anterior nasal fossae are drained mostly from the dorsal nasal vein. Other nasal structures also drain into deeper veins, such as the palatine, nasal septal, and sphenopalatine veins.
Surgical Approaches
There is limited detailed information about the surgical approaches to the rabbit nasal fossae. Sarnat and Wexler performed several studies in which the nasal septum was removed from growing rabbits using a sublabial incision between the upper incisors and lip. The nasal fossae were accessed by elevating the soft tissue off the anterior snout. This anterior approach effectively removed the nasal septum and all covering mucoperichondrium. Fuchs used a similar technique, but removed only the mucoperichondrium from selected regions of the septal cartilage. Other researchers investigating maxillary sinus physiology used a skin incision placed over the nasal dorsum and a cutting burr to drill away the anterior maxillary wall. Kennedy and Shaalan demonstrated a similar endoscopic method that created an anterior entry into the maxillary sinus using a curette and small punch. Other methods used to create osteotomies included chisels, rongeurs, scissors, scalpels, and punch forceps.
In attempting a complete mucoperichondrium-sparing submucosal resection, Verwoerd et al approached the nasal fossa through a midline incision over the nasal dorsum. The nasal fossae were entered by using a small drill burr to remove the left nasal bone anterior to the frontonasal suture. The septum was dissected away from its mucoperichondrium on both sides and a segment of the septum was removed. This technique allowed for improved visualization and exposure to the posterior septum and was replicated in several other studies. However, this modified technique did not allow for exposure to both sides of the nasal septum or nasal cavity.
Methods
Four Pasteurella-free New Zealand white rabbits (Western Organ Rabbit Co), ranging in age from 9 to 12 months and weighing between 3.5 and 4.0 kg, were used in this study. All protocols and experiment design processes were reviewed and approved by the University of California, Irvine, Institutional Animal Care and Use Committee. The study was conducted from June 1, 2014, to December 1, 2014.
Induction anesthesia (3.0 mL of ketamine, 100 mg/mL, and xylazine, 20 mg/mL, in a 2:1 ratio) was given via an intramuscular injection. An intravenous catheter was then introduced into the rostral auricular vein. The rabbit was then intubated (3.0 cuffed endotracheal tube) using a pediatric #0 Miller laryngoscope and connected to a ventilator (isoflurane, 2%, gas). Temperature, heart rate, and oxygen saturation were monitored, with the rabbit placed prone over a heating pad.
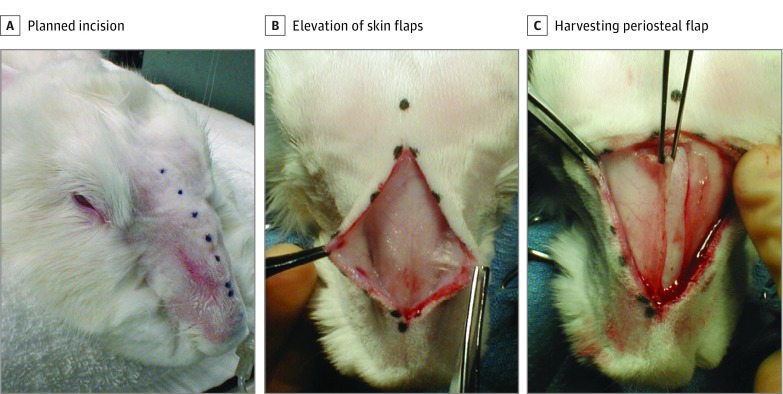
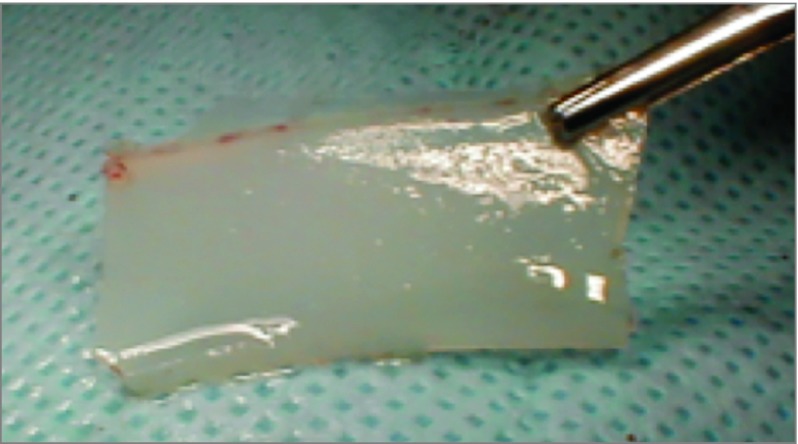
