Abstract
Shenmai injection (SMI) has been widely used as a therapy to treat a number of diseases. However, its anti-osteoarthritic properties have not yet been fully investigated. In the present study, the protective effect of SMI on knee articular cartilage of anterior cruciate ligament transected rabbits and interleukin-1β (IL-1β)-stimulated human chondrocytes was investigated. For the in vivo study, knee osteoarthritis (KOA) was induced in female New Zealand white rabbits by anterior cruciate ligament transection (ACLT) in the knee of right hind limb. Rabbits either underwent sham surgery or ACLT surgery. Out of the rabbits receiving ACLT surgery, half of the rabbits received one 0.3 ml Shenmai intra-articular injection in the knee per week for four weeks, following ACLT surgery. The other rabbits received the same volume of normal saline solution. The cartilage was subsequently collected for histological evaluation. For the in vitro study, cultured human chondrocytes were treated with 10 ng/ml IL-1β in the presence or absence of 5 and 2% (v/v) SMI for 24 h. Nitric oxide (NO) and prostaglandin E2 (PGE2) levels in cell culture supernatant were assessed using a Griess reaction and ELISA respectively. The mRNA expression of cyclooxgenase-2 (COX-2), inducible nitric oxide synthase (iNOS), matrix metalloproteinase (MMP)-1, MMP-13 and tissue inhibitors of metalloproteinase-1 (TIMP-1) in chondrocytes were detected by reverse transcription-quantitative polymerase chain reaction. The results of the current study revealed that treatment with SMI ameliorated cartilage degradation in the ACLT rabbit model, and decreased levels of NO and PGE2. Furthermore, treatment with SMI decreased levels of COX-2, iNOS, MMP-1 and MMP-13 mRNA expression and increased TIMP-1 mRNA expression in IL-1β-stimulated human chondrocytes. These results indicate that SMI suppresses inflammation and ameliorated cartilage degradation, making it a potential and promising therapeutic option to treat KOA.
Keywords: knee osteoarthritis, Shenmai injection, cartilage, anterior cruciate ligament transection, chondrocytes
Introduction
Knee osteoarthritis (KOA) is a common type of degenerative disease in middle-aged and elderly individuals globally, which typically causes uncomfortable knee pain and lower limb movement disorder, impairing quality of life. It is among the most common causes of pain and disability in European countries (1). Its estimated prevalence is 35% among people aged 50–59 years, and 55% for people over 70 years of age (2), while the lifetime risk for KOA is 45% (3). In addition, because of the limited capacity of articular cartilage for self-repair and the growing aging population, the incidence of KOA is increasing in many countries (4), which makes KOA a substantial burden for societies. Clinical KOA is not one single disease, but a final outcome secondary to a number of predisposing factors, notably age, altered biomechanics, deficient sex hormones, joint trauma and obesity. Furthermore, KOA is primarily characterized by the destruction of the articular cartilage, which is accompanied by inflammation due to the activation of certain inflammatory cytokines including interleukin-1β (IL-1β). IL-1β triggers a cascade of inflammation events of articular cartilage in KOA progression (5). It may also induce the expression and production of matrix metalloproteinases (MMPs) including MMP-1 and MMP-13, cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2), nitric oxide (NO) and inducible NO synthase (iNOS) (6–8). Therefore, IL-1β has become a target for novel therapeutic strategies.
Non-surgical modalities for KOA management include weight loss, exercise, activity modification, assistive devices, oral administration of non-steroidal anti-inflammatory drugs, analgesics and intra-articular injections of certain drugs, such as hyaluronic acid preparation (9). Intra-articular injections are the most commonly used approach in the management of KOA to deliver the therapeutic agent directly into the joint space. In September 2000, the American college of rheumatology guidelines for the treatment of knee osteoarthritis recommended that one therapeutic option to be considered is the use of intra-articular injections of hyaluronic acid in the knee joint to relieve osteoarthritic pain (10). This therapeutic strategy has since become popular in the non-operative management of patients with osteoarthritis (11–14). However, recent reviews conclude that intra-articular injection of hyaluronic acid has only minimal benefits on patient outcomes, and so its clinical relevance and cost-utility should be reassessed (15,16). Furthermore, a decrease in drug efficacy after the first treatment may also occur (17). Therefore, due to uncertainty regarding the appropriateness of hyaluronic acid in the clinical non-surgical management of KOA (18), more suitable alternatives for the treatment of KOA are required.
Shenmai injection (SMI) is widely used to clinically treat coronary heart disease, chronic pulmonary heart disease, viral myocarditis, heart and respiratory failure, cerebral infarction and malignant diseases (19–24). In addition, the toxicity of SMI has been evaluated and it is generally considered to be safe to use (25,26). ‘Shen’ and ‘mai’ are Chinese abbreviations for red ginseng and Ophiopogon japonicas. SMI contains a number of gensenosides from red ginseng, considered to be beneficial in the protection of articular cartilage, thus it is worth investigating whether SMI has the potential to treat KOA (27–32). The aim of the present study was to evaluate the anti-KOA activities of SMI on knee articular cartilage in an experimental rabbit KOA model induced by anterior cruciate ligament transection (ACLT), as well as in IL-1β-stimulated human chondrocytes.
