Introduction
Atrial fibrillation (AF) is the commonest cardiac rhythm disorder, affecting about 5% of elderly patients.[1] Despite the wide spread prevalence of AF, treatment options for the condition up until recently, were limited. Antiarrhythmic drug therapy which for a long time had been and to some extent still is the cornerstone for treating these patients, has shown a disappointing (≤ 40%) efficacy for long-term maintenance of sinus rhythm.[2] The seminal observations by Haissaguerre and colleagues demonstrating AF initiation from electrical depolarizations in the pulmonary veins (PV) and cure of AF in these patients by radiofrequency ablation (RFA) of the PV focus, has led to the emergence of percutaneous catheter based AF ablation.[3] Since its original description in 1998, the AF ablation procedure has evolved considerably.[4-7] This evolution was the result of several short comings of the original technique (focal ablation) including the inability to expeditiously target multiple evanescent PV foci effectively and the long term consequences of delivering RF energy within the PVs i.e., occurrence of vein stenosis.[8-12] Thus an alternative approach was developed where instead of ablating individual foci within the veins, RF lesions are delivered around the circumference of the PV ostium in order to interrupt PV-left atrial (LA) electrical connections and thus achieve PV isolation (PVI).[8] Compared with focal AF ablation, PVI has shown a significant enhancement in long term arrhythmia control while dramatically reducing the occurrence of PV stenosis.[11-13] both of which are welcome developments. A potential reason for the enhanced success of PVI may be that the triggers of AF are clustered in close proximity to the vein ostia. However, an alternative explanation for the enhanced success of PVI is that the procedure inadvertently targets LA tissue (around PV os) and so results in modification of the underlying substrate. This hypothesis has been supported by the observation that anatomically guided circumferential PV ostial ablation without necessarily achieving true PVI (entry / exit block) is equally efficacious in achieving long term AF control.[5][6] In fact, this observation has led to the development of an anatomically guided incremental ablation strategy which attempts to substantially modify the AF substrate by extensive LA ablation.[14-16] However, such an approach has not consistently demonstrated a significant improvement in long term AF control rates as compared with PVI alone, which would suggest that PVs may be critical to the initiation and / or maintenance of AF.[18][19] The purpose of this editorial is to provide the readers with a concise overview on the arrhythmogenic potential of PVs and to discuss how best we can identify and target such veins and whether that translates into long term control of AF.
Are the Pulmonary Veins Arrhythmogenic by Design?
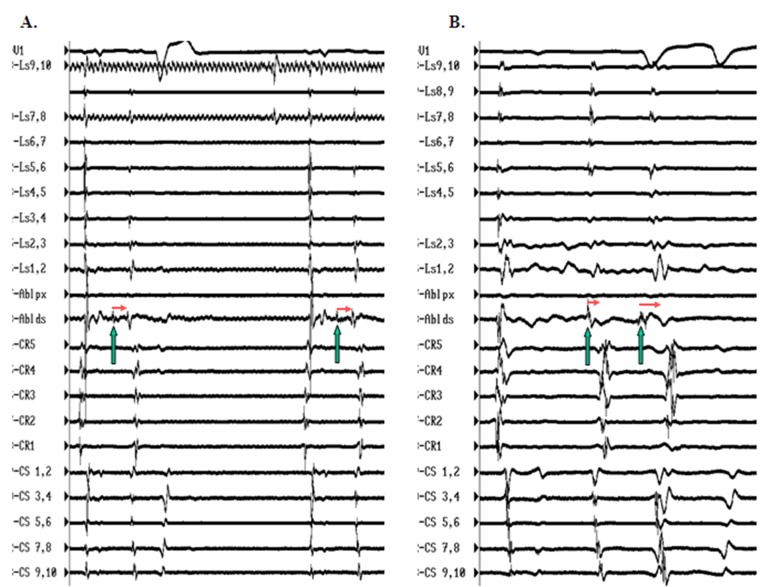
Both anatomical and electrical abnormalities can result in cardiac tissue arrhythmogenecity.[20-23] Anisotropy which, can be defined as lack of shared regional directionality amongst muscle fibers, is commonly observed in the right atrium along the crista terminalis, in the cavo-tricuspid isthmus region, near the coronary sinus ostium / eustachian ridge area, and these locations have frequently been identified as sites of atrial premature complexes and / or atrial tachycardias.[24-26] Data from autopsied human hearts has also shown marked tissue anisotropy in the form of complex looping posterior LA musculature encircling individual PV ostia and extending distally along the PV walls where it interdigitates with thevein musculature.[27] Such anisotropy may result in variable conduction velocities and unidirectional blocks that could potentiate wave front fractionation and AF initiation. Cardiac tissue arrhythmogenecity can also be an electrical phenomenon, due to alterations in the action potential profile of individual myocardial cells and / or cell groups resulting in abnormalities of impulse formation and / or conduction.[21-23] The PVs have been shown, both in animal models and human studies to have shorter refractory periods as compared with the rest of the LA.[28,29] Also, within the confines of the PVs, interdigitating LA musculature that is relatively isolated from the main LA syncitium, may manifest a different resting membrane potential which in turn can alter its action potential characteristics thus promoting arrhythmogenecity. Developmentally too, in the mouse model, cells constituting PV tissue have demonstrated properties akin to the AV node.[30] In support of the latter hypothesis, in some patients undergoing AF ablation where frequent spontaneous PV depolarizations have been identified, using a combination of circular mapping and a quadripolar ablation catheter, we have noted decremental conduction across the PVs such that the more closely coupled PV depolarizations manifest a longer exit time out of the vein. In these patients, isoproterenol infusion appears to facilitate PV conduction [[Figure 1]].
Figure 1. Panel A shows pulmonary vein depolarization (PVD; green arrows) recorded on the distal pole of ablation catheter (Abl ds) positioned in the left inferior (LI) PV with constant exit time out of the vein (interrupted line to generate atrial premature complexes (APCs). Panel B shows PVD response to isoproterenol infusion which causes increased frequency and the first PVD demonstrates shorter exit time compared with the second more closely coupled PVD that takes longer to exit out of the vein. Such behavior is consistent with facilitated and decremental conduction. From top to bottom the tracings are arranged as follows: ECG lead V1, Lasso (Ls; poles 9,10 to 1,2) positioned in the right superior PV, proximal (px) and distal (ds) poles of ablation catheter positioned in LIPV, decapolar catheter positioned in the posterior right atrium (CR5 – CR1) and decapoler catheter positioned in the coronary sinus (CS1,2 – CS 9,10).
