Abstract
Bombyx batryticatus is a traditional Chinese medicine. To understand apoptotic effect of B. batryticatus ethanol extract (BBE), we investigated the role of BBE in inducing apoptosis of human gastric cancer cells SGC-7901. Cells treated with BBE and apoptosis was assessed by methyl thiazolyl tetrazolium (MTT) assay, morphological changes, DNA fragmentation and flow cytometry assays. The expression of Bcl-2, Bax and P21 were evaluated by western blot analysis and real time polymerase chain reaction. MTT assay showed that the cytotoxicity of BBE extract on SGC-7901 cells was correlated with treatment time and concentration. After treatment with 6 mg/mL of BBE the microscopy showed that, the majority of SGC-7901 cells were obviously reduced, distorted and grew slowly. Annexin-V/propidium iodide double-staining assay emerge the early apoptosis and the late apoptosis after treatment with different times by laser confocal fluorescence microscopy and flow cytometer. Cell cycle analysis of SGC 79 cells showed that BBE induced cell cycle arrest in the G1 and G2 phases. DNA fragmentation indicated the trend of BBE inducing apoptosis on SGC-7901 cells. The qRT-PCR and western blot analysis indicated that the mRNA and protein expressions of Bax and P21 were significantly up-regulated whereas that of Bc1-2 was down-regulated after treatment with BBE for 24 h. Our results revealed a correlation between gene regulation and BBE-induced apoptosis, which might indicate the potential of BBE in cancer therapy.
Keywords: Apoptosis, Bax, Bcl-2, Bombyx batryticatus, P21, SGC-790 cells
Introduction
Apoptosis is a vital process of programmed cell death for maintaining the normal operation of tissues and cancer prevention. Recently, the researchers have paid attention to the potential role of traditional Chinese medicinal herbs extracts as alternative and complementary drug for cancer treatment (Li et al. 2009). In the case of Scutellaria baicalensis, a traditional Chinese herbal, apoptosis has been induced in lymphoma and myeloma cell lines by increasing level of the p27 “CDK inhibitor” and decreasing the level of c-myc oncogene (Kumagai et al. 2007). Albizia gummifera roots extract revealed cytotoxicity against various cancer cell lines in vitro (Cao et al. 2007). Honokiol is a natural product that could induce the apoptosis of B-CLL cells (Battle et al. 2005). The traditional Chinese herbal Ganoderma has a strong inhibition of cell proliferation and induces the apoptosis of breast and prostate cancer cells (Hu et al. 2002; Jiang et al. 2004; Lu et al. 2004). Cupids rhizome induced apoptosis in SNU-C4 human colorectal cancer cells through caspase pathways (Kim et al. 2004).
Bombyx batryticatus is a valuable crude drug, composed of dried silkworm larvae, Bombyx mori larvae that were stiffened by infection with Beauveria bassiana. In application of traditional Chinese medicine, B. batryticatus is used for treatment of headaches, convulsions, tonsillitis, Bell’s palsy, asthma, and thyroid adenoma (Jiang et al. 2014). In human tumor cell line Hela the BBE obviously inhibited the growth and induced the apoptosis by regulating the Bcl-2 and Bax mediated apoptotic pathways (Wu et al. 2015). The purpose of this study is to evaluate the effect of BBE extract on gastric cancer SGC-7901 cells.
Materials and methods
Preparation of BBE extract from Bombyx batryticatus
The BBE is a compound derived by extraction from dry B. batryticatus was bought from Xi’an Chinese Medicine Co. Ltd. (Xi’an, China). The B. batryticatus were dried at 60 °C for 10 h in an oven and then grounded to powder using mechanical grinder. The B. batryticatus powder was extracted repeatedly for three times for 12 h with 95% Ethanol at room temperature and repeated three times. The extracts were vacuum filtered through filter papers and the ethanol extract was collected. Then leach liquor was removed from the combined extracts using a vacuum rotary evaporator. 50 g of the extract was dissolved in 500 mL distilled water and 500 mL petroleum ether was added to the crude extract to remove grease with a separator funnel. The aqueous phase was transferred to a vacuum rotary evaporator to evaporate the water and the refined extract was obtained and stored at −20 °C. Finally the extract was dissolved in RPMI-1640 medium without fetal bovine serum (FBS) to a concentration of 100 mg/mL and stored in 4 °C.
