Abstract
Metastatic liver disease is a major cause of cancer-related morbidity and mortality. Surgical resection is considered the only curative treatment, yet only a minority is eligible. Patients who present with unresectable disease are treated with systemic agents and/or locoregional therapies. The latter include thermal ablation and catheter-based transarterial interventions. Thermal ablation is reserved for those with limited tumor burden. It is used to downstage the disease to enable curative surgical resection, as an adjunct to surgery, or in select patients it is potentially curative. Transarterial therapies are indicated in those with more diffuse disease. The goals of care are to palliate symptoms and prolong survival. The indications and supporting data for thermal ablation and transarterial interventions are reviewed, technical and tumor factors that need to be considered prior to intervention are outlined, and finally several cases are presented.
Keywords: interventional radiology, liver metastases, locoregional therapy, thermal ablation, radioembolization, chemoembolization
Objectives : Upon completion of this article, the reader will be able to describe the differential indications and supporting evidence for thermal ablation and catheter-based interventions in the treatment of the more common metastatic liver tumors.
Accreditation : This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™ . Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Liver metastases are 18 to 40 times more common than primary liver tumors. 1 The incidence and pattern of disease is dependent on the source of the primary tumor, the histologic tumor type, the duration of the disease, as well as patient's gender and age. Approximately half of all liver metastases originate in the gastrointestinal tract. More than 75% of patients present with bilobar disease with a solitary lesion identified in only 10%. 2 While about a third of patients with colorectal metastases are confined to the liver, 3 the majority of cancers present with synchronous extrahepatic metastases.
The National Comprehensive Cancer Network (NCCN) provides guidelines for the multidisciplinary treatment of cancer by site ( https://www.nccn.org/professionals/physician_gls/f_guidelines.asp ). Locoregional therapies including transarterial endovascular therapy and percutaneous ablation do not feature in the treatment algorithms for the majority of metastatic cancers. Unlike hepatocellular cancer for which treatment strategies are linked to clinical staging systems, for example, Barcelona Clinic Liver Cancer classification, 4 the appropriateness and timing of locoregional therapy for metastatic liver disease has not been well defined and its use is highly variable.
In cancers where surgical resection of liver metastases offers the best chance for long-term survival, the disease can be categorized as resectable, potentially resectable, unresectable with the intention to actively treat, or unresectable with initiation of supportive care. For resectable, or potentially resectable metastases, locoregional therapies (tumor ablation or catheter-based therapies) may be indicated to downstage the disease for subsequent surgical resection or used as an adjunct to surgery. In the vast majority of cancers, locoregional therapies are used alone or in combination with systemic chemotherapy to improve palliative outcomes.
This review includes a brief overview of the locoregional therapies used in the treatment of liver metastases, followed by the indications and data supporting the use of locoregional therapy in some commonly encountered cancers including colorectal, neuroendocrine, breast, and uveal melanoma. Tumor and technical factors that need to be considered prior to intervention will be outlined. Finally, several cases and the approach to treatment are presented.
Locoregional Therapy
The goal of thermal ablation is to treat the tumor and a margin of tissue around it. It is a local treatment. In general, ablation therapy achieves the best results when applied to small volume disease. It may be considered an alternative to surgery and, in some instances, is considered a potentially curative intervention. On the other hand, catheter-based embolization procedures are usually applied to one or more segments of the liver and therefore are best considered regional treatments. They are usually indicated where there is more diffuse disease. The goals of care are to slow down tumor progression and prolong survival. Local and regional therapies could also be considered complimentary to each other and by combining them, it may be possible to improve local tumor control and at the same time reduce the toxicity/side effects of each individual treatment.
It is incumbent on the interventional radiologist to be familiar with the technologies available and understand the pros and cons of each.
Thermal Ablation
Radiofrequency and microwave are the two most common thermal ablation modalities used in the liver. Radiofrequency ablation (RFA) is the ablation modality with the largest number of publications in the literature and, thus, is considered the reference ablation technique. While interventional radiologists are very experienced in the use of RFA, there are several well-documented limitations associated with this technology. Active heat production is limited at temperatures greater than 100°C due to desiccation of the tissues and charring at the applicator tip, thus increasing impedance and cutting off the current. Heat conduction to surrounding tissues is a passive process that is heavily dependent on the local tissue microenvironment. Heat-sink phenomenon involves the dissipation of heat away from the ablation zone by vessels greater than 3 mm in size, thus limiting the effectiveness of RFA in the immediate vicinity. Microwave ablation (MWA) offers several advantages over RFA in that heat production is almost an entirely active process, heat conduction is independent of the thermal and electric conduction properties of the tissues, and MWA is less susceptible to heat sink. In vivo, there is some evidence that faster, larger, more consistent ablations across a wide range of tissues can be achieved with MWA compared with RFA. 5 While outcome data relating to MWA use in metastatic liver disease is very limited, MWA has become the ablation tool of choice for treating primary and metastatic liver tumors in many centers in the United States.
Catheter-Based Therapies
Catheter-based therapies involve the administration of toxic agents to the capillary bed of the tumor via the hepatic artery. Damage to noncancerous liver is limited by the fact that normal hepatocytes are predominantly supplied by the portal venous system. Conventional transarterial chemoembolization (cTACE) is composed of one or more chemotherapeutic agents mixed with Ethiodol. This is followed by bland embolization with Gelfoam slurry or particles. There is considerable variability in the drug regimens and the type and size of embolic material used with cTACE. Drug-eluting beads (DEB) are chemotherapeutic-loaded microspheres delivered transarterially. This technique ensures a slow sustained release of the drug into the tumor while prolonging the ischemic effect. While DEB-TACE has been shown to be safe and effective for both primary and secondary liver cancers, a survival benefit over cTACE has not been demonstrated. 6 Radioembolization involves the injection of particles loaded with a radioisotope via transarterial approach. Ytrium-90 is the isotope used to treat primary and secondary liver cancers and comes in two forms—glass microspheres with diameter of 20 to 30 µm and resin microspheres with diameter of 20 to 60 µm. Radioembolization can be safely performed on an outpatient basis and has demonstrated benefit in a wide range of liver metastases.
Colorectal Cancer Metastases—Indications for Locoregional Therapy and Supporting Data
Colorectal cancer is the third most common malignancy worldwide. 7 Fifty percent of patients will develop liver metastases during the lifetime of their disease. The presence of liver metastases has significant prognostic implications. In those with isolated liver metastases, complete surgical resection is the gold standard and offers the best chance for long-term survival with 5-year overall survival (OS) rates approaching 50%. 8 9 Unfortunately, the majority of patients with liver metastases are ineligible for curative resection at presentation. While 5-year survival rates remain close to zero in those with incurable disease, recent advances in systemic chemotherapy and biological agents have improved the median survival (20–22 months) in this patient population. 10 11 12 13 14 15 16 17 There is now increasing demand for minimally invasive, nonsurgical locoregional therapies that can be used alone or in combination with systemic agents to control the disease and prolong survival while maintaining quality of life.
Thermal Ablation of Metastatic Colorectal Carcinoma
Thermal ablation is a potentially curative therapy in patients with a small number and size of tumors in whom an adequate (>1 cm) ablation margin can be achieved. There are multiple advantages of thermal ablation compared with surgery: it is minimally invasive, procedures may be out-patient based, damage to surrounding healthy liver tissue is reduced, it does not limit future therapies, mortality is rare, and major complication rates below 2.5% have been reported from several experienced centers. 18 19 20 21 While mortality rates following liver resection is less than 5% in experienced centers, major morbidity is reported at approximately 25 to 30% and may be up to 40% in those older than 70 years ( Fig. 1 ). 22
Fig. 1.
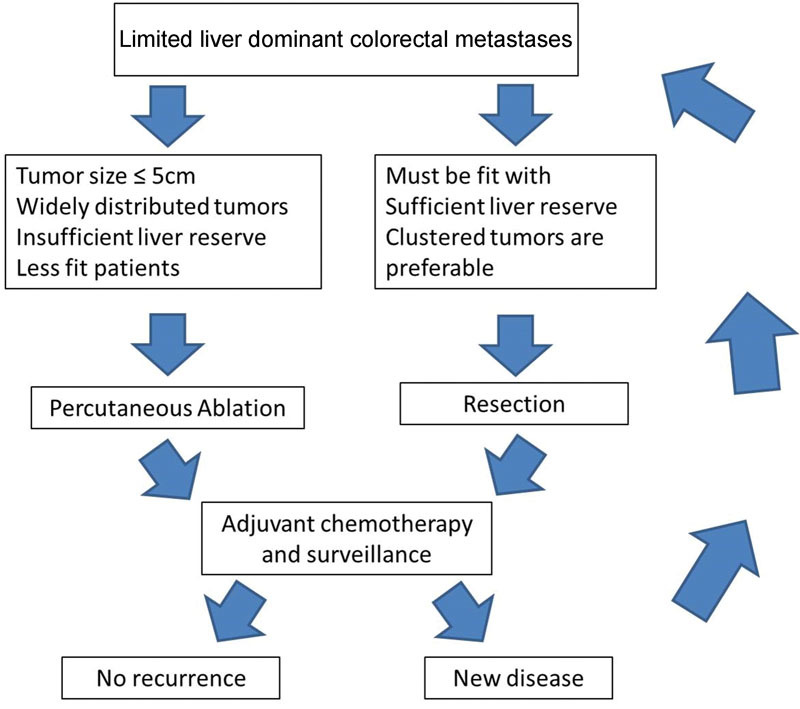
Management algorithm for patients presenting with limited liver-dominant colorectal metastases (image courtesy: Gillams et al 32 ).
Thermal Ablation versus Surgical Resection
Ablation is considered an alternative local treatment option in those who are ineligible for surgery, yet many patients who are unsuitable for surgical resection are also not the ideal candidate for ablation therapy. A review of the literature will reveal that patients who have undergone ablation often have larger number and size of tumors, centrally located tumors, extrahepatic disease, aggressive tumor biology, poor performance status, and/or significant comorbidities. This selection bias combined with a lack of prospective randomized trials comparing these two “potentially” curative therapies makes it difficult to draw meaningful conclusion regarding the true merits and limitations of thermal ablation. Reported outcomes for RFA are wide ranging—complete ablation rates range from 50 to 98%, 23 24 25 26 local progression rates range from 5 to 39%, 24 25 26 27 28 and 3-year OS rates range from 37 to 53%. 23 27 29 30 31
In a review by an international panel of ablation experts, eight studies of ablation performed in patients with unresectable small volume liver disease reported a mean 3-year survival from the date of first thermal ablation of 50% (37–77%) and a mean 5-year survival of 31% (17–51%). 32 Many patients had extrahepatic disease. When ablation was confined to patients with potentially resectable disease, the 5-year survival increased to 50%, comparable with reported surgical outcomes. A retrospective review of solitary colorectal metastases treated with hepatic resection or RFA showed tumor size larger than 3 cm to be the most important prognostic factor for both local recurrence and OS in those who underwent ablation. 33 Solbiati et al demonstrated favorable long-term outcomes in a select patient population who underwent RFA combined with chemotherapy. 20 The study group included 99 patients with 202 small (range: 0.8–4 cm, mean: 2.2 cm) metachronous colorectal liver metastases. OS rates were 98.0, 69.3, 47.8, 25.0, and 18.0% (median: 53.2 months) at 1, 3, 5, 7, and 10 years, respectively, and were comparable with survival outcomes following hepatic resection. Local tumor progression occurred in 11.9% of metastases, of which 54.2% were retreated. All patients received systemic chemotherapy. They attributed the improvements in progression-free survival (PFS) and OS to close monitoring with contrast-enhanced imaging following surgery, improved patient/tumor selection criteria for ablation, the creation of larger ablation zones, prompt retreatment of residual disease, and the routine administration of systemic chemotherapy.
The only randomized trial comparing hepatic resection and MWA in 30 patients reported 1-, 2-, and 3-year survival rates for the MWA group of 71, 57, and 14%, respectively, compared with 69, 56, and 23%, respectively, for the resection group. The mean survival time was longer in the MWA group (27 vs. 25 months), but the disease-free survival was greater in the resection group (11.3 vs. 13.3 months). The study was underpowered to demonstrate a significant difference between the two groups. 34
Thermal Ablation in the Palliative Setting
Thermal ablation with or without systemic chemotherapy should be considered in patients with unresectable small volume liver disease. Three-year survival rates of 37 to 77% and 5-year survival rates of 17 to 51% have been reported with this combination. 32
There is a single randomized controlled trial that evaluated the impact of adding ablation to systemic chemotherapy in patients with initially inoperable metastases. The study was closed early due to poor accrual and while it was not sufficiently powered to evaluate differences in OS, it did show significant differences in PFS at 3 years: 27.6% in the ablation arm versus 10.6% in the non-ablation arm ( p = 0.025). OS at 30 months was 61.7% in the combined RFA and systemic chemotherapy group compared with 57.6% in the systemic chemotherapy group. 35
Abdalla et al reported significantly greater median survival in unresectable patients who underwent ablation compared with chemotherapy alone in unablatable patients (25 vs. 16.5 months). Metastases were confined to the liver in all patients and all had potentially curable disease on preoperative staging studies. 27
In summary, thermal ablation should be considered in the following circumstances:
-
Resectable disease in a patient clinically unfit for surgery:
Patient preference : Occasionally, even after discussion of risks and benefits of each intervention, a patient may decline to undergo surgery and opt for ablation. When ablation is used as a first-line treatment in patients with resectable disease, the 5-year survival outcomes are very similar to surgical series.
