Abstract
End resection of DNA double-strand breaks (DSBs) to generate 3′-single-stranded DNA facilitates DSB repair via error-free homologous recombination (HR) while stymieing repair by the error-prone non-homologous end joining (NHEJ) pathway. Activation of DNA end resection involves phosphorylation of the 5′ to 3′ exonuclease EXO1 by the phosphoinositide 3-kinase-like kinases ATM (ataxia telangiectasia-mutated) and ATR (ATM and Rad3-related) and by the cyclin-dependent kinases 1 and 2. After activation, EXO1 must also be restrained to prevent over-resection that is known to hamper optimal HR and trigger global genomic instability. However, mechanisms by which EXO1 is restrained are still unclear. Here, we report that EXO1 is rapidly degraded by the ubiquitin-proteasome system soon after DSB induction in human cells. ATR inhibition attenuated DNA-damage-induced EXO1 degradation, indicating that ATR-mediated phosphorylation of EXO1 targets it for degradation. In accord with these results, EXO1 became resistant to degradation when its SQ motifs required for ATR-mediated phosphorylation were mutated. We show that upon the induction of DNA damage, EXO1 is ubiquitinated by a member of the Skp1-Cullin1-F-box (SCF) family of ubiquitin ligases in a phosphorylation-dependent manner. Importantly, expression of degradation-resistant EXO1 resulted in hyper-resection, which attenuated both NHEJ and HR and severely compromised DSB repair resulting in chromosomal instability. These findings indicate that the coupling of EXO1 activation with its eventual degradation is a timing mechanism that limits the extent of DNA end resection for accurate DNA repair.
Keywords: chromosomes, DNA-damage response, DNA-damage response, DNA repair, homologous recombination, ATR, DNA double-strand break, DNA end resection, EXO1, Genomic stability
Introduction
DNA double-strand breaks (DSBs)3 are arguably the most dangerous of all types of DNA lesions that can arise in the cell. These breaks arise as a result of both exogenous (for e.g. ionizing radiation and chemotherapeutic drugs) and endogenous (for e.g. reactive oxygen species and stalled replication forks) insults. DSBs can be repaired by one of two major pathways in eukaryotes: 1) non-homologous end joining (NHEJ), an error-prone process wherein the DNA ends are directly rejoined after limited end processing (1), and 2) homologous recombination (HR), an error-free pathway that uses the undamaged sister chromatid as a template for repair (2). Correct “repair pathway choice” is critical for the maintenance of genomic integrity (for review, see Refs. 3–5). Recent evidence suggests that cyclin-dependent kinases (CDKs) that are active in S and G2 phases regulate repair pathway choice by promoting DNA end resection that stymies NHEJ and facilitates HR (for review, see Ref. 6). End resection results in the generation of 3′-ended single-stranded DNA (ssDNA) that is rapidly coated by replication protein A (RPA), which is then replaced with Rad51 to generate a nucleoprotein filament that copies information from the sister chromatid. DNA end resection occurs in a two-step manner (for review, see Refs. 7 and 8). First, resection is initiated by the removal of ∼50–100 bases of DNA from the 5′ end by the MRX/MRN complex (Mre11-Rad50-Xrs2 in yeast and MRE11-RAD50-NBS1 in mammals) in concert with Sae2/CtIP (9–13). Next, long range resection is carried out by two alternate pathways involving either EXO1 alone or the helicase Sgs1/BLM working in conjunction with EXO1 or the nuclease DNA2 (14–16). Research from a number of laboratories has established that CDKs 1 and 2 promote the initiation of resection by phosphorylating Sae2/CtIP (12, 17–21) and NBS1 (22), thereby coupling HR to S and G2 phases of the cell cycle.
Recent results from our laboratory established that CDK1 and CDK2 also promote long-range resection via phosphorylation of EXO1 (23; for review, see Refs. 8 and 24). EXO1 is a 5′ to 3′ exonuclease with key roles in DNA mismatch repair, mitotic and meiotic recombination, replication, and telomere homeostasis (for review, see Refs. 25–27). Research from our laboratory has established that EXO1 plays a major role in DNA end resection in human cells and not only promotes a switch from NHEJ to HR but also facilitates a transition from ATM- to ATR-mediated checkpoint signaling (15, 16, 23, 28, 29). The nuclease domain of EXO1 is highly conserved (30), whereas its C-terminal region is divergent and unstructured and mediates interactions with multiple DNA repair proteins (25, 31–34). The C terminus of EXO1 is phosphorylated at four (S/T)P sites by CDKs 1 and 2 in the S/G2 phases of the cell cycle (23). Phosphorylation of EXO1 by CDKs stimulates DNA end resection by promoting the recruitment of EXO1 to DNA breaks via interactions with BRCA1 (23). The C terminus is also phosphorylated at serine 714 by ATM (35) and ATR (36), which are the central kinases triggering the DNA-damage response to DSBs and DNA replication stress, respectively (37, 38). The functional consequences of serine 714 phosphorylation are not well understood.
Given that EXO1 is a key HR exonuclease in eukaryotic cells, it is important to understand how such an enzyme is kept on a tight rein after it is activated to prevent excessive DNA end resection. Excessive ssDNA would pose a threat to genomic integrity as they would be prone to breakage and could even trigger global genomic instability by exhausting the existing pool of RPA (39, 40). Furthermore, extensive DNA end resection would also cause a switch in the DSB repair mode from error-free HR to the highly deleterious single-strand-annealing pathway (41, 42). Here, we describe a mechanism by which resection is restrained in human cells that involves the degradation of EXO1 after DNA damage in a phosphorylation- and ubiquitination-dependent manner.
Results
EXO1 is rapidly degraded after DNA damage
In the course of our studies on EXO1 we were intrigued to find that EXO1 levels rapidly declined in HEK-293 cells treated with the topoisomerase I poison camptothecin (CPT), which induces replication fork-associated DSBs (43) (Fig. 1a). A reduction in EXO1 levels was also seen in additional human cell lines treated with CPT (supplemental Fig. 1a) or other genotoxic agents (supplemental Fig. 1b). By following EXO1 levels over a longer time period after CPT treatment, we found that the decrease in EXO1 was transient, showing recovery by ∼16 h post-CPT treatment (supplemental Fig. 1c). By synchronizing cells in different phases of the cell cycle, we determined that EXO1 levels were cell cycle-dependent, with the highest levels observed in phases where HR is operative, i.e. in S and G2 (supplemental Fig. 1d). However, the rapid reduction in EXO1 levels after DNA damage could not be attributed to cell-cycle changes, as no major differences in cell-cycle distribution were observed for at least 8 h post-CPT treatment (supplemental Fig. 1e). No changes in EXO1 transcript levels were seen after CPT treatment (supplemental Fig. 1f), indicating that post-transcriptional mechanisms were involved in the decrease in EXO1 levels. Indeed, the decrease in EXO1 appeared to be due to proteasomal degradation as this could be inhibited by treatment of cells with the proteasomal inhibitor MG-132 (Fig. 1b). By treating cells with the protein synthesis inhibitor cycloheximide (CHX), we found that the half-life of EXO1 was considerably shortened upon the induction of DNA damage by ionizing radiation (IR) or CPT (Fig. 1c). Interestingly, treatment of cells with the phosphatase inhibitors okadaic acid or calyculin A in the absence of DNA damage also resulted in destabilization of EXO1, indicating that phosphorylation of EXO1 might trigger its degradation (Fig. 1c).
Figure 1.
EXO1 was rapidly degraded after DNA damage. a, HEK-293 cells were treated with CPT for the indicated times, and EXO1 protein levels were assessed by Western blotting. Phosphorylation of KAP-1 and p53 were assayed by Western blotting with phospho-specific antibodies to confirm DNA-damage induction. Actin served as a loading control. b, HEK-293 cells were pretreated with the proteasome inhibitor MG-132 or with DMSO as control for 4 h, then treated with CPT for the indicated times. EXO1 levels were assessed by Western blotting. c, HEK-293 cells were treated with DMSO, ionizing radiation (IR), CPT, okadaic acid, or calyculin A as indicated immediately after the addition of the protein synthesis inhibitor CHX. EXO1 levels were assessed by Western blotting at the indicated times after CHX treatment. The plot shows relative EXO1 protein levels after CHX treatment (y axis) versus time (x axis) as determined by scanning and quantifying EXO1 bands and normalizing to actin bands using ImageJ software. All experiments were replicated three times. Error bars depict S.E.