Materials and methods
Materials
SMI was purchased from Sanjiu Pharmaceutical Co., Ltd. (Ya'an, China). Ginsenosides Rb1, Rb3, Rg1 Rg3, Rd and Ro were purchased from the National Institutes for Food and Drug Control (Beijing, China). Sodium pentobarbital was purchased from China National Pharmaceutical Group (Shanghai, China). Trypsin, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and Penicillin-Streptomycin (Pen-Strep) were purchased from Mediatech Inc., (Manassas, VA, USA). Recombinant human IL-1β was purchased from PeproTech, Inc (Rocky Hill, NJ, USA). Collagenase II and MTT were purchased from Sigma-Aldrich; Merck kGaA (Darmstadt, Germany). TRIzol® reagent, RevertAid™ First Strand cDNA Synthesis kit and the Maxima™ SYBR-Green/Fluorescein qPCR Master Mix (2x) were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Ethanol and isopropanol of analytical grade were purchased from Dikma Co. (Beijing, China).
High performance liquid chromatography (HPLC) analysis of SMI
HPLC fingerprint analysis was performed using a Waters Alliance 2695–2998 system (Waters Corporation, Milford, MA, USA). Accurately weighted reference standard compounds, ginsenoside Rb1, Rb3, Rg1, Rg3, Ro and Rd were dissolved in methanol to prepare the mixed standard solution. SMI was directly injected into the HPLC for analysis. The injection volume of the standard and sample solutions was 10 µl. A Waters XBridge C18 column (4.6×250 mm, 5 µm) was used. The mobile phase consisted of acetonitrile and 0.1% phosphate solutions and was consecutively programmed as the following gradient with a flow rate of 1.0 ml/min: 19% (0–12 min) A, 19–36% (12–65 min). The column temperature was maintained at 35°C and the detection wavelength was 203 nm. All modules and data collection were controlled by Empower 2 software (Waters Corporation).
Establishment of ACLT surgery-induced KOA and drug treatment
A total of 18 female New Zealand white rabbits weighing (age, 4–6 months; weight, 2.0–2.5 kg) were purchased from Guangdong Medical Laboratory Animal Center (Guangdong, China). All rabbits were kept in a specific pathogen-free room under controlled conditions. A 12 h light-dark cycle was maintained, with lights on between 6:00 a.m. and 6:00 p.m. The temperature was maintained at 21–25°C and the relative humidity was maintained at 40–70%. The rabbits were provided with standard laboratory food and water ad libitum. All 18 rabbits underwent surgery; 12 rabbits were used as the ACLT experimental rabbits and had KOA induced in the knee of the right hind limb, the left knee was left untreated. The ACLT rabbit model was induced as previously described (4,33). In brief, after rabbits were anesthetized with 40 mg/kg sodium pentobarbital (3%) by intravenous administration, a medial arthrotomy was performed. With the right knee positioned in full flexion, the patella was dislocated laterally, and the anterior cruciate ligament was transected. The other 6 rabbits underwent sham surgery and were used as a control group. For the sham surgery, the anterior cruciate ligament of the right knee was exposed but not transected. Finally, wounds of rabbits were closed and the rabbits were allowed unrestricted cage activity following surgery. Wound healing and infection or any other complications were monitored continuously over 1 week. The 12 ACLT experimental rabbits were divided into 2 groups: 6 rabbits received 0.3 ml Shenmei intra-articular injection once a week for 4 weeks (KOA+SMI group) and another group received the same volume of normal saline injection once a week for 4 weeks (KOA group). At the end of 4 weeks, all rabbits were sacrificed with 90 mg/kg sodium pentobarbital (3%) by intravenous administration. Care and experimental treatments of the rabbits were approved by the Ethical Committee of Guangdong Second Traditional Chinese Medicine Hospital (Guangdong, China).
Histological examination
Following sacrifice, the distal femur and proximal tibia of the right side of each rabbit was resected and fixed at 4°C for 48 h in a 10% formalin solution, prior to decalcification in formic acid for 3 days. Following neutralization with 10% sodium sulfate for 24 h, samples were embedded in paraffin. Serial sections, 4–6 µm in thickness, were prepared in the coronal plane through the middle of the femoral and tibia condyles and one section from each sample was used for each of the histological analyses. Specimens were stained with hematoxylin and eosin and Safranin O-Fast Green following standard procedures. All results presented in the present study were acquired using an Olympus BX51 fluorescence microscope (Olympus Corporation, Tokyo, Japan). The histological grade of cartilage degeneration was evaluated according to the modified Mankin's scoring system (17,34,35). Final results represent the combined scoring data of two experienced researchers.
