Abstract
Differentiation of human pluripotent stem cells into cardiomyocytes (hPS-CMs) holds promise for myocardial regeneration therapies, drug discovery, and models of cardiac disease. Potential cardiotoxicities may affect hPS-CM mechanical contraction independent of calcium signaling. Herein, a method using an image capture system is described to measure hPS-CM contractility and intracellular calcium concurrently, with high spatial and temporal resolution. The image capture system rapidly alternates between brightfield and epifluorescent illumination of contracting cells. Mechanical contraction is quantified by a speckle tracking algorithm applied to brightfield image pairs, whereas calcium transients are measured by a fluorescent calcium reporter. This technique captured changes in contractile strain, calcium transients, and beat frequency of hPS-CMs over 21 days in culture, as well as acute responses to isoproterenol and Cytochalasin D. The technique described above can be applied without the need to alter the culture platform, allowing for determination of hPS-CM behavior over weeks in culture for drug discovery and myocardial regeneration applications.
Keywords: : high-speed imaging, pluripotent stem cell-derived cardiomyocytes, drug testing, calcium transients, mechanical contraction
Introduction
Investigation within the past decade has yielded the ability to reprogram somatic cells into induced pluripotent stem (iPS) cells.1,2 iPS cells can be differentiated into a multitude of cellular phenotypes, while maintaining the original genetic blueprint from the donor.3 Maintaining the donor's genetic information within these reprogrammable cells allows investigators to model disease states,4 many of which focus on cardiac specific pathologies.5 Generation of cardiomyocytes from iPS cells and embryonic stem cells1,6–8 presents great promise for the generation of patient-specific cardiac disease models, myocardial regeneration therapies, and drug discovery.9–11 However, human pluripotent stem cell-derived cardiomyocytes (hPS-CMs) are most phenotypically similar to fetal cardiomyocytes in terms of force production, adrenergic response, and sarcomere organization,12 which may not be ideal for drug development or regenerative therapies. Therefore, recent research has focused on improving the maturation of hPS-CMs to yield a more adult-like phenotype.13–15 However, there are limited tools available to effectively measure the cellular physiological response to different stimuli over time, which would significantly aid investigations seeking mature hPS-CMs and utilize them in disease modeling and regenerative therapies.
Many cardiac diseases manifest themselves as changes in cardiac tissue, which directly affect mechanical strain, electrical propagation, beat frequency, beat consistency (arrhythmia), and delays between calcium flux and initiation of mechanical contraction.16 Current techniques to monitor these changes in single cells or clusters of hPS-CMs include integrating microelectrode arrays (MEA), flexible posts, and force transducers, incorporation of fluorescent beads into the culture platform, patch-clamping techniques, and calcium-sensitive dyes.17–21 However, the incorporation of these devices into a culture platform is costly, low throughput, time-consuming, and may have secondary effects on cell performance, such as changes in beat frequency or magnitude of contraction.22,23 Thereby, the development of cost-effective and less-invasive modalities able to concurrently measure mechanical and electrical components of contraction are needed for long-term experimentation.
In this study, we describe a method to study the physiology of cardiomyocyte contractility with high spatial and temporal resolution using an image capture system. The system works by rapidly alternating between brightfield and epifluorescent illumination for each frame, to record mechanical contraction and calcium transients of contracting cardiomyocytes, respectively. A speckle tracking algorithm24 applied to the sequence of brightfield images determines the deformation of subpixel regions of cells and clusters in the field of view. From this information, the contractile strain and beat frequency can be calculated for the cell or cluster of interest that corresponds to the calcium transient frame that lies in between. As a result, the temporal relationship between the calcium transients and mechanical components leading to the contraction of a cardiomyocyte can be investigated. This technique can be applied aseptically and without the need to alter the culture platform, allowing for determination of cardiomyocyte behavior over time for use in drug discovery and functional tissues for myocardial regeneration strategies.
Materials and Methods
Generation and culture of pluripotent stem cell-derived cardiomyocytes
Human induced pluripotent (iPS-CM) and embryonic (hES-CM) stem cells were differentiated into cardiomyocytes using a previously described guided differentiation protocol involving the serial application of activin-A and bone morphogenetic protein-47,9,25 and were cryopreserved as previously reported.26 hES-CMs modified to express the genetically encoded protein calcium sensor, GCaMP, were used.27 All employed cell preparations were confirmed to contain >70% cardiac troponin T+ cardiomyocytes by flow cytometry. hPS-CM cells were thawed and seeded at a density of 150,000 cells/cm2 onto collagen IV-coated 96-well plates (10 μg/mL; Sigma). Cells were cultured at 37°C in 5% CO2 and media (RPMI-B27, Pen Strep, L-Glutamine) were changed every 2–3 days. Upon thawing, cells adhered to the coated plates within 24 h. After 3 days in culture, regions within the monolayer of cells appeared to form clusters of cells, which began to spontaneously contract. Over the next 18 days, clusters did not appear to change in size, although some of the clusters detached from the surface of the culture plate.