Phosphorylation of EXO1 by ATR triggers its degradation
EXO1 is known to be phosphorylated at serine 714 by ATM and ATR in response to ionizing radiation and DNA replication inhibitors, respectively (35, 36). CPT-induced EXO1 degradation was attenuated by pretreatment of cells with caffeine, a broad-specific inhibitor of both ATM and ATR kinases (44) (Fig. 2a). KU55933, a specific inhibitor ATM (45), or NU7026, a specific inhibitor of DNA-PKcs (46), had no effect on EXO1 degradation (supplemental Fig. 2a). On the other hand, pretreatment of cells withVE822, a specific inhibitor of ATR (47), or siRNA-mediated knockdown of ATR resulted in stabilization of EXO1 (Fig. 2, b and c). Taken together, these observations indicated that EXO1 degradation might be triggered by ATR-mediated phosphorylation events. Knockdown of second-tier kinases that function downstream of ATR and ATM, CHK1 and CHK2, respectively (48), had no effect on EXO1 levels (supplemental Fig. 2b), implying that direct phosphorylation of EXO1 by ATR might trigger its degradation. To test this possibility, we mutated serine 714 of EXO1 to alanine, and expressed siRNA-resistant V5-tagged S714A-EXO1 in HEK-293 cells with siRNA-mediated knockdown of endogenous EXO1 (23). However, upon challenging these cells with CPT, we found that mutation of the serine 714 phosphorylation site did not affect EXO1 degradation appreciably (Fig. 2d). We reasoned that EXO1 might be phosphorylated at additional SQ sites upon DNA damage. This line of reasoning was based upon the observation that EXO1 with mutated serine 714 (S714A-EXO1) was still recognized by anti-phospho-(Ser/Thr) ATM/ATR substrate antibody in immunoprecipitation/Western blotting, whereas EXO1 with mutation of all six potential SQ phosphorylation sites in its C-terminal tail (S432A/S652A/S674A/S676A/S694A/S714A; henceforth referred to as 6A-EXO1) was no longer recognized by this antibody (supplemental Fig. 2c). Moreover, CPT-induced phosphorylation of S714A-EXO1 was attenuated by pretreatment of cells with the ATR inhibitor VE822 (47), thereby implicating ATR in EXO1 phosphorylation (supplemental Fig. 2d). Significantly, unlike wild-type EXO1, 6A-EXO1 was very stable after the induction of DNA damage (Fig. 2e). We, therefore, concluded that DNA-damage-induced phosphorylation of the C terminus of EXO1 triggers its degradation.
Figure 2.
Phosphorylation of EXO1 by ATR triggered its degradation. a, HEK-293 cells were treated with the ATM/ATR inhibitor caffeine or with DMSO as the control for 1 h before treatment with CPT for the indicated times. EXO1 protein levels were assessed by Western blotting. Phosphorylation of KAP-1 and p53 were assayed by Western blotting with phospho-specific antibodies to confirm DNA-damage induction. Phosphorylation of CHK1 was assessed to confirm ATR inactivation. b, HEK-293 cells were treated with the ATR inhibitor VE822 or with DMSO as the control for 1 h before treatment with CPT. EXO1 protein levels were assessed by Western blotting. c, EXO1 levels were assessed after CPT treatment of HEK-293 cells with siRNA-mediated knockdown of ATR. d, HEK-293 cells were depleted of endogenous EXO1 using siRNA and complemented with V5-tagged, siRNA-resistant wild-type EXO1 (WT) or EXO1 mutated at serine 714 (S714A). Cells were treated with CPT, and EXO1 levels were assessed by Western blotting with anti-V5 antibody. e, HEK-293 cells were depleted of endogenous EXO1 using siRNA and complemented with siRNA-resistant EXO1 mutated at all six SQ sites (Ser-432, Ser-652, Ser-674, Ser-676, Ser-694, and Ser-714) in its C-terminal domain (6A). EXO1 levels after CPT treatment were assessed by Western blotting. All experiments were replicated three times.
EXO1 is ubiquitinated in a phosphorylation-dependent manner
As EXO1 appears to be degraded by the ubiquitin-proteasome system (Fig. 1b), we next sought to determine if EXO1 phosphorylation upon DNA damage serves as trigger for its ubiquitination and subsequent degradation. To determine whether EXO1 was directly ubiquitinated in vivo, we co-expressed V5-EXO1 and His6-ubiquitin in HeLa cells (49). The cells were exposed to CPT, His6-ubiquitin-conjugated proteins were captured under denaturing conditions using Ni2+-agarose beads, and ubiquitinated forms of EXO1 were detected by Western blotting with anti-V5 antibody. We found that EXO1 was ubiquitinated at low levels in unstressed cells and that EXO1 ubiquitination increased dramatically in response to DNA damage (Fig. 3a). The modified forms of EXO1 were not seen in cells expressing conjugation-defective His6-ubiquitin (ΔGG) (50), confirming that they represented ubiquitinated EXO1. Importantly, the DNA-damage-induced increase in EXO1 ubiquitination was not seen in cells treated with the ATR inhibitor caffeine (Fig. 3b) or in cells expressing 6A-EXO1 (Fig. 3c), indicating that phosphorylation of EXO1 by ATR serves to target it for ubiquitination.
Figure 3.
EXO1 was ubiquitinated in a phosphorylation-dependent manner. a, HeLa cells expressing V5-tagged EXO1 (V5-EXO1) and His6-tagged wild-type (WT) or conjugation-defective (ΔGG) ubiquitin were treated with CPT for the indicated periods of time. His6-ubiquitin-conjugated proteins were captured under denaturing conditions using Ni2+-agarose beads, and ubiquitinated forms of EXO1 were detected by Western blotting with anti-V5 antibody. b, HeLa cells expressing V5-EXO1 and His-Ub were treated with caffeine or DMSO as control before the addition of CPT for 4 h. EXO1 ubiquitination was assessed by Ni2+-capture followed by Western blotting. c, HeLa cells expressing wild-type (WT) or phosphorylation site-mutant (6A) V5-EXO1 and His-Ub were treated with CPT for 4 h. EXO1 ubiquitination was assessed by Ni2+-capture followed by Western blotting. d, HeLa cells expressing V5-EXO1 were treated with the cullin-RING ubiquitin ligase inhibitor MLN4924 or DMSO for 4 h before the addition of CPT for 4 h. EXO1 ubiquitination was assessed by Ni2+-capture followed by Western blotting. e, HEK-293 cells were treated with DMSO or MLN4924 for 4 h before treatment with CPT for the indicated times. EXO1 protein levels were assessed by Western blotting. f, HeLa cells expressing V5-EXO1 and His-Ub in the presence or absence of dominant negative Cullin1 (DN-Cullin1) were treated with CPT for 4 h. EXO1 ubiquitination was assessed by Ni2+-capture followed by Western blotting. Cells were transfected with a V5-β-gal vector as control (Mock). g, HEK-293 cells expressing DN-Cullin1 were treated with CPT for the indicated times. EXO1 levels were assessed by Western blotting. All experiments were replicated three times.
In light of the apparent link between EXO1 phosphorylation and ubiquitination, we were interested in the Skp1-Cullin1-F-box (SCF) family of ubiquitin ligases, which typically recognize and ubiquitinate phosphorylated substrates (51, 52). SCF ubiquitin ligases regulate multiple DNA-damage-response pathways (51, 53). Notably, a Cullin1-containing SCF has been reported to target CHK1 for degradation after it is phosphorylated by ATR in a manner similar to that observed for EXO1 (54, 55). To examine if a cullin-based E3 ligase was involved in EXO1 ubiquitination, we first treated cells with MLN4924, which is a general inhibitor of the cullin-RING subtype of ubiquitin ligases (56). Treatment with MLN4924 ablated EXO1 ubiquitination (Fig. 3d) and resulted in failure of CPT-induced destabilization of EXO1 (Fig. 3e). To specifically examine the role of Cullin1 in EXO1 ubiquitination, we transfected HeLa cells with V5-EXO1 and His6-ubiquitin along with dominant negative Cullin1 (DN-Cul1) (57) and assayed for EXO1 ubiquitination by Ni2+-pulldown under denaturing conditions. Expression of DN-Cul1 attenuated EXO1 ubiquitination (Fig. 3f) and suppressed degradation of EXO1 protein triggered by CPT exposure (Fig. 3g). As a control, we co-expressed dominant negative Cullin4A and Cullin4B (DN-Cul4) (57) in HeLa cells but saw no effect on EXO1 stability (supplemental Fig. 3.). These results implicate a Cullin1-containing SCF complex in phosphorylation-dependent EXO1 degradation.