Isolation and culture of human articular chondrocytes
Cartilage was obtained from patients (age 66±10 years) with KOA undergoing total joint replacement surgery. All the patients provided informed consent to be part of the current study and surgery was performed at Guangdong Second Traditional Chinese Medicine Hospital. Cartilage collection was approved by the Ethical Committee of Guangdong Second Traditional Chinese Medicine Hospital. Under aseptic conditions, cartilage sections, 1–3 mm in thickness, were removed from the condyles, minced and treated with 0.25% Trypsin for 30 min followed by 0.02% collagenase II in DMEM mixed with 1% Pen-Strep (containing 10,000 Units/ml penicillin and 10,000 µg/ml streptomycin) at 37°C overnight. Extracted cells were then cultured in 25 cm2 culture flasks in complete DMEM with 10% FBS and 1% Pen Strep in 5% CO2 at 37°C for 4 days. Third-generation chondrocytes were used for the current study.
Examination of relative cell viability
An MTT assay was used to evaluate the cytotoxicity of SMI. Firstly, chondrocytes were seeded in culture medium including complete DMEM with 10% FBS and 1% Pen Strep in 96-well plates (1×104/well) overnight and tested with or without 20, 10, 5, 2, 1 and 0.5% (v/v) SMI for 24 h. Then, cells were incubated with 20 µl MTT solution (5 mg/ml in phosphate-buffered saline) for 4 h at 37°C. Each well was administered 150 µl dimethyl sulfoxide once the supernatant was removed by Finnpipette (Thermo Fisher Scientific, Inc.). In addition, cells were incubated with dimethyl sulfoxide without MTT as a control. Finally, the absorbance of each well was measured at 570 nm using a Varioskan™ Flash Multimode Reader (Thermo Fisher Scientific, Inc.).
Chondrocyte treatments
Chondrocytes were seeded in complete DMEM with 10% FBS and 1% Pen Strep in six-well plates (3×105/well) overnight. Experimental cells were treated with 10 ng/ml IL-1β in the presence or absence of 5 and 2% (v/v) SMI (these concentrations was determined by relative cell viability) and control cells were treated without IL-1β or SMI. All cells were harvested following 24 h incubation at 37°C. Following these processes, cell medium was collected in order to measure the level of NO and PGE2, while chondrocytes were collected for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis.
Measurement of NO and PGE2 levels
The cell supernatant was drawn off by Finnpipette to determine NO levels via the Griess method (36). A 40 µl sample was transferred into 96-well microplates and 160 µl Griess reagent (1% sulfanilamide, 0.1% N- (1-naphthyl) ethylenediamine hydrochloride, 2.5% H3PO4) was added at room temperature. Following 20 min, absorbance was detected using a Varioskan™ Flash Multimode Reader at 540 nm. PGE2 levels in supernatant were quantified using a Prostaglandin E2 parameter assay kit (catalogue no. KGE004B) according to the manufacturer's protocols (R&D Systems, Inc., Minneapolis, MN, USA).
RT-qPCR analysis
Total RNA was extracted from chondrocytes in six-well plates using TRIzol reagent according to the manufacturer's protocol. Total RNA (3 µg) was reverse-transcribed into cDNA using a First Strand cDNA Synthesis kit, according to the manufacturer's protocols (Thermo Fisher Scientific, Inc., Waltham, MA, USA). at 42°C for 1 h. PCR was performed using a IQ™ 5 real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and completed using 1 µl cDNA, 12.5 µl Maxima™ SYBR Green/Fluorescein qPCR Master Mix (2x), 1 µM forward primer and 1 µM reverse primer in a total reaction volume of 25 µl. cDNA was amplified using specific primers (Table I) with 45 cycles at 94°C for 30 sec, an annealing temperature of 55°C for 40 sec and 72°C for 50 sec, with a final incubation period at 72°C for 7 min. qPCR detection was performed on three replicates per cDNA sample. The products were quantified using a Cq value and all values were normalized to GAPDH mRNA concentration. The final result was calculated using the 2−ΔΔCq method (37).
Table I.
Information on PCR primers.
| Gene | Accession no. | Primers (5′-3′) | Product size, bps |
|---|---|---|---|
| GAPDH | M33197 | F: CCCATCACCATCTTCCAGGAG | 105 |
| R: CTTCTCCATGGTGGTGAAGACG | |||
| iNOS | NM_000625 | F: GGAGATGCTGAACTA | 194 |
| R: AGAGGATGGTGACT | |||
| COX-2 | M90100 | F: GAGAGATGTATCCTCCCACAGTCA | 117 |
| R: GACCAGGCACCAGACCAAAG | |||
| MMP-1 | NM_002421 | F: CTGCTGCTGTTCTG | 245 |
| R: GGCTGCTTCATCAC | |||
| MMP-13 | NM_002427 | F: TGCTGCATTCTCCTTCAGGA | 183 |
| R: ATGCATCCAGGGGTCCTGGC | |||
| TIMP-1 | NM_003254 | F: CAACCAGACCACCTT | 130 |
| R: GTATCCGCAGACACT |
PCR, polymerase chain reaction; bps, base pairs; F, forward; R, reverse; GAPDH, glyceraldehypde 3-phosphate dehydrogenase; iNOS, inducible nitric oxide synthase; COX-2, cyclooxgenase-2; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinase.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis of the data was performed using the SPSS 17.0 (SPSS Inc., Chicago, IL, USA) statistical package. One-way analysis of variance was applied to analyze differences in data of all parameters among the different groups, followed by Dunnett's significant post hoc test for pairwise multiple comparisons. Differences were considered statistically significant when P<0.05.