Mechanisms Underlying PV Triggers of AF
Despite ample proof of the arrhythmogenic potential of PVs, there remains lack of consensus on the mechanism(s) that underlie PV triggers of AF. In an acetylcholine mediated, pacing induced model of AF in isolated sheep hearts, using optical and electrical mapping techniques, dominant and highest frequencies during AF were observed in the PV / posterior LA, and micro reentry mediated wave fronts were proposed as “drivers” for AF maintenance in the model.[28,31] Consistent with this observation, during ongoing AF in human subjects, rapid electrical activity has been infrequently recorded from within the PVs which has also been attributed to micro-reentry.[3,6,8] However, such sustained fast and organized electrical activity or PV tachycardia during AF is rarely observed.
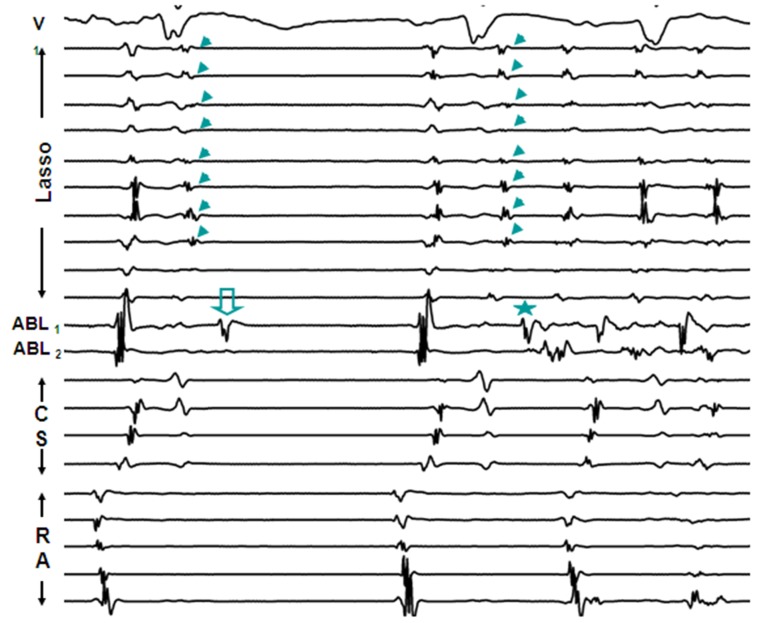
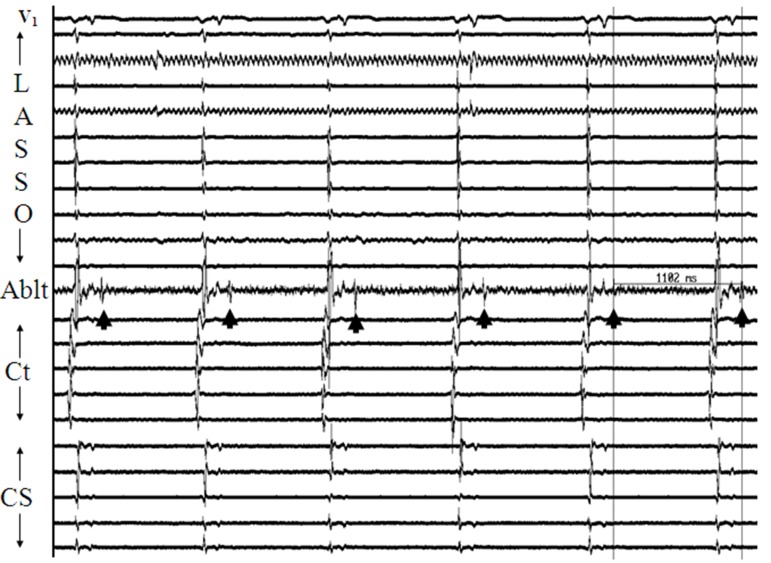
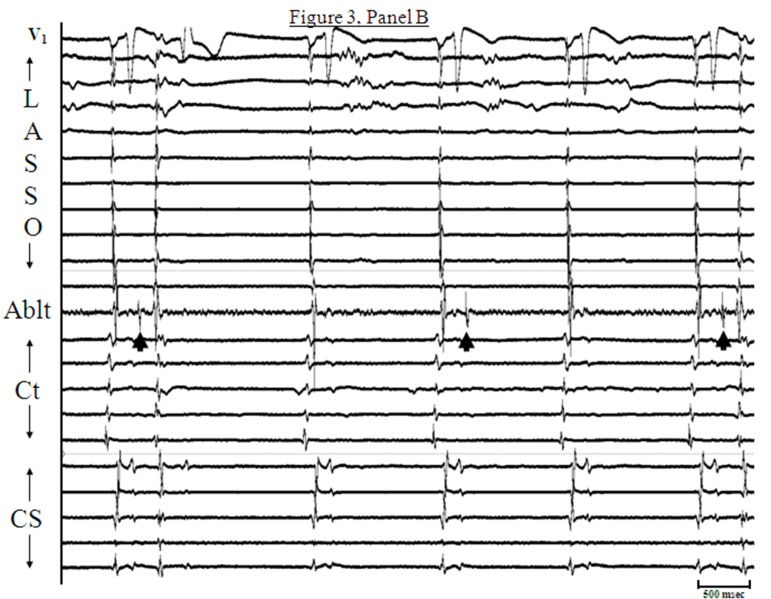
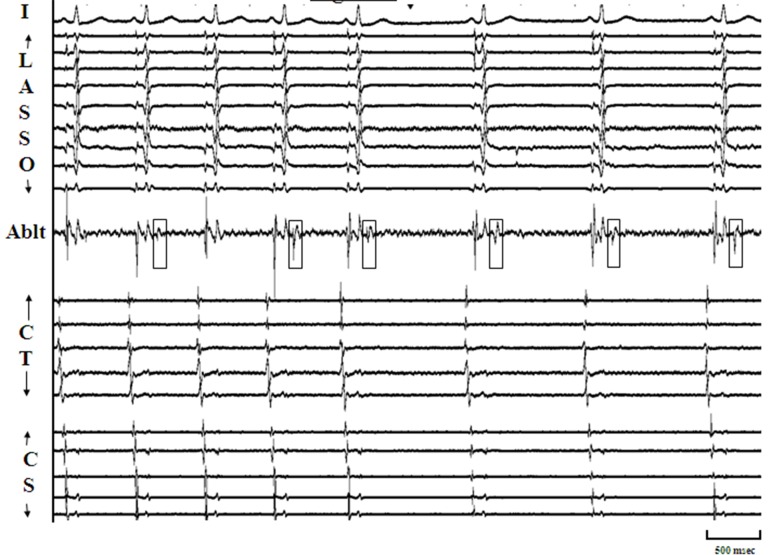
More frequently, AF is seen to initiate by atrial premature complexes (APCs) resulting from isolated PV depolarizations,[3,8] and these too are hard to characterize mechanistically [[Figure 2]]. On the other hand, sustained PV depolarizations or “firing” which can be defined as repetitive, discrete, multiphasic electrical activity recorded from catheters at/or distal to PV os [[Figure 3, Panels A]], that does not correlate in timing with other cardiac events (P, QRS and T waves) and persists at baseline for ≥ 5 minutes is an uncommon finding but when observed, it provides us a unique opportunity to explore mechanisms underlying PV arrhythmogenecity. In a series of 210 patients undergoing AF ablation at our center, using a combination of a decapolar circular mapping and quadripolar ablation catheters either simultaneously or separately we were able to identify sustained firing (PVF) in 15 subjects (7.1%; age 55±9 years; 14 males). Sustained PVF was observed in 16 veins including 6 right superior (RS), 4 left superior (LS), 1 right inferior (RI) and 5 left inferior (LI) PVs [20]. The relationship of sustained PVF to native sinus rhythm complexes showed a bigeminal pattern in the majority (n=11; 69%; [Figure 3; Panel A]), trigeminal pattern in 3 cases (19%; [Figure 3; Panels B]) and a variable pattern in 2 cases (13%). Once sustained PVF was confirmed, pacing was performed from coronary sinus and/or posterior right atrium at different cycle lengths (900 to 400ms; duration: 30 – 60 sec) following which, if PVF persisted, then in random order, isoproterenol and adenosine were administered and carotid sinus massage (CSM) was performed. PVF response was classified as: suppressed (complete quiescence during the maneuver; [Figure 4, Panels A] and [B]), augmented (increase in frequency of PVF / AF initiation; [Figure 5, Panels A] and [B]) and “no effect” [[Figure 6]]. In 13 pts (81%), PVF was suppressed during overdrive pacing with early recurrence (≤5seconds) post pacing regardless of the pacing cycle length in 11pts (85%). PVF was augmented by isoproterenol in the majority (88%), showed mixed response to adenosine (augmented-40%, suppressed-20 % and no effect-40%) and CSM appeared to have no effect on PVF. These observations suggest that PVF is unlikely due to sustained reentry and is more likely a result of triggered activity and / or abnormal automaticity.