EXO1 degradation prevents hyper-resection and genomic instability
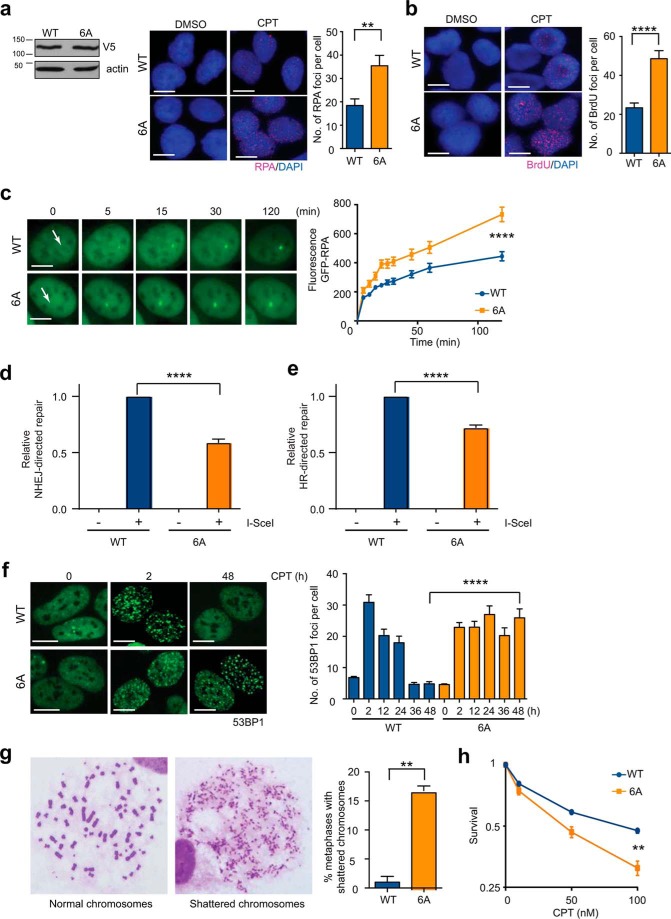
We next asked whether EXO1 degradation might serve to prevent unbridled DNA resection, as the generation of excessive ssDNA would not be conducive to optimal HR (41, 42) and could even contribute to global genomic instability by sequestering the nuclear pool of RPA (39, 40). The availability of a degradation-resistant version of EXO1 (6A-EXO1) (Fig. 2d) allowed us to directly answer this question. We first confirmed that these mutations did not affect the exonuclease activity of EXO1 per se (supplemental Fig. 4a) or its interactions with the resection proteins CtIP and BLM (23, 58, 59) (supplemental Fig. 4b). We next used three complementary assays to measure DNA end resection:quantification of RPA foci (an indirect measure of resection), enumeration of ssDNA/BrdU foci (a direct measure of resection), and live cell imaging of GFP-RPA accumulation at the sites of micro-laser-induced DSBs (which allows the visualization of end resection in real time) (16, 23, 60). We found that expression of 6A-EXO1 in U2OS cells resulted in increased numbers of CPT-induced RPA (Fig. 4a and supplemental Fig. 4c) and ssDNA/BrdU foci (Fig. 4b) as well as enhanced recruitment of GFP-RPA to micro-laser-induced DNA damage (Fig. 4c). Similar results were obtained by preventing degradation of endogenous wild-type EXO1 indirectly by expressing DN-Cul1 (supplemental Fig. 5, a–c). These two sets of results clearly demonstrate that EXO1 degradation prevents hyper-resection.
Figure 4.
EXO1 degradation prevented hyper-resection and genomic instability. a, U2OS cells were depleted of endogenous EXO1 using siRNA and complemented with V5-tagged, siRNA-resistant wild-type (WT), or phosphorylation site mutant (6A)-EXO1. Cells were treated with CPT or DMSO as control for 4 h and immunostained with anti-RPA antibody (red). Nuclei were stained with DAPI (blue). Representative images are shown. The plot shows average numbers of RPA foci per cell after subtracting background (average numbers of foci in DMSO-treated cells). V5-EXO1 expression was verified by Western blotting with anti-V5 antibody. b, U2OS cells expressing WT- or 6A-EXO1 were treated with CPT or DMSO for 4 h and immunostained for BrdU/ssDNA foci (red). Representative images are shown. The plot shows average numbers of BrdU foci per cell after subtracting background. c, U2OS cells expressing WT- or 6A-EXO1 along with GFP-RPA were laser-micro-irradiated to induce focal DSBs. Time-lapse images of accumulation of GFP-RPA at DSBs (arrows) are shown. The plot shows recruitment of GFP-RPA after DNA damage. d, NHEJ efficiency was measured by quantifying RFP expression (by flow cytometry) in HEK293 cells harboring a GFP/RFP reporter after transfection with an I-SceI plasmid. Cells were depleted of endogenous EXO1 using siRNA and complemented with WT- or 6A-EXO1. The plot shows NHEJ-directed repair relative to WT-EXO1-expressing cells. e, HR efficiency was measured by quantifying GFP expression in HEK-293 cells harboring a DR-GFP reporter after transfection with an I-SceI plasmid. Cells were depleted of endogenous EXO1 using siRNA and complemented with WT- or 6A-EXO1. The plot shows HR-directed repair relative to WT-EXO1 expressing cells. f, U2OS cells depleted of endogenous EXO1 and complemented with WT- or 6A-EXO1 were treated with CPT and immunostained for 53BP1 foci (green) at the indicated times post treatment. Representative images are shown. Rates of DSB repair were determined by scoring 53BP1 foci and plotting average number of 53BP1 foci per cell (y axis) versus time (x axis). g, percentages of metaphase spreads with shattered chromosomes are plotted for CPT-treated U2OS cells with knockdown of endogenous EXO1 and ectopic expression of WT- or 6A-EXO1. Representative metaphases are shown. Scale bar, 5 μm. h, the plot shows clonogenic survival of U2OS cells expressing WT- or 6A-EXO1. Cells were treated with the indicated doses of CPT for 4 h before plating for colony formation. All experiments were replicated three times. Error bars depict S.E. **, p < 0.005; ****, p < 0.0001.
To assess the effect of hyper-resection on DSB repair, we expressed WT- or 6A-EXO1 in cells harboring integrated reporters used to quantify the repair of I-SceI-induced DSBs by NHEJ (61) or HR (62). As expected, expression of 6A-EXO1 resulted in attenuation of NHEJ (Fig. 4d) because DNA end resection stymies NHEJ by preventing the binding of KU (7, 63). Interestingly, expression of 6A-EXO1 also resulted in reduced levels of HR relative to WT-EXO1 (Fig. 4e), presumably because excessive end resection hinders optimal HR (41, 42). Attenuation of both HR and NHEJ was also seen in cells expressing DN-Cul1 (supplemental Fig. 5, d and e). In light of the down-regulation of both NHEJ and HR, we predicted that cells expressing degradation-resistant EXO1 would be severely compromised in the repair of CPT-induced DSBs. We treated cells expressing WT- or 6A-EXO1 with CPT and quantified the formation and dissolution of 53BP1 or γH2AX foci (64, 65) to evaluate the effects of EXO1 stabilization on DSB repair. We found that cells expressing 6A-EXO1 displayed an almost complete block in the repair of CPT-induced DSBs (Fig. 4f and supplemental Fig. 4d). A similar block in repair was also seen in cells expressing DN-Cul1 (supplemental Fig. 5f). The lack of repair coupled with the generation of excessive ssDNA clearly compromised chromosomal stability, as cells expressing 6A-EXO1 or DN-Cul1 exhibited metaphases with shattered chromosomes after induction of DNA damage with CPT (Fig. 4g and supplemental Fig. 5g). Accordingly, cells expressing 6A-EXO1 were more sensitive to CPT compared with cells expressing WT-EXO1 in a colony survival assay (Fig. 4h). Taken together, these results demonstrate that DNA-damage-induced EXO1 degradation prevents excessive DNA end resection and promotes optimal DSB repair, which together contribute to the preservation of chromosomal integrity and cell survival in the face of genomic insults.