The rabbit’s face and snout were shaved and prepped with antiseptic solution. The nasal dorsum was then injected with 0.5 mL of lidocaine, 0.5%, mixed with 1:200 000 epinephrine solution for vasoconstriction and local anesthesia. Preoperative antibiotics consisted of an intramuscular injection of enrofloxacin, 2.5 mg/kg. A midline incision, as illustrated in Figure 2A, from the frontonasal suture to approximately 1.5 cm from the nasal tip was created over the dorsum. Right and left skin flaps were elevated with care to avoid injuring the lateral nasal artery and veins (Figure 2B). A right-based periosteal flap was created. An electric drill (Dremel; Robert Bosch Tool Corp) at 15 000 rpm with a 1-mm drill bit (model 106, Dremel) was used to create a bone flap (Figure 2C). Three osteotomies were created (Figure 3): 2 horizontal cuts just anterior to the frontonasal suture and 1 cm from the piriform aperture connected by a single parasagittal cut. The drill was then used to create a small starter cut at the base of the flap to facilitate the creation of a greenstick fracture at the base of bone flap. Once the osteotomies were complete, the nasal mucosa was elevated off the bone flap with a Cottle elevator (Medline Industries Inc) (Figure 4A). The bone flap was then outfractured, providing exposure into the nasal cavity. The mucoperichondrium was carefully dissected free from both sides of the septum with a Cottle elevator. The cartilage was freed from its inferior attachment to the septovomeral joint (Figure 4B). Vertical incisions were made into the middle portion of the septal cartilage, and the cartilage specimen was removed. The excised septal cartilage specimens removed from the middle segment of the septum were approximately 1.0 × 2.5 cm (Figure 5). A 1-cm segment of intact cartilage was preserved in the nasal vault anteriorly and posteriorly to maintain the general support of the airway. Both sides of the mucoperichondrium were reapproximated using 5-0 fast-absorbing gut quilting suture (Ethicon Inc). The bone flap was returned to its normal anatomic position and sutured into place after creation of 2 drill holes in the bone to allow suture fixation. The periosteum was reapproximated over the defect, and the skin incision was closed with 4-0 Vicryl suture with serial horizontal mattress fashion (eFigure in the Supplement). Reapproximation of the periosteal tissue layer was performed to promote bone growth over the nasal dorsum. A Dutch collar was placed around the rabbit’s neck to prevent it from scratching the surgical site.
Figure 2. Dissection of the Soft-Tissue Envelope.
A, Planned dorsal nasal incision. B, Elevation of skin flaps bilaterally. C, Harvesting a right-based periosteal flap.
Figure 3. Three-Osteotomy Window Illustration and Its Axis.
Figure 4. Access to the Nasal Cavity.
Cottle elevator to rotate and outfracture the nasofrontal osteoplastic flap (A), providing exposure to the nasal cavity and septal cartilage (B).
Figure 5. Rabbit Septal Cartilage.
Approximately 1 x 2.5 cm.
The rabbits were then extubated, and postoperative analgesia consisted of buprenorphine, 0.1 mg/kg, delivered subcutaneously every 8 to 12 hours as needed for observed pain or discomfort. The rabbits were observed daily in the vivarium for signs of pain, infection, or feeding abnormalities.
Results
All rabbits tolerated the operation without complication and were studied for 4 weeks. No intraoperative mortalities or postoperative morbidities were observed. Intraoperatively, there was no evidence of septal perforation. Furthermore, access to the nasal septum bilaterally was accomplished in 100% of the specimens.