[20] Our observations on the mechanisms underlying PV triggers have been corroborated by the work of other investigators.[32] Based on these observations, we have developed a standardized stimulation protocol to elicit PV triggers for AF. Our stimulation protocol consists of 1) isoproterenol infusion (starting at 3-5 mcg and incrementing by 3-5 mcg every 3 minutes till a maximum of 20 mcg) and, 2) cardioversion of AF induced by LA or RA pacing (15-beat runs at amplitude of 10mA and pulse width of 2msec; decrementing by 10 msec from 250 msec to 180 msec and / or failure to capture with and without isoproterenol infusion).[33] We define arrhythmogenic PVs as veins that are documented to initiate AF and / or atrial premature complexes based on direct intracardiac recordings and / or activation sequences of multiploar catheters located in the posterior RA and coronary sinus mimicking PV pace maps.[12]
Figure 2. From top to bottom are ECG lead V1, 10 bipolar recordings of circular mapping (Lasso) catheter located at LIPV Os, recordings from distal and proximal bipoles of ablation catheter located in LSPV and 5 bipolar recordings from catheters positioned in coronary sinus (CS) and right atrium (RA). Initial two beats represent sinus rhythm with delayed conduction within LIPV (arrow heads). The 3rd beat is an atrial premature complex (APC) which degenerates into AF. Note earliest electrical depolarization in the distal bipole of the ablation catheter (star) preceding APC. Similar electrical activity is noticed in the distal bipole of the ablation catheter after the 1st sinus beat (open arrow). This may represent localized “depolarization” within the LSPV that fails to propagate within or exit the vein.
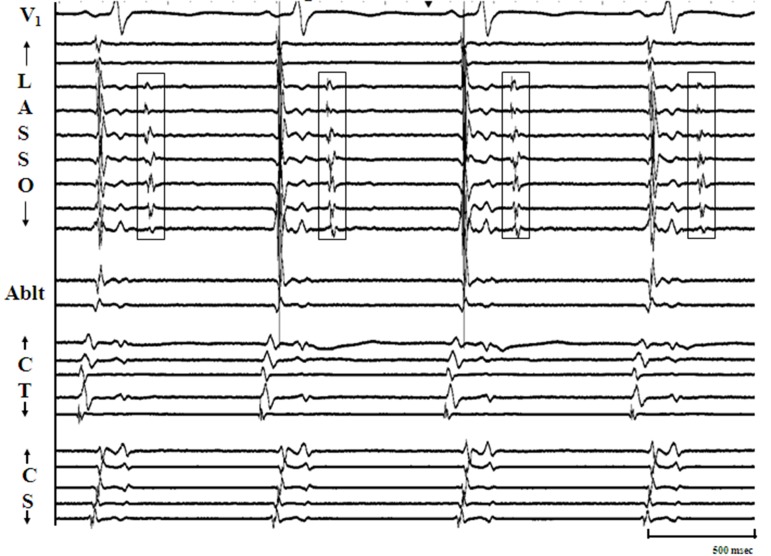
Figure 3A. Panel A: Illustrates the commonest pattern of sustained pulmonic vein firing (PVF) observed in our study. From top to bottom, the arrangement of tracings are: surface lead V1 , recordings from decapolar circular mapping catheter (Lasso) positioned at os of left superior pulmonic vein, distal bipole of ablation catheter positioned just beyond os of right superior pulmonary vein (RSPV), and recordings (distal to proximal) from decapolar catheters along the posterior right atrium (Ct) and in the coronary sinus (CS) respectively. Discrete multiphasic electrograms (Egm) representing spontaneous PVF are seen on the bipole of ablation catheter (arrow heads) that do not correspond in timing with P, QRS, or T waves. Since PVF occurs after each sinus beat, we define this pattern as bigeminal. Please note that PVF in this case does not exit out of the RSPV.
