Abstract
Background
Higher height and weight are known to be associated with higher risk of atrial fibrillation (AF); however, whether the risk of AF is related to abdominal obesity is unclear.
Methods and Results
We studied 501 690 adults (mean age: 47.6±14.3 years; 250 664 women [50.0%]) without baseline AF in the National Sample Cohort released by the National Health Insurance Service in Korea. Body mass index (underweight defined as <18.5; normal, 18.5 to <25.0; overweight, 25.0 to <30.0; and obese, ≥30.0) and waist circumference (abdominal obesity defined as ≥90 cm for men and ≥80 cm for women) were evaluated. During a mean follow‐up of 3.9±1.3 years, 3443 participants (1432 women [41.6%]) developed AF. In multivariable models adjusted for clinical variables, the AF risk of underweight, overweight, and obese individuals increased by 21% (95% confidence interval, 1.01–1.45, P=0.043), 14% (95% confidence interval, 1.06–1.23, P<0.001), and 52% (95% confidence interval, 1.30–1.78, P<0.001), respectively, compared with those with normal body mass index. AF risk with confounder‐adjusted hazards for abdominal obesity was 18% (95% confidence interval, 1.10–1.27, P<0.001). The increased AF risk was present in abdominally obese individuals regardless of body mass index except for the obese group. In subgroup analysis, abdominal obesity by waist circumference conferred increased risk of new‐onset AF, particularly in participants without comorbidities.
Conclusions
Abdominal obesity is an important, potentially modifiable risk factor for AF in nonobese Asian persons. These data suggest that interventions to decrease abdominal obesity may reduce the population burden of AF.
Keywords: Asians, atrial fibrillation, incidence, nationwide cohort, obesity
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Obesity
Clinical Perspective
What Is New?
In this Korean nationwide community‐based cohort, abdominal obesity and general obesity are associated with new‐onset AF after adjustment for other risk factors.
What Are the Clinical Implications?
These findings suggest that improvement of abdominal obesity may decrease the risk of AF in a nonobese Asian population. Further investigation regarding abdominal obesity and AF is needed.
Introduction
Atrial fibrillation (AF) is a public health problem because the arrhythmia affects millions of people and predisposes to heart failure, dementia, stroke, and death.1, 2, 3 Higher height, weight, and body mass index (BMI)4, 5, 6, 7 confer increased risk of AF. Importantly, general obesity, which is one of a limited number of modifiable risk factors for AF, and high BMI have been reported to be related to AF burden and severity.8, 9, 10, 11 The Framingham cohort study demonstrated a significant close relationship with increased risk of developing AF with increasing severity of general obesity.7 In particular, the dilated left atria observed in obese participants appear to be mechanistically important, as the association between BMI and AF was significantly weakened when adjustments were made for left atrial (LA) size in the Framingham study.7 LA dilatation and atrial dysfunction are known consequences of cardiomyopathy due to general obesity.12
Waist circumference (WC) and waist‐to‐hip circumference ratio are also associated with higher risk of AF.4, 13, 14, 15 Visceral adiposity is associated with incident cardiovascular disease after adjustment for clinical risk factors and generalized adiposity.16 Previous studies, however, did not show additional risk of AF by abdominal obesity beyond BMI. The prevalence of overweight status and obesity in Asian populations is lower than that in Western populations. Moreover, Asian persons tend to have higher amounts of abdominal fat at lower BMI compared with European persons.17 Nevertheless, assessments on whether the risk of AF is related to abdominal obesity in the Asian population are limited.17, 18 In this regard, we analyzed a recently developed Korean National Health Insurance sample cohort database, which includes >500 000 persons who had health examinations, to evaluate the association between abdominal obesity and AF between 2009 and 2013. We also evaluated whether the association was consistent regardless of participants’ BMI categories.
Methods
Study Population
A national health insurance system in Korea was established in 1963 according to the National Health Insurance Act, and participation is compulsory for all citizens in South Korea. Currently, the National Health Insurance Service (NHIS) maintains and manages all Korean health service databases. The NHIS is also in charge of the national health examination programs, which include a general health examination for all insured employees or self‐employed persons aged >40 years and their dependents. The examinations are recommended to be undertaken at least biennially.