Cell line and cell culture
Human gastric cancer cell line (SGC-7901, BGC 823) was provided by the Medical school in Xi’an Jiaotong University (Xi’an, China). Cells were grown as a monolayer in RPMI-1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated calf serum (Hyclone), 100 µg/mL penicillin and 50 µg/mL streptomycin in an incubator HF90 (Heal Force Bio-meditech Holdings Limited, Shanghai, China) which was maintained at a water-saturated atmosphere with 5% CO2 at 37 °C.
Cytotoxicity assay
Cytotoxicity assay of BBE was evaluated by MTT assay. Five thousand SGC-7901 and BGC 823 cells were seeded in a 96-well plate with 200 µL RPMI-1640 and incubated overnight in the incubator as described above. The culture medium was removed, and adherent cells were treated with different concentrations of BBE (1–10 mg/mL) in 200 µL of fresh medium for 24 and 48 h. Then 20 µL 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide in 200 µL fresh medium was added to each well for a 4-h period. The culture medium with MTT was then removed, and formazan was extracted with 150 µL of DMSO. Absorbance was measured at 570 nm (630 nm as a reference) by a Tecan Infinite® 200 PRO multimode micro-plate reader (Tecan, Männedorf, Switzerland). The experiments were repeated three times. Cell viability was expressed as a percentage of the value compared to control cultures.
Cell morphological assessment
The SGC-7901 cells were treated with different concentrations of BBE (2, 4 and 6 mg/mL) when the cell growth was in the mid-log phase. Then incubation was continued for a 24-h exposure. Finally, the morphology of cells was examined under a Nikon TE2000 inverted microscope (Nikon, Tokyo, Japan).
Apoptosis assessment by Annexin FITC-V/PI double staining assay
Apoptotic cells were identified by double staining with recombinant FITC (fluorescein isothiocyanate)-conjugated Annexin-V and PI. The Annexin V-FITC Apoptosis Detection kit (Bender Medsystems, Burlingame, CA, USA) was used to detect apoptosis according to the manufacturer’s instructions.
Laser confocal fluorescence microscopy analysis of apoptosis
The SGC-7901 cells were incubated overnight in confocal dish until the mid-log phase. The adherent cells were treated with 6 mg/mL BBE and incubated for 3, 6 and 12 h. The culture medium was removed and cells were washed thrice with ice-cold PBS. 5 µL Annexin V-FITC and 10 µL PI were added to all samples and then treated cells were incubated at room temperature for 15 min without light. Apoptotic cells were observed by laser confocal fluorescence microscopy.
Flow cytometric analysis of apoptosis
The SGC-7901 cells were plated in six-well plates until the mid-log phase. The adherent cells were treated with different concentrations of BBE (2, 4, 6 mg/mL), and incubation was continued for a 24-h. Cells were collected, washed and re-suspended in ice-cold PBS. Cells were stained with 5 µL Annexin V-FITC and 10 µL PI following the manufacturer’s instructions. Flow cytometric analysis was performed immediately after staining for 15 min without light. Apoptosis of cells was assayed using Becton-Dickinson FACSCalibur flow cytometer.
DNA fragmentation assay
The SGC-7901 cells were plated in six-well plates until the mid-log phase. The adherent cells were treated with different concentrations of BBE (2, 4, 6 mg/mL), and incubation was continued for a 24-h. DNA was extracted according to the protocol of the DNA ladder detection kit (Billerica, MA, Millipore). DNA was separated through a 1.5% gel electrophoresis, stained with ethidium bromide and fragments were assayed and recorded using a Gene Genius Bioimaging System Universal Hood II (Bio-Rad, Hercules, CA, USA).