Discussion
It is now well understood that several mechanisms exist to promote DNA end resection, a critical step that is essential for HR repair of DSBs, especially replication-associated DSBs like those induced by CPT (66). However, how resection is restrained or terminated is not well understood. Our results uncover a novel mechanism by which resection is negatively regulated via the degradation of EXO1, an exonuclease with critical functions in long-range resection (25, 26). Our results indicate that EXO1, although vital for resection and HR, is rapidly degraded after DNA damage in a phosphorylation- and ubiquitination-dependent manner. The rapid degradation of EXO1 serves to prevent hyper-resection. Hyper-resection could promote genomic instability because 1) extensive resection would shift DSB repair from RAD51-dependent HR to RAD52-dependent single-strand annealing, a highly mutagenic DNA repair pathway (41, 42), and 2) excessive ssDNA would exhaust the existing pool of RPA thereby triggering global genomic instability (39, 40). The net effect of these two phenomena would be the accumulation of CPT-induced DNA breaks and chromosome shattering that we observe in cells expressing degradation-resistant EXO1.
In light of these data and our previously published results (23), we envisage that phosphorylation of EXO1 by CDKs in S/G2 primes EXO1 to respond to DSBs, especially replication-associated breaks that can be correctly repaired only by HR. However, upon the induction of such breaks, EXO1 is phosphorylated by ATR, which marks it for SCF-mediated ubiquitination and subsequent degradation (Fig. 5). A delay between EXO1 phosphorylation and its degradation possibly creates a temporal window that is sufficient for EXO1 to optimally resect DNA ends before it is destroyed to prevent excessive resection or collateral damage to other regions of the genome.
Figure 5.
Proposed model of EXO1 regulation. As cells enter S phase, EXO1 is primed to function in resection by CDK-mediated phosphorylation (circles). The transduction of DNA damage signals by ATR is followed by EXO1 recruitment to DNA breaks and DNA end resection, which promotes DSB repair by HR (23) and cell-cycle checkpoint implementation via ATR (16). Once resection is under way, EXO1 is rapidly ubiquitinated (triangles) and targeted for degradation to restrict its activity and prevent over-resection. Potential phosphorylation sites are numbered.
The degradation of EXO1 that we observe appears to be mediated by a Cullin1-containing SCF ubiquitin ligase complex (51, 52). Ubiquitination along with SUMOylation are emerging as important regulators of DNA-damage response (for review, see Refs. 67 and 68). Most published studies have focused on the role of ubiquitination and SUMOylation in the recruitment of proteins to DSBs, notably CtIP, 53BP1, RIF1, RAD18, and BRCA1 (68–70). Interestingly, recent reports also implicate these modifications in the degradation of cell-cycle checkpoint or DNA-repair proteins, notably CHK1, CtIP, 53BP1, MDC1, RPA, BRCA1, and FANCA (54, 71–77), and in the release of the NHEJ factor KU from DSBs (78–80). Among these proteins, a very close analogy can be drawn between the degradation of EXO1 and that of the cell-cycle checkpoint kinase CHK1 (54, 55). Phosphorylation of CHK1 by ATR serves to initiate cell-cycle checkpoint arrest but also limits the duration of checkpoint signaling via SCF-mediated degradation of the kinase. In a similar manner, phosphorylation of EXO1 by ATR limits the extent of DNA end resection by SCF-mediated degradation of the potentially genotoxic nuclease. Of relevance to our findings, SCFs have been implicated in the phosphorylation-triggered degradation of a number of additional DNA repair and cell-cycle checkpoint proteins including BRCA1 (76), KU (78, 79), and Cdc25A (81).
Lending credence to our observation of DNA-damage-induced EXO1 ubiquitination, EXO1 was recently identified as a SCF target in a quantitative proteomic screen for UV-induced ubiquitination events in human cells (82). This screen and others (57, 83, 84) identified the following ubiquitination sites on EXO1 (Lys-214, Lys-292, Lys-391, Lys-548, and Lys-796). However, mutation of these five sites, singly or in combination, was not sufficient to affect EXO1 ubiquitination.4 It is possible that there are additional, yet unidentified, ubiquitination sites on EXO1 or that mutation of the above sites simply resulted in promiscuous ubiquitination on nearby lysines (85). Future studies will hopefully shed more light on the exact ubiquitination site(s) on EXO1 that trigger its degradation.
Our results showing that ATR triggers EXO1 degradation in response to replication-associated DSBs are of interest in light of a previous report showing that ATR triggers EXO1 degradation upon treatment of cells with the DNA replication inhibitor hydroxyurea (36). Given that treatment with hydroxyurea induces DSBs due to replication fork collapse (86, 87), it is plausible that the actual trigger for EXO1 degradation in the previous study might have been replication fork-associated DSBs. Also, in the previous study, as in ours, mutation of serine 714 was not sufficient to stabilize EXO1 (36), which bolsters our assessment that multiple phosphorylation sites on the C terminus of EXO1 are needed for its ubiquitination and subsequent degradation. In a recent study from the same group, it was reported that EXO1 is also SUMOylated in response to DNA replication stress, although mutation of the identified SUMO sites did not affect EXO1 stability (88). It is, therefore, clear that EXO1 stability is probably regulated by an interplay of post-translational modifications (PTM)–phosphorylation, SUMOylation, and ubiquitination–but the exact choreography of these PTMs and the exact roles of specific modifications in EXO1 regulation still need to be elucidated.
Regulatory mechanisms impinging on EXO1 are of significance from a cancer standpoint, as multiple SNPs in EXO1 have been identified in patients with colorectal cancers (89–91), and a specific polymorphism, K589E (rs1047840), has been linked with an increased risk of breast (92), gastric (93), oral (94), lung (95), and brain (96) cancer development. Another polymorphism, N279S (rs4149909), was recently linked to an increased risk of breast cancer in a genome-wide association analysis of >120,000 individuals (97). It would be of interest to determine in the future if some of these SNPs affect the degradation of EXO1 elucidated in our study and whether blocking EXO1 degradation might be a viable cancer therapeutic strategy.
Experimental procedures
Cell culture
Cell lines were obtained from the American Type Culture Collection. U2OS cells were maintained in α-MEM (α-minimum Eagle's medium) and HEK-293 and HeLa cells in DMEM medium supplemented with 10% fetal bovine serum and penicillin/streptomycin in a humidified atmosphere with 5% CO2. All cells were mycoplasma free.
Drug treatments
Cells were treated with 1 μm camptothecin (Sigma), 10 μm etoposide (Selleckchem), or 50 μm hydroxyurea (Sigma) for the indicated periods of time to induce DNA damage. Cells were treated with 10 μm MG-132 (Selleckchem) for 4 h before treatment with camptothecin to block proteasomal degradation of EXO1. Cells were treated with 50 μg/ml cycloheximide (EMD Millipore) to block protein synthesis. To inhibit protein phosphatases, cells were treated with 1 μm okadaic acid or 100 nm calyculin A (Santa Cruz) before treatment with cycloheximide. For inhibition of PI3K-like kinases, cells were treated with 5 mm caffeine (Sigma), 10 μm KU55933 (Sigma), 10 μm NU7026 (Sigma), or 1 μm VE822 (VX970) (Selleckchem) for 1 h before camptothecin treatment. To inhibit Cullin-RING ubiquitin ligases, cells were treated with 5 μm MLN4924 (R&D Systems) for 4 h before treatment with camptothecin.
Irradiation of cells
Cells were irradiated with 10 gray of gamma rays from a cesium source (JL Shepherd and Associates). Cells were irradiated with 10 J/m2 of UV-C (254 nm) at a dose rate of 0.5 J/m2/s.
Cell cycle synchronization and flow cytometry
HEK-293 cells were synchronized in G1 by treatment with 2 μg/ml aphidicolin (Sigma) for 16 h and then released into regular media for either 5 h (S phase) or 10 h (G2 phase) as described before (29). Cell cycle stage was determined by flow cytometry using a BD CYTOMICS FC500 Flow Cytometer (BD Biosciences).
Western blotting and antibodies
Nuclear extracts for Western blotting were prepared by lysing cells in hypotonic lysis buffer (10 mm Tris-HCl, pH 7.5, 1.5 mm MgCl2, 5 mm KCl, and protease and phosphatase inhibitors) followed by extraction in nuclear extraction buffer (50 mm Tris-HCl, pH 7.5, 0.5 m NaCl, 2 mm EDTA, 10% sucrose, 10% glycerol, and protease and phosphatase inhibitors) (29). The following primary antibodies were used: phospho-EXO1 (Ser-714) (15); EXO1, phospho-KAP-1 (Ser-824), KAP-1, CtIP, BLM (Bethyl); phospho-p53 (Ser-15), CHK1, phospho-CHK1 (Ser-317), phospho-ATM/ATR substrate (Cell Signaling); p53, actin, 53BP1 (H-300), Cullin1, Cullin4 (Santa Cruz); V5 (Invitrogen); RPA (Calbiochem); CyclinA (Abcam); BrdU (BD Biosciences); γH2AX (Millipore). The following secondary antibodies were used: horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) and Alexa 488/568-conjugated secondary antibodies (Thermo Fisher Scientific).