The NHIS released the National Sample Cohort (2002–2013) database (NHIS‐NSC [2002–2013]) in 2015. It consists of 1 025 340 Koreans as an initial 2002 cohort and follows the participants until 2013. The cohort data represent about 2.2% of the source population in 2002 (46 605 433). This study enrolled 501 509 patients aged >18 years without AF from the NHIS‐NSC in 2009. These patients had physical examination data including WC and analyzed follow‐up data until December 2014. The database is a semidynamic cohort database; the cohort has been followed up to either the time of the participant's disqualification from health services because of death or emigration or the end of the study period. Samples of newborn infants are included annually. The database contains eligibility and demographic information about health insurance and medical aid beneficiaries, medical bill details, medical treatment, disease histories, and prescriptions. Such data are constructed after converting insurance claim information to the first day of medical treatment.
In the cohort, the disease information of the participants was classified according to the International Classification of Diseases, 10th Revision (ICD‐10) codes, and the mortality information along with cause of death of the participants was obtained from the Korean National Statistical Office. Definitions of diagnoses, comorbidity, and outcomes were evaluated based on information from NHIS‐NSC/Yonsei University (Table S1).
AF was diagnosed using ICD‐10 codes (ICD‐10:I48). To ensure accurate diagnosis, we defined patients with AF only when it was a discharge diagnosis or was confirmed more than twice in the outpatient department.19, 20, 21 Furthermore, the laboratory and survey questionnaire data of general and life‐transition health examinations for all cohort members were combined. This study was based on NHIS data. Informed consent was not obtained individually. Data were completely anonymized and deidentified for analysis. Height, weight, and WC were measured by a trained nurse during the physical examination at a primary care unit or hospital clinic in charge of the national health examination programs. WC was measured between the lower margin of the last palpable rib and the top of the iliac crest, at the level of maximum circumference, using a flexible inelastic fiberglass tape with the tape parallel to the floor.22 BMI is defined as the body weight divided by the square of the body height (kg/m2).
Additional analysis was performed in 1037 consecutive AF patients who were admitted to Severance Hospital from 2009 to 2015 to evaluate the relationship between LA size and BMI or WC. LA enlargement was defined as LA diameter >45 mm, according to a previous study.23 Data from the NHIS and Severance Hospital were used after approval by the institutional review board of Yonsei University Hospital.
Statistical Analysis
BMI was analyzed as both continuous and categorical variables using the World Health Organization/National Institutes of Health classification scheme (underweight defined as <18.5; normal, 18.5 to <25.0; overweight, 25.0 to <30.0; and obese, >30.0).24 WC was also evaluated as both continuous and categorical variables (abdominal obesity defined as ≥90 cm for men and ≥80 cm for women).25 Data are presented as mean±SD for continuous variables and as proportions for categorical variables. Continuous variables were compared using the Student t test. Analysis of categorical variables was performed with the χ2 test. Continuous variables were compared using 1‐way ANOVA and χ2 test or Fisher exact test as a post hoc test for each BMI group. The relationship among BMI, WC, and the incidence of AF is graphically illustrated with smooth curves fitted to a Cox proportional hazards model using a penalized B‐spline method. The splines among BMI, WC, and log hazard rate of new‐onset AF were adjusted for the relevant covariates in the overall population and a sex‐stratified population.
We examined the association among BMI, WC, and the risk of developing new‐onset AF using Cox proportional hazards regressions. We estimated the age‐adjusted and multivariable models. Covariates selected for adjustment included age, sex, history of hypertension, diabetes mellitus, dyslipidemia, congestive heart failure, chronic kidney disease or end‐stage renal disease, previous myocardial infarction (MI), transient ischemic attack or stroke, heavy alcohol use, and status as a former or current smoker.26, 27 We defined interim heart failure and MI as events that occur after the baseline examination and before the onset of AF and examined a Cox regression model adjusted for them. The risk of new‐onset AF was assessed according to patients’ abdominal obesity using 4 BMI categories, and the risk of AF incidence for BMI or WC was estimated using a model adjusted according to age, sex, and clinical variables in the total population. To examine the proportional assumption for the Cox regression models, we analyzed the proportional hazards assumption tested based on the scaled Schoenfeld residuals.