High-density mapping analysis of cardiomyocyte contraction
A high-speed camera (HiSpec4; Fastec, Inc.) attached to an inverted microscope (Leica, DMIL) was used to record contracting myocytes at 60 frames per second (fps). High-density mapping (HDM), a speckle tracking algorithm previously developed to measure strains on cardiac tissues24 was applied to the raw images to calculate strain fields and beat frequency (Fig. 1). Briefly, HDM divides a selected region of interest (ROI) into variable window sizes (e.g., 16 × 16 pixel windows) (Fig. 1B) and tracks displacements of each window between two sequential frames over a series of images. Displacement calculations are based on the phase correlation algorithm with subpixel accuracy first described by Foroosh et al.28 and are theoretically accurate to within 0.2 pixels.24 Briefly, a hamming window is applied to the pixel windows, which are then transformed to the Fourier domain and combined by a cross power spectrum function (Fig. 1C, D). Computation of the inverse Fourier transform of the cross power spectrum yields an impulse function marking the x and y displacement of the region between the two frames (Fig. 1D, E). This process is repeated across the entire ROI with windows overlapping in both directions by a spacing equal to half the window size (e.g., 8 pixels for a 16 × 16 window, see Fig. 1B) for each pair of frames yielding displacement fields over time. From displacement fields, regional strain tensors were computed by the Green-Lagrange definition29 using the start of contraction as a reference state. All strain fields reported herein are the maximum contractile strain (minimum E2) from the initiation of contraction to peak contraction. To isolate regions with significant contraction from noise, only regions contracting with greater than 0.5% strain were analyzed. Overlapping windows were applied to obtain a spatial map of contractile strain across the cardiomyocyte cluster for each contraction cycle. From these data, heat maps were generated to indicate the location and magnitude of contractile strain spatially within a cluster at peak contraction (Figs. 3A and 5A). From this map, the spatial average (reported as contractile strain) and maximum contractile strains were calculated across a cluster, and plotted over time (Figs. 5 and 6). Finally, to compare effects of culture duration on cardiomyocyte contractility, these spatial average and maximum strains were temporally averaged over three contraction cycles (Fig. 4). For display of strain waveforms, baseline drift (due to frame-to-frame error propagation in the HDM algorithm) was removed by subtracting a spline curve fit to the minimum (spatial average) strain just before each contraction beat. Average principal strain across the entire ROI over time was used to determine beat frequency by Fourier analysis. To determine the parameters to be used for HDM analysis, different window sizes (8 × 8, 16 × 16, and 32 × 32 pixels), each with a half window spacing, and different number of averaging windows3–9 were examined.
FIG. 1.
Use of HDM to determine displacement fields of contracting hPS-CMs. HDM was applied by acquiring a high-speed video of contracting hPS-CMs to obtain a stack of images (A) from which an ROI could be selected (B) and subdivided into 16 × 16 pixel windows. Each 16 × 16 pixel window is transformed to the Fourier domain (C) where phase correlation between frames is applied (D) and an inverse Fourier transform is taken, resulting in the peak displacement between the two frames (E). HDM, high-density mapping; hPS-CM, human pluripotent stem cell-derived cardiomyocyte; ROI, region of interest.
FIG. 3.
The effect of window size and window spacing on reported contractile strain values. The resulting strain magnitude and location was used to generate heat maps (A) with increasing average spatial contractile strain at peak contraction in red. Pixel width for boxes in (A) was 325 wide and 272 pixels in height. Using different window sizes and spacings (1/2 window size) can affect reported contractile strain values (B), area used for contractile strain calculations reported as a change from the original selected ROI (C), and computing times (D). A 16 × 16 pixel window spacing with an 8 pixel window shift and an average contractile strain calculated over five windows was used to report contractile strain (A, B). Mean ± SD.
FIG. 5.
Overlay of mechanical contraction and calcium transients. Alternating brightfield and fluorescent images of contracting induced pluripotent stem cell-derived cardiomyocytes (iPS-CMs) loaded with Fluo-4 AM on day 21 resulted in spatial heat maps for peak mechanical contraction (A) and peak calcium flux (B). The location of peak calcium intensity did not correspond with location of peak contractile strains. (A, B, white arrows) The resulting cyclic contractile strain (blue) and calcium signal (black) are presented over 8 s (C) with an isolated cycle indicated by the red box in C (E). Cells treated with Cytochalasin-D exhibit loss of mechanical contraction, while calcium transients are maintained (D).
FIG. 6.
Isoproterenol stimulation of hPS-CMs. Adding isoproterenol to hPS-CMs increased beat frequency (A, B) up to the addition of 10 μM isoproterenol, with representative traces of Fluo-4 AM loaded contracting iPS-CMs (A) and frequency data averaged in (B). Addition of isoproterenol did not affect contractile strain for any condition compared to baseline (C). Mean ± SD for frequency, mean ± SEM for contractile strain, n = 5, *p < 0.05.
FIG. 4.
HDM can be used to quantify contraction of hPS-CMs seeded on collagen IV-coated plates over 21 days. hPS-CMs significantly increased beat frequency by 1.5× between day 7 and 21 (A). A significant decrease between day 7 and 21 was observed for contractile strain (B) and maximum contractile strain (C). Mean ± SD for frequency, mean ± SEM for contractile and maximum strain, n = 6, *p < 0.05.