RT-PCR
HEK-293 cells were treated with 1 μm camptothecin for 4 h. Cells were harvested and RNA was isolated using the RNeasy kit (Qiagen). The RNA was then reverse-transcribed to make cDNA using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). qRT-PCR of the cDNA was performed using SYBR® Green PCR Master Mix (Applied Biosystems) and primers against EXO1, p21, and TFRC. Primers are listed in supplemental Table 1.
Cloning and mutagenesis
EXO1b was subcloned from Flag2-EXO1 (15) into pDONR221 (Invitrogen) using the Gateway BP Clonase II enzyme mix (Invitrogen). Site-directed mutagenesis of EXO1 was carried out using Pfx50 polymerase (Invitrogen) and Taq ligase (New England BioLabs); see supplemental Table 2 for primer sequences. EXO1 was then transferred to pLenti6.3/V5-DEST (Invitrogen) using the Gateway LR Clonase II enzyme mix (Invitrogen) for C-terminal V5-tagged fusion protein expression, as described before (23). Serines 432, 652, 674, 676, 694, and 714 of EXO1 were mutated to alanine to generate 6A-EXO1.
Transfection of cells
Depletion of EXO1, ATR, CHK1, or CHK2 was carried out by transfection with the appropriate siRNAs (supplemental Table 3; Invitrogen) using electroporation (Lonza) for U2OS cells and Lipofectamine2000 (Invitrogen) for HEK-293 cells; cells were harvested 48 h later to verify knockdown by Western blotting. Cells were transfected with scrambled siRNA as control; see supplemental Table 3 for siRNA sequences. For ectopic expression of proteins, cells were transfected with the appropriate expression plasmid and assayed after 24–48 h. pHAGE DN Cullin1, pHAGE DN Cullin4A, and pHAGE DN Cullin4B were gifts from Stephen Elledge (Addgene plasmid #41911, 41914, 41915). For ectopic expression of siRNA-resistant EXO1 in cells with knockdown of endogenous EXO1, cells were transfected with the expression plasmid 24 h after siRNA transfection.
Ubiquitination assay
HeLa cells were transfected with His-Ub (WT or ΔGG) (49) and V5-EXO1 (WT or 6A) expression plasmids using Effectene (Qiagen). After 48 h, cells were treated with 10 μm MG-132 for 4 h before the addition of 1 μm camptothecin. Cells were harvested at the indicated times post-camptothecin treatment. Harvested cells were resuspended in denaturing lysis buffer (6 m guanidine-HCl, 100 mm Na2HPO4/NaH2PO4, pH 8, 10 mm Tris-HCl, pH 8, 5 mm imidazole, and 10 mm β-mercaptoethanol). Extracts were clarified and incubated with 100 μl of nickel-nitrilotriacetic acid beads for 4 h with rotation. Beads were serially washed with lysis buffer without imidazole, wash buffer A (8 m urea, 100 mm Na2HPO4/NaH2PO4, pH 8, 10 mm Tris-HCl, pH 8, and 10 mm β-mercaptoethanol), wash buffer B (8 m urea, 100 mm Na2HPO4/NaH2PO4, pH 6.8, 10 mm Tris-HCl, pH 6.8, 0.2% Triton-X, and 10 mm β-mercaptoethanol) and wash buffer C (8 m urea, 100 mm Na2HPO4, NaH2PO4, pH 6.8, 10 mm Tris-HCl, pH 6.8, 0.1% Triton-X, and 10 mm β-mercaptoethanol). Bound proteins were eluted with elution buffer (200 mm imidazole, 150 mm Tris-HCl, pH 6.8, 30% glycerol, 5% SDS, and 720 mm β-mercaptoethanol) and analyzed by Western blotting.
Immunoprecipitation
Whole cell extracts of HEK293 cells expressing V5-tagged EXO1 were made in lysis buffer (20 mm Tris-HCl, pH 7.5, 80 mm NaCl, 2 mm EDTA, 10% glycerol, and 0.2% Nonidet P-40) supplemented with protease and phosphatase inhibitors. Anti-V5 antibodies were bound to Dynabeads (Invitrogen), and beads were incubated for 4 h with cell extracts following the manufacturer's protocol. Beads were washed extensively in lysis buffer and then boiled in 1× SDS loading buffer before Western blotting. Normal mouse IgG (Santa Cruz) was used for control immunoprecipitations.
Exonuclease assay
V5-tagged EXO1 proteins were immunoprecipitated from HEK293 cells using Dynabeads as described previously (23). Nuclease activity of wild-type or mutant EXO1 was assessed by analyzing the degradation of a linearized 3′- radioactively labeled 7.8-kb plasmid (pLVX-Tight Puro). Each nuclease reaction contained EXO1 protein equivalent to the immunoprecipitate from 2 mg of cell lysate and 50 ng of substrate in a final volume of 35 μl of nuclease assay buffer (20 mm HEPES, pH 7.5, 40 mm KCl, 5 mm MgCl2, 0.05% Triton X-100, 5% glycerol, 100 μg/μl BSA, 0.5 mm DTT, and 1 mm ATP). Reactions were incubated at 37 °C, and samples were removed at the indicated intervals. Samples were resolved on 0.8% agarose gels and transferred onto a Hybond-XL nylon membrane (Amersham Biosciences). Images were acquired using phosphorimaging screens and a Fujifilm Starion scanner. Quantitation was carried out using Multi Gauge software (Fujifilm). Gel lanes were marked and used to generate line traces, and peaks were detected automatically using default settings. Area under the curve (AUC) for the 7.8-kb reagent peak was calculated for each lane. The fraction of substrate remaining was calculated by dividing AUC of each sample by AUC of the starting material. Three independent time courses were quantified for each protein.
Immunofluorescence staining
To stain for RPA foci, cells were seeded onto glass chamber slides (Lab-Tek) and treated with 0.1 μm camptothecin for 4 h. The cells were fixed with 4% paraformaldehyde/PBS and permeabilized with 0.5% Triton-X/PBS before incubation with antibodies (60). To obtain clear RPA foci, cells were subject to in situ fractionation (98). The average number of RPA foci per nucleus was determined after scoring at least 50 nuclei and subtracting background (average numbers of foci in mock-treated cells). For quantifying DSB repair kinetics, cells were treated with 0.1 μm camptothecin for 2 h after which the media were replaced with camptothecin-free media. Cells were co-immunostained with anti-53BP1 or γH2AX antibody at different time points post-camptothecin treatment as described previously (61). Images were captured using a Leica DH5500B fluorescence microscope (X40 objective lens) coupled to a Leica DFC340 FX camera using Leica Application Suite v4 acquisition software.
BrdU/ssDNA assay
Cells grown in the presence of 10 μm BrdU (Sigma) for 16 h were treated with 0.1 μm camptothecin for 4 h and immunofluorescence stained with anti-BrdU antibody under non-denaturing conditions (to detect BrdU incorporated into ssDNA) (60). For clarifying BrdU/ssDNA foci, cells were subject to in situ fractionation (98). The average number of BrdU/ssDNA foci per nucleus was determined after scoring at least 50 nuclei and subtracting background (average numbers of foci in mock-treated cells).
Laser live cell imaging
Cells were transfected with GFP-RPA construct, laser micro-irradiated and time-lapse-imaged, and the fluorescence intensities of micro-irradiated areas were plotted after background subtraction (fluorescence intensities of un-irradiated areas) as described before (16). Cells were irradiated, and live cell images were taken with a pulsed nitrogen laser (Spectra-Physics; 365 nm, 10 Hz) coupled to a Carl Zeiss Axiovert 200M microscope (×63 oil immersion objective). Mean fluorescence intensities for each time point were determined using Axiovision software v4 from at least 30 independent measurements, and total increase in fluorescence signal was plotted versus time.