Results
HPLC analysis of SMI
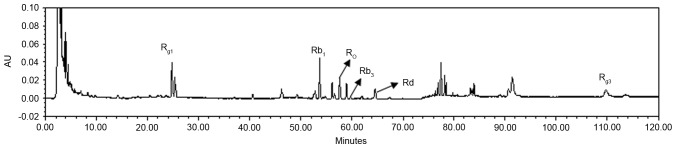
The ginsenosides in SMI were analyzed and quantified as 186.1 µg/ml Rb1, 15.6 µg/ml Rb3, 50.62 µg/ml Rd, 181.6 µg/ml Rg1, 32.34 µg/ml Rg3 and 111.9 µg/ml Ro (Fig. 1).
Figure 1.
Chemical profile of Shenmai injection analyzed by high performance lipid chromatography.
Histological evaluation
H&E staining of articular cartilage is shown in Fig. 2. The nucleus was stained purple-blue and cytoplasm was stained red. Normal articular cartilage was observed in the control group. The KOA group exhibited a marked loss of chondrocytes. In the KOA+SMI group, SMI protected the articular cartilage from loss of chondrocytes. Furthermore, Safranin O-fast green staining of articular cartilage is also shown in Fig. 2. Cartilage was stained red and subchondral bone was stained blue. Normal articular cartilage was shown in control group. KOA group exhibited severe loss of Safranin O-fast green staining and loss of cartilage. In the KOA+SMI group, SMI protected articular cartilage from loss of cartilage. Mankin's scoring was evaluated based on structural changes, cellular changes, Safranin-O staining and tidemark integrity. Final results of Mankin's scoring were shown as sum of scores. Compared with the control group, cartilage lesions in the KOA group were more severe and exhibited a significantly higher Mankin's scoring (P<0.01). However, SMI treatment ameliorated the cartilage lesions and significantly decreased the Mankin's scoring compared with the KOA group (P<0.01; Table II).
Figure 2.
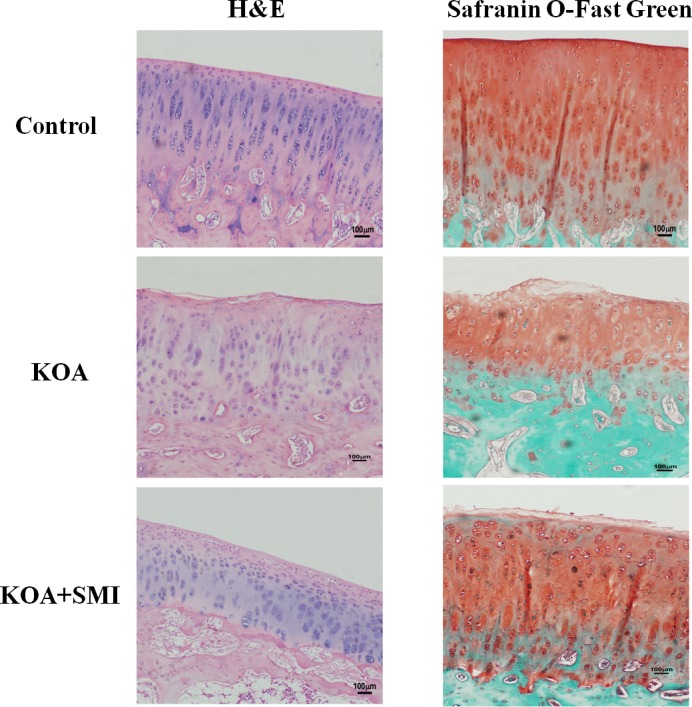
H&E and Safranin O-Fast Green staining of articular cartilage in different groups (magnification ×100). In H&E staining, the nucleus was stained purple-blue and cytoplasm was stained red. In Safranin O-Fast Green staining, cartilage was stained red and subchondral bone was stained blue. H&E, hematoxylin and eosin.
Table II.
Mankin's scoring of articular cartilage in different groups.
| Articular cartilage | Control | KOA | KOA+SMI |
|---|---|---|---|
| Structural changes | 1.83±0.75 | 4.00±0.00b | 3.33±0.82c |
| Cellular changes | 0.67±0.52 | 2.33±1.03b | 1.83±0.75 |
| Safranin-O staining | 0.83±0.75 | 2.17±0.75b | 1.67±0.52 |
| Tidemark integrity | 0.33±0.52 | 1.00±0.00a | 0.83±0.41 |
| Sum of scores | 3.67±1.03 | 9.50±0.55b | 7.67±1.37d |
Results are presented as the mean ± standard deviation obtained from 6 rabbits in each group.