Brightfield and fluorescent imaging acquisition
Dual brightfield and fluorescent cardiomyocyte imaging was performed using a Zeiss AxioObserver.A1 inverted microscope with Zeiss Fluar objective lens (20 × , Numerical Aperture: 0.5) and a Hamamatsu Orca Flash 4.0 sCMOS camera with CameraLink board mounted with a 1.0 × c-Mount adapter. A digital illumination controller (Nobska Imaging) synchronized image capture with alternating pulses of either (1) epifluorescence excitation illumination from a 50 W blue LED (Mightex) coupled to the microscope by a liquid light guide or (2) brightfield illumination from a green 3 W LED (Luxeon Rebel with Spot optics; LEDsupply) mounted above the stage in place of the condenser. MicroManager software controlled image streaming parameters and illumination timing (pulse duration and delay relative to start of frame capture) through programmable TTL logic from the camera (Fig. 2A). The controller activated either the epifluorescence LED (blue), for a pulse duration as specified by the user in MicroManager, or the brightfield LED (green), for a fixed 16 μs pulse (Fig. 2B). Time-series videos were acquired in free-running streaming mode with a minimum exposure time defined by
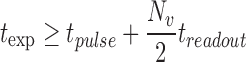 |
FIG. 2.
Image acquisition schematic with temporal control of fluorescent or brightfield illumination. Microscope schematic, a digital microscope controller for image capture of contracting cardiomyocytes using an Orca Flash 4.0 sCMOS camera with alternating pulses of fluorescent and brightfield illumination (A). Timing chart indicating sensor readout and the start of pixel exposure; when all lines are exposed, the controller initiates a pulse of fluorescent (blue) or brightfield (green) illumination (B). The cycle is started over after the total exposure time (7 ms) with alternating brightfield and fluorescent illuminations.
where tpulse is the excitation pulse duration, Nv is the vertical images size (pixels), and treadout is the readout time per vertical line = 9.744 μs. For a typical 2048 (h) × 1024 (v) pixel image, the readout time is ∼5 ms, limiting total frame rate to ∼200 fps and rate of brightfield/fluorescent frame pairs to ∼100 fps. Reducing vertical pixel number proportionally increases frame rate, with 1000 fps possible with a narrowed vertical image size (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tec). Image stacks were de-interlaced using ImageJ (Supplementary Video S1).
Loading iPS-CMs with Fluo-4 AM
Fluo-4 AM dye (Invitrogen) was loaded into iPS-CMs for calcium transient analysis. Cells were incubated with Fluo-4 AM (5 μM dissolved in Pluronic F-127 (20% solution in DMSO; Invitrogen) in RPMI-B27 medium) for 10 min at 37°C. Before recordings were obtained, fresh prewarmed RPMI-B27 medium was added and cells were allowed to sit for 20 min to allow the dye to de-esterify inside the cells. All fluorescence imaging of calcium transients were recorded at 71 fps within 2 h of dye addition. After recording, cells were rinsed in the RPMI-B27 medium and returned to the incubator for continued culture in the RPMI-B27 medium. To assess if the calcium signal occurred independent of mechanical contraction, cells were treated with 10 μM Cytochalasin-D (Sigma) for 10 min before imaging.
Calcium transient analysis
Intracellular calcium levels were quantified using a custom MATLAB code that determined average fluorescent intensity change with respect to baseline intensity values across a selected ROI and reported as ΔF/F0. F0, the baseline fluorescent value, was defined as the minimum intensity value in the region, while ΔF is the average change in intensity for the selected region at each given time point. A deconvolution algorithm was implemented in MATLAB (“deconv” function with exponential decay kernel) to correct for the dynamics of the fluorescent calcium reporters, using time constants of 4 ms for the Fluo-4 AM indicator30 and 50 ms for GCaMP (representing the “on”-kinetics).31
Infusion of chemical stimulation by isoproterenol
Isoproterenol hydrochloride (Sigma) was used to examine the cardiomyocyte response to an adrenergic stimulant. Baseline recordings were taken with cells cultured in the normal medium; the medium was then removed and a recording was obtained with cells incubated in the medium supplemented with isoproterenol at 0.1, 1, and 10 μM. The same contracting region was analyzed for each increasing drug addition with a total of five regions analyzed.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). Comparisons for contractile parameters of hPS-CMs between time points were done using a one-way analysis of variance with a Tukey post-hoc analysis for multiple comparisons. All measurements were averaged over the entire selected region and then averaged over three contraction cycles. All data are reported as mean ± SEM, with the exception of strain data (Fig. 3B), and frequency data (Figs. 4A and 6B) are reported as mean ± SD. Significance was considered at p < 0.05.
Results
Effect of spatial resolution on determining contraction in hPS-CMs
Using the dual image acquisition system, high-speed videos were captured of regions of beating hPS-CMs at 142 fps (71 fps). A computational algorithm based on Fourier transforms (HDM) was applied to the recorded image sequences to determine contractile strain. Different window sizes (8–32 pixels), window spacing (half of window size), and number of windows (three to nine; used to obtain an averaged contractile strain) were examined to determine how these parameters affected contractile strain quantification across a cluster of contracting hPS-CMs (Fig. 3). The smallest window size (8 × 8 pixels) gave the widest range of contractile strains (4.5%–11.3%) and highest standard deviation using three or five averaging windows (Fig. 3B), and the finest spatial resolution and largest strain map (Fig. 3C), but also the longest computation time (Fig. 3D). The largest window size (32 × 32 pixels) resulted in a coarser and smaller map of the strain distribution due to overlapping window regions, showed no difference in the contractile strain output when averaged over a range of windows, and was computed five times faster than an 8 × 8 window for the same region. Overall, the number of windows used to calculate strain did not have a significant effect on computational time. A 16 × 16 pixel window with an 8 pixel window spacing and 5 window averaging was chosen for further applications of HDM, based on its narrower range of contractile strain values (4.5–7.6%), subcellular spatial resolution, and reasonable computation time. In addition, spatial distribution of strain for four independent contracting clusters was examined and found to follow a normal distribution (Supplementary Fig. S1).