Following extubation, all operative sites appeared similar to the preoperative nasal dorsum, without any skin or soft-tissue lesions. The incision sites appeared well and intact. Postoperative, peri-incisional edema was present in all animals, although this did not obstruct resumption of postextubation nasal breathing in any of the rabbits. By postoperative day 3, there was no evidence of postoperative hematoma or airway obstruction.
At 4 weeks, no signs of surgical site or upper respiratory tract infection were present in any of the specimens. The rabbit snout retained its native shape without distortions to the lateral nasal wall in all specimens.
Discussion
The obvious differences in anatomy and size between the rabbit and human nasal vault dictate different approaches to surgery. The small snout is almost completely enveloped by a bony framework; in contrast, the human nose is more flush with the face and is only approximately one-third covered by the bony pyramid. The rabbit nostril aperture is too narrow to permit any conventional septal surgery to be performed let alone introduction of an endoscope. Septoplasty in humans can be performed entirely via classic anterior approaches without disrupting the overall structure of the nose or adjacent soft tissues. Such approaches will not work in rabbits because of poor anterior exposure, smaller anatomy, and difficulty in sparing injury to delicate structures, such as the mucoperichondrium.
It is difficult to imagine performing a classic submucosal resection in rabbits where the mucoperichondrium is spared. Septal cartilage harvest in rabbits requires access to the nasal vault with osteotomies. In preliminary investigations on ex vivo rabbit crania, we attempted to develop an endoscopic anterior septoplasty technique but were unable to access the nose because of the narrow aperture and short dimensions of the rabbit nasal fossae. Other methods of accessing the nose from an anterior degloving approach, similar to that of Sarnat and Wexler, also proved to be impractical owing to the nasal passageway being too narrow to allow successful dissection of the mucoperichondrial layer of the septum. Sarnat and Wexler, like many of the earlier investigators, were able to remove only en bloc septal cartilage without regard for the mucoperichondrial layer, thus producing perforations. Verwoerd and colleagues first documented that the mucoperichondrium could be preserved in survival studies. Nevertheless, they did not mention whether the mucosa was reapproximated, increasing the risk of potential postoperative septal perforations and hematomas.
Although it is theoretically possible to dissect the mucosa from septal cartilage using the single-sided approach, we found that our approach allowed for better access and reduced the chance of inadvertent injury to the delicate mucosa. Furthermore, both sides of the mucoperichondrium were reapproximated with a quilting stitch to prevent septal perforations or hematoma formation, again reducing the likelihood of acute postoperative airway obstruction.
In addition, osteotomies were made along parasagittal lines to preserve the lateral buttresses of the nasal bones and thus minimize potential distortion of the rabbit snout. By greenstick fracturing the bone flap, we sought to minimize distortions to the nasal dorsum once the bone flap was replaced. In contrast, the technique used by Verwoerd et al cut the left nasal bone in a midsagittal plane to allow lateral removal. Although there is no mention of postoperative deformities of the nasal dorsum, one can infer that some degree of distortion occurred, potentially leading to airway compromise and structural deformity. Furthermore, although postoperative sinonasal infection has not been documented in rabbit surgical models, precautions while accessing the nasal vault must be discussed.
Significant morbidity and mortality due to Pasteurella-associated upper and lower respiratory tract infections have been observed in the rabbit model. The absence of upper respiratory tract complications in our study may in part be explained by our institution’s strict Pasteurella precautions, which require all animal caregivers and research staff to wear a surgical cap, clean gown, gloves, and shoe covers when entering the controlled ventilation environment in which the animals are housed. The study rabbits were also quarantined from rabbits of other studies to reduce the chance of cross contamination. In addition, the rabbits were obtained from a supplier that guaranteed Pasteurella-free status.
Limitations
Similar to human specimens, rabbit septal cartilage grows and matures with the developing rabbit snout and crania. Variability in size, texture, and amount harvested en bloc will vary with rabbit age and weight. In theory, the technique described may be limited by a septal size minimum, though this was not encountered in the study.