Test of time : Thermal ablation may be used to successfully treat some tumors while simultaneously building an interval into the patient's treatment program to observe and better understand the natural history of their disease. This test-of-time approach was first described in 2003. 36 In that study, of the 88 patients treated with RFA, 26% remained tumor free, negating the need for resection; 50% developed more widespread disease and became unsuitable for resection; and 24% underwent resection. This approach allows for cytoreduction, but can also avoid an unnecessary procedure in many patients.
-
Unresectable disease due to number and distribution of the metastases:
Ablation with or without chemotherapy should be considered in all patients with limited liver-dominant disease. Ablation should also be considered in those who are potentially resectable but are unsuccessfully downsized following neoadjuvant chemotherapy and/or portal vein embolization. The 5-year survival rates postablation compare favorably with the reported 25% 5-year survival reported following resection after portal vein embolization. 37
Unresectable disease : Insufficient liver reserve due to widely scattered tumors or previous liver resection. Forty to 70% of patients will develop new metastases after a R0 resection. 38 With insufficient hepatic reserve to undergo repeat resection in the majority of these patients, ablation with or without chemotherapy should be considered.
Catheter-Based Therapy of Metastatic Colorectal Carcinoma
cTACE
Data on cTACE combining chemotherapy with ethiodized oil with or without the addition of bland embolic particles is heterogeneous. There is no standard treatment regimen; however in the United States, triple combination therapy with doxorubicin, cisplatin, and mitomycin has traditionally been administered, while monotherapy with doxorubicin is most frequently used worldwide. 39 A prospective study of 463 patients evaluating three different chemoembolic protocols as second-line therapy for metastatic colorectal liver metastases showed no statistically significant difference in OS between the three protocols. The median OS was 38 months from the date of diagnosis of liver metastases and 14 months from the date of the first chemoembolization. 40 A retrospective study by Albert et al evaluated 121 patients who received traditional triple combination chemoembolization as second-line therapy or salvage therapy and showed a median survival of 27 months from the diagnosis of liver metastases. Median survival was 9 months from the time of first cTACE. This same study showed that the presence of limited extrahepatic disease did not adversely impact survival after chemoembolization, and chemoembolization after first- or second-line systemic therapy resulted in better survival than after third- to fifth-line systemic therapy. 41 The differences in OS between the two aforementioned studies may be related to differences in patient population characteristics with the former study excluding patients with poorer performance status. A smaller retrospective study of second-line therapy with cTACE by Hong et al showed an overall median survival of 7.7 months after cTACE. 42 Two small prospective studies evaluating cTACE as second-line therapy support findings by Albert et al, with median OS after chemoembolization between 8.6 and 10 months. 43 44 Only one of these prospective studies reported median OS after initial diagnosis of liver metastases and found it to be similar to that of Albert et al at 29 months. 44
cTACE safety : Multiple studies report that the majority of patients receiving cTACE have some element of postembolization syndrome (pain, nausea, vomiting, fever, leukocytosis, fatigue). The major complication rate has been reported between 2.7 and 11%. 41 42 43 44
Transarterial Hepatic Chemoembolization with Irinotecan-Loaded Drug-Eluting Beads (DEBIRI)
DEBIRI in combination with systemic therapy as first-line treatment was evaluated by Martin et al in a prospective randomized multi-institutional study of 72 patients. 45 OS was not evaluated; however, there was a statistically significant improvement in liver PFS in the DEBIRI arm of the trial compared with the control arm with systemic therapy alone (17 vs. 12 months, p = 0.05). Overall extrahepatic PFS was similar, despite the DEBIRI arm population having statistically more extrahepatic disease compared with the control arm at baseline. More importantly, the study showed a significantly higher rate of downstaging to resection with the DEBIRI arm compared with the control arm (35 vs. 16%, p = 0.05) and no significant augmentation in chemotherapy-associated adverse events. The study results suggest that DEBIRI in combination with systemic therapy as first-line treatment is a safe option for patients who are not hepatectomy candidates. 45 The potential for downstaging is supported by a prospective multi-institutional registry of 55 patients with liver-dominant colorectal metastases treated with DEBIRI. Twenty percent of the patients in the registry were either successfully downstaged or achieved disease stability without extrahepatic progression enabling resection, ablation, or a combination of resection and ablation. 46
As salvage therapy in patients with liver metastases but no radiologic evidence of extrahepatic disease, a prospective multi-institutional double-arm randomized control trial of 74 patients comparing DEBIRI versus FOLFIRI treatment showed a significantly higher median OS in the DEBIRI arm compared with the chemotherapy arm (20 vs. 15 months, p = 0.05). 47 A prospective multi-institutional single-arm treatment registry evaluating DEBIRI treatment as second-line or salvage therapy for 55 patients with liver-dominant colorectal metastases showed a median OS of 19 months from DEBIRI treatment. 48
DEBIRI safety . The most common adverse event with DEBIRI treatment is postembolization syndrome (40–63%) followed by hypertension coinciding with the immediate postembolic syndrome. 49 50 In a prospective registry of DEBIRI treatment for liver-dominant metastatic disease where 76% of the patients had metastatic colorectal carcinoma, the rate of adverse event was 19% with a median adverse event grade of 2. Risk factors for adverse events were shown to be lack of pretreatment with intra-arterial lidocaine, achievement of complete stasis, higher than 100 mg DEBIRI in one treatment, and total bilirubin level greater than 2 µg/dL with more than 50% liver volume involvement. 50 As first-line therapy, DEBIRI combined with systemic therapy versus systemic therapy alone showed no increase in systemic toxicity of chemotherapy and caused no treatment delays. 45 As salvage therapy, DEBIRI performs better on quality-of-life assessments compared with systemic therapy with better physical functioning at 1, 3, and 8 months and increased time to decline of quality of life compared with systemic therapy (3 vs. 8 months, p = 0.0002). 47
Transarterial Hepatic Radioembolization (TARE) with Yttrium-90 (Y90)-Loaded Microspheres
Data on TARE have been reported with both glass and resin microspheres. Glass microspheres were granted humanitarian device exemption by the U.S. Food and Drug Administration (FDA) for the treatment of unresectable hepatocellular carcinoma in patients with appropriately positioned hepatic arterial catheters and are used off-label for metastatic disease at institutions with approved internal review board protocols ( www.accessdata.fda.gov/cdrh_docs/pdf/h980006b.pdf ). Radioembolization with resin microspheres is the only FDA-approved transarterial therapy for metastatic colorectal carcinoma. The FDA granted full premarket approval of resin microspheres for the treatment of colorectal metastases in conjunction with intrahepatic floxuridine ( http://www.accessdata.fda.gov/cdrh_docs/pdf/p990065a.pdf ). The FDA approval of resin microspheres is primarily based on a phase III randomized control trial by Gray et al. 51 The trial evaluated 74 patients who had undergone resection of their primary adenocarcinoma and had unresectable colorectal metastases limited to the liver and porta hepatic lymph nodes. The patients were randomized to hepatic arterial chemotherapy with floxuridine alone or to combination therapy of hepatic arterial chemotherapy plus TARE. The radioembolization arm of the study had significantly better partial plus complete response, longer median time to disease progression, and significantly higher 1-, 2-, 3-, and 5-year survival rates. There was no increase in grade 3–4 treatment-related toxicities and no loss of quality of life in the radioembolization arm compared with the control arm. Although median survival was not statistically different (17 vs. 15.9 months, p = 0.18), Cox regression analysis suggested that patients had a survival advantage with combination therapy if they survived more than 15 months. 51
TARE as first-line therapy : As first-line treatment in combination with chemotherapy, a phase II randomized control trial of 21 patients receiving chemotherapy with fluorouracil/leucovorin with or without Y90 radioembolization showed that the combination therapy was well tolerated. The study reported a significant improvement in response rate, increased time to progression, and increased median OS in the combination group. 52 Fluorouracil and leucovorin with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) are the most common chemotherapy regimens used for metastatic colorectal carcinoma and provide a median OS of 16 to 20 months. Biologic agents (bevacizumab, cetuximab) have shown to improve upon both PFS and OS. 53 Since oxaliplatin is a radiosensitizer for cancer cells, 54 there is promise in the combination treatment of Y90 radioembolization plus FOLFOX as first-line therapy for colorectal liver metastases. Both a phase I study and a retrospective study confirmed tolerability of radioembolization combined with FOLFOX as first-line therapy 55 56 with the toxicity of neutropenia or neutropenic fever noted in both studies. Sharma et al suggested that the oxaliplatin dose be reduced in the first three chemotherapy cycles to improve treatment tolerability. 55 These results have subsequently led to the creation of three large ongoing phase III randomized controlled trials evaluating Y90 radioembolization with resin microspheres in combination with FOLFOX versus FOLFOX alone as first-line treatment for metastatic colorectal cancer with liver-only or liver-dominant disease. These studies include the SIRFLOX, the FOXFIRE, and the FOXFIRE Global studies.
Initial published results from the SIRFLOX study have not shown a statistically significant difference in median OS between the control group and the combination therapy group; however, PFS in the liver was higher in the combination group compared with the control group (20.5 vs. 12.6 months, p = 0.002). Extrahepatic metastases were present in 40% of the patient population and approximately 45% of the patients did not have resection of their primary tumor. 57 These findings suggest that the overall PFS may be due to progression of extrahepatic disease. Accrual has been completed in the SIRFLOX trial, the FOXFIRE trial (a UK-based randomized phase III trial), and the FOXFIRE Global trial (an international randomized phase 3 trial) with combined sample size of 1,103 patients. 58 OS analysis of combined data from the three trials will be released within the next few years.
TARE as salvage therapy : Systemic therapy has been shown to be an effective treatment for colorectal liver metastases; however, over time, these metastases become refractory to chemotherapy and biologic agents. Patients with chemorefractory colorectal metastases have median OS of 4 to 5 months. 59 Over the past decade, numerous retrospective and prospective studies of Y90 radioembolization with either resin or glass microspheres for the treatment of chemorefractory colorectal liver metastases have shown median OS of between 7.9 and 11.9 months. 60 61 62 63 64 65 66 67 68 69 70 71 The MORE study, a retrospective multicenter study, evaluated Y90 radioembolization with resin microspheres for salvage therapy in 606 consecutive patients with liver-dominant colorectal metastases. The patients had a median of two lines of prior chemotherapy. One-third had limited extrahepatic metastases. Median OS from first radioembolization was 9.6 months. 70 A large retrospective multicenter study of Y90 radioembolization with glass microspheres for colorectal liver metastases with progressive disease refractory to previous systemic or locoregional therapies evaluated 531 patients. Of these, 62% had extrahepatic disease. Median OS from first radioembolization was 10.6 months. 59 Both of the aforementioned studies showed that independent predictors of better survival included better performance status, smaller liver tumor burden, no extrahepatic metastases, and less prior chemotherapy. 59 70
TARE safety : The most commonly reported side effects of Y90 radioembolization include fatigue followed by abdominal discomfort, nausea, fevers, and vomiting. 59 70 The SIRFLOX study reported adverse events of grade higher than 3 in 85.4% of patients in the combination therapy arm compared with 73.3% of patients in the control arm ( p = 0.514). Neutropenia, febrile neutropenia, and thrombocytopenia were reported statistically higher in the combination group. Gastrointestinal ulceration rates were 3.7% in the SIRFLOX study, 3.5% in the MORE study, and 0% in the study by Hickey et al. 59 Radioembolization-induced liver disease was reported at 0.8% in the SIRFLOX study and 0.5% in the MORE study. Hepatic failure was reported at 1.2% in the SIRFLOX study and at 0.8% in the MORE study. Hickey et al reported grade 3 or 4 biochemical toxicity in 13% of patients but did not note the rate of radioembolization-induced liver disease or hepatic failure. 57 59 70
Liver-Dominant Metastatic Colorectal Cancer: How We Do It
All patients with metastatic colorectal carcinoma are reviewed by our multidisciplinary team which includes oncologists, surgeons, radiation oncologists, and interventional radiologists. Patients who are not resection candidates are evaluated for ablation. Based on the available data, transarterial therapies are generally reserved for salvage therapy at our institution. Given that Y90 radioembolization is the only FDA-approved transarterial therapy for liver-dominant colorectal metastases and there are numerous studies showing good radiologic responses as well as a potential survival benefit of Y90 radioembolization as a salvage therapy, Y90 radioembolization is the first transarterial therapy used in our patients with chemorefractory colorectal liver metastases. If there is progression after Y90 radioembolization, consideration for DEBIRI or cTACE is made. The preliminary results of the SIRFLOX study show no difference in overall PFS rate with Y90 radioembolization, suggesting that the first-line combination therapy may result in no difference in OS. However, patients with liver-isolated disease may benefit. Combined OS data from the three ongoing randomized controlled studies are shortly anticipated and will help delineate the role of Y90 radioembolization as first-line combination therapy.