Contractile mechanics of hPS-CMs can be monitored over 21 days
To test the ability of the HDM algorithm to evaluate cardiac contractile mechanics over time, hPS-CMs were cultured on collagen type IV-coated plates and high-speed videos of contracting hPS-CMs were taken on days 7, 14, and 21. Three clusters of contracting cells within two wells were analyzed for contraction over three contraction cycles per cluster (n = 6). HDM was applied to the cluster using a window size of 16 × 16 pixels, an 8 pixel window spacing, and 5 averaging windows. Beat frequency, contractile strain, and maximum strain were computed for each time point. hPS-CMs showed an increased beating frequency over time, where cells exhibited a significantly higher (p < 0.001) beat frequency on day 21 (1.52 ± 0.09 Hz) when compared to day 7 (1.06 ± 0.09 Hz; Fig. 4A). Cells seeded on collagen IV-coated plates had a contractile strain of 4.5 ± 0.4% on day 7, which significantly decreased (p < 0.05) to 2.9% ± 0.3% strain on day 21 (Fig. 4B). Maximum contractile strain calculated on day 7 for hPS-CMs was found to be 19.9% ± 1.4%, which decreased significantly (p < 0.001) to 10.8% ± 1.5% strain on day 21 (Fig. 4C). These results indicate the ability of the HDM algorithm to detect and quantify changes in cardiomyocyte contractility over 21 days.
Measurements of calcium transients and mechanical contraction in hPS-CMs
iPS-CM calcium transients were visualized by loading the cells with 5 μM Fluo-4 AM, a calcium indicator dye that changes fluorescence in proportion to intracellular free calcium. In addition, calcium transients were observed in ES-derived cardiomyocytes (ES-CMs) differentiated from a reporter line expressing the genetically encoded calcium indicator GCaMP3.9 The same image acquisition system was able to detect calcium changes over the contractile cycle by Fluo-4 AM in iPS-CMs (Fig. 5) and GCaMP in ES-CMs (Supplementary Fig. S2). A 1 ms fluorescent light pulse with 2 × 2 binning allowed for recordings at a frame rate of 142 fps (71 fps for each channel, brightfield, and fluorescent), spatial resolution of 0.65 μm/pixel, and field of view of 1024 × 512 pixels or 665 × 333 μm (Supplementary Table S1). These settings yielded a calcium signal-to-noise ratio of 21 dB as determined using MATLAB. Fluorescent traces were deconvolved to remove the effects of slow optical calcium sensor dynamics, using the “on”-kinetics of the Fluo-4 and GCaMP sensors, whereas no difference was seen before and after deconvolution for the faster Fluo-4 sensor (data not shown), the deconvolved GCaMP trace rose faster than the original trace; however, both traces began at the same time (Supplementary Fig. S3).
Brightfield and fluorescent images were alternatively acquired to examine the spatial and temporal relationships between mechanical contraction and calcium transients of contracting hPS-CMs. Regions of peak contractile strain (Fig. 5A) and peak calcium intensity change (Fig. 5B) during the contractile beat cycle were not well correlated across the iPS-CM cluster. The mechanical contraction and calcium transient tracings were plotted together to examine their temporal relationship (Fig. 5C). Calcium levels rose before the onset of mechanical contraction for Fluo-4 AM-loaded iPS-CMs (Fig. 5E) and GCaMP-expressing ES-CMs (Supplementary Fig. S2C), following expectations for cardiomyocyte physiology. Fluo-4 calcium traces peaked before peak strain in iPS-CMs, whereas GCaMP calcium traces peaked after peak ES-CM strain, reflecting differences in calcium sensor or cardiomyocyte dynamics. Cells treated with Cytochalasin-D, which eliminate mechanical function by inhibiting actin polymerization, retained similar calcium signal cycles (Fig. 5D), demonstrating that the calcium signal was due to calcium transients and not mechanical interferences.
Isoproterenol simulation increases beat frequency
Adrenergic stimulation (isoproterenol) was applied to Fluo-4 loaded-iPS-CMs to evaluate the ability of the algorithm to detect simultaneous changes in contractility in terms of contractile strain, beat frequency, and calcium transients. Isoproterenol, a β-adrenergic agonist, was chosen as it has been shown to increase beat frequency for adult cardiomyocytes.32 Isoproterenol was applied at concentrations of 0.1, 1, and 10 μM with recordings taken immediately after each addition (n = 5). Beat frequency was found to be 0.66 ± 0.13 Hz with cells cultured in RPMI-B27 media without isoproterenol (Fig. 6A, B). The addition of isoproterenol at concentrations up to 10 μM significantly increased beat frequency to 0.88 ± 0.09 Hz (Fig. 6A, B, p < 0.05). Contractile strain began at 3.1% ± 0.45% with RPMI-B27 media (Fig. 6C). The addition of isoproterenol did not significantly change strain levels as all strain levels for stimulated cells were between 3.2–3.6% (Fig. 6C, p > 0.05). These results indicate that this system is capable of detecting changes in the contractile properties of cells when isoproterenol is applied and could be used for drug screening studies.