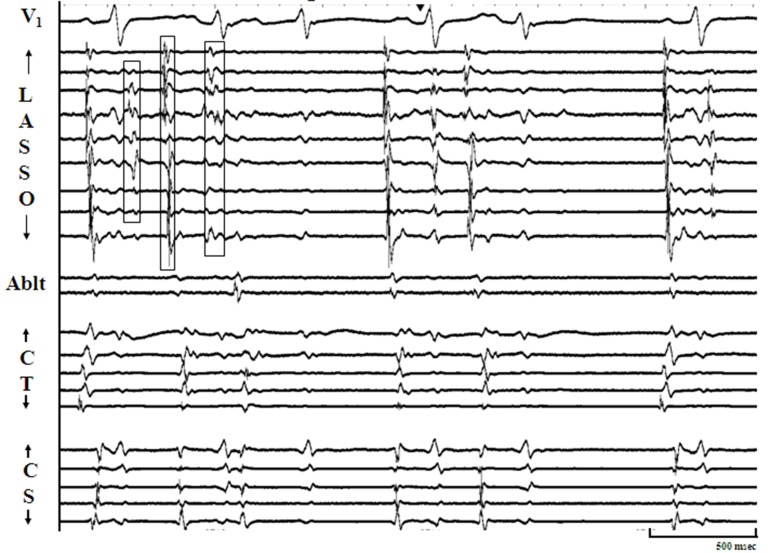
Figure 3B. Panel B : Illustrates another pattern of sustained pulmonic vein firing (PVF). The arrangement of tracings are identical to Panel A. Discrete multiphasic electrograms (Egm) representing spontaneous PVF are seen on the bipole of ablation catheter (arrow heads) and do not correspond in timing with P, QRS, or T waves. In this case, PVF occurs after every two sinus beat, and so the pattern is trigeminal. It can be appreciated from the figure that the 1st and last PVF exit out of the vein, generating atrial premature complexes (APCs) with expected alteration of intracardiac activation patterns and surface P wave morphology.
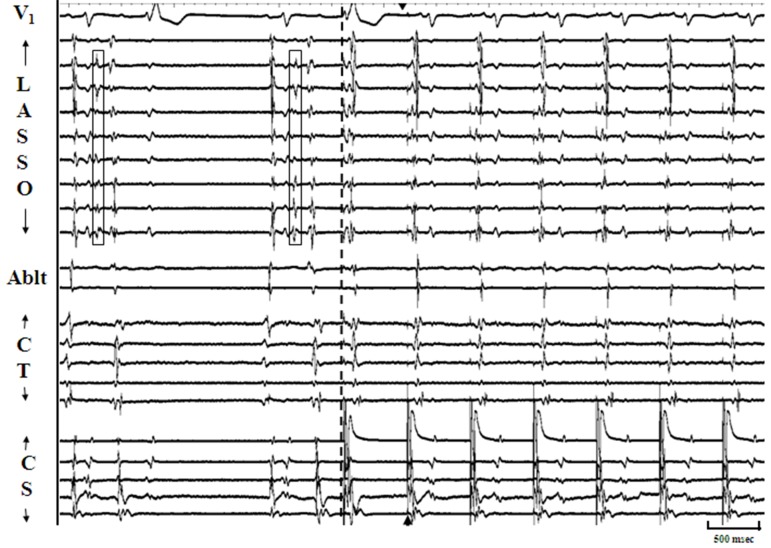
Figure 4A. Panel A: Illustrates response of sustained pulmonic vein firing (PVF) to overdrive pacing. The arrangement of tracings are similar to figure 1 and decapolar circular mapping catheter (Lasso) is positioned at os of right superior pulmonic vein (RSPV). Following both the initial sinus beats, discrete multiphasic electrograms (Egm) representing spontaneous pulmonic vein PVF (open boxes) are seen on multiple bipoles of Lasso catheter and result in atrial premature complexes. Pacing is initiated from distal most bipole of CS catheter(interrupted line) and capture of both atria is achieved with the second pacing drive (arrow head). Almost immediately with capture of overdrive pacing no further PVF is seen for the rest of the recording. Such early PVF suppression (within 5 seconds) in response to overdrive pacing was observed in the majority of cases in our study.
Figure 4B. Panel B: Is the continuation of the example in Figure 1, Panel A (with identical arrangement of tracings) and represents termination of the 60-second pacing drive from the distal CS bipole (cycle length 600 msec). Recurrence of PVF post pacing (open box) is seen after the 2nd sinus beat and occurs within 5 seconds of pacing drive termination (early recurrence). This pattern of early PVF recurrence post pacing was a commonly observed response in our study.
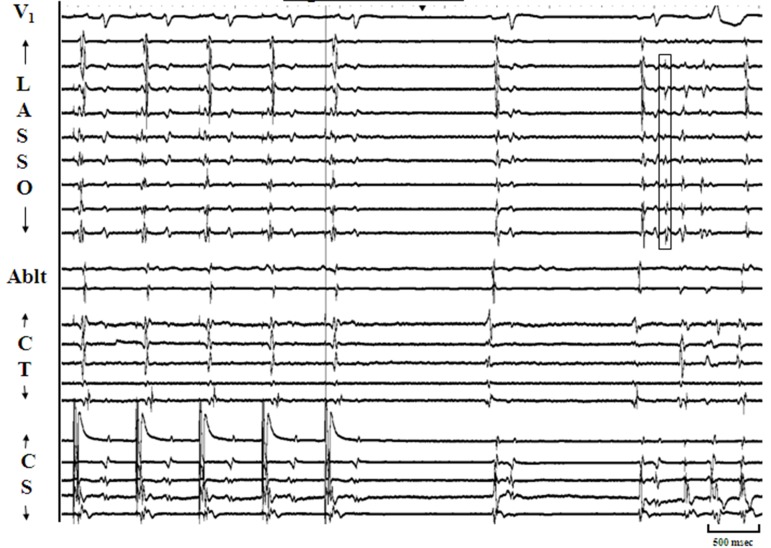
Figure 5A. Panel A: IIllustrates sustained pulmonic vein firing (PVF) at baseline seen as discrete multiphasic electrograms (Egm) on multiple poles of Lasso catheter (open boxes) positioned at os of left inferior pulmonary vein (LIPV). The arrangement of tracings areas in previous figures. PVF in this case does not exit out of the LIPV.