To evaluate the HR of abdominal obesity for new‐onset AF in patients with different comorbidities and habits of smoking and alcohol, we analyzed the effect of abdominal obesity on incident AF in subgroups. A heavy alcohol habit was defined as >1 drink per day for women and >2 drinks per day for men.7 Linear regression analysis was performed to identify relationships among BMI, WC, and LA size. The risk of BMI and WC for LA enlargement was assessed using an age‐ and sex‐adjusted model of multivariate logistic regression analysis.
Kaplan–Meier survival curves were plotted for incidental AF risk across categories of BMI and WC and compared by means of a log‐rank test. The population‐attributable risks of overweight status or obesity and abdominal obesity were calculated by subtracting the rate of AF in the entire cohort from the rate of AF in those who carried the risk factor and dividing it by the rate of AF in the entire cohort.28, 29
Statistical analysis using the Cox proportional hazards model with a penalized B‐spline method (Figure S1) and the required proportional hazards assumption tested based on the scaled Schoenfeld residuals was performed using R version 3.2.2 software. Other statistical analyses were performed with SPSS 21.0 statistical software. All P values were 2‐tailed, and values <0.05 were considered statistically significant.
Results
Study Participants
The mean age of the participants was 47.6 years (range: 18–98 years). Of the 251 026 men, 83 955 (33.4%) were overweight and 10 018 (4.0%) were obese; of the 250 664 women, 57 174 (22.8%) were overweight and 9078 (3.6%) were obese. Mean BMI was 23.7±3.3, and mean WC was 80.0±9.4 cm. The baseline characteristics of the study population by BMI level and WC category are presented in Table 1. An increase in BMI resulted in increased proportions of participants with hypertension, diabetes mellitus, and/or heart failure (all P<0.001). Participants without abdominal obesity had lower proportions of hypertension, diabetes mellitus, and/or heart failure compared with those who had abdominal obesity, as defined by WC level (all P<0.001). There were more men in the overweight and obese groups. In our cohorts, of the 19 096 obese participants, 734 (3.8%) did not have abdominal obesity and 18 331 (96.2%) did; of the 482 594 nonobese subjects, 358 182 (74.2%) did not have abdominal obesity and 124 263 (25.8%) did.
Table 1.
Baseline Characteristics of Participants by the Level of BMI and WC Category Among Koreans
| Overall (n=501 690) | BMI (kg/m2) | WC (cm) | |||||
|---|---|---|---|---|---|---|---|
| Underweight (n=20 987) | Normal (n=320 478) | Overweight (n=141 129) | Obese (n=19 096) | Abdominal Obesity (−) (n=358 916) | Abdominal Obesity (+) (n=142 594) | ||
| Age, y | 47.6±14.3 | 41.5±17.3 | 47.1±14.4 | 49.8±13.4 | 46.6±14.0 | 45.5±13.9 | 53.1±13.9 |
| Male, n (%) | 250 940 (50.0) | 6140 (29.3) | 150 913 (47.1) | 83 955 (59.5) | 10 018 (52.5) | 193 850 (54.0) | 57 091 (40.0) |
| Height, cm | 163.4±9.2 | 162.3±8.1 | 163.2±9.0 | 164.0±9.7 | 163.8±10.6 | 164.0±8.8 | 161.8±10.0 |
| Weight, kg | 63.5±11.8 | 46.4±5.1 | 59.2±8.2 | 72.2±9.2 | 86.3±12.5 | 60.7±10.1 | 70.6±12.8 |