Discussion
Recent advances in stem cell technology have yielded high purity (>90%) populations of contracting cardiomyocytes derived from both ES and iPS cells.7,8 These cardiomyocytes have the potential to be used in a wide variety of applications, including myocardial regeneration therapies, disease modeling, drug screening, and development. To fulfill the potential of these applications, cost-effective, high-throughput techniques to concurrently monitor contractile and calcium transient changes in single cells or clusters of hPS-CMs are needed. By concurrently monitoring the relationship between contractile and ionic changes over time, physiological relationships can be better understood, which is valuable for applications such as drug screening for off-target changes in cardiomyocyte contractility.
Current techniques to monitor cardiomyocyte electrophysiological function include the use of MEA, patch clamping, and voltage- or ion-sensitive fluorescent dyes. MEA give measurements of beat frequency and field potential, but require cells to be cultured on a specific surface connected to the appropriate data acquisition system.33 Patch clamping records action potential and ion channel information of single cells, and is low-throughput, time-consuming, and requires expertise.34 Voltage and ionic dyes, such as di-8-ANEPPS and Fluo-4 AM, or their genetically encoded counterparts, such as ArcLight and GCaMP, are good for noninvasive measurements of cardiomyocyte electrophysiology or intracellular calcium concentrations at the single-cell level or in multicellular preparations.35,36
Contractile force can be evaluated using force transducers or traction force microscopy (TFM).37–39 Both these techniques require known substrate mechanical properties and necessitate changes to the culture platform that may have secondary effects on cell function.22,23 Force transducers are best suited for measurements on the macrotissue scale, which is not appropriate for studies investigating cellular contractility. TFM can measure contractility on the cellular level, but requires cells to be cultured on a substrate with incorporated microbeads. Bead displacement is used to quantify cell contraction; however, microbead incorporation can severely limit measurements by having random bead distributions and densities that might not accurately depict cellular contractility.
Optical methods can be used to overcome the challenges of integrative culture platforms for monitoring cardiomyocyte function. Current image processing techniques focus on edge detection techniques, such as with laser diffraction and microscopic cell images, to obtain information about cell contractility.40,41 However, these methods are limited to the spatial resolution of the imaging systems, as well as to changes in cardiomyocyte contraction from unexpected movement, asynchronous contraction, or cardiomyocyte rotation, which would result in an inaccurate contractile output. Imaging methods that focus on changes in sarcomere striations42 are limited to single isolated cardiomyocytes with well-defined striated patterns. However, hPS-CMs are comparatively immature,43 exhibit less defined sarcomere patterns, and are cultured in monolayers instead of single cells; thus, these imaging techniques may not be sufficient to measure contractile mechanics of hPS-CMs.
More recent techniques to monitor contraction apply an optical flow algorithm with macroblocks to estimate motion of a set of images of contracting cardiomyocytes,22 or particle image velocimetry, a cross-correlation procedure to determine displacement fields of cardiomyocytes visualized using video microscopy.23 Both methods quantify beating speed, beating velocity, and beat pattern, but not the magnitude of contraction. Neither technique gives quantitative information about cardiomyocyte contraction combined with information about calcium signaling. While calcium signaling alone has been shown to predict in vitro cardiotoxicity,19 the combination of calcium signaling and mechanical contraction provides a more robust method to detect cardiotoxicities that may affect mechanical contraction apart from calcium signaling.
In this study, we describe a technique for concurrent monitoring of mechanical contraction and calcium transients of hPS-CMs over several weeks using a system that alternates high-speed imaging of brightfield and fluorescence. The system allows frames to be captured at >60 fps over a 1024 × 512 pixel ROI or up to 1000 fps by decreasing the ROI to 96 vertical pixels. Our results demonstrate the ability to characterize mechanical contraction by applying HDM, a speckle tracking algorithm, to brightfield images to calculate beat frequency as well as quantitative information about the magnitude of contraction (contractile strain). Calcium transients were calculated by analyzing the acquired fluorescent signals from Fluo-4 AM or GCaMP calcium reporters. Calcium transients and contractile strain waveforms were plotted together; for Fluo-4 AM-loaded iPS-CMs, the calcium waveform was initiated and peaked before strain, as expected for cardiomyocyte physiology.44 For GCaMP ES-CMs, the calcium signal began before the strain signal, but the calcium signal peaked after strain began to decline, highlighting the differences between the cardiomyocyte sources and kinetics of the calcium reporters.36,45 Deconvolution was performed on both Fluo-4 and GCaMP fluorescent traces to remove any sensor-induced delays. At the frame rates used (14 ms interval), deconvolution had little effect on Fluo-4 traces (time constant 4 ms), but resulted in GCaMP traces that rose faster than the original traces (“on” time constant 50 ms). However, “off” kinetics of GCaMP sensors are often much slower than “on” kinetics.31 Deconvolution algorithms that address different on- and off-kinetics can better correct for these effects.