NHEJ and HR assays
To measure HR, GFP expression in HEK-293 cells with an integrated DR-GFP reporter was quantified by flow cytometry (62). To measure NHEJ, red fluorescent protein (RFP) expression in HEK-293 cells with an integrated GFP-to-RFP reporter was quantified by flow cytometry (61). For both assays, cells were depleted of endogenous EXO1 using siRNA and transfected with the V5-EXO1 (WT or 6A) expression plasmid; after 24 h, cells were transfected with an I-SceI expression vector, and GFP or RFP expression was quantified by flow cytometry after an additional 72 h. GFP-positive or RFP-positive frequencies were corrected for transfection efficiencies (quantified by parallel transfection with a wild-type GFP expression vector).
Metaphase chromosome preparations
Cells were treated with 0.1 μm camptothecin for 2 h after which the media was replaced with camptothecin-free media. Colcemid (Sigma), along with 1 mm caffeine (Sigma) to bypass G2/M arrest, was added to cells 24 h after camptothecin treatment. Metaphase chromosome spreads were prepared 16 h later as described before (99).
Colony formation assay
Cells were treated with the indicated doses of camptothecin for 4 h and plated in triplicate onto 60-mm dishes (1000 cells/dish). Surviving colonies were stained with crystal violet ∼10–14 days later as described before (100).
Statistical analyses
Statistical analyses were performed using GraphPad Prism7 software. Two-tailed Student's t test was used to determine statistical significance (ns, not significant; *, p < 0.01; **, p < 0.005; ***, p < 0.001; ****, p < 0.0001).
Author contributions
N. T., B. M., M. C. H., F. L. B., J. L. H., and S. B. conducted the experiments. N. T., P. R. P., J. H., K. K. K., and S. B. designed the experiments and interpreted the results. B. M. and S. B. wrote the paper.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants RO1CA197796, RO1CA149461, and R21CA202403 (to S. B). This work was also supported by National Aeronautics and Space Administration Grant NNX16AD78G. The authors declare that they have no conflict of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Tables 1–3 and Figs. 1–5.
N. Tomimatsu, B. Mukherjee, J. L. Harris, F. L. Boffo, M. C. Hardebeck, P. R. Potts, K. K. Khanna, and S. Burma, unpublished data; HR, homologous recombination.
- DSB
- double-strand break
- NHEJ
- non-homologous end joining
- ssDNA
- single-stranded DNA
- RPA
- replication protein A
- ATM
- ataxia telangiectasia-mutated
- ATR
- ATM and Rad3-related
- CPT
- camptothecin
- CHX
- cycloheximide
- SCF
- Skp1-Cullin1-F-box
- DN
- dominant negative
- AUC
- area under the curve
- RFP
- red fluorescent protein
- Ub
- ubiquitin
- CDK
- cyclin-dependent kinase.
References
- 1. Burma S., Chen B. P., and Chen D. J. (2006) Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair 5, 1042–1048 [DOI] [PubMed] [Google Scholar]
- 2. Heyer W. D., Ehmsen K. T., and Liu J. (2010) Regulation of homologous recombination in eukaryotes. Annu. Rev. Genet. 44, 113–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shrivastav M., De Haro L. P., and Nickoloff J. A. (2008) Regulation of DNA double-strand break repair pathway choice. Cell Res. 18, 134–147 [DOI] [PubMed] [Google Scholar]
- 4. Chapman J. R., Taylor M. R., and Boulton S. J. (2012) Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 47, 497–510 [DOI] [PubMed] [Google Scholar]
- 5. Mladenov E., Magin S., Soni A., and Iliakis G. (2016) DNA double-strand-break repair in higher eukaryotes and its role in genomic instability and cancer: Cell cycle and proliferation-dependent regulation. Semin. Cancer Biol. 37, 51–64 [DOI] [PubMed] [Google Scholar]
- 6. Ferretti L. P., Lafranchi L., and Sartori A. A. (2013) Controlling DNA-end resection: a new task for CDKs. Front. Genet. 4, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Symington L. S., and Gautier J. (2011) Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 45, 247–271 [DOI] [PubMed] [Google Scholar]
- 8. Daley J. M., Niu H., Miller A. S., and Sung P. (2015) Biochemical mechanism of DSB end resection and its regulation. DNA Repair 32, 66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gravel S., Chapman J. R., Magill C., and Jackson S. P. (2008) DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 22, 2767–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mimitou E. P., and Symington L. S. (2008) Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455, 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Z., Chung W. H., Shim E. Y., Lee S. E., and Ira G. (2008) Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134, 981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makharashvili N., Tubbs A. T., Yang S. H., Wang H., Barton O., Zhou Y., Deshpande R. A., Lee J. H., Lobrich M., Sleckman B. P., Wu X., and Paull T. T. (2014) Catalytic and noncatalytic roles of the CtIP endonuclease in double-strand break end resection. Mol. Cell 54, 1022–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nicolette M. L., Lee K., Guo Z., Rani M., Chow J. M., Lee S. E., and Paull T. T. (2010) Mre11-Rad50-Xrs2 and Sae2 promote 5′-strand resection of DNA double-strand breaks. Nat. Struct. Mol. Biol. 17, 1478–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nimonkar A. V., Genschel J., Kinoshita E., Polaczek P., Campbell J. L., Wyman C., Modrich P., and Kowalczykowski S. C. (2011) BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 25, 350–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bolderson E., Tomimatsu N., Richard D. J., Boucher D., Kumar R., Pandita T. K., Burma S., and Khanna K. K. (2010) Phosphorylation of Exo1 modulates homologous recombination repair of DNA double-strand breaks. Nucleic Acids Res. 38, 1821–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomimatsu N., Mukherjee B., Deland K., Kurimasa A., Bolderson E., Khanna K. K., and Burma S. (2012) Exo1 plays a major role in DNA end resection in humans and influences double-strand break repair and damage signaling decisions. DNA Repair 11, 441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huertas P., and Jackson S. P. (2009) Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J. Biol. Chem. 284, 9558–9565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huertas P., Cortés-Ledesma F., Sartori A. A., Aguilera A., and Jackson S. P. (2008) CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature 455, 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yun M. H., and Hiom K. (2009) CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 459, 460–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang H., Shi L. Z., Wong C. C., Han X., Hwang P. Y., Truong L. N., Zhu Q., Shao Z., Chen D. J., Berns M. W., Yates J. R. 3rd, Chen L., and Wu X. (2013) The interaction of CtIP and Nbs1 connects CDK and ATM to regulate HR-mediated double-strand break repair. PLoS Genet. 9, e1003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buis J., Stoneham T., Spehalski E., and Ferguson D. O. (2012) Mre11 regulates CtIP-dependent double-strand break repair by interaction with CDK2. Nat. Struct. Mol. Biol. 19, 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falck J., Forment J. V., Coates J., Mistrik M., Lukas J., Bartek J., and Jackson S. P. (2012) CDK targeting of NBS1 promotes DNA-end resection, replication restart, and homologous recombination. EMBO Rep. 13, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomimatsu N., Mukherjee B., Catherine Hardebeck M., Ilcheva M., Vanessa Camacho C., Louise Harris J., Porteus M., Llorente B., Khanna K. K., and Burma S. (2014) Phosphorylation of EXO1 by CDKs 1 and 2 regulates DNA end resection and repair pathway choice. Nat. Commun. 5, 3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng X. F., Kalev P., and Chowdhury D. (2015) Emerging role of protein phosphatases changes the landscape of phospho-signaling in DNA damage response. DNA Repair 32, 58–65 [DOI] [PubMed] [Google Scholar]
- 25. Tran P. T., Erdeniz N., Symington L. S., and Liskay R. M. (2004) EXO1-A multi-tasking eukaryotic nuclease. DNA Repair 3, 1549–1559 [DOI] [PubMed] [Google Scholar]
- 26. Keijzers G., Liu D., and Rasmussen L. J. (2016) Exonuclease 1 and its versatile roles in DNA repair. Crit. Rev. Biochem. Mol. Biol. 51, 440–451 [DOI] [PubMed] [Google Scholar]