| BMI, kg/m2 | 23.7±3.3 | 17.6±0.8 | 22.2±1.7 | 26.8±1.3 | 32.1±2.4 | 22.5±2.5 | 26.7±3.0 |
| WC, cm | 80.0±9.4 | 66.0±5.7 | 76.6±7.0 | 87.2±6.3 | 97.0±7.8 | 76.2±7.3 | 89.6±6.8 |
| HF, n (%) | 10 937 (2.2) | 286 (1.4) | 5442 (1.7) | 4351 (3.1) | 858 (4.5) | 4861 (1.4) | 6072 (4.3) |
| HTN, n (%) | 107 925 (21.5) | 1974 (9.4) | 56 401 (17.6) | 42 661 (30.2) | 6889 (36.1) | 55 692 (15.5) | 52 192 (36.6) |
| DM, n (%) | 64 132 (12.8) | 1537 (7.3) | 35 216 (11.0) | 23 593 (16.7) | 3786 (19.8) | 34 505 (9.6) | 29 600 (20.8) |
| Previous MI, n (%) | 4555 (0.9) | 152 (0.7) | 2504 (0.8) | 1648 (1.2) | 251 (1.3) | 2565 (0.7) | 1988 (1.4) |
| CKD or ESRD, n (%) | 29 264 (5.8) | 1000 (4.8) | 17 538 (5.5) | 9532 (6.8) | 1194 (6.3) | 17 268 (4.8) | 11 984 (8.4) |
| Ischemic stroke/TIA, n (%) | 18 461 (3.7) | 557 (2.7) | 10 317 (3.2) | 6668 (4.6) | 919 (4.7) | 9547 (2.7) | 8907 (6.2) |
| Vascular disease, n (%) | 39 295 (7.8) | 974 (4.8) | 21 784 (6.9) | 14 421 (10.0) | 2116 (10.8) | 20 660 (5.8) | 18 620 (13.1) |
| CHA2DS2‐VASc | 1.19±1.38 | 1.20±1.20 | 1.13±1.30 | 1.28±1.52 | 1.43±1.59 | 0.96±1.17 | 1.75±1.67 |
| SBP, mm Hg | 122±15 | 113±14 | 120±15 | 127±15 | 131±15 | 120±15 | 127±15 |
| DBP, mm Hg | 76±10 | 71±10 | 75±10 | 79±10 | 82±11 | 75±10 | 79±10 |
| Fasting glucose, mg/dL | 97.9±24.8 | 91.2±21.3 | 96.1±23.4 | 101.8±26.5 | 106.0±31.1 | 95.7±22.8 | 103.2±28.6 |
| Total cholesterol, mg/dL | 194.9±37.3 | 178.4±33.0 | 192.1±36.3 | 202.2±38.1 | 205.2±39.3 | 191.6±36.0 | 203.4±39.1 |
| Triglyceride, mg/dL | 132.0±93.9 | 84.0±55.6 | 118.3±82.3 | 162.6±106.9 | 181.5±118.4 | 121.1±87.1 | 159.5±104.2 |
| HDL‐cholesterol, mg/dL | 56.4±27.2 | 64.2±29.0 | 57.9±27.5 | 52.6±26.1 | 51.2±24.6 | 57.6±27.6 | 53.3±26.2 |
| LDL‐cholesterol, mg/dL | 113.7±37.2 | 98.7±33.5 | 111.9±36.4 | 118.8±38.3 | 119.5±39.6 | 111.2±36.2 | 119.9±39.0 |
| Hb, g/dL | 13.9±1.7 | 13.1±1.5 | 13.7±1.6 | 14.3±1.6 | 14.3±1.7 | 13.9±1.6 | 13.8±1.6 |
| Creatinine, mg/dL | 1.10±1.12 | 1.07±1.06 | 1.10±1.10 | 1.11±1.21 | 1.07±0.94 | 1.11±1.15 | 1.07±1.06 |
| AST, mg/dL | 25.3±17.2 | 23.0±20.4 | 24.1±16.9 | 27.3±16.5 | 31.4±20.1 | 24.4±17.0 | 27.4±17.5 |
| ALT, mg/dL | 24.8±22.10 | 16.7±17.1 | 21.8±19.3 | 30.6±24.4 | 40.7±32.9 | 22.8±20.3 | 29.9±25.4 |
Underweight defined as BMI <18.5; normal, 18.5 to <25.0; overweight, 25.0 to <30.0; and obese, >30.0. Abdominal obesity defined as WC ≥90 cm for men, ≥80 cm for women in Asian. All P values of 1‐way ANOVA and the χ2 test for underweight vs normal vs overweight vs obese were <0.001. All P values of the Student t test or χ2 test for abdominal obesity (−) vs abdominal obesity (+) were <0.001. ALT indicates alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CHA2DS2‐VASc, congestive heart failure, hypertension, age 75 years or older, diabetes mellitus, previous stroke/transient ischemic attack, vascular disease, age 65–74 years, female; CKD, chronic kidney disease; DBP, diastolic blood pressure; DM, diabetes mellitus; ESRD, end‐stage renal disease; Hb, hemoglobin; HDL, high‐density lipoprotein; HF, heart failure; HTN, hypertension; LDL, low‐density lipoprotein; MI, myocardial infarction; SBP, systolic blood pressure; TIA, transient ischemic attack; WC, waist circumference.