Due to the aseptic nature of this analysis technique, recordings were obtained for several time points over a period of 21 days. Mechanical contraction of hPS-CM clusters was quantified using HDM in terms of beat frequency, contractile strain, and maximum contractile strain. Over 21 days, iPS-CMs exhibited a significant increase in beat frequency, similar to reports from other groups,46,47 accompanied by a significant decrease in contractile strain. Isoproterenol stimulation demonstrated the system's ability to capture chemically induced changes in mechanical contraction, which will be valuable for drug screening studies. With the addition of isoproterenol, we demonstrated a significant increase in beat frequency along a similar order reported by others,14,20,48 but no significant changes in contractile strain. While isoproterenol has been shown to increase twitch forces in mature adult cardiomyocytes, predicting an increase in contractile strain levels, studies have shown that immature cardiomyocytes, such as the hPS-CMs used in this study, have different responses to drug additions than adult cardiomyocytes. For example, immature hPS-CMs produce less force in response to isoproterenol compared with adult cardiomyocytes;49 this may describe why no significant change in strain was demonstrated with isoproterenol addition.
Another application of this system could be to measure functional differentiation of cultures over time. Although cardiac differentiation is a well-established technique, purity remains a major issue. Our system may be able to elucidate changes due to differentiation protocols or batch variability. In a computational model of fibroblasts and cardiomyocytes, Zhan et al. demonstrated changes in deformation resulting from changes in the ratio of fibroblasts to cardiomyocytes.50 A lower purity of cardiomyocytes may result in decreased contraction. In addition, the whole-field property of our system could be used to determine if regions of cells are not contracting under whole-field stimulation, suggesting undifferentiated or dysfunctional cells. Together, this information may continuously inform investigators on the functional differentiation of their cultures.
The system developed in this study allows brightfield and fluorescent images to be acquired in rapid succession, for concurrent measurements of strain and intracellular calcium. Strain data are generated by analyzing the displacement between two brightfield images, which span each fluorescent image used to quantify calcium levels. Thus, the calcium information is obtained temporally at the midpoint of strain information, and the temporal relationship between calcium activity and the corresponding mechanical contraction can be obtained by overlaying the two waveforms. In addition, by alternating image acquisition, this system avoids the expense and alignment required by dual-view or dual-camera setups, which spectrally split fluorescence (blue-green) and brightfield (red or infrared), but capture both images simultaneously. Another advantage of this system is that as it uses a 96-well format, with the appropriate alterations, the system could be automated to provide for real-time information, which would be ideal for high-throughput screening.
Different window sizes and spacings were used to compute contractile strain to determine if the calculated strain reached an optimum point, suggesting a minimum number of pixels needed for calculating strain. A 16 × 16 pixel window with an 8 pixel spacing averaged over five windows was used for strain calculations as these parameters maintained spatial information with less strain variance and decreased computation time. By averaging over five windows, strain calculations are likely not overestimated, as regions of strain artifacts are removed, or underestimated, as using five windows maintains spatial information. The 8 × 8 pixel window with a 4 pixel spacing maintained the most spatial information, but had the highest computation time and the largest degree of strain variance when fewer averaging windows were used (3 and 5) due to the small regions being analyzed. A 32 × 32 pixel window with a 16 pixel spacing had the fastest computation time; however, spatial information was lost due to large regions being analyzed. One of the advantages of this method is spatial resolution, as the smaller pixel window sizes allow the regions analyzed to be the size of a cell. The larger spacing results in displacement being determined in fewer locations, while the larger window size may miss inhomogeneous deformation that may occur within the window. A key benefit to this technique is that HDM enables different combinations of window sizes, window spacings, and number of windows averaged, which can be tailored to the desired application.
The use of HDM provides high temporal resolution of quantitative information about cardiomyocyte contraction at a subpixel resolution. In this study, this technique was demonstrated at a cellular level, but can also be applied at a tissue level as demonstrated on hPS-CM-seeded myocardium fibers.51 As this technique can be easily applied to high-speed videos, it is a powerful tool to examine cardiomyocyte contractility over time. This technique can be used to obtain information about how different drug compounds affect cardiomyocyte contractility, both mechanically and at the intracellular calcium level, which would enhance the quality of current drug testing systems. Herein, we demonstrated the ability of the system to determine increased contractile frequencies with the administration of isoproterenol. In addition, for hPS-CM potential to be realized for cell replacement strategies, hPS-CMs need to exhibit a more adult-like phenotype to couple and contract with human myocardium. The use of this optical system to monitor contraction as an indicator for maturity, for example through adrenergic stimulation, would provide a convenient and economic solution to examine cell maturation without having to sacrifice cells for use in terminal analysis techniques.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants RO1-HL115282 (G.R.G.), P01-HL094374, and RO1-HL117991 (M.A.L.) and National Science Foundation grants DGE 1144804 (K.J.H., J.R.G., G.R.G., and D.R.A.) and CBET 1605679 (D.R.A.). D.R.A. is supported by a Burroughs Wellcome Career Award at the Scientific Interface.