Neuroendocrine Liver Metastases—Indications for Locoregional Therapy and Supporting Data
Neuroendocrine tumors are a heterogeneous group of malignancies with variable histologies, symptomology, and clinical courses. Over half of all patients with neuroendocrine tumors of gastroenteropancreatic origin present with liver metastases. A systematic evaluation of the Surveillance, Epidemiology, and End Results (SEER) showed that survival was significantly associated with the presence of liver metastases and histologic grade. Median OS for G1 neuroendocrine tumors was reported at 124 months, for G2 neuroendocrine tumors at 64 months, and for both G3 and G4 neuroendocrine tumors at 10 months. Median OS for G1 and G2 tumors with distant metastases was 33 months. 71 Long-term survival rate for patients with gastrinoma without liver metastases is reported as 95% at 20 years but only 15% at 10 years if liver metastases are present. 72 In patients with carcinoid tumors, the 5-year survival rate ranges from 75 to 88% in those without liver metastases compared to only 30% for those with liver metastases. 73 74 75
The management of neuroendocrine liver metastases requires multidisciplinary input. Treatment is determined by the extent and distribution of the liver metastases, the grade of the tumor, and the natural history of the disease ( Fig. 2 ). The goals of locoregional therapy include symptom palliation, tumor bulk reduction, and potential downstaging prior to resection.
Fig. 2.
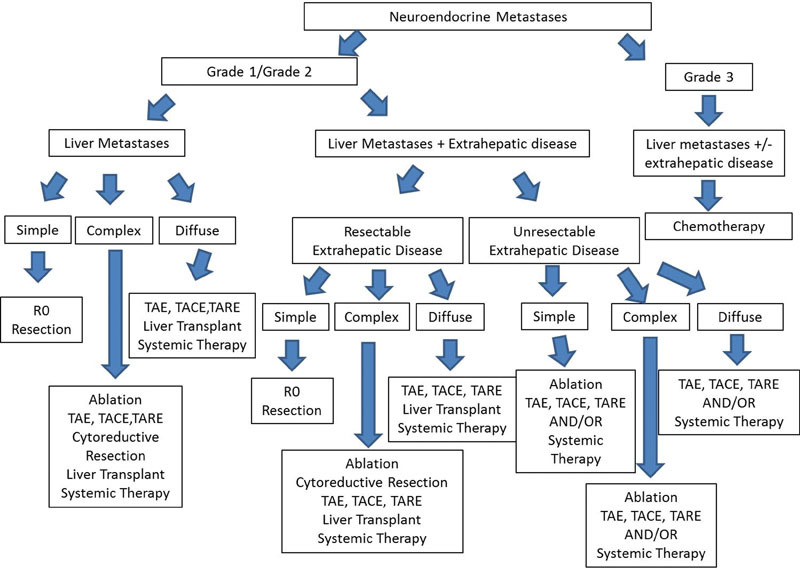
Management algorithm for neuroendocrine liver metastases (image courtesy: Frilling and Clift 157 ).
Liver resection is currently considered the only potentially curative treatment option. It is indicated in those with grade 1 or 2 liver metastases that are unilobar or confined to two adjacent segments (simple). Thermal ablation may be considered an alternative to surgery in those unfit for hepatic resection. In patients with disease primarily involving one lobe but with small satellite lesions in the contralateral lobe (complex), ablation may be considered as an adjunct to surgery. While surgical resection has demonstrated improved survival as well as symptom control, the data are almost entirely derived from retrospective single-center studies in which there is no nonoperative control group for comparison. In those with diffuse, bilobar, (diffuse) low- and intermediate-grade disease, transarterial therapies and/or systemic chemotherapy are the mainstay of treatment. Chemotherapy has low efficacy for low-grade neuroendocrine tumors, although results are slightly better for islet cell carcinomas compared with carcinoid tumors. On the other hand, systemic chemotherapy is the standard of care for high-grade tumors. The introduction of somatostatin analogs has improved OS due to improved hormonal symptoms and cytostatic activity. Over time, however, tumors can become refractory to these drugs. The “test-of-time” approach is frequently employed in those who are asymptomatic with stable limited tumor burden.
Thermal Ablation of Metastatic Neuroendocrine Tumor
RFA is the most frequently used ablation technology for patients with neuroendocrine liver metastases. It is indicated in patients who are ineligible for complete resection (R0) and can be used alone or in conjunction with surgery. Unlike other liver metastases, neuroendocrine liver metastases are usually multiple and bilobar. Ablation is frequently performed intraoperatively as an adjunct to surgery in patients undergoing cytoreductive surgery, and, in this setting, ablation of more than five tumors is not unusual. The goal of treatment is to debulk the disease and control functional syndromes due to hormone excess. In a study of 119 laparoscopic RFAs without liver resection performed in 89 patients with mean tumor size of 3.6 cm, 44 patients had hormonal symptoms prior to the procedure. One week postablation, 97% reported partial symptom relief and 73% had significant or complete symptom relief. The symptom relief lasted for a median of 14 ± 5 months. 76
In a systematic review of the literature that included eight studies (301 patients treated with percutaneous or intraoperative RFA) by Mohan et al, overall 5-year survival rates ranged from 37 to 57%. 77 Recurrence was common, ranging from 63 to 87%. Risk factors for recurrence include tumor size, proximity to major blood vessels, and the width of the ablation margin. Ablation is most effective in patients with low tumor volume, tumor number less than 5, lesions less than 3.5 cm diameter, and in the setting of multiple tumors a sum of the diameters less than 8 cm. 78 79
Catheter-Based Therapy of Metastatic Neuroendocrine
The data on locoregional therapies for neuroendocrine liver metastases are predominantly limited to retrospective studies using heterogeneous treatment protocols and statistical methods. Transarterial therapies are most commonly performed in those with diffuse bilobar disease and can provide effective symptom relief in patients with hormonally active tumors.
cTACE, Transarterial Bland Embolization (TAE), and DEBDOX
Drugs used in cTACE of neuroendocrine liver metastases include doxorubicin, streptozotocin, mitomycin C, cisplatin, and gemcitabine (alone or in combination). No one drug regimen has been shown to be superior. Some centers use doxorubicin for intestinal tumors and streptozotocin for pancreatic tumors. The modality of transarterial therapy has not been shown to have a significant effect on outcomes. Two separate retrospective studies have shown no difference in median OS in patients treated with cTACE compared with TAE. 80 81 Gupta et al showed a trend toward better OS with chemoembolization compared with TAE in patients with islet cell tumors, although the finding was not statistically significant. 82 Theoretically, patients with islet cell tumors should benefit more from chemoembolization compared with those with carcinoid tumors, as islet cell tumors are also more susceptible to systemic chemotherapy. The efficacy data for DEBDOX is limited and there are no large comparative studies with other locoregional modalities. Five-year survival rates for TAE and TACE have been reported between 11.1 and 50%. Multiple studies have shown that there are worse outcomes in patients with large liver tumor burden. Studies by both Gupta et al and Kress et al showed that patients with more than 75% tumor burden within the liver had higher major complication rates after chemoembolization. 82 83 Patients with high liver tumor burden do get some response to treatment; therefore to avoid adverse events, consider dividing the liver volume between multiple selective segmental treatment sessions.
Safety of cTACE, TAE, and DEBDOX : Numerous studies have shown that almost all patients experience some degree of postembolic syndrome. Carcinoid crisis is a rare adverse event due to prophylactic treatment with somatostatin analogs. Large variation in the reported rate of severe adverse events after treatment is due to the heterogeneity of the studies. The larger studies report serious adverse event rates ranging between 8.5 and 25%. 80 82
In the United States, a multicenter randomized trial to evaluate hepatic PFS in patients with liver-dominant neuroendocrine metastases undergoing treatment with TAE, cTACE, or DEBDOX has just got underway (ClinicalTrials.gov Identifier: NCT02724540). Estimated primary completion date is March 2020.
TARE with Y90-Loaded Microspheres
In a retrospective review of 148 patients from 10 institutions treated with resin microspheres, complete and partial tumor response rates were seen in 2.7 and 60.5% of cases, respectively. 84 Stable disease was observed in 22.7% of the cases and progressive disease occurred in only 4.9% of the cases. Symptomatic responses were observed in 55 to 100%. Three-year survival rate of 45 to 57% and 5-year survival rate of 46% have been reported.
A multicenter retrospective review of outcomes following embolotherapy (TAE, cTACE, TARE) in 202 patients with metastatic neuroendocrine tumors showed higher tumor grade and hepatic tumor burden more than 50% to be significant negative prognostic factors for PFS and OS. 85 Multivariate OS analysis showed a trend toward a worse prognosis in the TARE group, while OS was similar among the TAE and TACE groups. PFS was equivalent across all three treatments. The chemotherapy regimen, the type of microsphere particle (glass or resin), the total number of treatments, and bland embolization particle size (<150–300) did not significantly impact PFS or OS. Safety profiles were similar for all three groups. DEBDOX chemoembolization was not evaluated.
Liver-Dominant Neuroendocrine Metastases: How We Do It
Based on the aforementioned data, if patients are not surgical or ablation candidates, our multidisciplinary team refers lower-grade liver-dominant tumors for transarterial therapy after progression on somatostatin analogs or for symptoms not well controlled with somatostatin analogs. Since data relating to embolotherapies are inconclusive, the type of transarterial therapy to be administered is determined on a case-by-case basis. Results from prospective randomized trials, including the Randomized Embolization Trial for NeuroEndocrine Tumors (RETNET), will further elucidate which transarterial therapy may be appropriate for patients with neuroendocrine liver metastases.
Breast Cancer Liver Metastases—Indications for Locoregional Therapy and Supporting Data
Metastatic breast cancer is a heterogeneous disease. Anthracycline and taxane-based drugs are the mainstay of chemotherapy. Most patients undergo multiple sequential lines of systemic treatment until the disease finally becomes refractory to systemic agents. Liver metastases are usually indicative of disseminated disease and portend a poor prognosis. Overall, the median survival rate for those receiving palliative chemotherapy ranges from approximately 18 to 24 months. 86
Oligometastatic disease is defined by a limited number and size of metastases and accounts for up to 20% of all metastatic breast cancers. 87 It is an important subset of patients to identify, as aggressive multidisciplinary therapies (including liver-directed therapy) can improve local control and prolong survival. 88 89 In patients who received local surgical or image-guided therapies, factors that predicted a treatment benefit included longer disease-free interval between the treatment of the primary cancer and the diagnosis of liver metastases, small size and/or number of liver metastases, well-differentiated histopathology, and response to preinterventional or preoperative chemotherapy. 90 91 92 93 94 95 96
Liver-directed therapy represents a minimally invasive targeted treatment option that alone or in conjunction with systemic agents may improve local tumor control in subgroups of patients with breast cancer liver metastases. Both thermal ablation and catheter-based therapies are well tolerated with very acceptable risk–benefit profiles.
Thermal Ablation of Metastatic Breast Cancer
Less than 1% of breast cancer patients with liver metastases are eligible for surgical resection. 97 Despite this, retrospective studies have demonstrated a survival benefit of surgery over palliative chemotherapy —median survival of 20 to 67 months versus 18 to 24 months and a 5-year survival rate of 21 to 61% versus 5 to 10%, respectively. 98 Unfortunately, recurrence after liver resection is common, occurring after a median time of 13 months. Thermal ablation may be used as an alternative to or as an adjunct to surgery. Similar to other tumor types, ablation is best used to treat limited disease burden. Indications for its use include resectable disease in a patient unfit for surgery, patient preference, unresectable disease due to insufficient liver reserve such as previous resection, and as an adjunct to surgery.