- 27. Liao S., Toczylowski T., and Yan H. (2011) Mechanistic analysis of Xenopus EXO1's function in 5′-strand resection at DNA double-strand breaks. Nucleic Acids Res. 39, 5967–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costelloe T., Louge R., Tomimatsu N., Mukherjee B., Martini E., Khadaroo B., Dubois K., Wiegant W. W., Thierry A., Burma S., van Attikum H., and Llorente B. (2012) The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature 489, 581–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomimatsu N., Mukherjee B., and Burma S. (2009) Distinct roles of ATR and DNA-PKcs in triggering DNA damage responses in ATM-deficient cells. EMBO Rep. 10, 629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orans J., McSweeney E. A., Iyer R. R., Hast M. A., Hellinga H. W., Modrich P., and Beese L. S. (2011) Structures of human exonuclease 1 DNA complexes suggest a unified mechanism for nuclease family. Cell 145, 212–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goellner E. M., Putnam C. D., and Kolodner R. D. (2015) Exonuclease 1-dependent and independent mismatch repair. DNA Repair 32, 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen X., Kim I. K., Honaker Y., Paudyal S. C., Koh W. K., Sparks M., Li S., Piwnica-Worms H., Ellenberger T., and You Z. (2015) 14-3-3 proteins restrain the exo1 nuclease to prevent over-resection. J. Biol. Chem. 290, 12300–12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X., Paudyal S. C., Chin R. I., and You Z. (2013) PCNA promotes processive DNA end resection by Exo1. Nucleic Acids Res. 41, 9325–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang F., Shi J., Chen S. H., Bian C., and Yu X. (2015) The PIN domain of EXO1 recognizes poly(ADP-ribose) in DNA damage response. Nucleic Acids Res. 43, 10782–10794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolderson E., Richard D. J., Edelmann W., and Khanna K. K. (2009) Involvement of Exo1b in DNA damage-induced apoptosis. Nucleic Acids Res. 37, 3452–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El-Shemerly M., Hess D., Pyakurel A. K., Moselhy S., and Ferrari S. (2008) ATR-dependent pathways control hEXO1 stability in response to stalled forks. Nucleic Acids Res. 36, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiloh Y., and Ziv Y. (2013) The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 14, 197–210 [PubMed] [Google Scholar]
- 38. Flynn R. L., and Zou L. (2011) ATR: a master conductor of cellular responses to DNA replication stress. Trends Biochem. Sci. 36, 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toledo L. I., Altmeyer M., Rask M. B., Lukas C., Larsen D. H., Povlsen L. K., Bekker-Jensen S., Mailand N., Bartek J., and Lukas J. (2013) ATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell 155, 1088–1103 [DOI] [PubMed] [Google Scholar]
- 40. Fernandez-Capetillo O., and Nussenzweig A. (2013) Naked replication forks break apRPArt. Cell 155, 979–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ochs F., Somyajit K., Altmeyer M., Rask M. B., Lukas J., and Lukas C. (2016) 53BP1 fosters fidelity of homology-directed DNA repair. Nat. Struct. Mol. Biol. 23, 714–721 [DOI] [PubMed] [Google Scholar]
- 42. Zong D., Chaudhuri A. R., and Nussenzweig A. (2016) More end resection is not merrier. Nat. Struct. Mol. Biol. 23, 699–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsiang Y. H., Lihou M. G., and Liu L. F. (1989) Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 49, 5077–5082 [PubMed] [Google Scholar]
- 44. Sarkaria J. N., Busby E. C., Tibbetts R. S., Roos P., Taya Y., Karnitz L. M., and Abraham R. T. (1999) Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375–4382 [PubMed] [Google Scholar]
- 45. Hickson I., Zhao Y., Richardson C. J., Green S. J., Martin N. M., Orr A. I., Reaper P. M., Jackson S. P., Curtin N. J., and Smith G. C. (2004) Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 [DOI] [PubMed] [Google Scholar]
- 46. Veuger S. J., Curtin N. J., Richardson C. J., Smith G. C., and Durkacz B. W. (2003) Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 63, 6008–6015 [PubMed] [Google Scholar]
- 47. Leszczynska K. B., Dobrynin G., Leslie R. E., Ient J., Boumelha A. J., Senra J. M., Hawkins M. A., Maughan T., Mukherjee S., and Hammond E. M. (2016) Preclinical testing of an Atr inhibitor demonstrates improved response to standard therapies for esophageal cancer. Radiother. Oncol 121, 232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stracker T. H., Usui T., and Petrini J. H. (2009) Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair 8, 1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hao Y. H., Doyle J. M., Ramanathan S., Gomez T. S., Jia D., Xu M., Chen Z. J., Billadeau D. D., Rosen M. K., and Potts P. R. (2013) Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell 152, 1051–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doyle J. M., Gao J., Wang J., Yang M., and Potts P. R. (2010) MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol. Cell 39, 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silverman J. S., Skaar J. R., and Pagano M. (2012) SCF ubiquitin ligases in the maintenance of genome stability. Trends Biochem. Sci. 37, 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang X., Orlicky S., Liu Q., Willems A., Sicheri F., and Tyers M. (2005) Genome-wide surveys for phosphorylation-dependent substrates of SCF ubiquitin ligases. Methods Enzymol. 399, 433–458 [DOI] [PubMed] [Google Scholar]
- 53. Hannah J., and Zhou P. (2009) Regulation of DNA damage response pathways by the cullin-RING ubiquitin ligases. DNA Repair 8, 536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Y. W., Brognard J., Coughlin C., You Z., Dolled-Filhart M., Aslanian A., Manning G., Abraham R. T., and Hunter T. (2009) The F box protein Fbx6 regulates Chk1 stability and cellular sensitivity to replication stress. Mol. Cell 35, 442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y. W., Otterness D. M., Chiang G. G., Xie W., Liu Y. C., Mercurio F., and Abraham R. T. (2005) Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol. Cell 19, 607–618 [DOI] [PubMed] [Google Scholar]
- 56. Soucy T. A., Smith P. G., Milhollen M. A., Berger A. J., Gavin J. M., Adhikari S., Brownell J. E., Burke K. E., Cardin D. P., Critchley S., Cullis C. A., Doucette A., Garnsey J. J., Gaulin J. L., Gershman R. E., et al. (2009) An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 [DOI] [PubMed] [Google Scholar]
- 57. Emanuele M. J., Elia A. E., Xu Q., Thoma C. R., Izhar L., Leng Y., Guo A., Chen Y. N., Rush J., Hsu P. W., Yen H. C., and Elledge S. J. (2011) Global identification of modular cullin-RING ligase substrates. Cell 147, 459–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eid W., Steger M., El-Shemerly M., Ferretti L. P., Peña-Diaz J., König C., Valtorta E., Sartori A. A., and Ferrari S. (2010) DNA end resection by CtIP and exonuclease 1 prevents genomic instability. EMBO Rep. 11, 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nimonkar A. V., Ozsoy A. Z., Genschel J., Modrich P., and Kowalczykowski S. C. (2008) Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc. Natl. Acad. Sci. U.S.A. 105, 16906–16911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mukherjee B., Tomimatsu N., and Burma S. (2015) Immunofluorescence-based methods to monitor DNA end resection. Methods Mol. Biol. 1292, 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mukherjee B., Tomimatsu N., Amancherla K., Camacho C. V., Pichamoorthy N., and Burma S. (2012) The dual PI3K/mTOR inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and DNA-PKCs-mediated DNA damage responses. Neoplasia 14, 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Potts P. R., Porteus M. H., and Yu H. (2006) Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. EMBO J. 25, 3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Symington L. S. (2014) End resection at double-strand breaks: mechanism and regulation. Cold Spring Harb. Perspect. Biol. 6, a016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Burma S., Chen B. P., Murphy M., Kurimasa A., and Chen D. J. (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462–42467 [DOI] [PubMed] [Google Scholar]
- 65. Mukherjee B., Camacho C. V., Tomimatsu N., Miller J., and Burma S. (2008) Modulation of the DNA-damage response to HZE particles by shielding. DNA Repair 7, 1717–1730 [DOI] [PubMed] [Google Scholar]
- 66. Symington L. S. (2016) Mechanism and regulation of DNA end resection in eukaryotes. Crit. Rev. Biochem. Mol. Biol. 51, 195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ulrich H. D. (2012) Ubiquitin and SUMO in DNA repair at a glance. J. Cell Sci. 125, 249–254 [DOI] [PubMed] [Google Scholar]
- 68. Jackson S. P., and Durocher D. (2013) Regulation of DNA damage responses by ubiquitin and SUMO. Mol. Cell 49, 795–807 [DOI] [PubMed] [Google Scholar]