Figure 5B. Panel B: This figure represents pulmonic vein firing (PVF) in the same patient as in Panel A. In response to isoproterenol, there is increase in the heart rate. Also evident is an increase in the frequency of PVF (multiple discrete electrograms on several bipoles of Lasso catheter – open boxes) with and without conduction out of the vein resulting in atrial premature complexes. This constitutes augmentation of sustained PVF in response to isoproterenol.
Figure 6. This figure represents pulmonic vein firing (PVF) in the same patient as in Panel A. In response to isoproterenol, there is increase in the heart rate. Also evident is an increase in the frequency of PVF (multiple discrete electrograms on several bipoles of Lasso catheter – open boxes) with and without conduction out of the vein resulting in atrial premature complexes. This constitutes augmentation of sustained PVF in response to isoproterenol.
Is Isolation of Arrhythmogenic PVs Sufficient for the Long-Term Efficacy of AF Ablation?
Since its original description in 1998,3 atrial fibrillation (AF) ablation procedure has undergone several modifications. Many operators performing AF ablation utilize an anatomical approach that involves creation of circumferential radiofrequency ablation (RFA) lesions encircling ipsilateral pulmonary veins (PVs) with or without additional left atrial (LA) linear lesions.[4-7,14,17,18] Practitioners of this approach do not seek proof of PV “arrhythmogenecity” and may not consistently document isolation of the targeted veins. While this strategy has shown good AF control rates, it involves extensive LA ablation.[15,17,18] An alternative approach is to target only the arrhythmogenic PVs. This latter technique has not gained wide acceptance because PV triggers of AF can be evanescent and there is no established protocol which has been consistently shown to reproducibly elicit them. The advantage of this approach may be the ability to achieve AF control by limiting ablations to only the arrhythmogenic veins. We conducted a prospective, randomized study to, 1) assess the ability of our stimulation protocol to consistently identify arrhythmogenic PVs and, 2) compare the efficacy of isolating thus identified arrhythmogenic PVs versus empiric isolation of all PVs on long term control of AF.[34,35] The primary end-point was the efficacy of either strategy in achieving long-term control of AF which, was defined as complete freedom and / or >90% reduction in AF burden either off or on previously ineffective antiarrhythmic drugs (AAD) at 1 year after a single ablation procedure. All patients with drug refractory AF undergoing their first ablation procedure were eligible to participate. Thus over a 20-month period 105 subjects were enrolled: 53 subjects to the all vein arm and 52 subjects to the arrhythmogenic vein arm. Using the stimulation protocol, PV triggers were reproducibly identified in all but one patient randomized to the arrhythmogenic PV arm based on which mean of 2.9±0.9 veins were isolated per patient in this arm [≤2 veins were isolated in 15 patients (29%), 3 veins were isolated in 21 patients (40%) and 4 veins were isolated in 16 patients (31%)]. Total procedure and fluoroscopy times (secondary end-points) showed a trend towards being longer in the all PV (327±97 minutes and 97±36 minutes, respectively) as compared with the arrhythmogenic PV arm (317±88 minutes and 85±33 minutes, p=0.57 and 0.14, respectively). As expected, time taken to elicit triggers was longer in the arrhythmogenic as compared to the all PV arm (50±23 minutes Vs 31±14 minutes; p<0.05). Other parameters to assess acute outcomes including PV isolation time, number of lesions delivered per vein and acute PV reconnection rates were comparable between the two groups ([Table 1]). Of the 105 subjects enrolled, 103 (98%) completed the one year follow-up visit. Long-term control of AF after a single ablation procedure (primary end-point) was achieved in 75 patients (73%) and was not different between patients randomized to undergo isolation of all versus arrhythmogenic veins [38 patients (75%) versus 37 patients (71%), respectively; OR 1.18; 95% CI 0.50, 2.83; p = 0.70; [Table 2]]. The secondary end point of freedom from AF at 1 year off AADs after a single procedure was achieved in 61 patients (59%) and was also not different between patients randomized to undergo isolation of all versus arrhythmogenic veins [30 patients (59%) versus 31 patients (61%), respectively; OR 1.03; 95% CI 0.47, 2.27; p = 0.935; [Table 3]]. Furthermore, long-term AF control and freedom from AF off AAD at 1 year after a single ablation procedure were comparable in patients who had all 4 veins isolated (72% and 58% respectively) versus those who had ≤3 veins isolated (75% and 61% respectively; p = 0.715 and 0.775 respectively). Serious adverse events were observed in 2 subjects (small right subcortical thromboembolic stroke and LA-esophageal fistula) who were both randomized to undergo isolation of all veins. Thus our study showed that in a mixed population of patients with predominantly paroxysmal AF, isolation of arrhythmogenic veins identified using a comprehensive stimulation protocol was as efficacious as empiric isolation of all veins in achieving long-term arrhythmia control after a single ablation procedure.[35]
Table 1. Comparison of acute outcomes between patients randomized to isolation of all versus arrhythmogenic veins. PV – Pulmonary vein; * p < 0.05.
| All Veins (n = 53) | Arrhythmogenic Veins (n = 52) | |
|---|---|---|
| Veins Isolated / Patient | 4 | 2.9 ± 0.9 |
| Procedure Time (minutes) | 327 ± 93 | 317 ± 88 |
| Total Fluoroscopy Time (minutes) | 97 ± 36 | 85 ± 33 |
| Time for Isolation of Veins (minutes) | 50 ± 30 | 40 ± 23 |
| Time to Elicit Triggers (minutes) | 31 ± 14 | 50 ± 23* |
| Number of Lesions / Vein | 17 ± 9 | 20 ± 10 |
| Acute PV Reconnect | 49 / 185 (26%) | 39 / 129 (30%) |
Table 2. Unadjusted odds ratios for various factors vis-à-vis endpoint of freedom from AF at 1 year off AADs after single ablation procedure. Co-morbidities include hypertension, chronic pulmonary disease (obstructive or restrictive), diabetes and sleep apnea. AF – atrial fibrillation, AADs – antiarrhythmic drugs, CI – confidence interval.