RFA outcome data also compare favorably with systemic chemotherapy with median survival of 30 to 60 months and 5-year survival of 20 to 32% reported. 99 100 101 102 Local tumor progression has been demonstrated in 13.5 to 58% of treated lesions using RFA. 98 99 100 101 102
Vogl et al reported a median survival of 37.6 months ( n = 276) following MRI-guided laser-induced interstitial thermotherapy (LITT). 103 Mack et al reported median survival of 51.6 months and 1-, 2-, and 3-year survival rates of 96, 80, and 63%, respectively, in 232 women treated with LITT. 104
Catheter-Based Therapy of Metastatic Breast Cancer
TACE
Chemoembolization data are difficult to interpret due to widely varying techniques used across institutions. In patients who are refractory to systemic chemotherapy, outcome measures following cTACE range from 3.3 to 7 months for PFS, 10.2 to 21.8 months for OS, and 7 to 13% for tumor response rates. 105 106 107 In selected patients, cTACE in combination with systemic therapy has shown favorable results. A retrospective study by Duan et al reported a 1-year survival rate of 76.2% in those treated with cTACE combined with systemic chemotherapy compared to 48.1% in those treated with systemic therapy alone ( p = 0.027). 108 In a similar retrospective review comparing cTACE and systemic chemotherapy with chemotherapy alone, Li et al also showed a survival benefit with combined therapy (63 vs. 33.9%). 109
Data relating to the use of DEBDOX are limited. A pilot study involving 23 patients with liver-dominant metastases, who had failed three lines of chemotherapy, underwent lobar treatments with DEBDOX (75–100 µm). 110 While survival data (median PFS was 8 months, median OS was 17 months) were comparable with that reported for cTACE, safety concerns were raised. Almost half of patients suffered a biliary injury. A quarter of patients were unable to undergo a second treatment due to severe adverse events that occurred following the first procedure. It is unclear as to the cause of these events, but the Doxorubicin dose administered in a bead as small as 75 µm may be a contributing factor. In another study by Martin et al, 40 patients were treated with DEBDOX (100–300 µm) and no severe adverse events were recorded. Favorable survival data including median PFS of 26 months and median OS of 47 months were reported. The majority of patients had less than 25% liver involvement. 111
TARE
Radioembolization with both glass and resin microspheres has been used as salvage therapy for the treatment of breast cancer liver metastases. The treatment has been shown to be safe and effective in slowing down the progression of disease in a majority of patients (disease control rate: 70–98.5%). 112 113 114 115 116 117 118 In a retrospective review by Pieper et al of 44 women who underwent radioembolization, the histology of the primary tumor and baseline serum gamma-GT were identified as independent predictors of time to progression in the liver (median: 101 days) and OS (median: 6 months). 119 In other studies, effective control of tumor growth in the liver was not always accompanied by a survival benefit (OS: 2–14 months). 112 113 114 115 116 117 118 Factors associated with prolonged survival included ECOG 0, liver involvement less than 25%, low bilirubin levels, liver-only disease, morphologic response, and chemotherapy after radioembolization. 112 113 117 118 The data would suggest that patients who are treated with radioembolization earlier in the course of the disease prior to multiple lines of chemotherapy are more likely to tolerate and benefit from the treatment.
Liver-Dominant Breast Cancer Metastases—How We Do It
At our center, thermal ablation is reserved for patients with a small number and size of tumors (<3–5 cm), limited and stable extrahepatic disease, and who are ineligible for surgical resection. Most patients with diffuse liver disease up to 50% tumor load and limited stable extrahepatic disease are offered transarterial therapy. While there is a paucity of data comparing the various transarterial therapies, radioembolization is our preferred therapy, as it is well tolerated with an acceptable side-effect profile. Liver function blood work and the patient's chemotherapy history are given careful consideration. Lobar treatments are separated in time by at least 1 month. The decision to treat with systemic agents concurrent with radioembolization is made on a case-by-case basis.
Uveal Melanoma Liver Metastases—Indications for Locoregional Therapy and Supporting Data
Uveal melanoma is the most common primary intraocular malignant tumor in adults. Up to 50% of patients will develop hematogenous metastases. 119 120 There is no proven standard of care for those with systemic metastases nor has any adjuvant therapy been shown to improve outcomes in those at high risk of developing metastatic disease. The liver is involved in more than 90% and is the only site of metastatic disease in 50%. 121 In the majority of patients, survival is determined by the intrahepatic tumor burden and its response to treatment. Liver-directed therapies play an important role in the control of the disease.
Thermal Ablation of Metastatic for Uveal Melanoma
While there is some retrospective data demonstrating a survival benefit in patients who undergo surgical resection, less than 10% are eligible. Thermal ablation can be used as an alternative or an adjunct to surgery. An intraoperative, rather than percutaneous, approach enables the operator to evaluate the surface of the liver for miliary disease (lesions <5 mm), before proceeding with thermal ablation. A retrospective review evaluating 57 patients who underwent surgical resection alone and 5 patients who underwent RFA plus surgical resection showed equivalent OS and PFS. Clinical and pathologic features were similar in the two groups. The mean tumor size for those treated with RFA was 13.5 mm (range: 3–25 mm). 122 In another review of 32 patients who underwent surgical resection and 16 patients who underwent percutaneous thermal ablation, median OS was 26 and 18 months, respectively ( p > 0.2). Patients in the ablation group were more likely to have extrahepatic disease and to have received systemic chemotherapy prior to intervention. 123 The presence of extrahepatic disease was a negative prognostic factor in those who underwent resection. In conclusion, thermal ablation may be considered an appropriate alternative or adjunct to surgery that can enable more patients to benefit from local therapy while sparing the surrounding liver parenchyma in a disease that has very limited systemic treatment options.
Catheter-Directed Therapy of Metastatic Uveal Melanoma
Chemoembolization
Chemoembolization has been used effectively for more than two decades in patients with uveal melanoma liver metastases; however, no one treatment regimen has been shown to be superior. 124 125 126 127 128 129 A survival benefit has been reported when cTACE was compared with systemic chemotherapy. In a review of 201 patients with uveal melanoma liver metastases who received systemic chemotherapy, intra-arterial chemotherapy infusion, or cTACE at MD Anderson Cancer Center, a response rate of 36% was reported following chemoembolization compared with less than 1% for systemic chemotherapy. Median OS in those who responded to chemoembolization was significantly longer than that in patients who did not respond to chemoembolization (14.5 vs. 5 months; p = 0.003) or those who received systemic chemotherapy (14.5 vs. 5 months; p = 0.003). 127 The survival benefit in those who responded to TACE has been echoed by other studies. 126 130 131 132 Additional factors that may impact treatment response and survival include liver tumor burden at the time of initial treatment, tumor vascularity, and angiographic pattern of disease (nodular vs. infiltrative disease). 129 131 133 In relation to DEB-TACE, both doxorubicin and irinotecan have been used to treat uveal melanoma liver metastases. Reported experience is derived from small retrospective studies. OS using DEBIRI has been reported between 9.4 and 15.5 months. 134 135 Higher liver tumor burden, high serum gamma-GT, and lactate dehydrogenase at the time of initial treatment were associated with poorer outcomes.
Immunoembolization
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is the cytokine used in immunoembolization of metastatic uveal melanoma. An emulsion containing GM-CSF and Ethiodol is injected into the tumor-feeding arteries. The aim is to induce ischemia of the local tumor tissue, attract and stimulate antigen presenting cells to uptake antigen released from necrotic tumor cells, and to enhance systemic immunity against tumor cells. The treatment has been shown to be safe and well tolerated up to doses of 2,000 µg GM-CSF. When low (≤1,000 µg) and high (≥1,500 µg) dose immunoembolization was compared with BCNU chemoembolization, the high-dose immunoembolization group demonstrated a longer median OS (20.4 vs. 9.8 months; p = 0.005) and a longer median systemic PFS (12.4 vs. 4.8 months; p = 0.001). 136 No significant improvement in hepatic PFS was reported. There was no significant difference in OS or PFS between patients who received BCNU chemoembolization and low-dose immunoembolization. A double-blinded, randomized phase II study using embolization with or without GM-CSF in 52 patients demonstrated a greater proinflammatory cytokine production following immunoembolization. 137 Levels of interleukin-6 at 1 hour postprocedure ( p = 0.001) and interleukin-8 at 18 hours postprocedure ( p > 0.001) were significant predictors of longer systemic PFS. There was a trend toward greater OS in those treated with immunoembolization compared with those who underwent bland embolization (21.5 vs. 17.2 months, respectively).
Radioembolization
There are several small studies reporting outcomes following radioembolization used as salvage therapy. In 2011, a review of 32 patients treated with resin microspheres after failure of immunoembolization and chemoembolization was performed at our institution. 138 As these patients had undergone multiple prior embolization procedures, a 25% dose reduction was applied. OS for the 32 patients ranged from 1.0 to 29.0 months (median: 10 months). Patients with less than 25% tumor burden had a significantly longer OS compared with those with 25% or more tumor burden (10.5 vs. 3.9 months; p = 0.0003). Median PFS of hepatic metastasis was 4.7 months (range: 1.0–26.5 months). Once again, patients with tumor burden less than 25% had significantly longer intrahepatic PFS than did patients with 25% or more tumor burden (6.4 vs. 3.0 months; p = 0.03). Klingenstein et al reported outcomes in 13 patients treated with salvage radioembolization. Median OS after radioembolization was 7 months and achieved local tumor control in 77%. 139 Another retrospective review of eight patients who failed first-line therapy and treated with glass microspheres showed that median hepatic PFS and OS were 4.3 weeks (range, 3.4–28.6 weeks) and 12.3 weeks (range, 3.7–62.6 weeks), respectively. 140 In this study, patients were heavily pretreated prior to radioembolization and the median time interval between initial diagnosis of uveal melanoma and radioembolization was considerably longer than those treated by Klingenstein et al (17.1 vs. 5 months). A phase II clinical trial to prospectively investigate the safety and efficacy of radioembolization with Y90 resin microspheres as first-line therapy for hepatic metastasis of uveal melanoma is underway.
Isolated Hepatic Perfusion
Isolated hepatic perfusion was initially developed as a surgical technique for the treatment of liver-dominant metastases. The blood supply to the liver is isolated to allow direct delivery of high-dose chemotherapy into the hepatic artery. Veno-veno bypass perfuses the remainder of the body. The nonsurgical alternative to isolated hepatic perfusion is percutaneous hepatic perfusion. A phase III trial of percutaneous hepatic perfusion with melphalan compared with best alternative care in 93 patients with ocular (88%) or cutaneous (12%) melanoma demonstrated significantly improved hepatic PFS (8 vs. 1.6 months) and overall response rate with percutaneous hepatic perfusion therapy. 141 The median OS was similar between the two arms.
Liver-Dominant Uveal Melanoma Cancer Metastases—How We Do It
Patients are managed by a multidisciplinary team. For those who are ineligible for surgical resection with less than 50% of the liver replaced by tumor and good performance status, immune- or radioembolization is initiated. For patients with large tumors greater than 5 cm or those with more than 50% tumor replacement, treatment begins with cTACE or DEB-TACE. Chemoembolization is also used as salvage therapy in those who progress following immunoembolization or radioembolization.
Combination Therapies for Metastatic Liver Disease
In both primary and secondary liver lesions treated with RFA, tumor size and location are strong predictors of success, with lesions smaller than 3 cm demonstrating less local recurrence compared with larger lesions. In an effort to improve survival in patients with larger hepatocellular tumors, outcomes following combination therapies have been evaluated.
The instillation of alcohol into the tumor and TACE both reduce blood flow in the immediate vicinity of the tumor, enabling a larger ablation zone to be achieved with RFA. TACE will also treat small satellite lesions. Meta-analyses of five randomized controlled trials involving 570 patients showed that the combination of RFA and TACE was associated with a significantly higher OS rate and recurrence-free survival rate. 142
Tumor/Technical Factors to be Considered Prior to the Use of Locoregional Therapy
When considering a patient for locoregional therapy, the interventional radiologist must first review recent imaging studies to determine if intervention is technically feasible, safe, and appropriate. The tumor characteristics and technical factors that need to be considered in the evaluation and planning of treatment are different for thermal ablation and catheter-based therapies, and are outlined below.
Thermal Ablation
-
Tumor size
For primary liver cancer and for some liver metastases, thermal ablation is potentially curative and, if feasible, is preferred to other locoregional therapies. There are no clinical practice guidelines that define an upper limit on the size of metastatic tumors that can be treated. In the case of colorectal cancer metastases, some centers limit ablation to less than 5 cm, others to less than 3 cm. This decision is based on lower local recurrence rates reported for tumors less than 3 cm. Recurrence rates for tumors larger than 5 cm have been reported between 27 and 45%. 143 144 There is some support for ablation of larger lesions depending on location and ability to achieve a sufficient ablation margin around the tumor. Combination therapies such as TACE prior to RFA may be used to improve local control for larger lesions.
-
Tumor number
In colorectal metastases, the best outcomes are reported for solitary liver lesions with 3-year survival more than 80% and 5-year survival of 51%. 145 146 Many centers will treat up to five tumors.
-
Overall liver tumor volume
A crude overall liver tumor volume may be calculated as the product of the mean maximum tumor size and mean number of tumors. Gillams et al 32 reviewed 5-year survival in four studies where patients without extrahepatic disease were treated with RFA. A direct correlation between crude liver tumor volume and survival was demonstrated with the best results in those with small volume disease.
-
Tumor location
Thermal ablation in close proximity to central bile ducts carries a higher risk of bile duct injury—bile duct stricture, cholangitis, or liver abscess. While there has been some success reported in cooling the ducts via nasobiliary tube, it is recommended that these lesions be treated with nonthermal ablation modalities, such as irreversible electroporation or transarterial therapies.