Real-time RT-PCR
The SGC-7901 cells were plated in a six-well plate until the mid-log phase. The adherent cells were treated with different concentrations of BBE (2, 4, 6 mg/mL), and incubated for a 36-h. Total RNA was extracted from the treated cells using RNAiso Plus (TaKaRa, Shiga, Japan) following manufacturer instructions. RNA was dissolved in DEPC water. The quality of RNA was evaluated on agarose gel. RNA was reverse transcribed for 15 min at 37 °C and 85 °C for 5 s using PrimeScript® RT reagent Kit Perfect Real Time (TaKaRa). The reaction was carried out in a total volume of 20 µL containing 4 µL 5× PrimeScript® buffer, 1 µL PrimeScript® RT enzyme mix, 1 µL Oligo dT primer and 14 µL total RNA. The cDNA was stored at −80 °C. Real-time PCR was performed using an iCycler iQ2 Real time PCR detection system. Reaction mixtures contained 10 µL SYBR® Premix Ex Taq™ II, 0.8 µL of each primer (10 µM), 2.0 µL DNA template and 6.4 µL ddH2O up to 20 µL total volume. The reaction had two steps: stage 1: 94 °C for 30 s; stage 2: forty cycles of 94 °C for 5 s, 60 °C for 30 s. β-actin was used as endogenous control. Data were analyzed using the comparative 2−∆∆CT method.
The primer sequences of β-actin were 5′-CGGGAAATCGTGCGTGAC-3′ (forward) and 5′-GGAAGGAAGGCTGGAAGAGTG-3′ (reverse). The primers for Bcl-2 were 5′-ATGTGTGTGGAGAGCGTCAAC-3′ (forward) and 5′-ATGTGTGTGGAGAGCGTCAAC-3′ (reverse). The primers for Bax were 5′-AGGATGCGTCCACCAAGAAGC-3′ (forward) and 5′-CTGCCACTCGGAAAAAGACCT-3′ (reverse). The primers for P21 were 5′-GTCACTGTCTTGTACCCTTGT-3′ (forward) and 5′-GGAGTGGTAGAAATCTGTCAT-3′ (reverse).
Western blot analysis
The SGC-7901 cells were plated in a six-well plate until the mid-log phase. The adherent cells were treated with different concentrations of BBE (2, 4 and 6 mg/mL) and incubation was continued for a 36-h. The six-well plates were placed on ice and washed twice with ice-cold PBS. Proteins were isolated by RIPA buffer at 4 °C for 15 min. Protein samples were centrifuged at 12,000 rpm for 10 min at 4 °C, mixed with sample loading buffer and boiled for 15 min. The protein concentration in supernatant was determined by Bradford Assay (Bio-Rad). Equal amounts of protein were separated on 12% SDS–polyacrylamide gel and transferred onto the PVDF membranes (Millipore). After being infiltrated, the membranes were blocked in washing buffer (20 mM Tris–HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween-20) containing 5% non-fat milk for 2 h at room temperature, and incubated overnight at 4 °C with different primary antibodies for different target proteins (mouse: anti-β-actin; rabbit polyclonal: anti-Bax, anti-Bcl-2 and anti-P21 (Abcam, Cambridge, England). Membranes were washed with washing buffer three times and incubated with different secondary antibodies (Abcam) at room temperature for 1 h. Blots were developed with an enhanced chemiluminescent kit (Thermo Scientific Pierce ECL, Waltham, MA, USA) according to the manufacturer’s protocol and were photographed by a ChemiScope 2950 MI (Clinx Science Instruments, Shanghai, China).
Cell cycle analysis
Proportion of cells at different cell cycle phases was also monitored by cell cycle and apoptosis analysis kit (Jiangsu, China). SGC7901 cells were treated with different concentrations (4 and 6 mg/mL) and collected as described in the previous section “analysis of apoptosis”. Cells were resuspended in 1 mL cold PBS, centrifuged at 1000g/5 min. 1 mL chilled ethanol 70% was added slowly for incubation to fix the cells at 4 °C for 16 h. Ethanol was removed by centrifugation 1000g/5 min, and cells were washed with PBS. Cells were resuspended in 0.5 mL mixture of buffer, PI and RNase A. Cells were incubated at 37 °C for another 30 min in the dark. Finally the distribution of cells at various cell cycle stages was determined by FACSCalibur flow cytometer.