| Predictor / Covariate | Freedom from AF at 1 year off AADs | Odds Ratio | 95% C.I. | P-value |
|---|---|---|---|---|
| Ablation Strategy | 1.03 | (0.47, 2.27) | 0.935 | |
| All Veins | 30 (59%) | |||
| Arrhythmogenic Veins | 31 (60%) | |||
| Gender | 1.42 | (0.54, 3.73) | 0.476 | |
| Male | 50 (61%) | |||
| Female | 11 (52%) | |||
| Paroxysmal AF | 2.53 | (1.04, 6.10) | 0.042 | |
| Yes | 49 (65%) | |||
| No | 12 (43%) | |||
| Number of Veins Isolated | 0.816 | |||
| ≤ 2 Veins | 10 / 15 (67%) | 1.45 | (0.46, 4.61) | 0.527 |
| ≤ 3 Veins | 22 / 36 (61%) | 1.13 | (0.49, 2.58) | 0.775 |
| 4 Veins | 39 / 67 (58%) | ref. group | ref. group | ref. group |
| Co-Morbidities | 1.38 | (0.56, 3.36) | 0.481 | |
| Yes | 31 (53%) | |||
| No | 30 (67%) | |||
| Early AF Recurrence | 0.04 | (0.01, 0.20) | <0.001 | |
| Yes | 2 (10%) | |||
| No | 57 (72%) |
Table 3. Unadjusted odds ratios for various factors vis-à-vis endpoint of long term AF control at 1 year after single ablation procedure. Co-morbidities include hypertension, chronic pulmonary disease (obstructive or restrictive), diabetes and sleep apnea. AF – atrial fibrillation, CI – confidence interval.
| Predictor / Covariate | Long-Term AF Control | Odds Ratio | 95% C.I. | P-value |
|---|---|---|---|---|
| Ablation Strategy | 1.18 | (0.50, 2.83) | 0.702 | |
| All Veins | 38 (75%) | |||
| Arrhythmogenic Veins | 37 (71%) | |||
| Gender | 1.45 | (0.52, 4.08) | 0.479 | |
| Male | 61 (74%) | |||
| Female | 14 (67%) | |||
| Paroxysmal AF | 2.76 | (1.09, 7.01) | 0.032 | |
| Yes | 59 (79%) | |||
| No | 16 (57%) | |||
| Number of Veins Isolated | Overall 0.919 | |||
| ≤ 2 Veins | 11 / 15 (73%) | 1.03 | (0.30, 3.57) | 0.961 |
| ≤ 3 Veins | 27 / 36 (75%) | 1.18 | (0.47, 2.99) | 0.715 |
| 4 Veins | 48 / 67 (72%) | ref. group | ref. group | ref. group |
| Co-Morbidities | 1.28 | (0.53, 3.11) | 0.582 | |
| Yes | 41 (71%) | |||
| No | 34 (76%) | |||
| Early AF Recurrence | 0.14 | (0.05, 0.42) | <0.001 | |
| Yes | 8 (40%) | |||
| No | 65 (82%) |
Challenges to Identifying and Targeting Arrhythmogenic Pulmonary Veins
Although our randomized study supports the utility of a standardized stimulation protocol in successfully identifying arrhythmogenic PVs, there are several limitations inherent to this approach:
Performing the stimulation protocol can be tedious and timeconsuming.
Because the mechanisms underlying abnormalities of impulse formation, especially triggered activity can be evanescent, inability to demonstrate arrhythmogenicity does not necessarily exclude PV triggers of AF.
Mechanisms underlying PV triggers may not be consistent amongst the veins. Therefore after the initially identified arrhythmogenic PVs have been successfully isolated, repeat stimulation may be required to identify additional veins.
Since only a limited number of catheters are deployed in the LA, inability to sample all veins simultaneously may potentially preclude identification of an arrhythmogenic vein due to lack of direct recordings. To overcome this limitation, we have developed a methodology for using activation patterns of multipolar catheters in locations distant from the PVs which can help in localizing the culprit vein.[36,37] However, this may not be as definitive as direct catheter recordings of AF triggers from within the veins.
Despite our ability to successfully identify and isolate arrhythmogenic veins, in patients demonstrating arrhythmia recurrences subsequently, up to 40% of previously targeted PVs have been documented to reconnect in one or more segments.[38,39] Whether this is a limitation of the currently used energy sources or a reflection of the unique regenerative potential of PV musculature, remains to be answered. Nevertheless, in patients experiencing arrhythmia recurrences post AF ablation, on the repeat procedure in our experience, reisolating the reconnected veins alone can achieve long-term AF control rates of ≥90%.[38] Thus, research efforts towards developing alternative energy sources that can achieve more lasting PV isolation may be worthwhile. Similarly, strategies that can better identify dormant PV conduction (adenosine infusion, etc) during the initial or redo ablation procedure may also be useful.[40,41]
Conclusions
From the above narrative, one may conclude that the PVs are arrhythmogenic by design. In our experience, PV triggers of AF can be reproducibly identified using a standardized stimulation protocol and targeting thus identified arrhythmogenic PVs alone can be sufficient in achieving long term arrhythmia control in the majority of patients with AF. If done correctly, the advantage of this limited approach to AF ablation is the potential for reducing procedure complications that may be associated with more extensive AF ablation strategies.
Disclosures
None.