- 69. Galanty Y., Belotserkovskaya R., Coates J., Polo S., Miller K. M., and Jackson S. P. (2009) Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462, 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morris J. R., Boutell C., Keppler M., Densham R., Weekes D., Alamshah A., Butler L., Galanty Y., Pangon L., Kiuchi T., Ng T., and Solomon E. (2009) The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 462, 886–890 [DOI] [PubMed] [Google Scholar]
- 71. Han X., Zhang L., Chung J., Mayca Pozo F., Tran A., Seachrist D. D., Jacobberger J. W., Keri R. A., Gilmore H., and Zhang Y. (2014) UbcH7 regulates 53BP1 stability and DSB repair. Proc. Natl. Acad. Sci. U.S.A. 111, 17456–17461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yin Y., Seifert A., Chua J. S., Maure J. F., Golebiowski F., and Hay R. T. (2012) SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes Dev. 26, 1196–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Galanty Y., Belotserkovskaya R., Coates J., and Jackson S. P. (2012) RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 26, 1179–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luo K., Zhang H., Wang L., Yuan J., and Lou Z. (2012) Sumoylation of MDC1 is important for proper DNA damage response. EMBO J. 31, 3008–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xie J., Kim H., Moreau L. A., Puhalla S., Garber J., Al Abo M., Takeda S., and D'Andrea A. D. (2015) RNF4-mediated polyubiquitination regulates the Fanconi anemia/BRCA pathway. J. Clin. Investig. 125, 1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parameswaran B., Chiang H. C., Lu Y., Coates J., Deng C. X., Baer R., Lin H. K., Li R., Paull T. T., and Hu Y. (2015) Damage-induced BRCA1 phosphorylation by Chk2 contributes to the timing of end resection. Cell Cycle 14, 437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ferretti L. P., Himmels S. F., Trenner A., Walker C., von Aesch C., Eggenschwiler A., Murina O., Enchev R. I., Peter M., Freire R., Porro A., and Sartori A. A. (2016) Cullin3-KLHL15 ubiquitin ligase mediates CtIP protein turnover to fine-tune DNA-end resection. Nat. Commun. 7, 12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Postow L., and Funabiki H. (2013) An SCF complex containing Fbxl12 mediates DNA damage-induced Ku80 ubiquitylation. Cell Cycle 12, 587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brown J. S., Lukashchuk N., Sczaniecka-Clift M., Britton S., le Sage C., Calsou P., Beli P., Galanty Y., and Jackson S. P. (2015) Neddylation promotes ubiquitylation and release of Ku from DNA-damage sites. Cell Rep. 11, 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ismail I. H., Gagné J. P., Genois M. M., Strickfaden H., McDonald D., Xu Z., Poirier G. G., Masson J. Y., and Hendzel M. J. (2015) The RNF138 E3 ligase displaces Ku to promote DNA end resection and regulate DNA repair pathway choice. Nat. Cell Biol. 17, 1446–1457 [DOI] [PubMed] [Google Scholar]
- 81. Jin J., Shirogane T., Xu L., Nalepa G., Qin J., Elledge S. J., and Harper J. W. (2003) SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17, 3062–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Elia A. E., Boardman A. P., Wang D. C., Huttlin E. L., Everley R. A., Dephoure N., Zhou C., Koren I., Gygi S. P., and Elledge S. J. (2015) Quantitative proteomic atlas of ubiquitination and acetylation in the DNA damage response. Mol. Cell 59, 867–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wagner S. A., Beli P., Weinert B. T., Nielsen M. L., Cox J., Mann M., and Choudhary C. (2011) A proteome-wide, quantitative survey of in vivo ubiquitinylation sites reveals widespread regulatory roles. Mol. Cell. Proteomics 10, M111.013284 ( 10.1074/mcp.M111.013284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kim W., Bennett E. J., Huttlin E. L., Guo A., Li J., Possemato A., Sowa M. E., Rad R., Rush J., Comb M. J., Harper J. W., and Gygi S. P. (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xu G., and Jaffrey S. R. (2013) Proteomic identification of protein ubiquitination events. Biotechnol. Genet. Eng. Rev. 29, 73–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Saintigny Y., Delacôte F., Varès G., Petitot F., Lambert S., Averbeck D., and Lopez B. S. (2001) Characterization of homologous recombination induced by replication inhibition in mammalian cells. EMBO J. 20, 3861–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Petermann E., Orta M. L., Issaeva N., Schultz N., and Helleday T. (2010) Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol. Cell 37, 492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bologna S., Altmannova V., Valtorta E., Koenig C., Liberali P., Gentili C., Anrather D., Ammerer G., Pelkmans L., Krejci L., and Ferrari S. (2015) Sumoylation regulates EXO1 stability and processing of DNA damage. Cell Cycle 14, 2439–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sun X., Zheng L., and Shen B. (2002) Functional alterations of human exonuclease 1 mutants identified in atypical hereditary nonpolyposis colorectal cancer syndrome. Cancer Res. 62, 6026–6030 [PubMed] [Google Scholar]
- 90. Wu Y., Berends M. J., Post J. G., Mensink R. G., Verlind E., Van Der Sluis T., Kempinga C., Sijmons R. H., van der Zee A. G., Hollema H., Kleibeuker J. H., Buys C. H., and Hofstra R. M. (2001) Germline mutations of EXO1 gene in patients with hereditary nonpolyposis colorectal cancer (HNPCC) and atypical HNPCC forms. Gastroenterology 120, 1580–1587 [DOI] [PubMed] [Google Scholar]
- 91. Yamamoto H., Hanafusa H., Ouchida M., Yano M., Suzuki H., Murakami M., Aoe M., Shimizu N., Nakachi K., and Shimizu K. (2005) Single nucleotide polymorphisms in the EXO1 gene and risk of colorectal cancer in a Japanese population. Carcinogenesis 26, 411–416 [DOI] [PubMed] [Google Scholar]
- 92. Wang H. C., Chiu C. F., Tsai R. Y., Kuo Y. S., Chen H. S., Wang R. F., Tsai C. W., Chang C. H., Lin C. C., and Bau D. T. (2009) Association of genetic polymorphisms of EXO1 gene with risk of breast cancer in Taiwan. Anticancer Res. 29, 3897–3901 [PubMed] [Google Scholar]
- 93. Bau D. T., Wang H. C., Liu C. S., Chang C. L., Chiang S. Y., Wang R. F., Tsai C. W., Lo Y. L., Hsiung C. A., Lin C. C., and Huang C. Y. (2009) Single-nucleotide polymorphism of the Exo1 gene: association with gastric cancer susceptibility and interaction with smoking in Taiwan. Chin. J. Physiol. 52, 411–418 [DOI] [PubMed] [Google Scholar]
- 94. Tsai M. H., Tseng H. C., Liu C. S., Chang C. L., Tsai C. W., Tsou Y. A., Wang R. F., Lin C. C., Wang H. C., Chiu C. F., and Bau D. T. (2009) Interaction of Exo1 genotypes and smoking habit in oral cancer in Taiwan. Oral Oncol. 45, e90–94 [DOI] [PubMed] [Google Scholar]
- 95. Jin G., Wang H., Hu Z., Liu H., Sun W., Ma H., Chen D., Miao R., Tian T., Jin L., Wei Q., Huang W., Lu D., and Shen H. (2008) Potentially functional polymorphisms of EXO1 and risk of lung cancer in a Chinese population: a case-control analysis. Lung Cancer 60, 340–346 [DOI] [PubMed] [Google Scholar]
- 96. Chang J. S., Yeh R. F., Wiencke J. K., Wiemels J. L., Smirnov I., Pico A. R., Tihan T., Patoka J., Miike R., Sison J. D., Rice T., and Wrensch M. R. (2008) Pathway analysis of single-nucleotide polymorphisms potentially associated with glioblastoma multiforme susceptibility using random forests. Cancer Epidemiol. Biomarkers Prev. 17, 1368–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Michailidou K., Beesley J., Lindstrom S., Canisius S., Dennis J., Lush M. J., Maranian M. J., Bolla M. K., Wang Q., Shah M., Perkins B. J., Czene K., Eriksson M., Darabi H., Brand J. S., et al. (2015) Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat. Genet. 47, 373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cuadrado M., Martinez-Pastor B., Murga M., Toledo L. I., Gutierrez-Martinez P., Lopez E., and Fernandez-Capetillo O. (2006) ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J. Exp. Med. 203, 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McEllin B., Camacho C. V., Mukherjee B., Hahm B., Tomimatsu N., Bachoo R. M., and Burma S. (2010) PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 70, 5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mukherjee B., McEllin B., Camacho C. V., Tomimatsu N., Sirasanagandala S., Nannepaga S., Hatanpaa K. J., Mickey B., Madden C., Maher E., Boothman D. A., Furnari F., Cavenee W. K., Bachoo R. M., and Burma S. (2009) EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 69, 4252–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.