Statistics and data presentation
All data are presented as the mean ± standard deviation (SD). Statistical analysis was performed using the one-way analysis of variance (ANOVA). A value of probability (p) < 0.01 was considered to be statistically significant. Data were analyzed and plotted using GraphPad Prism version 5.0d for PC (GraphPad Software, La Jolla, CA, USA).
Results
Cytotoxicity of BBE
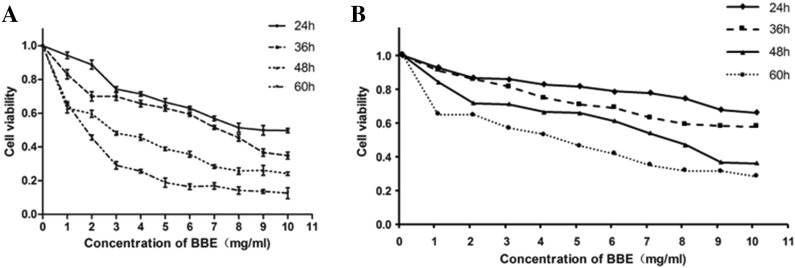
MTT assay indicated that the cytotoxicity of BBE on SGC-7901 and BGC-823 cells was correlated with treatment time and concentration (Fig. 1a, b). The IC50 values of BBE were 8.647, 7.091, 3.175 and 1.316 mg/mL for 24, 36, 48 and 60 h BBE treatment for SGC-7901, respectively; and >10 mg/mL for 24/48 h BBE treatment, while it was 4.5, 7.8 mg/mL 48 and 60 h BBE treatment for BGC-823, respectively. These results showed that BBE could significantly inhibit the growth of SGC-7901 and BGC-823 cells in vitro.
Fig. 1.
Inhibition of proliferation by BBE. SGC-7901 cells (a) and BGC-823 cells (b). Cells were treated with 1.0–10.0 mg/mL BBE for 24, 36, 48 and 60 h. Cell viability was determined by MTT assay. Results are expressed as percentages of proliferation compared to the untreated control (mean ± SE, n = 3)
Morphological characteristics of apoptosis by inverted microscope
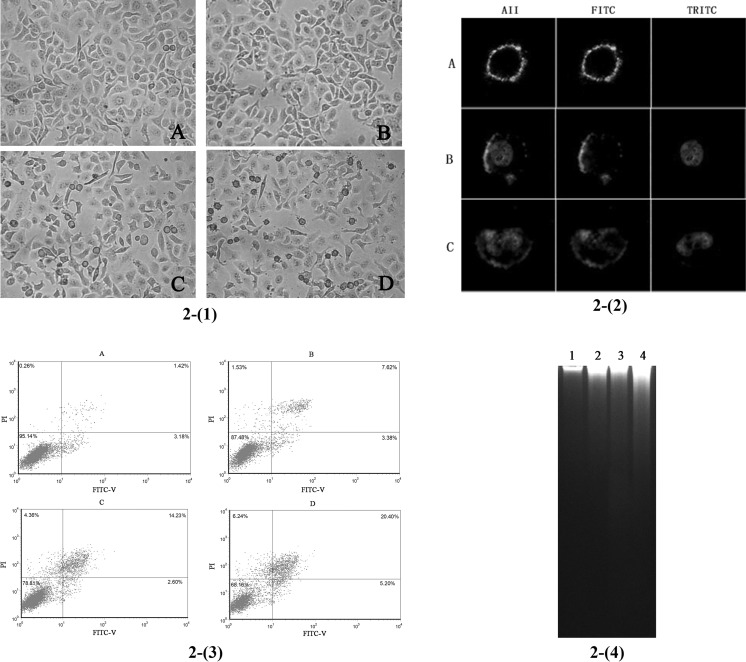
The characteristics of SGC-7901 cells treated with the BBE were drastically different from the controls observed by inverted microscope. Untreated cells displayed normal, healthy growth and had a distinct outline observed by inverted microscopy (Fig. 2(1)a). Conversely, the majority of SGC-7901 cells treated with BBE were obviously reduced, distorted and grew slowly. When the concentration of BBE was 6 mg/mL, the bodies of SGC-7901 cells became smoother.