AF Incidence
During a mean follow‐up of 3.9 years, 3443 participants (0.7%), including 2011 men (58.4%), developed AF. The overall AF incidence for follow‐up duration was 1.78 per 1000 person‐years. The incidence rates of AF by BMI or WC categories are presented in Table 2. AF incidence in the obese and overweight groups was higher compared with that in the underweight and normal groups (P<0.001). AF incidence was also higher among participants with abdominal obesity than in those without (P<0.001).
Table 2.
Incidence of Atrial Fibrillation by BMI and WC Categories
| BMI (kg/m2) | WC (cm) | |||||
|---|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | Abdominal Obesity (−) | Abdominal Obesity (+) | |
| Numbers of events/person‐y | 121/75 358 | 1907/1 225 534 | 1231/559 989 | 183/72 002 | 1963/1 385 832 | 1479/547 052 |
| AF incidence per 1000 person‐y | 1.61 | 1.56 | 2.20 | 2.54 | 1.42 | 2.70 |
Underweight defined as BMI <18.5; normal, 18.5 to <25.0; overweight, 25.0 to <30.0; and obese, >30.0 of BMI. Abdominal obesity defined as WC ≥90 cm for men and ≥80 cm for women in Asians. AF indicates atrial fibrillation; BMI, body mass index; WC, waist circumference.
Figure 1 shows the Kaplan–Meier curves for new‐onset AF in patients with different categories of BMI (Figure 1A) or WC (Figure 1B). Patients in the obese and overweight groups showed higher cumulative incidence of new‐onset AF than those in the normal and underweight groups (all P<0.001). Patients with abdominal obesity had higher cumulative incidence of new‐onset AF than those without abdominal obesity (P<0.001).
Figure 1.

Kaplan–Meier curves for incidental AF risk across categories of body mass index (A) and waist circumference (B). AF indicates atrial fibrillation.
Risk of New‐Onset AF According to BMI and WC
Adjusted results of the multivariable Cox proportional hazards regressions for age, sex, and relevant cardiovascular risk factors are shown in Table 3. After adjustment for age and sex, each 1‐SD increase in BMI (+3.7) or WC (+9.4 cm) was associated with an increase of 17% (P<0.001) or 23% (P<0.001), respectively. These relationships remained significant in multivariable‐adjusted models, with 8% and 12% increases in the risk of AF per 1‐SD increase in BMI (P<0.001) and WC (P<0.001), respectively.
Table 3.