P<0.05
P<0.01 vs. Control group
P<0.05
P<0.01 vs. KOA group. KOA, knee osteoarthritis; SMI, Shenmai injection.
Effect of SMI on relative cell viability
The MTT assay was applied to measure the cytotoxicity of 20, 10, 5, 2, 1 and 0.5% (v/v) SMI in normal chondrocytes. No significant differences were observed in relative cell viability compared with the control group with the exception of 20 and 10% (v/v) SMI, which exhibited significant decreases in relative cell viability compared with the control (P<0.01; Fig. 3). Thus, 5 and 2% (v/v) were selected as the optimal concentrations for further study.
Figure 3.
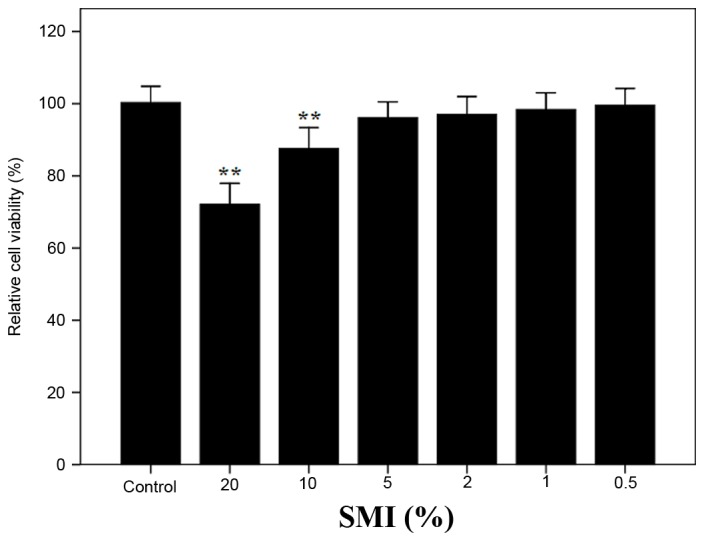
Effect of SMI on relative cell viability. The results are presented as mean ± standard deviation obtained from the 6 wells in each group. **P<0.01 vs. Control group. SMI, shenmai injection.
Effect of SMI on NO and PGE2 levels
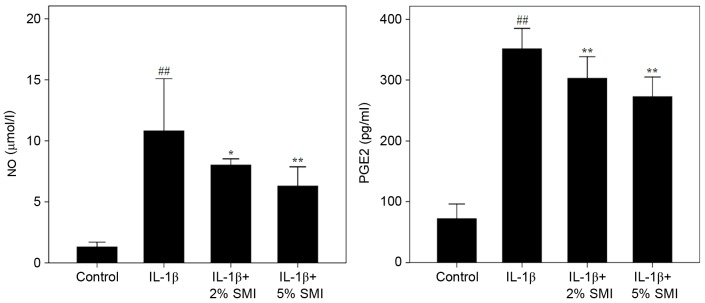
IL-1β treatment resulted in elevated NO and PGE2 levels in the cell culture supernatant compared with controls (P<0.01; Fig. 4). The production of NO and PGE2 was significantly inhibited following treatment with 2% (v/v) SMI, compared with untreated cells (P<0.05 and P<0.01, respectively) and 5% (v/v) SMI (both P<0.01; Fig. 4).
Figure 4.
Effect of SMI on NO and PGE2 levels in human chondrocytes. Results are presented as mean ± standard deviation obtained from 3 wells in each group. ##P<0.01 vs. Control group; *P<0.05, **P<0.01 vs. IL-1β group. SMI, Shenmai injection; NO, nitric oxide; PGE2, prostaglandin; IL-1β, interleukin-1β.
Effect of SMI on mRNA expression of human chondrocytes
RT-qPCR was performed to quantify mRNA expression in human chondrocytes. Compared with the control group, IL-1β caused a significant increase of COX-2, iNOS, MMP-1, MMP-13 mRNA expression (P<0.01) and a significant decrease of TIMP-1 mRNA expression in the IL-1β group (P<0.05; Table III). However, treatment with 2 and 5% (v/v) SMI significantly decreased mRNA expression of COX-2, iNOS, MMP-1 and MMP-13 (P<0.05), and significantly increased TIMP-1 mRNA expression compared with untreated IL-1β-induced cells (P<0.05; Table III).
Table III.
Effect of SMI on mRNA expressions in human chondrocytes.
| Gene | Control | IL-1β | IL-1β+2% SMI | IL-1β+5% SMI |
|---|---|---|---|---|
| COX-2 | 1.00±0.21 | 11.78±2.16b | 7.81±0.86c | 6.37±0.63c |
| iNOS | 1.00±0.37 | 6.43±0.70b | 5.08±0.46c | 3.88±0.40d |
| MMP-1 | 1.00±0.36 | 5.85±0.85b | 4.40±0.22c | 3.86±0.72c |
| MMP-13 | 1.00±0.54 | 9.79±1.05b | 5.63±1.48c | 3.40±0.68d |
| TIMP-1 | 1.00±0.23 | 0.54±0.08a | 0.69±0.03c | 0.81±0.09c |
The results are presented as mean ± standard deviation obtained from 3 wells in each group.