References
- 1.Feinberg W M, Blackshear J L, Laupacis A, Kronmal R, Hart R G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Intern. Med. 1995 Mar 13;155 (5):469–73. [PubMed] [Google Scholar]
- 2.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Packer Douglas, Skanes Allan. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005 Mar 08;111 (9):1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 5.Pappone Carlo, Manguso Francesco, Vicedomini Gabriele, Gugliotta Filippo, Santinelli Ornella, Ferro Amedeo, Gulletta Simone, Sala Simone, Sora Nicoleta, Paglino Gabriele, Augello Giuseppe, Agricola Eustachio, Zangrillo Alberto, Alfieri Ottavio, Santinelli Vincenzo. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation. 2004 Nov 09;110 (19):3036–42. doi: 10.1161/01.CIR.0000147186.83715.95. [DOI] [PubMed] [Google Scholar]
- 6.Oral Hakan, Scharf Christoph, Chugh Aman, Hall Burr, Cheung Peter, Good Eric, Veerareddy Srikar, Pelosi Frank, Morady Fred. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003 Nov 11;108 (19):2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 7.Oral Hakan, Chugh Aman, Lemola Kristina, Cheung Peter, Hall Burr, Good Eric, Han Jihn, Tamirisa Kamala, Bogun Frank, Pelosi Frank, Morady Fred. Noninducibility of atrial fibrillation as an end point of left atrial circumferential ablation for paroxysmal atrial fibrillation: a randomized study. Circulation. 2004 Nov 02;110 (18):2797–801. doi: 10.1161/01.CIR.0000146786.87037.26. [DOI] [PubMed] [Google Scholar]
- 8.Haïssaguerre M, Jaïs P, Shah D C, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng J T, Roudaut R, Clémenty J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000 Mar 28;101 (12):1409–17. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi Athar M, Prieto Lourdes R, Latson Larry A, Lane Geoffrey K, Mesia C Igor, Radvansky Penelope, White Richard D, Marrouche Nassir F, Saad Eduardo B, Bash Dianna L, Natale Andrea, Rhodes John F. Transcatheter angioplasty for acquired pulmonary vein stenosis after radiofrequency ablation. Circulation. 2003 Sep 16;108 (11):1336–42. doi: 10.1161/01.CIR.0000086322.21781.6A. [DOI] [PubMed] [Google Scholar]
- 10.Ren Jian-Fang, Marchlinski Francis E, Callans David J, Zado Erica S. Intracardiac Doppler echocardiographic quantification of pulmonary vein flow velocity: an effective technique for monitoring pulmonary vein ostia narrowing during focal atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2002 Nov;13 (11):1076–81. doi: 10.1046/j.1540-8167.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- 11.Saad Eduardo B, Rossillo Antonio, Saad Cynthia P, Martin David O, Bhargava Mandeep, Erciyes Demet, Bash Dianna, Williams-Andrews Michelle, Beheiry Salwa, Marrouche Nassir F, Adams James, Pisanò Ennio, Fanelli Raffaele, Potenza Domenico, Raviele Antonio, Bonso Aldo, Themistoclakis Sakis, Brachmann Joannes, Saliba Walid I, Schweikert Robert A, Natale Andrea. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation. 2003 Dec 23;108 (25):3102–7. doi: 10.1161/01.CIR.0000104569.96907.7F. [DOI] [PubMed] [Google Scholar]
- 12.Dixit Sanjay, Ren Jian-Fang, Callans David J, Gerstenfeld Edward P, Zado Erica, Vanderhoff Mark E, Marchlinski Francis E. Favorable effect of pulmonic vein isolation by partial circumferential ablation on ostial flow velocity. Heart Rhythm. 2004 Sep;1 (3):262–7. doi: 10.1016/j.hrthm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Marchlinski Francis E, Callans David, Dixit Sanjay, Gerstenfeld Edward P, Rho Robert, Ren Jian-Fang, Zado Erica. Efficacy and safety of targeted focal ablation versus PV isolation assisted by magnetic electroanatomic mapping. J. Cardiovasc. Electrophysiol. 2003 Apr;14 (4):358–65. doi: 10.1046/j.1540-8167.2003.02468.x. [DOI] [PubMed] [Google Scholar]
- 14.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 02;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Haïssaguerre Michel, Sanders Prashanthan, Hocini Mélèze, Takahashi Yoshihide, Rotter Martin, Sacher Frederic, Rostock Thomas, Hsu Li-Fern, Bordachar Pierre, Reuter Sylvain, Roudaut Raymond, Clémenty Jacques, Jaïs Pierre. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Yoshihide, O'Neill Mark D, Hocini Mélèze, Dubois Rémi, Matsuo Seiichiro, Knecht Sébastien, Mahapatra Srijoy, Lim Kang-Teng, Jaïs Pierre, Jonsson Anders, Sacher Frédéric, Sanders Prashanthan, Rostock Thomas, Bordachar Pierre, Clémenty Jacques, Klein George J, Haïssaguerre Michel. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J. Am. Coll. Cardiol. 2008 Mar 11;51 (10):1003–10. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 17.Rostock Thomas, Steven Daniel, Hoffmann Boris, Servatius Helge, Drewitz Imke, Sydow Karsten, Müllerleile Kai, Ventura Rodolfo, Wegscheider Karl, Meinertz Thomas, Willems Stephan. Chronic atrial fibrillation is a biatrial arrhythmia: data from catheter ablation of chronic atrial fibrillation aiming arrhythmia termination using a sequential ablation approach. Circ Arrhythm Electrophysiol. 2008 Dec;1 (5):344–53. doi: 10.1161/CIRCEP.108.772392. [DOI] [PubMed] [Google Scholar]
- 18.Oral Hakan, Chugh Aman, Good Eric, Wimmer Alan, Dey Sujoya, Gadeela Nitesh, Sankaran Sundar, Crawford Thomas, Sarrazin Jean F, Kuhne Michael, Chalfoun Nagib, Wells Darryl, Frederick Melissa, Fortino Jackie, Benloucif-Moore Suzanne, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Morady Fred. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007 May 22;115 (20):2606–12. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 19.Dixit Sanjay. Evolving strategies in catheter ablation of long-standing atrial fibrillation. Circ Arrhythm Electrophysiol. 2008 Dec;1 (5):324–6. doi: 10.1161/CIRCEP.108.834879. [DOI] [PubMed] [Google Scholar]
- 20.Ebner Erich, Krätschmer Hannes, Danilovic Dejan, Hribernigg Martin, Hutten Helmut. Ventricular evoked response as clinical marker for hemodynamic changes in dilative cardiomyopathy. Pacing Clin Electrophysiol. 2004 Feb;27 (2):166–74. doi: 10.1111/j.1540-8159.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 21.Dixit Sanjay, Sauer William H, Callans David J, Marchlinski Francis E. Arrhythmogenic potential of pulmonary venous tissue: triggers for atrial fibrillation identified within the remnant of a vein. J. Cardiovasc. Electrophysiol. 2009 Apr;20 (4):441–4. doi: 10.1111/j.1540-8167.2008.01338.x. [DOI] [PubMed] [Google Scholar]