Surface lesions pose an increased risk of tumor seeding and hemorrhage. When embarking on thermal ablation of these lesions, a no-touch technique should be used, whereby the antennae are placed on either side of the lesion parallel or perpendicular to each other to bracket the tumor. Ultrasound may offer the best visualization of the tumor boundaries and facilitate precise placement of the antennae in these lesions. Hydrodissection may be necessary to prevent injury to the abdominal wall. While ascites have also been cited as a relative contraindication to percutaneous ablation, the fluid can provide a suitable sonographic window for probe placement and act as a buffer between the ablation zone and the abdominal wall. The ascites should be drained before removal of the antenna.
RFA of tumors close to major blood vessels (>3 mm) is more likely to require a second treatment or result in a recurrence in this region due to the heat-sink phenomenon. Efforts to counteract this limitation of RFA include placing more probes on the vessel side of the lesion, or to use increased power and longer burn times in these lesions. Alternatively, MWA and nonthermal ablation modalities may be used.
Hydrodissection or CO 2 dissection should be employed when ablating tumors close to (<1 cm) the bowel or gallbladder to protect these structures from thermal injury.
In patients who have undergone liver resection and have a single remaining hepatic vein, portal vein or major bile duct, caution should be exercised when ablating a lesion in close proximity to these structures.
-
Sphincter of Oddi disruption
There is no consensus on the effectiveness of prophylactic antibiotics for patients undergoing thermal ablation to reduce the risk of postprocedural infection. Potential pathogens include Staphylococcus aureus , Staphylococcus epidermidis , Streptococcus species, Escherichia coli , Proteus species, Klebsiella species, and Enterococcus species. Ampicillin/sulbactam IV within 1 hour of starting the procedure—or for penicillin-allergic patients, vancomycin or clindamycin for gram-positive coverage and an aminoglycoside (e.g., gentamicin) for gram-negative coverage—has been proposed. Prior biliary tract intervention is a significant risk factor for infectious complications following percutaneous thermal ablation—the risk of liver abscess postablation is reported at 40% in those with bilioenteric anastomosis. 147 The authors placed this high-risk patient group on oral Moxifloxacin for 2 days prior and 17 days postablation.
-
Local recurrence
While there are many advantages to using thermal ablation over surgical resection to treat liver metastases, the biggest disadvantage is its inferior local control rates. If a lesion is to be treated with curative intent, then the interventional radiologist should aim to achieve a sufficient circumferential ablation margin that will minimize the risk of local recurrence. Factors that can improve local control include the use of contrast-enhanced imaging to delineate the tumor prior to ablation, a circumferential margin of at least 1 cm, an understanding and familiarity with the ablation technology, the device in use, and the use of general anesthesia. Finally operator experience with a minimum of 100 liver tumors has also been correlated with decreased recurrence rates. 148
Catheter-Based Therapies
-
1. Portal vein thrombus
Patients with portal vein thrombus have higher liver parenchyma dependency on arterial supply; therefore, additional consideration should be made in treatment of patients with portal vein thrombus. Theoretically, the least embolic treatment would preserve the most perfusion to the liver parenchyma. In our practice, the first transarterial treatment consideration for patients with portal vein thrombus is radioembolization due to its relative microembolic effect, a practice supported by prospective data. 149 150 Recently, a retrospective study of patients with portal vein thrombus showed statistically better toxicity profile and improved OS after radioembolization treatment with glass microspheres compared with resin microspheres, 151 also supporting the theory that lower embolic treatment may be better. Note that despite the theoretic contraindication of chemoembolization in patients with portal vein thrombus, chemoembolization has also been shown to be safe and effective. 152 153
-
2. Arterioportal shunting
Arterioportal shunting may be seen in patients with portal vein tumor thrombus or with hypervascular tumors. If the portal shunting flows toward the treatment zone, treatment is performed with a selective segmental approach. If lobar treatment is required, the liver volume is divided between multiple selective segmental treatment sessions. However, if there is significant arterioportal shunting with portal flow away from the treatment zone, more caution should be taken. In these cases, initial treatment may be performed with larger particles to close the shunt and spare nontargeted liver parenchyma.
-
3. Lung shunt fraction
In patients with elevated lung shunt fractions, radioembolization may be contraindicated or limited. For these patients, we perform bland embolization or chemoembolization as the first-line transarterial therapy. If further therapy is required, repeat evaluation of the lung shunt fraction is made. Often after initial particle embolization, the lung shunt fraction decreases to a value safe for radioembolization. It must be noted that elevated lung shunt fraction is an independent prognostic indicator of poorer survival for patients with colorectal metastases and those with neuroendocrine liver metastases. 154 155
-
4. Sphincter of Oddi dysfunction
There is higher risk of biloma formation and infection after transarterial therapies in patients with prior biliary interventions. For these patients, we prefer transarterial therapy with radioembolization, again due to its relatively microembolic effect. Whichever transarterial therapy we use in these patients, antibiotic prophylaxis with Moxifloxacin is given. 156
Case Review
Case 1 : A 73-year-old man, background history of renal cell carcinoma with sarcomatoid features, was treated with radical nephrectomy. Seven years later, an enlarging liver mass was identified. A biopsy confirmed renal cell carcinoma metastatic disease. He was deemed unsuitable for liver resection due to cardiac comorbidities. Systemic treatment options were limited. He was referred to interventional radiology for an opinion regarding liver-directed therapy. The lesion was treated with bland embolization but recurred within 6 months. A decision was made to retreat this single 4.6-cm hypervascular lesion with a combination of conventional TACE followed by thermal ablation ( Fig. 3 ). The patient's postoperative course was uneventful. The patient has no documented recurrence or new metastases 18 months after liver-directed therapy.
Fig. 3.
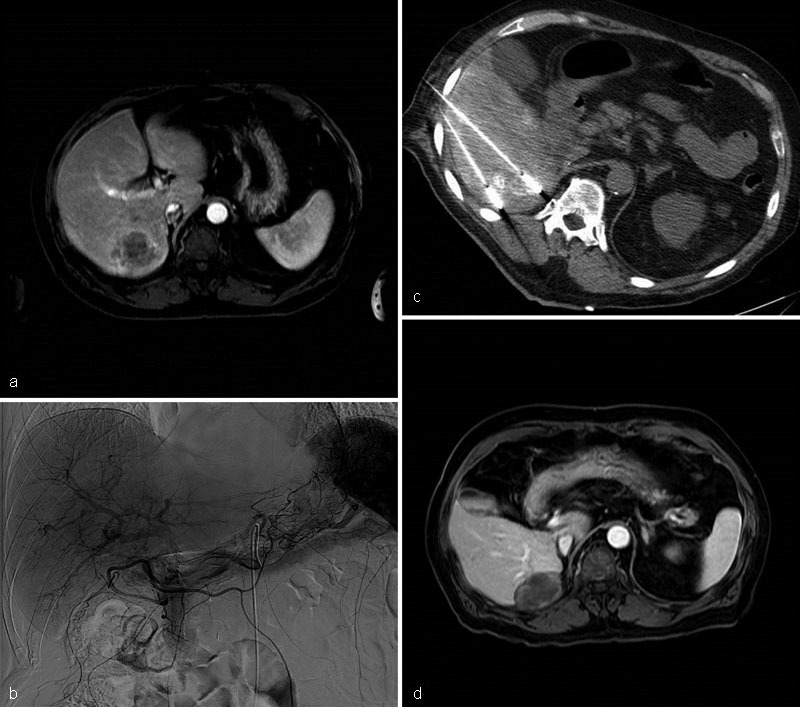
Case 1: MRI of the abdomen postcontrast T1-weighted sequence demonstrates an enhancing 4.6 cm × 4 cm mass in segments 6/7 ( a ). Celiac arteriogram: a large hypervascular mass in the right lobe of liver is identified. cTACE with Doxorubicin and Mitomycin was performed ( b ). A week later, the patient underwent MWA using 3 antennae ( c ). MRI of the abdomen postcontrast T1-weighted sequence 18 months posttreatment shows no evidence of recurrent disease. No extrahepatic metastases were identified ( d ).
Learning point : In the absence of evidence-based data to direct patient care, liver-directed therapy may be an appropriate minimally invasive nonsurgical option in patients with liver-dominant disease. It can easily be combined with other therapies and does not significantly delay or disrupt systemic treatment.
Case 2 : A 74-year-old woman was diagnosed with adenocarcinoma of the head of pancreas. She underwent Whipple procedure (T3N1) followed by adjuvant radiation and chemotherapy with Gemcitabine and Capecitabine. Two and a half years after completion of chemotherapy, CT imaging demonstrated a single liver lesion measuring 2.8 cm in segment 8 that remained stable over a 6-month period. A biopsy demonstrated metastatic adenocarcinoma. The following options were discussed with the patient—watchful waiting, recommence systemic therapy, or consider a liver-directed treatment. In light of other active comorbidities, the patient was keen to avoid further chemotherapy. Thermal ablation of the lesion and the significant risk of infection (cholangitis/liver abscess) in the setting of a bilioenteric anastomosis were discussed in detail with the patient. She accepted the risk and opted to proceed with MWA ( Fig. 4 ). She received a course of Moxifloxacin perioperatively. Her postoperative course was uneventful.
Fig. 4.
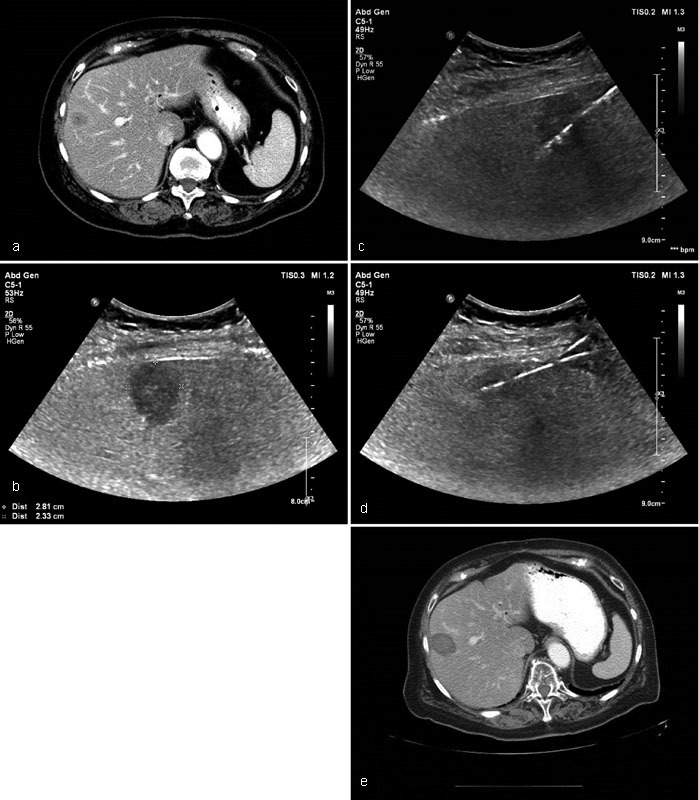
Case 2: Contrast-enhanced CT demonstrates a hypoattenuating mass in segment 8 of the liver consistent with metastatic disease. Air in the biliary tree status post bilioenteric anastomosis ( a ). Ultrasound confirmed a 2.8 × 2.3 cm hypoechoic mass at the periphery of segment 8 ( b ). Two antennae were placed with ultrasound guidance. Microwave ablation was performed ( c and d ). Contrast-enhanced CT abdomen 6 months posttreatment shows no evidence of recurrent disease. No new metastases were identified ( e ).
Learning point : While there is a paucity of evidence to support the use of locoregional therapy in the treatment of pancreatic adenocarcinoma metastatic disease, liver-directed therapy may be appropriate in patients who cannot tolerate chemotherapy, request a “chemotherapy holiday,” or for those in whom quality of life is significantly affected by other treatments.
Case 3 : A 52-year-old man was diagnosed with well-differentiated pancreatic neuroendocrine tumor metastatic to the liver (K i -67 < 20%). The patient had a background history of HIV, and poorly controlled diabetes mellitus. He was not eligible for resection of the primary tumor. An Octreotide scan showed diffuse metabolic activity within the liver. He was commenced on a somatostatin analog and systemic chemotherapy regimen of Capecitabine and Temozolomide. Cross-sectional imaging showed stable size of the primary tumor and a partial response in the liver to systemic therapy. The patient was referred for concomitant liver-directed therapy. Given the disseminated nature of the liver metastases, catheter-based embolotherapies and the risk–benefit profiles of each were discussed. The patient was keen to keep the number of procedures to a minimum. He opted to undergo bland embolization. Bilobar embolization was performed over three encounters. Follow-up MRI of the abdomen 6 months after the initial transarterial treatment showed significant decrease in size and vascularity of the tumors ( Fig. 5 ). Chromogranin A decreased from 18 nmol/L pretreatment to 2 nmol/L posttreatment (reference: 0–5). The patient is now maintained on systemic therapy.