P<0.05
P<0.01 vs. Control group
P<0.05
P<0.01 vs. IL-1β group. SMI, Shenmai injection; IL-1β, interleukin-1β; COX-2, cyclooxgenase-2; iNOS, inducible nitric oxide synthase; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinase.
Discussion
It is well understood that KOA is characterized by cartilage degradation, mild synovial lining inflammation, joint space narrowing, subchondral bone sclerosis and osteophyte formation (38). A network of cytokines promotes cartilage degradation and the catabolic cytokine IL-1β serves a crucial role in this (39). Activation of the IL-1β intracellular signaling pathway results in the upregulation of important genes encoding MMPs, iNOS and COX-2.
The MMP family serves a crucial role in tissue remodeling in addition to the destruction of the extracellular matrix of cartilage and bone in arthritic joints (40). Out of the MMP enzymes, MMP-1 and MMP-13 are the most relevant enzymes in KOA (41). The activity of all known MMPs is regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs). It has been demonstrated that the effect of MMPs is highly dependent on the MMP: TIMP ratio and excessive activity of MMPs compared with TIMPs results in pathological cartilage destruction (41). Imbalance between MMPs and TIMPs such as TIMP-1 is important in the incidence and progression of KOA (42).
Punzi et al (41) reported that oxidative stress serves an important role in the pathology of KOA. Excessive production of oxidants such as NO may occur due to the IL-1β-induced expression of iNOS (43–45). In addition, IL-1β stimulates COX-2 expression to increase synthesis of PGE2, which is responsible for joint pain in KOA (46,47). NO and PGE2 are capable of upregulating the production of MMPs and other inflammatory cytokines (48,49).
In the present study, ACLT was used to establish an experimental in vivo rabbit KOA model. Histological analysis indicated that intra-articular injection of SMI ameliorated cartilage degradation in KOA. An in vitro study was also conducted, in which the KOA microenvironment was mimicked using cultured IL-1β-stimulated human chondrocytes. In the chondrocytes treated with IL-1β, gene expression and production of COX-2, iNOS, MMP-1 and MMP-13 was upregulated, however the expression of TIMP-1 was downregulated and the over-production of NO and PGE2 was observed. SMI at a concentration of 2 and 5% (v/v) significantly decreased levels of COX-2, iNOS, MMP-1, MMP-13 mRNA expression and significantly elevated TIMP-1 mRNA expression (P<0.05), as well as suppressing over-production of NO and PGE2.
In order to analyze the active components of SMI, HPLC was performed. As presented in Fig. 1, the present study identified a number of ginsenosides contained in SMI, including 186.2 µg/ml Rb1, 181.6 µg/ml Rg1 and 111.9 µg/ml Ro. Ginsenoside Rb1 may inhibit the production of inflammatory agents including MMP-13, COX-2, iNOS and NO induced by IL-1β in human articular chondrocytes (28–30). Rg1 inhibited IL-1β induced chondrocyte apoptosis, promoted TIMP-1 expression and inhibited MMP-13 expression via its effects on the phosphatidylinositol-3-kinase/protein kinase B and mitochondrial signaling pathways (31). Furthermore, Ro evidently prevented IL-1β-stimulated chondrocyte apoptosis and inflammation by inhibiting nuclear factor-κB activation (32).
Therefore, gensenosides including Rb1, Rg1 and Ro in SMI may contribute to its protective effect on chondrocytes by regulating the balance between MMPs and TIMPs, inhibiting the production of NO and PGE2 and suppressing the expression of iNOS and COX-2 mRNA. In conclusion, the results of the current study demonstrate that SMI suppresses inflammation and ameliorates cartilage degradation, confirming that SMI has a protective effect on knee articular cartilage in vitro and in vivo. To the best of our knowledge, the current study is the first to demonstrate this protective effect of SMI, and suggests that SMI may be a potential and promising therapeutic option to treat KOA.
Acknowledgements
The present study was supported by Science and Technology Planning Project (Guangdong, China; Grant No. 2013B021800213 and No. 2013B021800214), the Natural Science Foundation of Guangdong Province, China (Grant No. 2014A030310128) and Breakthrough Project of Traditional Chinese Medicine Predominant Disease (Guangdong, China).