- 22.NS Peters, C Cabo, AL Wit. In Cardiac Electrophysiology: From Cell to Bedside. Edited by Zipes DP, Jalife J. Philadelphia: W.B Saunders Company. 1999:345–349. [Google Scholar]
- 23.Wit A L, Rosen M R, Hoffman B F. Electrophysiology and pharmacology of cardiac arrhythmias. II. Relationship of normal and abnormal electrical activity of cardiac fibers to the genesis of arrhythmias. A Automaticity. Am. Heart J. 1974 Oct;88 (4):515–24. doi: 10.1016/0002-8703(74)90214-2. [DOI] [PubMed] [Google Scholar]
- 24.Ho Siew Yen, Anderson Robert H, Sánchez-Quintana Damián. Atrial structure and fibres: morphologic bases of atrial conduction. Cardiovasc. Res. 2002 May;54 (2):325–36. doi: 10.1016/s0008-6363(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 25.Chen Jian, Hoff Per Ivar, Erga Knut Ståle, Rossvoll Ole, Ohm Ole-Jørgen. Three-dimensional noncontact mapping defines two zones of slow conduction in the circuit of typical atrial flutter. Pacing Clin Electrophysiol. 2003 Jan;26 (1 Pt 2):318–22. doi: 10.1046/j.1460-9592.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 26.Olgin J E, Kalman J M, Fitzpatrick A P, Lesh M D. Role of right atrial endocardial structures as barriers to conduction during human type I atrial flutter. Activation and entrainment mapping guided by intracardiac echocardiography. Circulation. 1995 Oct 01;92 (7):1839–48. doi: 10.1161/01.cir.92.7.1839. [DOI] [PubMed] [Google Scholar]
- 27.Ho S Y, Cabrera J A, Tran V H, Farré J, Anderson R H, Sánchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart. 2001 Sep;86 (3):265–70. doi: 10.1136/heart.86.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000 Jan 18;101 (2):194–9. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 29.Jaïs Pierre, Hocini Mélèze, Macle Laurent, Choi Kee-Joon, Deisenhofer Isabel, Weerasooriya Rukshen, Shah Dipen C, Garrigue Stéphane, Raybaud Florence, Scavee Christophe, Le Metayer Philippe, Clémenty Jacques, Haïssaguerre Michel. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation. 2002 Nov 05;106 (19):2479–85. doi: 10.1161/01.cir.0000036744.39782.9f. [DOI] [PubMed] [Google Scholar]
- 30.Levin Mark D, Lu Min Min, Petrenko Nataliya B, Hawkins Brian J, Gupta Tara H, Lang Deborah, Buckley Peter T, Jochems Jeanine, Liu Fang, Spurney Christopher F, Yuan Li J, Jacobson Jason T, Brown Christopher B, Huang Li, Beermann Friedrich, Margulies Kenneth B, Madesh Muniswamy, Eberwine James H, Epstein Jonathan A, Patel Vickas V. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J. Clin. Invest. 2009 Nov;119 (11):3420–36. doi: 10.1172/JCI39109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skanes A C, Mandapati R, Berenfeld O, Davidenko J M, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998 Sep 22;98 (12):1236–48. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 32.Chen S A, Hsieh M H, Tai C T, Tsai C F, Prakash V S, Yu W C, Hsu T L, Ding Y A, Chang M S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999 Nov 02;100 (18):1879–86. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 33.Dixit Sanjay, Callans David J, Gerstenfeld Edward P, Marchlinski Francis E. Reentrant and nonreentrant forms of atrio-ventricular nodal tachycardia mimicking atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006 Mar;17 (3):312–6. doi: 10.1111/j.1540-8167.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 34.Dixit Sanjay, Gerstenfeld Edward P, Callans David J, Cooper Joshua M, Lin David, Russo Andrea M, Verdino Ralph J, Patel Vickas V, Kimmel Stephen E, Ratcliffe Sarah J, Hsia Henry H, Nayak Hemal M, Zado Erica, Ren Jian-Fang, Marchlinski Francis E. Comparison of cool tip versus 8-mm tip catheter in achieving electrical isolation of pulmonary veins for long-term control of atrial fibrillation: a prospective randomized pilot study. J. Cardiovasc. Electrophysiol. 2006 Oct;17 (10):1074–9. doi: 10.1111/j.1540-8167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 35.Dixit Sanjay, Gerstenfeld Edward P, Ratcliffe Sarah J, Cooper Joshua M, Russo Andrea M, Kimmel Stephen E, Callans David J, Lin David, Verdino Ralph J, Patel Vickas V, Zado Erica, Marchlinski Francis E. Single procedure efficacy of isolating all versus arrhythmogenic pulmonary veins on long-term control of atrial fibrillation: a prospective randomized study. Heart Rhythm. 2008 Feb;5 (2):174–81. doi: 10.1016/j.hrthm.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Ashar M S, Pennington J, Callans D J, Marchlinski F E. Localization of arrhythmogenic triggers of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2000 Dec;11 (12):1300–5. doi: 10.1046/j.1540-8167.2000.01300.x. [DOI] [PubMed] [Google Scholar]
- 37.Dixit Sanjay, Gerstenfeld Edward P, Rho Robert W, Patel Vickas, Callans David J, Marchlinski Francis E. Change in distant atrial activation patterns during circumferential pacemapping of pulmonic vein ostium: implications for localizing triggers for atrial fibrillation. J Interv Card Electrophysiol. 2003 Jun;8 (3):187–94. doi: 10.1023/a:1023965104924. [DOI] [PubMed] [Google Scholar]
- 38.Callans David J, Gerstenfeld Edward P, Dixit Sanjay, Zado Erica, Vanderhoff Mark, Ren Jian-Fang, Marchlinski Francis E. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2004 Sep;15 (9):1050–5. doi: 10.1046/j.1540-8167.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 39.Mainigi Sumeet K, Sauer William H, Cooper Joshua M, Dixit Sanjay, Gerstenfeld Edward P, Callans David J, Russo Andrea M, Verdino Ralph J, Lin David, Zado Erica S, Marchlinski Francis E. Incidence and predictors of very late recurrence of atrial fibrillation after ablation. J. Cardiovasc. Electrophysiol. 2007 Jan;18 (1):69–74. doi: 10.1111/j.1540-8167.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki Shinsuke, Kuwahara Taishi, Kobori Atsushi, Takahashi Yoshihide, Takei Asumi, Sato Akira, Takahashi Atsushi, Isobe Mitsuaki. Adenosine triphosphate exposes dormant pulmonary vein conduction responsible for recurrent atrial tachyarrhythmias: importance of evaluating the dormant conduction during the re-do ablation procedure. Circ. J. 2009 Jun;73 (6):1160–2. doi: 10.1253/circj.cj-08-0450. [DOI] [PubMed] [Google Scholar]
- 41.Arentz Thomas, Macle Laurent, Kalusche Dietrich, Hocini Mélèze, Jais Pierre, Shah Dipen, Haissaguerre Michel. "Dormant" pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2004 Sep;15 (9):1041–7. doi: 10.1046/j.1540-8167.2004.04031.x. [DOI] [PubMed] [Google Scholar]