Author Contributions
K.J.H. designed the study, collected, analyzed, and interpreted data, and wrote the article. J.T.F. wrote the MATLAB codes for HDM and for calcium transient analysis, and contributed to article writing. J.R.G. contributed to study design and article editing. M.A.L. provided the hPS-CMs and contributed to study design, data interpretation, and article editing. D.R.A. built the microscope and illumination controller, provided study materials, and contributed to data interpretation and article writing. G.R.G. contributed to design study, data interpretation, article writing, and provided financial support. All authors read and approved the final article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Takahashi K., and Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., and Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bellin M., Marchetto M.C., Gage F.H., and Mummery C.L. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol 13, 713, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Soldner F., and Jaenisch R. iPSC disease modeling. Science 338, 1155, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Moretti A., Bellin M., Welling A., Jung C.B., Lam J.T., Bott-Flügel L., Dorn T., Goedel A., Höhnke C., Hofmann F., Seyfarth M., Sinnecker D., Schömig A., and Laugwitz K.-L. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 363, 1397, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Thomson J.A. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S., O'Sullivan C., Collins L., Chen Y., Minami E., Gill E.A., Ueno S., Yuan C., Gold J., and Murry C.E. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25, 1015, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Lian X., Hsiao C., Wilson G., Zhu K., Hazeltine L.B., Azarin S.M., Raval K.K., Zhang J., Kamp T.J., and Palecek S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A 109, E1848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiba Y., Fernandes S., Zhu W.Z., Filice D., Muskheli V., Kim J., Palpant N.J., Gantz J., Moyes K.W., Reinecke H., Van Biber B., Dardas T., Mignone J.L., Izawa A., Hanna R., Viswanathan M., Gold J.D., Kotlikoff M.I., Sarvazyan N., Kay M.W., Murry C.E., and Laflamme M.A. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen A., Eder A., Bonstrup M., Flato M., Mewe M., Schaaf S., Aksehirlioglu B., Schwoerer A.P., Uebeler J., and Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ Res 107, 35, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Godier-Furnemont A.F., Tiburcy M., Wagner E., Dewenter M., Lammle S., El-Armouche A., Lehnart S.E., Vunjak-Novakovic G., and Zimmermann W.H. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials 60, 82, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snir M., Kehat I., Gepstein A., Coleman R., Itskovitz-Eldor J., Livne E., and Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol 285, H2355, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Lundy S.D., Zhu W.Z., Regnier M., and Laflamme M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 22, 1991, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D., Shadrin I.Y., Lam J., Xian H.Q., Snodgrass H.R., and Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34, 5813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thavandiran N., Dubois N., Mikryukov A., Masse S., Beca B., Simmons C.A., Deshpande V.S., McGarry J.P., Chen C.S., Nanthakumar K., Keller G.M., Radisic M., and Zandstra P.W. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci U S A 49, E4698, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan F., Lee A.S., Liang P., Sanchez-Freire V., Nguyen P.K., Wang L., Han L., Yen M., Wang Y., Sun N., Abilez O.J., Hu S., Ebert A.D., Navarrete E.G., Simmons C.S., Wheeler M., Pruitt B., Lewis R., Yamaguchi Y., Ashley E.A., Bers D.M., Robbins R.C., Longaker M.T., and Wu J.C. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 12, 101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunes S.S., Miklas J.W., Liu J., Aschar-Sobbi R., Xiao Y., Zhang B., Jiang J., Masse S., Gagliardi M., Hsieh A., Thavandiran N., Laflamme M.L., Nanthakumar K., Gross G.J., Backx P.H., Keller G., and Radisic M. Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat Methods 10, 781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asakura K., Hayashi S., Ojima A., Taniguchi T., Miyamoto N., Nakamori C., Nagasawa C., Kitamura T., Osada T., Honda Y., Kasai C., Ando H., Kanda Y., Sekino Y., and Sawada K. Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. J Pharmacol Toxicol Methods 75, 17, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Sirenko O., Cromwell E.F., Crittenden C., Wignall J.A., Wright F.A., and Rusyn I. Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol Appl Pharmacol 273, 500, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bielawski K.S., Leonard A., Bhandari S., Murry C.E., and Sniadecki N. Real-time force and frequency analysis of engineered human heart tissue derived from induced pluripotent stem cells using magnetic sensing. Tissue Eng Part C Methods 22, 932, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grespan E., Martewicz S., Serena E., Le Houérou V., Rühe J., and Elvassore N. Analysis of calcium transients and uni-axial contraction force in single human embryonic stem cell-derived cardiomyocytes on microstructured elastic substrate with spatially controlled surface chemistries. Langmuir 32, 12190, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Huebsch N., Loskill P., Mandegar M.A., Marks N.C., Sheehan A.S., Ma Z., Mathur A., Nguyen T.N., Yoo J.C., Judge L.M., Spencer C.I., Chukka A.C., Russell C.R., So P.L., Conklin B.R., and Healy K.E. Automated video-based analysis of contractility and calcium flux in human-induced pluripotent stem cell-derived cardiomyocytes cultured over different spatial scales. Tissue Eng Part C Methods 21, 467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajasingh S., Thangavel J., Czirok A., Samanta S., Roby K.F., Dawn B., and Rajasingh J. Generation of functional cardiomyocytes from efficiently generated human iPSCs and a novel method of measuring contractility. PLoS One 10, e0134093, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly D.J., Azeloglu E.U., Kochupura P.V., Sharma G.S., and Gaudette G.R. Accuracy and reproducibility of a subpixel extended phase correlation method to determine micron level displacements in the heart. Med Eng Phys 29, 154, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Zhu W.Z., Van Biber B., and Laflamme M.A. Methods for the derivation and use of cardiomyocytes from human pluripotent stem cells. Methods Mol Biol 767, 419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C., Police S., Hassanipour M., Li Y., Chen Y., Priest C., O'Sullivan C., Laflamme M.A., Zhu W.Z., Van Biber B., Hegerova L., Yang J., Delavan-Boorsma K., Davies A., Lebkowski J., and Gold J.D. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med 6, 53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W.-Z., Filice D., Palpant N.J., and Laflamme M.A. Methods for assessing the electromechanical integration of human pluripotent stem cell-derived cardiomyocyte grafts. Methods Mol Biol 1181, 229, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foroosh H., Zerubia J.B., and Berthod M. Extension of phase correlation to subpixel registration. IEEE Trans Image Process 11, 188, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Fung Y.C. A First Course in Continuum Mechanics, 3rd edition. Englewood Cliffs, NJ: Prentice Hall, 1993 [Google Scholar]
- 30.Zahradníková A., Poláková E., Zahradník I., and Zahradníková A. Kinetics of calcium spikes in rat cardiac myocytes. J Physiol 578, 677, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helassa N., Zhang X.-H., Conte I., Scaringi J., Esposito E., Bradley J., Carter T., Ogden D., Morad M., and Török K. Fast-response calmodulin-based fluorescent indicators reveal rapid intracellular calcium dynamics. Sci Rep 5, 15978, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endoh M. The effects of various drugs on the myocardial inotropic response. Gen Pharmacol Vasc Syst 26, 1, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Navarrete E.G., Liang P., Lan F., Sanchez-Freire V., Simmons C., Gong T., Sharma A., Burridge P.W., Patlolla B., Lee A.S., Wu H., Beygui R.E., Wu S.M., Robbins R.C., Bers D.M., and Wu J.C. Screening drug-induced arrhythmia using human induced pluripotent stem cell–derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 128, S3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda M., Kiyokawa J., Tabo M., and Inoue T. Electrophysiological characterization of cardiomyocytes derived from human induced pluripotent stem cells. J Pharmacol Sci 117, 149, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Herron T.J., Lee P., and Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res 110, 609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guatimosim S., Guatimosim C., and Song L.-S. Imaging calcium sparks in cardiac myocytes. Methods Mol Biol 689, 205, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann W.H., Fink C., Kralisch D., Remmers U., Weil J., and Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng 68, 106, 2000 [PubMed] [Google Scholar]
- 38.Jacot J.G., McCulloch A.D., and Omens J.H. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95, 3479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engler A.J., Carag-Krieger C., Johnson C.P., Raab M., Tang H.Y., Speicher D.W., Sanger J.W., Sanger J.M., and Discher D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121, 3794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delbridge L.M.D., and Roos K.P. Optical Methods to Evaluate the contractile function of unloaded isolated cardiac myocytes. J Mol Cell Cardiol 29, 11, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Bazan C., Barba D.T., Blomgren P., and Paolini P. Image processing techniques for assessing contractility in isolated adult cardiac myocytes. Int J Biomed Imaging 2009, 352954, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bub G., Camelliti P., Bollensdorff C., Stuckey D.J., Picton G., Burton R.A.B., Clarke K., and Kohl P. Measurement and analysis of sarcomere length in rat cardiomyocytes in situ and in vitro. Am J Physiol Heart Circ Physiol 298, H1616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X., Pabon L., and Murry C.E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 114, 511, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bers D.M. Cardiac excitation-contraction coupling. Nature 415, 198, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Tallini Y.N., Ohkura M., Choi B.-R., Ji G., Imoto K., Doran R., Lee J., Plan P., Wilson J., Xin H.-B., Sanbe A., Gulick J., Mathai J., Robbins J., Salama G., Nakai J., and Kotlikoff M.I. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A 103, 4753, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hescheler J., Halbach M., Egert U., Lu Z.J., Bohlen H., Fleischmann B.K., and Reppel M. Determination of electrical properties of ES cell-derived cardiomyocytes using MEAs. J Electrocardiol 37, Supplement, 110, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Eng G., Lee B.W., Protas L., Gagliardi M., Brown K., Kass R.S., Keller G., Robinson R.B., and Vunjak-Novakovic G. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun 7, 10312, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayakawa T., Kunihiro T., Ando T., Kobayashi S., Matsui E., Yada H., Kanda Y., Kurokawa J., and Furukawa T. Image-based evaluation of contraction–relaxation kinetics of human-induced pluripotent stem cell-derived cardiomyocytes: correlation and complementarity with extracellular electrophysiology. J Mol Cell Cardiol 77, 178, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Pillekamp F., Haustein M., Khalil M., Emmelheinz M., Nazzal R., Adelmann R., Nguemo F., Rubenchyk O., Pfannkuche K., Matzkies M., Reppel M., Bloch W., Brockmeier K., and Hescheler J. Contractile properties of early human embryonic stem cell-derived cardiomyocytes: beta-adrenergic stimulation induces positive chronotropy and lusitropy but not inotropy. Stem Cells Dev 21, 2111, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Zhan H.-Q., Xia L., Shou G.-F., Zang Y.-L., Liu F., and Crozier S. Fibroblast proliferation alters cardiac excitation conduction and contraction: a computational study. J Zhejiang Univ Sci B 15, 225, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guyette J.P., Charest J.M., Mills R.W., Jank B.J., Moser P.T., Gilpin S.E., Gershlak J.R., Okamoto T., Gonzalez G., Milan D.J., Gaudette G.R., and Ott H.C. Bioengineering human myocardium on native extracellular matrix. Circ Res 118, 56, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