Fig. 2.
Apoptosis of SGC-7901 cells treated with BBE. 1 Morphologic changes include rupture, aggregation, swelling, of the membrane of cells observed under an inverted microscope (×25). SGC-7901 cells were treated with different concentrations of BBE for 24 h. a Control, b 2 mg/mL, c 4 mg/mL, d 6 mg/mL. 2 Morphology of apoptotic cells observed by laser confocal fluorescence microscopy. SGC-7901 cells were treated with 6 mg/mL BBE for 3, 6, 12 h. Cells were observed by confocal fluorescence microscopy. a Early apoptosis, b late apoptosis, c late apoptosis, more distortion in both cytomembrane and nucleus. 3 Fluorescence-activated cell sorter analysis for Annexin-V and propidium iodide (PI) staining of SGC-7901 cells incubated with BBE at different concentrations for 24 h. a Control, b 2 mg/mL, c 4 mg/mL, d 6 mg/mL. Upper right necrotic cells and late apoptotic cells. Lower left fully viable cells. Lower right early apoptotic cells. 4 DNA fragmentation influenced by BBE in SGC-7901 cells for 24 h. Lanes 1–4 stand for 0 mg/mL (control), 2, 4 and 6 mg/mL, respectively
Apoptosis detection by laser confocal fluorescence microscopy
The cytomembranes of SGC-7901 cells treated with 4 mg/mL BBE for 3 h were identified by Annexin V-FITC showing bright green fluorescence (Fig. 2(2)a). In longer treatment, up to 6 and 12 h, cell nucleus was identified by PI showing bright red fluorescence (Fig. 2(2)b, c). The cytomembranes were destroyed with prolonged treatment of BBE. These results demonstrated that BBE could induce apoptosis in SGC-7901 cells.
Apoptosis assessment by flow cytometry assay
Counts of apoptotic cells induced by increasing concentrations of BBE detected by flow cytometric analysis are presented in Fig. 2(3). The upper and lower right quadrants represented late apoptotic/necrotic and early apoptotic cells, respectively. The percentages of apoptotic cells were significantly increased following the increased concentrations of BBE, 11.00, 16.83 and 25.60% (Figure 2(3)b–d), respectively.
BBE induced DNA fragmentation
DNA fragmentation was evident in the cells treated with BBE in the second lane to the forth lane. Meanwhile, the untreated cells did not show any DNA fragmentation in the first line (Fig. 2(4)). More evidence of DNA fragmentation was observed with high concentrations of BBE.
BBE induced gene expression change
As shown in Fig. 3b, the transcription level of Bcl-2 significantly decreased when treated with BBE while that of Bax increased. The ratio of Bax/Bcl-2 obviously increased. At the same time, the transcription level of P21 increased with the increasing concentration of BBE (Fig. 3b). Western blot indicated that the expression of Bax and P21 protein was significantly up-regulated whereas the expression of Bc1-2 protein was down-regulated after treatment with BBE for 36 h (Fig. 3a).
Fig. 3.