Conclusions
Submucosal resection of the rabbit septal cartilage is feasible using a 3-osteotomy, dorsal approach. Our technique demonstrates minimal facial deformity and no significant operative or postoperative complications. By taking care to reduce injury to the mucoperichondrium, the remaining septum is preserved, as well as postoperative nasal function. Owing to the rabbit’s nasal anatomy, the need for adequate exposure to both sides of the nasal septum, as well as the cephalic portion, necessitates that osteotomies be performed. Our experience has demonstrated this technique to be safe, while preserving the rabbit nasal integrity, vascular anatomy, and airway dynamics.
eFigure. Periosteum and Skin Reapproximated, With Complete Wound Closure
References
- 1.Nolst Trenité GJ, Verwoerd CDA, Verwoerd-Verhoef HL. Reimplantation of autologous septal cartilage in the growing nasal septum: II—the influence of reimplantation of rotated or crushed autologous septal cartilage on nasal growth: an experimental study in growing rabbits. Rhinology. 1988;26(1):25-32. [PubMed] [Google Scholar]
- 2.Nolst Trenité GJ, Verwoerd CD, Verwoerd-Verhoef HL. Reimplantation of autologous septal cartilage in the growing nasal septum: I—the influence of resection and reimplantation of septal cartilage upon nasal growth: an experimental study in rabbits. Rhinology. 1987;25(4):225-236. [PubMed] [Google Scholar]
- 3.Verwoerd CDA, Urbanus NAM, Nijdam DC. The effects of septal surgery on the growth of nose and maxilla. Rhinology. 1979;17(2):53-63. [PubMed] [Google Scholar]
- 4.Verwoerd CD, Verwoerd-Verhoef HL. Developmental aspects of the deviated nose. Facial Plast Surg. 1989;6(2):95-100. [DOI] [PubMed] [Google Scholar]
- 5.Verwoerd CD, Urbanus NA, Mastenbroek GJ. The influence of partial resections of the nasal septal cartilage on the growth of the upper jaw and the nose: an experimental study in rabbits. Clin Otolaryngol Allied Sci. 1980;5(5):291-302. [DOI] [PubMed] [Google Scholar]
- 6.Tonneyck-Müller I, Van der Werf F. The development of the nasal septum in the rabbit: I—its relationship with the neighboring parts of the skull [in German]. Acta Morphol Neerl Scand. 1982;20(2):93-109. [PubMed] [Google Scholar]
- 7.Wexler MR, Sarnat BG. Rabbit snout growth: effect of injury to septovomeral region. Arch Otolaryngol. 1961;74:305-313. [DOI] [PubMed] [Google Scholar]
- 8.Verwoerd CD, Verwoerd-Verhoef HL, Meeuwis CA, vd Heul RO. Wound healing of the nasal septal perichondrium in young rabbits. ORL J Otorhinolaryngol Relat Spec. 1990;52(3):180-186. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein L. Early submucous resection of nasal septal cartilage: a pilot study in canine pups. Arch Otolaryngol. 1973;97(3):273-278. [DOI] [PubMed] [Google Scholar]
- 10.Sarnat BG, Wexler MR. Growth of the face and jaws after resection of the septal cartilage in the rabbit. Am J Anat. 1966;118(3):755-767. [DOI] [PubMed] [Google Scholar]
- 11.Sarnat BG, Wexler MR. The snout after resection of nasal septum in adult rabbits. Arch Otolaryngol. 1967;86(4):463-466. [DOI] [PubMed] [Google Scholar]
- 12.Sarnat BGWM, Wexler MR. Postnatal growth of the nose and face after resection of septal cartilage in the rabbit. Oral Surg Oral Med Oral Pathol. 1968;26(5):712-727. [DOI] [PubMed] [Google Scholar]
- 13.Jeffries DJ, Rhys Evans PH. Cartilage regeneration following septal surgery in young rabbits. J Laryngol Otol. 1984;98(6):577-583. [DOI] [PubMed] [Google Scholar]
- 14.Hilding AH. Experimental sinus surgery; some experiments on ventilation and sinusitis. Laryngoscope. 1948;58(10):1098-1102. [DOI] [PubMed] [Google Scholar]