References
- 1.Kingsbury SR, Gross HJ, Isherwood G, Conaghan PG. Osteoarthritis in Europe: Impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology (Oxford) 2014;53:937–947. doi: 10.1093/rheumatology/ket463. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Neogi T, Hochberg MC. Quality of osteoarthritis management and the need for reform in the US. Arthritis Care Res (Hoboken) 2011;63:31–38. doi: 10.1002/acr.20278. [DOI] [PubMed] [Google Scholar]
- 3.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang ER, Ma HL, Wang JP, Liu CL, Chen TH, Hung SC. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS One. 2016;11:e0149835. doi: 10.1371/journal.pone.0149835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aigner T, McKenna L, Zien A, Fan Z, Gebhard PM, Zimmer R. Gene expression profiling of serum- and interleukin-1 beta-stimulated primary human adult articular chondrocytes-a molecular analysis based on chondrocytes isolated from one donor. Cytokine. 2005;31:227–240. doi: 10.1016/j.cyto.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.El Mansouri FE, Chabane N, Zayed N, Kapoor M, Benderdour M, Martel-Pelletier J, Pelletier JP, Duval N, Fahmi H. Contribution of H3K4 methylation by SET-1A to interleukin-1-induced cyclooxygenase 2 and inducible nitric oxide synthase expression in human osteoarthritis chondrocytes. Arthritis Rheum. 2011;63:168–179. doi: 10.1002/art.27762. [DOI] [PubMed] [Google Scholar]
- 8.Chen WP, Wu LD. Chlorogenic acid suppresses interleukin-1β-induced inflammatory mediators in human chondrocytes. Int J Clin Exp Pathol. 2014;7:8797–8801. [PMC free article] [PubMed] [Google Scholar]
- 9.Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38:1541–1546. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 10.Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Zou J, Huang L, Shi Q, Zhu X, Wang G, Yang H. Efficacy of intra-articular injection of celecoxib in a rabbit model of osteoarthritis. Int J Mol Sci. 2010;11:4106–4113. doi: 10.3390/ijms11104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gigante A, Callegari L. The role of intra-articular hyaluronan (sinovial) in the treatment of osteoarthritis. Rheumatol Int. 2011;31:427–444. doi: 10.1007/s00296-011-1919-6. [DOI] [PubMed] [Google Scholar]
- 13.Iannitti T, Elhensheri M, Bingöl AO, Palmieri B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J Mol Histol. 2013;44:191–201. doi: 10.1007/s10735-012-9457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagnano M, Westrich G. Successful nonoperative management of chronic osteoarthritis pain of the knee: Safety and efficacy of retreatment with intra-articular hyaluronans. Osteoarthritis Cartilage. 2005;13:751–761. doi: 10.1016/j.joca.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis-meta-analysis. Osteoarthritis Cartilage. 2011;19:611–619. doi: 10.1016/j.joca.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann Intern Med. 2012;157:180–191. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 17.Webber TA, Webber AE, Matzkin E. Rate of adverse reactions to more than 1 series of viscosupplementation. Orthopedics. 2012;35:e514–e519. doi: 10.3928/01477447-20120327-26. [DOI] [PubMed] [Google Scholar]
- 18.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Wang NL, Chang CK, Liou YL, Lin CL, Lin MT. Shengmai San, a Chinese herbal medicine protects against rat heat stroke by reducing inflammatory cytokines and nitric oxide formation. J Pharmacol Sci. 2005;98:1–7. doi: 10.1254/jphs.FP0050018. [DOI] [PubMed] [Google Scholar]
- 20.Zhang GQ, Wang H, Liu WT, Dong H, Fong WF, Tang LM, Xiong YH, Yu ZL, Ko KM. Long-term treatment with a Chinese herbal formula, Sheng-Mai-San, improves cardiac contractile function in aged rats: The role of Ca (2+) homeostasis. Rejuvenation Res. 2008;11:991–1000. doi: 10.1089/rej.2008.0771. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Wu J, Zhang X, Kuang H, Guo Y, Ma L. The effect of Shenmai injection on the proliferation of Rat airway smooth muscle cells in asthma and underlying mechanism. BMC Complement Altern Med. 2013;13:221. doi: 10.1186/1472-6882-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YZ, Wu HY, Ren LW, Zhang HS, Jia X, Zhang YZ. Study on modified shengmai yin injection for prevention and treatment of brain impairment in endotoxin shock rats. J Tradit Chin Med. 2010;30:272–277. doi: 10.1016/S0254-6272(10)60055-6. [DOI] [PubMed] [Google Scholar]
- 23.Ni Q, Wang J, Li EQ, Zhao AB, Yu B, Wang M, Huang CR. Study on the protective effect of shengmai san (see text) on the myocardium in the type 2 diabetic cardiomyopathy model rat. J Tradit Chin Med. 2011;31:209–219. doi: 10.1016/S0254-6272(11)60044-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhao LM, Ma LJ, Zhang LX, Wu JZ. Shenmai injection inhibiting the extracellular signal regulated kinase-induced human airway smooth muscle proliferation in asthma. Chin J Integr Med. 2010;16:331–336. doi: 10.1007/s11655-010-0505-1. [DOI] [PubMed] [Google Scholar]