Possible pathway of apoptosis in SGC-7901 cells treated with BBE. a Relative expression levels of Bcl-2, Bax and P21 in SGC-7901 cells. Cells were treated with 0 mg/mL (control), 2, 4, 6, 8 mg/mL BBE for 36 h. Results were expressed as average of three replicates ± SD. β-actin was used as an internal reference gene. The relative expression was calculated based on the value of the lowest expression (expression levels of Bcl-2 at 8 mg/mL), which was ascribed an arbitrary value of 1. b Effects of BBE on β-actin, Bcl-2, Bax and P21 protein expression in SGC-7901 cells. Cells were treated with 0 mg/mL (control), 2, 4, 6, 8 mg/mL BBE for 36 h. The protein levels were monitored by Western blot analysis. The signal was normalized using β-actin as an internal standard
Cell cycle analysis
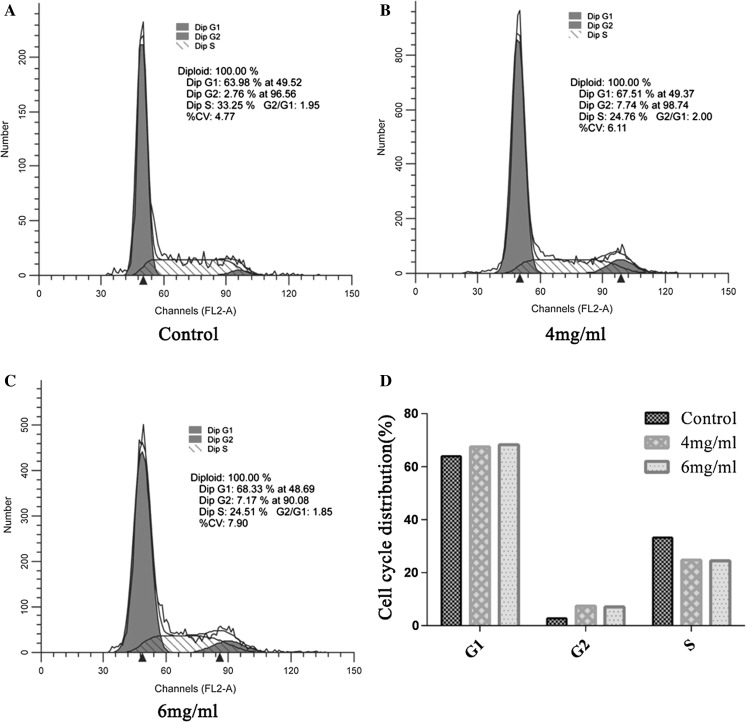
The cell cycle of SGC 7901 cells treated with BBE was differentiated into the respective phases (Fig. 4b, c). In comparison to control cells (Fig. 4d), there was reduction in the cells number in S phase and an increase in the cell numbers in G1 and G2 phase after exposure to BBE.
Fig. 4.
Cell cycle analysis (b, c) of SGC 7901 cells treated with 4 and 6 mg/ml BBE for 24h. Cells distribution was increased in G1 and G2 phase, when it was decreased in S phase. The difference in G1, G2 and S phase summarized in (d), while (a) is the control treatment
Discussion
One of the potential strategies in cancer treatment is to induce apoptosis. BBE is a traditional Chinese herbal which proved to prevent the growth and induce apoptosis in Hela cancer cell line (Wu et al. 2015).
In this study, the molecular mechanisms of BBE inducing the SGC-7901 cells apoptosis have been evaluated. The results showed that the BBE effect of inducing apoptosis is related to the concentration of BBE and time of the treatment. The SGC-7901 cells presented typical apoptotic characteristics, irregular shape and DNA fragmentation.
Programmed cell death (PCD) is characterized by a sequence of tightly regulated events. Early event in PCD can be detected by Annexin V, while Late apoptotic events can be determined by flow cytometry (Clodi et al. 2000). Previous studies indicated that Annexin V and Flow cytometry could assist in the determination of the general feature of apoptosis (Martin et al. 1995; Wang et al. 2006). Herein, the laser confocal fluorescence microscopy result using Annexin-V has indicated the apoptosis of SGC-7901 cells treated by BBE. The Flow cytometry distinguished early apoptotic and late apoptotic cells and further demonstrated that BBE prominently induced apoptosis in SGC-7901 cell.