Fig. 5.
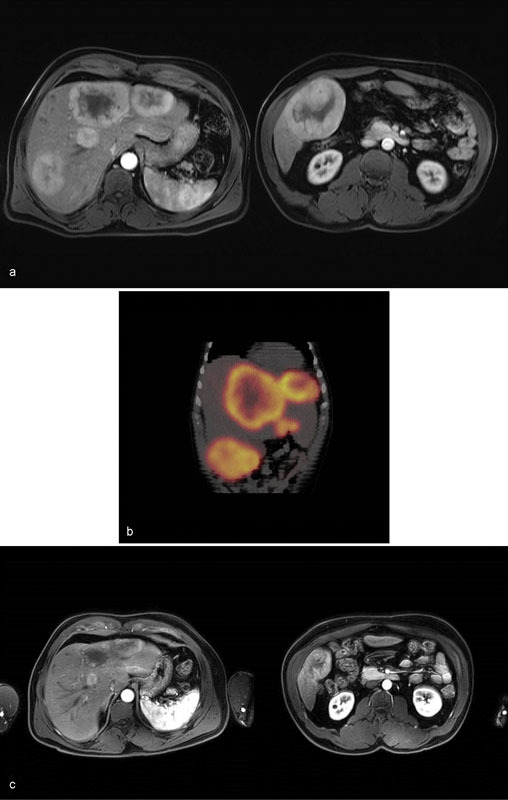
Case 3: MRI of the liver postcontrast T1-weighted sequence shows multiple bilobar hypervascular liver masses ( a ). Octreotide SPECT CT coronal reconstruction demonstrates significant metabolic activity within the known neuroendocrine liver metastases ( b ). MRI of the liver T1-weighted sequence postcontrast performed 6 months following the initial bland embolization shows significant decrease in size of the liver metastases. No new lesions are seen ( c ).
Learning point : Liver metastases are an important prognostic factor in patients with neuroendocrine tumors. In this young patient in whom the primary cancer cannot be resected and there are diffuse liver metastases, the goals of care are to limit the progression of disease and to prolong survival. If liver-directed therapy is to be integrated with systemic chemotherapy, multidisciplinary cooperation is crucial to coordinate the treatments and to monitor closely for signs of hepatotoxicity.
Case 4 : A 65-year-old man diagnosed with carcinoid tumor of the lung was treated with right upper lobectomy. Four years later, he developed a single liver metastasis associated with facial flushing and diaphoresis. Percutaneous RFA successfully treated the tumor and alleviated his symptoms. Over the next 2 years, he was managed with an octreotide analog. He then developed further liver metastases, his symptoms recurred, and serum chromogranin A rose progressively. He underwent cTACE (Doxorubicin, Mitomycin, and Cisplatin) to tumors in segments II and III of the liver. He reported improvement in symptoms and a decrease in serum chromogranin from 585 to 78 ng/mL (reference: 1.9–15). In the 4 years to follow, he developed new bilobar liver metastases accompanied by a steady rise in serum chromogranin A. He underwent further cTACE (Doxorubicin, Mitomycin, and Cisplatin) to both lobes of the liver that successfully palliated the hormone- and bulk-related symptoms. Serum chromogranin decreased from 558 to 161 ng/mL (reference: 1.9–15). One year after cTACE and 12 years since the initial diagnosis was made, the patient is asymptomatic, and the liver tumor burden is stable by cross-sectional imaging. He is not undergoing any active treatment at present ( Fig. 6 ).
Fig. 6.
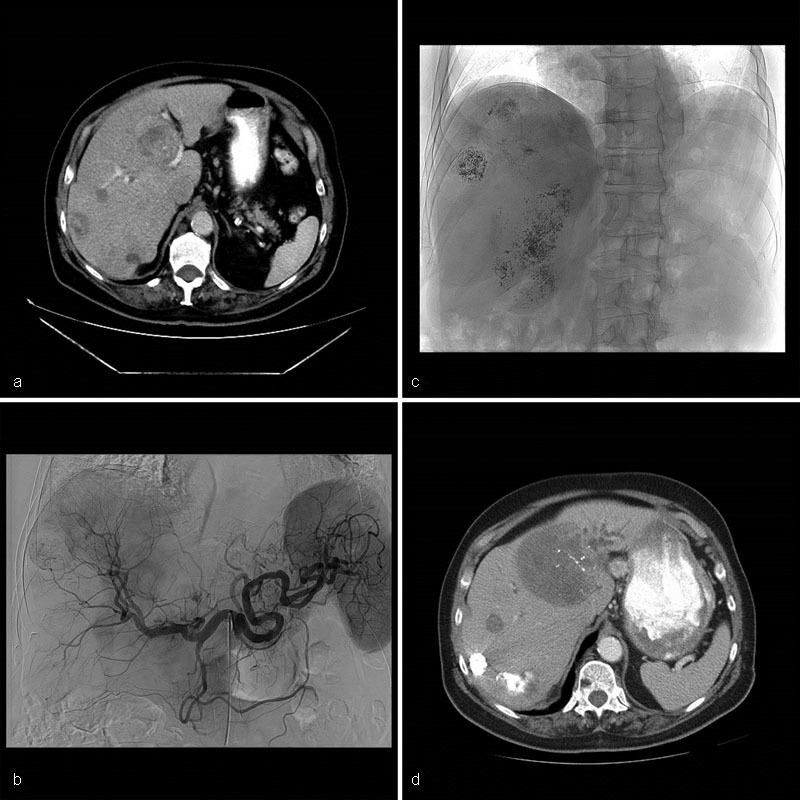
Case 4: CT of the abdomen portal venous phase shows bilobar liver metastases ( a ). Celiac arteriography shows multiple hypervascular masses within both lobes of the liver ( b ). Fluoroscopic spot image shows Ethiodol staining within the tumors following cTACE with Doxorubicin and Mitomycin ( c ). CT of the abdomen portal venous phase one year later shows decreased vascularity and Ethiodol staining within multiple liver masses. New left lateral segment bile duct dilation secondary to mass effect by the metastases ( d ).
Learning point : This case highlights the chronic natural history of low-grade neuroendocrine tumors, the need for multidisciplinary input at different times in the patient's cancer history, and the appropriateness of watchful waiting when patients are asymptomatic and the disease is stable.
Case 5 : A 49-year-old woman with a background history of splenic flexure colon cancer, liver metastases, and alcoholic liver disease underwent left hemicolectomy and received adjuvant FOLFOX and Bevacizumab. The liver metastases were successfully downsized and subsequently resected. Fifteen months later, she developed a new liver lesion that was biopsy-confirmed metastatic disease. She was commenced on FOLFIRI and Cetuximab. After six cycles, PET-CT showed interval growth of the liver mass with no extrahepatic disease. She was referred for radioembolization. A single treatment was performed from the posterior right hepatic artery. The 6-month follow-up MRI of the abdomen showed tumor necrosis and a significant reduction in the size of the lesion ( Fig. 7 ).
Fig. 7.
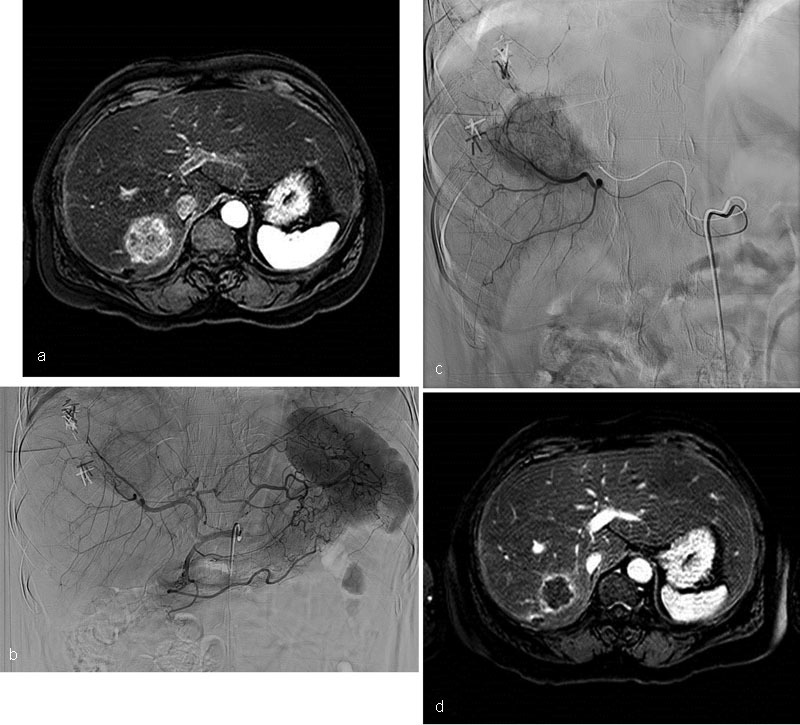
Case 5: MRI of the abdomen postcontrast fat-saturated T1-weighted sequence demonstrates a heterogeneously enhancing 5 × 5 × 3.9 cm mass in segment 7 consistent with metastases ( a ). Celiac arteriogram shows a large enhancing mass in the posterior right lobe ( b and c ). MRI of the abdomen postcontrast fat-saturated T1-weighted sequence 6 months post-radioembolization shows gross necrosis and reduction in size of the segment 7 mass. It now measures 3.6 × 2.6 × 2.7 cm ( d) .
Learning point : New metastases after liver resection are not uncommon—locoregional therapy is an important treatment option in those with limited liver reserve.
Conclusion
As longevity increases and personalized medicine evolves, the need for minimally invasive, targeted, nonsurgical interventions will increase. Locoregional therapies represent an important tool in the treatment of liver metastases that are currently used to palliate symptoms, control disease, and in some cancers have been shown to prolong survival. Clinical trials are needed to determine how locoregional therapies should be integrated into the current treatment paradigms.