- 25.Lu LY, Zheng GQ, Wang Y. An overview of systematic reviews of shenmai injection for healthcare. Evid Based Complement Alternat Med. 2014;2014:840650. doi: 10.1155/2014/840650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Xin YF, Gu LQ, Gao HY, Xia LJ, You ZQ, Xie F, Ma ZF, Wang Z, Xuan YX. One-month toxicokinetic study of SHENMAI injection in rats. J Ethnopharmacol. 2014;154:391–399. doi: 10.1016/j.jep.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Gu LQ, Xin YF, Bai YS, Zhang S, Gao HY, Xu PS, Ma ZF, You ZQ, Wang Z, Xuan YX. Potential accumulation of protopanaxadiol-type ginsenosides in six-months toxicokinetic study of SHENMAI injection in dogs. Regul Toxicol Pharmacol. 2017;83:5–12. doi: 10.1016/j.yrtph.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Na JY, Song KB, Choi DS, Kim JH, Kwon YB, Kwon J. Protective effect of ginsenoside Rb1 on hydrogen peroxide-induced oxidative stress in rat articular chondrocytes. J Ginseng Res. 2012;36:161–168. doi: 10.5142/jgr.2012.36.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng W, Wu D, Zuo Q, Wang Z, Fan W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int Orthop. 2013;37:2065–2070. doi: 10.1007/s00264-013-1990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Na JY, Kim S, Song K, Lim KH, Shin GW, Kim JH, Kim B, Kwon YB, Kwon J. Anti-apoptotic activity of ginsenoside Rb1 in hydrogen peroxide-treated chondrocytes: Stabilization of mitochondria and the inhibition of caspase-3. J Ginseng Res. 2012;36:242–247. doi: 10.5142/jgr.2012.36.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y, Wu D, Fan W. Protection of ginsenoside Rg1 on chondrocyte from IL-1β-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol Cell Biochem. 2014;392:249–257. doi: 10.1007/s11010-014-2035-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XH, Xu XX, Xu T. Ginsenoside Ro suppresses interleukin-1β-induced apoptosis and inflammation in rat chondrocytes by inhibiting NF-κB. Chin J Nat Med. 2015;13:283–289. doi: 10.1016/S1875-5364(15)30015-7. [DOI] [PubMed] [Google Scholar]
- 33.Jo H, Ahn HJ, Kim EM, Kim HJ, Seong SC, Lee I, Lee MC. Effects of dehydroepiandrosterone on articular cartilage during the development of osteoarthritis. Arthritis Rheum. 2004;50:2531–2538. doi: 10.1002/art.20368. [DOI] [PubMed] [Google Scholar]
- 34.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. doi: 10.2106/00004623-197153030-00009. [DOI] [PubMed] [Google Scholar]
- 35.Youssef A Rehan, Longino D, Seerattan R, Leonard T, Herzog W. Muscle weakness causes joint degeneration in rabbits. Osteoarthritis Cartilage. 2009;17:1228–1235. doi: 10.1016/j.joca.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Ricart-Jané D, Llobera M, López-Tejero MD. Anticoagulants and other preanalytical factors interfere in plasma nitrate/nitrite quantification by the Griess method. Nitric Oxide. 2002;6:178–185. doi: 10.1006/niox.2001.0392. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11:227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The Role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: Role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 41.Punzi L, Oliviero F, Plebani M. New biochemical insights into the pathogenesis of osteoarthritis and the role of laboratory investigations in clinical assessment. Crit Rev Clin Lab Sci. 2005;42:279–309. doi: 10.1080/10408360591001886. [DOI] [PubMed] [Google Scholar]
- 42.Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84:678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abramson SB. Inflammation in osteoarthritis. J Rheumatol Suppl. 2004;70:70–76. [PubMed] [Google Scholar]
- 44.Maneiro E, López-Armada MJ, de Andres MC, Caramés B, Martin MA, Bonilla A, Del Hoyo P, Galdo F, Arenas J, Blanco FJ. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann Rheum Dis. 2005;64:388–395. doi: 10.1136/ard.2004.022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvatierra J, Escames G, Hernandez P, Cantero J, Crespo E, Leon J, Salvatierra D, Acuña-Castroviejo D, Vives F. Cartilage and serum levels of nitric oxide in patients with hip osteoarthritis. J Rheumatol. 1999;26:2015–2017. [PubMed] [Google Scholar]
- 46.Miyaura C, Inada M, Suzawa T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Biol Chem. 2000;275:19819–19823. doi: 10.1074/jbc.M002079200. [DOI] [PubMed] [Google Scholar]
- 47.Dannhardt G, Kiefer W. Cyclooxygenase inhibitors-current status and future prospects. Eur J Med Chem. 2001;36:109–126. doi: 10.1016/S0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki K, Hattori T, Fujisawa T, Takahashi K, Inoue H, Takigawa M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J Biochem. 1998;123:431–439. doi: 10.1093/oxfordjournals.jbchem.a021955. [DOI] [PubMed] [Google Scholar]
- 49.Tung JT, Arnold CE, Alexander LH, Yuzbasiyan-Gurkan V, Venta PJ, Richardson DW, Caron JP. Evaluation of the influence of prostaglandin E2 on recombinant equine interleukin-1beta-stimulated matrix metalloproteinases 1, 3, and 13 and tissue inhibitor of matrix metallo-proteinase 1 expression in equine chondrocyte cultures. Am J Vet Res. 2002;63:987–993. doi: 10.2460/ajvr.2002.63.987. [DOI] [PubMed] [Google Scholar]