To further understanding the molecular mechanism of apoptosis of SGC-7901 cells induced by the BBE, we studied the expression levels of Bcl-2 and Bax in cells. It is well confirmed that cell apoptosis is a complex biological process which involved many pathways (Chen et al. 2002; Twomey and McCarthy 2005). Bcl-2-family proteins play an important role in regulation all major types of cell death including apoptosis (Yip and Reed 2008; Zha and Reed 1997). The proteins family BCL-2 are the regulators of apoptosis (Hardwick and Soane 2013). In mammalian cells, 15 Bcl-2 family members have been identified so far. They function as either anti-apoptotic Bcl-2 or pro-apoptotic Bax regulators (Saeedi Borujeni et al. 2016). The ratio between Bcl-2 and Bax plays a more important role than the quantity of Bcl-2 alone (Heimlich et al. 2004; Pettersson et al. 2002). Previous studies revealed a negative correlation between the ratio of Bax/Bcl-2 and the size of the tumor suggesting a role of this ratio in the control of tumor growth, and showed that the ratio of intracellular Bax/Bcl-2 is elevated in case of increased cell death. One possible mechanism for the BBE-induced apoptosis was suggested: gene expression was regulated by upregulating Bax and downregulating Bcl-2 (Wu et al. 2015; Chen et al. 2002). The results indicated that the expression of Bax protein increased significantly while that of Bcl-2 protein remarkably decreased in SGC-7901 cells treated with BBE. It is possible that BBE induced apoptosis on SGC-7901 cell by up-regulating the expression of Bax/Bcl-2 at mRNA and protein levels.
The cyclin-dependent kinase (CDK) inhibitor P21 that is activated due to different stress stimuli could act as cell cycle suppressor. P21 can bind and inhibit CDK/cyclin complexes to mediate the arrest of the growth in G1 and G2 phases. This situation enables DNA repair and suggests that p21 could have a role of tumor suppressor (Crispi and Piccolo 2012). In some human cancers, the expression of p21 suggesting that p21 acts as a tumor suppressor (Gartel 2006). As an inhibitor of proliferation, p21 plays an important role in preventing the development of tumors (Tyner and Gartel 2002). The present study showed that the expression level of P21 was significantly increased at both mRNA and protein levels after treatment with BBE. Cell cycle analysis showed that BBE could inhibit cell proliferation and promote cell cycle G1/G2 phase arrest of SGC7901 cells. We propose that the up-regulated expression of P21 may maintain more cells in G1/G2 phase, therefore influencing the proliferation of SGC-7901. However, the specific mechanism needs further investigation.
In conclusion, our results illustrate that BBE induced apoptosis in human gastric cell line SGC-7901 by up-regulation the expression of Bax/P21 and down-regulating that of Bcl-2. A correlation between gene regulation and BBE induced apoptosis was demonstrated. This study suggests that BBE may exhibit a potential role in gastric cancer therapy.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 31270691, 31170609).
Author contributions
DW conceived and designed the experiments; JYW, AS HM and TL performed the experiments; YQZ, YLZ and TL analyzed the data; DW contributed reagents/materials/analysis tools; JYW, DW and AS wrote the paper.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Footnotes
Jin-yi Wu and Almutamad Sheikho have contributed equally to this work.
References
- Battle TE, Arbiser J, Frank DA. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood. 2005;106:690–697. doi: 10.1182/blood-2004-11-4273. [DOI] [PubMed] [Google Scholar]
- Cao S, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R, Rasamison VE, TenDyke K, Suh T, Kingston DG. Cytotoxic triterpenoid saponins of Albizia gummifera from the Madagascar rain forest. J Nat Prod. 2007;70:361–366. doi: 10.1021/np060506g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GG, Lai BSP, Hu X, Lam KYI, Chak CWE, Chun SY, Lau YW. Negative correlation between the ratio of Bax to Bcl-2 and the size of tumor treated by culture supernatants from Kupffer cells. Clin Exp Metastasis. 2002;19:457–464. doi: 10.1023/A:1016336724463. [DOI] [PubMed] [Google Scholar]