- 15.Hilding A. Experimental sinus surgery: effects of operative windows on normal sinuses. Ann Otol Rhinol Laryngol. 1941;50:379-392. [Google Scholar]
- 16.Forsgren K, Stierna P, Kumlien J, Carlsöö B. Regeneration of maxillary sinus mucosa following surgical removal: experimental study in rabbits. Ann Otol Rhinol Laryngol. 1993;102(6):459-466. [DOI] [PubMed] [Google Scholar]
- 17.Cöloğlu H, Uysal A, Koçer U, Kankaya Y, Oruç M, Uysal S. Rhinoplasty model in rabbit. Plast Reconstr Surg. 2006;117(6):1851-1859. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy DW, Shaalan H. Reevaluation of maxillary sinus surgery: experimental study in rabbits. Ann Otol Rhinol Laryngol. 1989;98(11):901-906. [DOI] [PubMed] [Google Scholar]
- 19.Melgarejo-Moreno PJ, Ribera-Cortada I, Sarroca-Capell E. Radical or partial maxillary sinus surgery: a dilemma today? an experimental study. Rhinology. 1996;34(2):110-113. [PubMed] [Google Scholar]
- 20.Wong BJ, Chao KK, Kim HK, et al. The porcine and lagomorph septal cartilages: models for tissue engineering and morphologic cartilage research. Am J Rhinol. 2001;15(2):109-116. [DOI] [PubMed] [Google Scholar]
- 21.Protsenko DE, Ho K, Wong BJF. Survival of chondrocytes in rabbit septal cartilage after electromechanical reshaping. Ann Biomed Eng. 2011;39(1):66-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu EC, Protsenko DE, Khan AZ, Dubin S, Karimi K, Wong BJF. Needle electrode-based electromechanical reshaping of rabbit septal cartilage: a systematic evaluation. IEEE Trans Biomed Eng. 2011;58(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills RP, Christmas HE. Applied comparative anatomy of the nasal turbinates. Clin Otolaryngol Allied Sci. 1990;15(6):553-558. [DOI] [PubMed] [Google Scholar]
- 24.Forsgren K, Otori N, Stierna P, Kumlien J. Microvasculature, blood flow, and vasoreactivity in rabbit sinus mucosa after surgery. Laryngoscope. 1999;109(4):562-568. [DOI] [PubMed] [Google Scholar]
- 25.Kumlien J, Schiratzki H. The vascular arrangement of the sinus mucosa: a study in rabbits. Acta Otolaryngol. 1985;99(1-2):122-132. [DOI] [PubMed] [Google Scholar]
- 26.Godynicki S. Blood vessels of the nasal cavity in the rabbit. Folia Morphol (Warsz). 1975;34(1):69-76. [PubMed] [Google Scholar]
- 27.Evans HE, de Lahunta A. Miller's Anatomy of the Dog. 4th ed. St Louis, MO: Elsevier Saunders; 2013. [Google Scholar]
- 28.Levine P. Certain aspects of the growth pattern of the rabbit’s skull as revealed by alizarine and metallic implants. Angle Orthod. 1948;18(27). [Google Scholar]
- 29.Babler WJPJ. Relationships between cranial suture growth and basicranial and midfacial growth in the young-rabbit. Anat Rec. 1984;208(3):11A-12A. [Google Scholar]
- 30.Fuchs P. Experimental production of growth disturbance by using a caudally based vomerine flap in rabbits In: Sanvanero-Rosseilli G, ed. Transactions of the Fourth International Congress of Plastic Surgery. Amsterdam, the Netherlands: Excerpta Medical Foundation, 1969:484-488. [Google Scholar]
- 31.Gehani NC, Houser SM. Septoplasty, turbinate reduction, and correction of nasal obstruction In: Johnson J, Rosen C, eds. Bailey’s Head and Neck Surgery: Otolaryngology. 5th ed Baltimore, MD: Lippincott Williams & Wilkins; 2014:e1716-e1744. [Google Scholar]
- 32.Deeb BJ, DiGiacomo RF, Bernard BL, Silbernagel SM. Pasteurella multocida and Bordetella bronchiseptica infections in rabbits. J Clin Microbiol. 1990;28(1):70-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass LS, Beasley JN. Infection with and antibody response to Pasteurella multocida and Bordetella bronchiseptica in immature rabbits. Lab Anim Sci. 1989;39(5):406-410. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Periosteum and Skin Reapproximated, With Complete Wound Closure