References
- 1.Namasivayam S, Martin D R, Saini S. Imaging of liver metastases: MRI. Cancer Imaging. 2007;7:2–9. doi: 10.1102/1470-7330.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson P J. Imaging liver metastases: current limitations and future prospects. Br J Radiol. 2000;73(867):234–241. doi: 10.1259/bjr.73.867.10817037. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(01):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet J M, Bruix J.Hepatocellular carcinoma Lancet 2012379(9822):1245–1255. [DOI] [PubMed] [Google Scholar]
- 5.Brace C L. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38(01):65–78. doi: 10.1615/critrevbiomedeng.v38.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammer J, Malagari K, Vogl T et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(01):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steward B W, Kleihues P, eds.Colorectal Cancer. World Cancer Report IACR Press; 2003198–202. [Google Scholar]
- 8.Choti M A, Sitzmann J V, Tiburi M F et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(06):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornprat P, Jarnagin W R, Gonen M et al. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007;14(03):1151–1160. doi: 10.1245/s10434-006-9068-y. [DOI] [PubMed] [Google Scholar]
- 10.Douillard J Y, Cunningham D, Roth A Det al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial Lancet 2000355(9209):1041–1047. [DOI] [PubMed] [Google Scholar]
- 11.de Gramont A, Figer A, Seymour M et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 12.Saltz L B, Cox J V, Blanke C et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 13.Kabbinavar F, Hurwitz H I, Fehrenbacher L et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21(01):60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 14.Tournigand C, André T, Achille E et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(02):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg R M, Sargent D J, Morton R F et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24(21):3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 17.Saltz L B, Clarke S, Díaz-Rubio E et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 18.Hamada A, Yamakado K, Nakatsuka A et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30(07):567–574. doi: 10.1007/s11604-012-0089-0. [DOI] [PubMed] [Google Scholar]
- 19.Jakobs T F, Hoffmann R T, Trumm C, Reiser M F, Helmberger T K.Radiofrequency ablation of colorectal liver metastases: mid-term results in 68 patients Anticancer Res 200626(1B):671–680. [PubMed] [Google Scholar]
- 20.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg S N. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(03):958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 21.Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31(05):948–956. doi: 10.1007/s00270-008-9362-0. [DOI] [PubMed] [Google Scholar]
- 22.de Liguori Carino N, van Leeuwen B L, Ghaneh P, Wu A, Audisio R A, Poston G J. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol. 2008;67(03):273–278. doi: 10.1016/j.critrevonc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Solbiati L, Livraghi T, Goldberg S N et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(01):159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 24.de Baere T, Elias D, Dromain C et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175(06):1619–1625. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 25.Solbiati L, Goldberg S N, Ierace T et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205(02):367–373. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 26.White T J, Roy-Choudhury S H, Breen D J et al. Percutaneous radiofrequency ablation of colorectal hepatic metastases - initial experience. An adjunct technique to systemic chemotherapy for those with inoperable colorectal hepatic metastases. Dig Surg. 2004;21(04):314–320. doi: 10.1159/000080886. [DOI] [PubMed] [Google Scholar]
- 27.Abdalla E K, Vauthey J N, Ellis L Met al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases Ann Surg 200423906818–825., discussion 825–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleicher R J, Allegra D P, Nora D T, Wood T F, Foshag L J, Bilchik A J. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10(01):52–58. doi: 10.1245/aso.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Oshowo A, Gillams A, Harrison E, Lees W R, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90(10):1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- 30.Gillams A R, Lees W R. Radiofrequency ablation of colorectal liver metastases. Abdom Imaging. 2005;30(04):419–426. doi: 10.1007/s00261-004-0256-6. [DOI] [PubMed] [Google Scholar]
- 31.Joosten J, Jager G, Oyen W, Wobbes T, Ruers T. Cryosurgery and radiofrequency ablation for unresectable colorectal liver metastases. Eur J Surg Oncol. 2005;31(10):1152–1159. doi: 10.1016/j.ejso.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Gillams A, Goldberg N, Ahmed M et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25(12):3438–3454. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko S, Jo H, Yun S, Park E, Kim S, Seo H I. Comparative analysis of radiofrequency ablation and resection for resectable colorectal liver metastases. World J Gastroenterol. 2014;20(02):525–531. doi: 10.3748/wjg.v20.i2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89(02):276–284. [PubMed] [Google Scholar]
- 35.Ruers T, Punt C, Van Coevorden F et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23(10):2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg S N, Gazelle G S. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer. 2003;97(12):3027–3035. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
- 37.Pamecha V, Glantzounis G, Davies N, Fusai G, Sharma D, Davidson B. Long-term survival and disease recurrence following portal vein embolisation prior to major hepatectomy for colorectal metastases. Ann Surg Oncol. 2009;16(05):1202–1207. doi: 10.1245/s10434-008-0269-4. [DOI] [PubMed] [Google Scholar]
- 38.Viganò L, Capussotti L, Lapointe R et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21(04):1276–1286. doi: 10.1245/s10434-013-3421-8. [DOI] [PubMed] [Google Scholar]
- 39.Xing M, Kooby D A, El-Rayes B F, Kokabi N, Camacho J C, Kim H S. Locoregional therapies for metastatic colorectal carcinoma to the liver--an evidence-based review. J Surg Oncol. 2014;110(02):182–196. doi: 10.1002/jso.23619. [DOI] [PubMed] [Google Scholar]
- 40.Vogl T J, Gruber T, Balzer J O, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250(01):281–289. doi: 10.1148/radiol.2501080295. [DOI] [PubMed] [Google Scholar]
- 41.Albert M, Kiefer M V, Sun W et al. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, Ethiodol, and polyvinyl alcohol. Cancer. 2011;117(02):343–352. doi: 10.1002/cncr.25387. [DOI] [PubMed] [Google Scholar]
- 42.Hong K, McBride J D, Georgiades C S et al. Salvage therapy for liver-dominant colorectal metastatic adenocarcinoma: comparison between transcatheter arterial chemoembolization versus yttrium-90 radioembolization. J Vasc Interv Radiol. 2009;20(03):360–367. doi: 10.1016/j.jvir.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Sanz-Altamira P M, Spence L D, Huberman M S et al. Selective chemoembolization in the management of hepatic metastases in refractory colorectal carcinoma: a phase II trial. Dis Colon Rectum. 1997;40(07):770–775. doi: 10.1007/BF02055430. [DOI] [PubMed] [Google Scholar]
- 44.Tellez C, Benson A B, III, Lyster M T et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998;82(07):1250–1259. doi: 10.1002/(sici)1097-0142(19980401)82:7<1250::aid-cncr7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Martin R C, II, Scoggins C R, Schreeder M et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121(20):3649–3658. doi: 10.1002/cncr.29534. [DOI] [PubMed] [Google Scholar]
- 46.Bower M, Metzger T, Robbins K et al. Surgical downstaging and neo-adjuvant therapy in metastatic colorectal carcinoma with irinotecan drug-eluting beads: a multi-institutional study. HPB (Oxford) 2010;12(01):31–36. doi: 10.1111/j.1477-2574.2009.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiorentini G, Aliberti C, Tilli M et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32(04):1387–1395. [PubMed] [Google Scholar]
- 48.Martin R C, Joshi J, Robbins K et al. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011;18(01):192–198. doi: 10.1245/s10434-010-1288-5. [DOI] [PubMed] [Google Scholar]
- 49.Richardson A J, Laurence J M, Lam V W. Transarterial chemoembolization with irinotecan beads in the treatment of colorectal liver metastases: systematic review. J Vasc Interv Radiol. 2013;24(08):1209–1217. doi: 10.1016/j.jvir.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 50.Martin R C, Howard J, Tomalty D et al. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc Intervent Radiol. 2010;33(05):960–966. doi: 10.1007/s00270-010-9937-4. [DOI] [PubMed] [Google Scholar]
- 51.Gray B, Van Hazel G, Hope M et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12(12):1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 52.Van Hazel G, Blackwell A, Anderson J et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88(02):78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 53.Gibbs P, Gebski V, Van Buskirk M, Thurston K, Cade D N, Van Hazel G A; SIRFLOX Study Group.Selective Internal Radiation Therapy (SIRT) with yttrium-90 resin microspheres plus standard systemic chemotherapy regimen of FOLFOX versus FOLFOX alone as first-line treatment of non-resectable liver metastases from colorectal cancer: the SIRFLOX study BMC Cancer 201414897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kjellström J, Kjellén E, Johnsson A. In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncol. 2005;44(07):687–693. doi: 10.1080/02841860500247552. [DOI] [PubMed] [Google Scholar]
- 55.Sharma R A, Van Hazel G A, Morgan B et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25(09):1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 56.Kosmider S, Tan T H, Yip D, Dowling R, Lichtenstein M, Gibbs P. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol. 2011;22(06):780–786. doi: 10.1016/j.jvir.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 57.van Hazel G A, Heinemann V, Sharma N K et al. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(15):1723–1731. doi: 10.1200/JCO.2015.66.1181. [DOI] [PubMed] [Google Scholar]
- 58.Dutton S J, Kenealy N, Love S B, Wasan H S, Sharma R A; FOXFIRE Protocol Development Group and the NCRI Colorectal Clinical Study Group.FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer BMC Cancer 201414497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hickey R, Lewandowski R J, Prudhomme T et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: safety and survival outcomes from a 531-patient multicenter study. J Nucl Med. 2016;57(05):665–671. doi: 10.2967/jnumed.115.166082. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy A S, Coldwell D, Nutting C et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65(02):412–425. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 61.Jakobs T F, Hoffmann R T, Dehm K et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol. 2008;19(08):1187–1195. doi: 10.1016/j.jvir.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Mulcahy M F, Lewandowski R J, Ibrahim S M et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer. 2009;115(09):1849–1858. doi: 10.1002/cncr.24224. [DOI] [PubMed] [Google Scholar]
- 63.Cianni R, Urigo C, Notarianni E et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol. 2009;32(06):1179–1186. doi: 10.1007/s00270-009-9658-8. [DOI] [PubMed] [Google Scholar]
- 64.Cosimelli M, Golfieri R, Cagol P P et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103(03):324–331. doi: 10.1038/sj.bjc.6605770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans K A, Richardson M G, Pavlakis N, Morris D L, Liauw W, Bester L. Survival outcomes of a salvage patient population after radioembolization of hepatic metastases with yttrium-90 microspheres. J Vasc Interv Radiol. 2010;21(10):1521–1526. doi: 10.1016/j.jvir.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Bester L, Meteling B, Pocock N et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol. 2012;23(01):96–105. doi: 10.1016/j.jvir.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 67.Lewandowski R J, Memon K, Mulcahy M F et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41(10):1861–1869. doi: 10.1007/s00259-014-2799-2. [DOI] [PubMed] [Google Scholar]
- 68.Saxena A, Meteling B, Kapoor J, Golani S, Morris D L, Bester L. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann Surg Oncol. 2015;22(03):794–802. doi: 10.1245/s10434-014-4164-x. [DOI] [PubMed] [Google Scholar]
- 69.Golfieri R, Mosconi C, Giampalma E et al. Selective transarterial radioembolisation of unresectable liver-dominant colorectal cancer refractory to chemotherapy. Radiol Med (Torino) 2015;120(08):767–776. doi: 10.1007/s11547-015-0504-6. [DOI] [PubMed] [Google Scholar]
- 70.Kennedy A S, Ball D, Cohen S J et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol. 2015;6(02):134–142. doi: 10.3978/j.issn.2078-6891.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao J C, Hassan M, Phan A et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 72.Norton J A, Jensen R T. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240(05):757–773. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Modlin I M, Lye K D, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(04):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 74.Janson E T, Holmberg L, Stridsberg M et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8(07):685–690. doi: 10.1023/a:1008215730767. [DOI] [PubMed] [Google Scholar]
- 75.Soga J. Statistical evaluation of 2001 carcinoid cases with metastases, collected from literature: a comparative study between ordinary carcinoids and atypical varieties. J Exp Clin Cancer Res. 1998;17(01):3–12. [PubMed] [Google Scholar]
- 76.Akyildiz H Y, Mitchell J, Milas M, Siperstein A, Berber E.Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up Surgery 2010148061288–1293., discussion 1293 [DOI] [PubMed] [Google Scholar]
- 77.Mohan H, Nicholson P, Winter D C et al. Radiofrequency ablation for neuroendocrine liver metastases: a systematic review. J Vasc Interv Radiol. 2015;26(07):935–9420. doi: 10.1016/j.jvir.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Steinmüller T, Kianmanesh R, Falconi M et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87(01):47–62. doi: 10.1159/000111037. [DOI] [PubMed] [Google Scholar]
- 79.de Baere T, Deschamps F, Tselikas L et al. GEP-NETS update: Interventional radiology: role in the treatment of liver metastases from GEP-NETs. Eur J Endocrinol. 2015;172(04):R151–R166. doi: 10.1530/EJE-14-0630. [DOI] [PubMed] [Google Scholar]
- 80.Ruutiainen A T, Soulen M C, Tuite C M et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18(07):847–855. doi: 10.1016/j.jvir.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 81.Pitt S C, Knuth J, Keily J M et al. Hepatic neuroendocrine metastases: chemo- or bland embolization? J Gastrointest Surg. 2008;12(11):1951–1960. doi: 10.1007/s11605-008-0640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta S, Johnson M M, Murthy R et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104(08):1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 83.Kress O, Wagner H J, Wied M, Klose K J, Arnold R, Alfke H.Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors--a retrospective single-center analysis Digestion 200368(2-3):94–101. [DOI] [PubMed] [Google Scholar]
- 84.Kennedy A S, Dezarn W A, McNeillie P et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31(03):271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 85.Chen J X, Rose S, White S B et al. Embolotherapy for neuroendocrine tumor liver metastases: prognostic factors for hepatic progression-free survival and overall survival. Cardiovasc Intervent Radiol. 2017;40(01):69–80. doi: 10.1007/s00270-016-1478-z. [DOI] [PubMed] [Google Scholar]