- Clodi K, Kliche KO, Zhao S, Weidner D, Schenk T, Consoli U, Jiang S, Snell V, Andreeff M. Cell-surface exposure of phosphatidylserine correlates with the stage of fludarabine-induced apoptosis in chronic lymphocytic leukemia and expression of apoptosis-regulating genes. Cytometry. 2000;40:19–25. doi: 10.1002/(SICI)1097-0320(20000501)40:1<19::AID-CYTO3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Crispi S, Piccolo TM. The dual role played by p21 may influence the apoptotic or anti-apoptotic fate in cancer. J Can Res Updates. 2012;1:189–202. [Google Scholar]
- Gartel AL. Is p21 an oncogene? Mol Cancer Ther. 2006;5:1385–1386. doi: 10.1158/1535-7163.MCT-06-0163. [DOI] [PubMed] [Google Scholar]
- Hardwick MJ, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5:2. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimlich G, McKinnon AD, Bernardo K, Brdiczka D, Reed JC, Kain R, Kronke M, Jurgensmeier JM. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem J. 2004;378:247–255. doi: 10.1042/bj20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ahn NS, Yang X, Lee YS, Kang KS. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Cancer. 2002;102:250–253. doi: 10.1002/ijc.10707. [DOI] [PubMed] [Google Scholar]
- Jiang J, Slivova V, Valachovicova T, Harvey K, Sliva D. Ganoderma lucidum inhibits proliferation and induces apoptosis in human prostate cancer cells PC-3. Int J Oncol. 2004;24:1093–1099. [PubMed] [Google Scholar]
- Jiang X, Zhang Z, Chen Y, Cui Z, Shi L. Structural elucidation and in vitro antitumor activity of a novel oligosaccharide from Bombyx batryticatus. Carbohydr Polym. 2014;103:434–441. doi: 10.1016/j.carbpol.2013.12.039. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kang SA, Hong MS, Park HJ, Kim MJ, Park HJ, Kim HK. Coptidis rhizoma induces apoptosis in human colorectal cancer cells SNU-C4. Am J Chin Med. 2004;32:873–882. doi: 10.1142/S0192415X0400248X. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Muller CI, Desmond JC, Imai Y, Heber D, Koeffler HP. Scutellaria baicalensis a herbal medicine: anti-proliferative and apoptotic activity against acute lymphocytic leukemia lymphoma and myeloma cell lines. Leuk Res. 2007;31:523–530. doi: 10.1016/j.leukres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Li W, Chan SW, Guo DJ, Chung MK, Leung TY, Yu PH. Water extract of Rheum officinale Baill induces apoptosis in human lung adenocarcinoma A549 and human breast cancer MCF-7 cell lines. J Ethnopharmacol. 2009;124:251–256. doi: 10.1016/j.jep.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Lu QY, Sartippour MR, Brooks MN, Zhang Q, Hardy M, Go VL, Li FP, Heber D. Ganoderma lucidum spore extract inhibits endothelial and breast cancer cells in vitro. Oncol Rep. 2004;12:659–662. [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555–561. doi: 10.1038/sj.bjc.6600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi Borujeni MJ, Hami J, Haghir H, Rastin M, Sazegar G. Evaluation of Bax and Bcl-2 proteins expression in the rat hippocampus due to childhood febrile seizure. Iran J Child Neurol. 2016;10:53–60. [PMC free article] [PubMed] [Google Scholar]
- Twomey C, McCarthy JV. Pathways of apoptosis and importance in development. J Cell Mol Med. 2005;9:345–359. doi: 10.1111/j.1582-4934.2005.tb00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner AL, Gartel AL. Review: the role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- Wang W, Guo Q, You Q, Zhang K, Yang Y, Yu J, Liu W, Zhao L, Gu H, Hu Y, Tan Z, Wang X. Involvement of bax/bcl-2 in wogonin-induced apoptosis of human hepatoma cell line SMMC-7721. Anticancer Drugs. 2006;17:797–805. doi: 10.1097/01.cad.0000217431.64118.3f. [DOI] [PubMed] [Google Scholar]
- Wu WP, Cao J, Wu JY, Chen H, Wang D. Anticancer activity of Bombyx batryticatus ethanol extract against the human tumor cell line hela. Genet Mol Res. 2015;14:79–88. doi: 10.4238/2015.January.15.10. [DOI] [PubMed] [Google Scholar]
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- Zha H, Reed CJ. Heterodimerization-independent functions of cell death regulatory proteins Bax and Bcl-2 in yeast and mammalian cells. J Biol Chem. 1997;272:31482–31488. doi: 10.1074/jbc.272.50.31482. [DOI] [PubMed] [Google Scholar]