- 86.Stockler M, Wilcken N R, Ghersi D, Simes R J. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev. 2000;26(03):151–168. doi: 10.1053/ctrv.1999.0161. [DOI] [PubMed] [Google Scholar]
- 87.Jain S K, Dorn P L, Chmura S Jet al. Incidence and implications of oligometastatic breast cancer J Clin Oncol 201230(Suppl):e11512 [Google Scholar]
- 88.Hanrahan E O, Broglio K R, Buzdar A U et al. Combined-modality treatment for isolated recurrences of breast carcinoma: update on 30 years of experience at the University of Texas M.D. Anderson Cancer Center and assessment of prognostic factors. Cancer. 2005;104(06):1158–1171. doi: 10.1002/cncr.21305. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi T, Ichiba T, Sakuyama T et al. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19(03):218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 90.Weinrich M, Weiß C, Schuld J, Rau B M. Liver resections of isolated liver metastasis in breast cancer: results and possible prognostic factors. HPB Surg. 2014;2014:893829. doi: 10.1155/2014/893829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Walsum G A, de Ridder J A, Verhoef C et al. Resection of liver metastases in patients with breast cancer: survival and prognostic factors. Eur J Surg Oncol. 2012;38(10):910–917. doi: 10.1016/j.ejso.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 92.Hoffmann K, Franz C, Hinz U et al. Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surg Oncol. 2010;17(06):1546–1554. doi: 10.1245/s10434-010-0931-5. [DOI] [PubMed] [Google Scholar]
- 93.Caralt M, Bilbao I, Cortés J et al. Hepatic resection for liver metastases as part of the “oncosurgical” treatment of metastatic breast cancer. Ann Surg Oncol. 2008;15(10):2804–2810. doi: 10.1245/s10434-008-0072-2. [DOI] [PubMed] [Google Scholar]
- 94.Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotté M. Liver resection for breast cancer metastasis: does it improve survival? Surg Today. 2008;38(04):293–299. doi: 10.1007/s00595-007-3617-2. [DOI] [PubMed] [Google Scholar]
- 95.Adam R, Aloia T, Krissat Jet al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 200624406897–907., discussion 907–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pagani O, Senkus E, Wood W et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102(07):456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bergenfeldt M, Jensen B V, Skjoldbye B, Nielsen D. Liver resection and local ablation of breast cancer liver metastases--a systematic review. Eur J Surg Oncol. 2011;37(07):549–557. doi: 10.1016/j.ejso.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 98.Sofocleous C T, Nascimento R G, Gonen M et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189(04):883–889. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 99.Meloni M F, Andreano A, Laeseke P F, Livraghi T, Sironi S, Lee F T., Jr Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology. 2009;253(03):861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jakobs T F, Hoffmann R-T, Schrader A et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32(01):38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 101.Carrafiello G, Fontana F, Cotta E et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med (Torino) 2011;116(07):1059–1066. doi: 10.1007/s11547-011-0697-2. [DOI] [PubMed] [Google Scholar]
- 102.Gunabushanam G, Sharma S, Thulkar Set al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients J Vasc Interv Radiol 200718(1, Pt 1):67–72. [DOI] [PubMed] [Google Scholar]
- 103.Vogl T J, Freier V, Nour-Eldin N E, Eichler K, Zangos S, Naguib N N. Magnetic resonance-guided laser-induced interstitial thermotherapy of breast cancer liver metastases and other noncolorectal cancer liver metastases: an analysis of prognostic factors for long-term survival and progression-free survival. Invest Radiol. 2013;48(06):406–412. doi: 10.1097/RLI.0b013e31828328d7. [DOI] [PubMed] [Google Scholar]
- 104.Mack M G, Straub R, Eichler K, Söllner O, Lehnert T, Vogl T J. Breast cancer metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;233(02):400–409. doi: 10.1148/radiol.2332030454. [DOI] [PubMed] [Google Scholar]
- 105.Eichler K, Jakobi S, Gruber-Rouh T, Hammerstingl R, Vogl T J, Zangos S. Transarterial chemoembolisation (TACE) with gemcitabine: phase II study in patients with liver metastases of breast cancer. Eur J Radiol. 2013;82(12):e816–e822. doi: 10.1016/j.ejrad.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 106.Giroux M F, Baum R A, Soulen M C. Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol. 2004;15(03):289–291. doi: 10.1097/01.rvi.0000116190.44877.04. [DOI] [PubMed] [Google Scholar]
- 107.Vogl T J, Naguib N N, Nour-Eldin N E, Eichler K, Zangos S, Gruber-Rouh T. Transarterial chemoembolization (TACE) with mitomycin C and gemcitabine for liver metastases in breast cancer. Eur Radiol. 2010;20(01):173–180. doi: 10.1007/s00330-009-1525-0. [DOI] [PubMed] [Google Scholar]
- 108.Duan X F, Dong N N, Zhang T, Li Q. Treatment outcome of patients with liver-only metastases from breast cancer after mastectomy: a retrospective analysis. J Cancer Res Clin Oncol. 2011;137(09):1363–1370. doi: 10.1007/s00432-011-1008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li X P, Meng Z Q, Guo W J, Li J. Treatment for liver metastases from breast cancer: results and prognostic factors. World J Gastroenterol. 2005;11(24):3782–3787. doi: 10.3748/wjg.v11.i24.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin Y T, Medioni J, Amouyal G et al. Doxorubicin-loaded 70–150 µm microspheres for liver-dominant metastatic breast cancer: results and outcomes of a pilot study. Cardiovasc Intervent Radiol. 2017;40(01):81–89. doi: 10.1007/s00270-016-1465-4. [DOI] [PubMed] [Google Scholar]
- 111.Martin R C, Robbins K, Fagés J F et al. Optimal outcomes for liver-dominant metastatic breast cancer with transarterial chemoembolization with drug-eluting beads loaded with doxorubicin. Breast Cancer Res Treat. 2012;132(02):753–763. doi: 10.1007/s10549-011-1926-z. [DOI] [PubMed] [Google Scholar]
- 112.Saxena A, Kapoor J, Meteling B, Morris D L, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol. 2014;21(04):1296–1303. doi: 10.1245/s10434-013-3436-1. [DOI] [PubMed] [Google Scholar]
- 113.Bangash A K, Atassi B, Kaklamani V et al. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18(05):621–628. doi: 10.1016/j.jvir.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 114.Coldwell D M, Kennedy A S, Nutting C W. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2007;69(03):800–804. doi: 10.1016/j.ijrobp.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 115.Jakobs T F, Hoffmann R T, Fischer T et al. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19(05):683–690. doi: 10.1016/j.jvir.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Haug A R, Tiega Donfack B P, Trumm C et al. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53(03):371–377. doi: 10.2967/jnumed.111.096230. [DOI] [PubMed] [Google Scholar]
- 117.Gordon A C, Gradishar W J, Kaklamani V Get al. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy J Vasc Interv Radiol 201425101523–1532., 1532.e1–1532.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cianni R, Pelle G.Evidence-based integration of selective internal radiation therapy into the management of breast cancer liver metastases Future Oncol 201410(15, Suppl):93–95. [DOI] [PubMed] [Google Scholar]
- 119.Pieper C C, Meyer C, Wilhelm K E et al. Yttrium-90 radioembolization of advanced, unresectable breast cancer liver metastases—a single-center experience. J Vasc Interv Radiol. 2016;27(09):1305–1315. doi: 10.1016/j.jvir.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 120.Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 121.Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012;38(05):549–553. doi: 10.1016/j.ctrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Mariani P, Almubarak M M, Kollen M et al. Radiofrequency ablation and surgical resection of liver metastases from uveal melanoma. Eur J Surg Oncol. 2016;42(05):706–712. doi: 10.1016/j.ejso.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 123.Doussot A, Nardin C, Takaki H et al. Liver resection and ablation for metastatic melanoma: a single center experience. J Surg Oncol. 2015;111(08):962–968. doi: 10.1002/jso.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dayani P N, Gould J E, Brown D B, Sharma K V, Linette G P, Harbour J W. Hepatic metastasis from uveal melanoma: angiographic pattern predictive of survival after hepatic arterial chemoembolization. Arch Ophthalmol. 2009;127(05):628–632. doi: 10.1001/archophthalmol.2009.45. [DOI] [PubMed] [Google Scholar]
- 125.Gupta S, Bedikian A Y, Ahrar J et al. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: response, survival, and prognostic factors. Am J Clin Oncol. 2010;33(05):474–480. doi: 10.1097/COC.0b013e3181b4b065. [DOI] [PubMed] [Google Scholar]
- 126.Mavligit G M, Charnsangavej C, Carrasco C H, Patt Y Z, Benjamin R S, Wallace S. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA. 1988;260(07):974–976. [PubMed] [Google Scholar]
- 127.Bedikian A Y, Legha S S, Mavligit G et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76(09):1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 128.Sato T, Nathan F E, Berd D et al. Lack of effect from chemoembolization for liver metastasis from uveal melanoma. Proc Am Soc Clin Oncol. 1995;14:415. [Google Scholar]
- 129.Patel K, Sullivan K, Berd D et al. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a phase II study. Melanoma Res. 2005;15(04):297–304. doi: 10.1097/00008390-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 130.Huppert P E, Fierlbeck G, Pereira P et al. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol. 2010;74(03):e38–e44. doi: 10.1016/j.ejrad.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 131.Vogl T, Eichler K, Zangos S et al. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol. 2007;133(03):177–184. doi: 10.1007/s00432-006-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Edelhauser G, Schicher N, Berzaczy D et al. Fotemustine chemoembolization of hepatic metastases from uveal melanoma: a retrospective single-center analysis. AJR Am J Roentgenol. 2012;199(06):1387–1392. doi: 10.2214/AJR.11.7748. [DOI] [PubMed] [Google Scholar]
- 133.Sharma K V, Gould J E, Harbour J W et al. Hepatic arterial chemoembolization for management of metastatic melanoma. AJR Am J Roentgenol. 2008;190(01):99–104. doi: 10.2214/AJR.07.2675. [DOI] [PubMed] [Google Scholar]
- 134.Valpione S, Aliberti C, Parrozzani R et al. A retrospective analysis of 141 patients with liver metastases from uveal melanoma: a two-cohort study comparing transarterial chemoembolization with CPT-11 charged microbeads and historical treatments. Melanoma Res. 2015;25(02):164–168. doi: 10.1097/CMR.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 135.Carling U, Dorenberg E J, Haugvik S P et al. Transarterial chemoembolization of liver metastases from uveal melanoma using irinotecan-loaded beads: treatment response and complications. Cardiovasc Intervent Radiol. 2015;38(06):1532–1541. doi: 10.1007/s00270-015-1093-4. [DOI] [PubMed] [Google Scholar]
- 136.Yamamoto A, Chervoneva I, Sullivan K L et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology. 2009;252(01):290–298. doi: 10.1148/radiol.2521081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Valsecchi M E, Terai M, Eschelman D J et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol. 2015;26(04):523–3200. doi: 10.1016/j.jvir.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gonsalves C F, Eschelman D J, Sullivan K L, Anne P R, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011;196(02):468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 139.Klingenstein A, Haug A R, Zech C J, Schaller U C. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovasc Intervent Radiol. 2013;36(01):158–165. doi: 10.1007/s00270-012-0373-5. [DOI] [PubMed] [Google Scholar]
- 140.Schelhorn J, Richly H, Ruhlmann M, Lauenstein T C, Theysohn J M. A single-center experience in radioembolization as salvage therapy of hepatic metastases of uveal melanoma. Acta Radiol Open. 2015;4(04):2.047981615570417E15. doi: 10.1177/2047981615570417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pingpank J F, Hughes M S, Faries M B et al. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma. J Clin Oncol. 2010;28:18s. [Google Scholar]
- 142.Liu Z, Gao F, Yang G et al. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: an up-to-date meta-analysis. Tumour Biol. 2014;35(08):7407–7413. doi: 10.1007/s13277-014-1976-z. [DOI] [PubMed] [Google Scholar]
- 143.Hammill C W, Billingsley K G, Cassera M A, Wolf R F, Ujiki M B, Hansen P D. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol. 2011;18(07):1947–1954. doi: 10.1245/s10434-010-1535-9. [DOI] [PubMed] [Google Scholar]
- 144.Nielsen K, van Tilborg A A, Meijerink M R et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg. 2013;37(06):1340–1347. doi: 10.1007/s00268-013-1997-6. [DOI] [PubMed] [Google Scholar]
- 145.Gillams A R, Lees W R. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol. 2008;19(05):712–717. doi: 10.1016/j.jvir.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 146.Kim K H, Yoon Y S, Yu C S et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011;81(01):25–34. doi: 10.4174/jkss.2011.81.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Elias D, Di Pietroantonio D, Gachot B, Menegon P, Hakime A, De Baere T.Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure Gastroenterol Clin Biol 200630(6-7):823–827. [DOI] [PubMed] [Google Scholar]
- 148.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(02):158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kulik L M, Carr B I, Mulcahy M F et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(01):71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 150.Mazzaferro V, Sposito C, Bhoori S et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(05):1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 151.Biederman D M, Titano J J, Tabori N E et al. Outcomes of radioembolization in the treatment of hepatocellular carcinoma with portal vein invasion: resin versus glass microspheres. J Vasc Interv Radiol. 2016;27(06):812–82100. doi: 10.1016/j.jvir.2016.01.147. [DOI] [PubMed] [Google Scholar]
- 152.Kothary N, Weintraub J L, Susman J, Rundback J H.Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk J Vasc Interv Radiol 200718121517–1526., quiz 1527 [DOI] [PubMed] [Google Scholar]
- 153.Chung G E, Lee J H, Kim H Y et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(02):627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 154.Narsinh K H, Van Buskirk M, Kennedy A S et al. Hepatopulmonary shunting: a prognostic indicator of survival in patients with metastatic colorectal adenocarcinoma treated with (90)Y radioembolization. Radiology. 2017;282(01):281–288. doi: 10.1148/radiol.2016152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ludwig J M, Ambinder E M, Ghodadra A, Xing M, Prajapati H J, Kim H S. Lung shunt fraction prior to Yttrium-90 radioembolization predicts survival in patients with neuroendocrine liver metastases: single center prospective analysis. Cardiovasc Intervent Radiol. 2016;39(07):1007–1014. doi: 10.1007/s00270-016-1323-4. [DOI] [PubMed] [Google Scholar]
- 156.Khan W, Sullivan K L, McCann J W et al. Moxifloxacin prophylaxis for chemoembolization or embolization in patients with previous biliary interventions: a pilot study. AJR Am J Roentgenol. 2011;197(02):W343-5. doi: 10.2214/AJR.10.6019. [DOI] [PubMed] [Google Scholar]
- 157.Frilling A, Clift A K. Therapeutic strategies for neuroendocrine liver metastases. Cancer. 2015;121(08):1172–1186. doi: 10.1002/cncr.28760. [DOI] [PubMed] [Google Scholar]