AF Risk of BMI or WC According to the Age‐, Sex‐, and Clinical Variable–Adjusted Model in the Total Population
| Model | Overall | |
|---|---|---|
| HR (95% CI) | P Value | |
| With BMI as a 1‐SD (3.7) increase | ||
| Age, sex adjusted | 1.17 (1.13–1.21) | <0.001 |
| Age, sex, and WC adjusted | 1.04 (0.99–1.10) | 0.121 |
| Adjusted for clinical variablesa | 1.08 (1.05–1.12) | <0.001 |
| With BMI as a categorical variable | ||
| Age, sex adjusted | ||
| Underweight (<18.5) | 1.10 (0.92–1.32) | 0.308 |
| Normal (18.5 to <25) | 1 | |
| Overweight (25 to <30) | 1.29 (1.20–1.38) | <0.001 |
| Obese (≥30) | 1.93 (1.65–2.24) | <0.001 |
| Age, sex, and WC adjusted | ||
| Underweight (<18.5) | 1.14 (0.94–1.37) | 0.182 |
| Normal (18.5 to <25) | 1 | |
| Overweight (25 to <30) | 1.17 (1.07–1.27) | <0.001 |
| Obese (≥30) | 1.65 (1.40–1.94) | <0.001 |
| Adjusted for clinical variablesa | ||
| Underweight (<18.5) | 1.21 (1.01–1.46) | 0.041 |
| Normal (18.5 to <25) | 1 | |
| Overweight (25 to <30) | 1.14 (1.06–1.23) | <0.001 |
| Obese (≥30) | 1.52 (1.30–1.78) | <0.001 |
| With WC as a 1‐SD (9.4‐cm) increase | ||
| Age, sex adjusted | 1.23 (1.18–1.27) | <0.001 |
| Age, sex, and BMI adjusted | 1.18 (1.11–1.26) | <0.001 |
| Adjusted for clinical variablesa | 1.12 (1.08–1.17) | <0.001 |
| With WC as a categorical variable | ||
| Age, sex adjusted | ||
| Abdominal obesity, without | 1 | |
| Abdominal obesity, with | 1.35 (1.26–1.45) | <0.001 |
| Age, sex, and BMI adjusted | ||
| Abdominal obesity, without | 1 | |
| Abdominal obesity, with | 1.20 (1.10–1.31) | <0.001 |
| Adjusted for clinical variablesa | ||
| Abdominal obesity, without | 1 | |
| Abdominal obesity, with | 1.18 (1.10–1.26) | <0.001 |
BMI indicates body mass index; CI, confidence interval; HR, hazard ratio; WC, waist circumference.
Clinical variables were age, sex, hypertension, diabetes mellitus, dyslipidemia, congestive heart failure, chronic kidney disease or end‐stage renal disease, history of previous myocardial infarction, transient ischemic attack or stroke history, smoking history, and heavy alcohol use.
Spline curves between BMI and WC and the hazard ratio (HR) of new‐onset AF are presented in Figure S1. A nonlinear J‐shaped association was found between continuous BMI variables and AF risk; AF risk positively increased with the increase of WC. Interestingly, the overall J‐shaped and linear patterns in the association seem to be driven mainly by the patterns for men.
To assess the influence of different degrees of BMI, we estimated regressions with 4 BMI categories (underweight, normal, overweight, and obese; Table 3). Age‐ and sex‐adjusted HRs for AF progressively increased across these 4 BMI categories (HR 1.10, 1.29, and 1.93 for underweight, overweight, and obese, respectively; normal was reference); after adjustment for clinical variables, the HRs were 1.21, 1.14, and 1.52, respectively. In addition to the baseline covariates, the adjusted HRs for AF were not attenuated in models adjusted for interim MI or heart failure (HR: 1.20 [95% confidence interval [CI], 0.99‐1.44; P=0.052]; HR: 1; 1.14 [95% CI, 1.06–1.23; P<0.001]; HR: 1.53 [95% CI, 1.31–1.79; P<0.001] for underweight, normal, overweight, and obese, respectively). To assess the influence of different degrees of WC, we estimated regressions with 2 WC categories (normal and abdominal obesity). Age‐ and sex‐adjusted HRs (1.35) and clinical variable–adjusted HRs (1.18) for WC increased across these 2 WC categories. General overweight or obese status and abdominal obesity accounted for 12.4% and 20.2%, respectively, of the population‐attributable risk.
In Cox proportional hazards models using covariates including age, sex, and BMI as a 1‐SD increase, WC as a 1‐SD (9.4 cm) increase independently increased the risk of new‐onset AF, whereas BMI as a 1‐SD (3.7) increase was not significant after adjusting for age, sex, and WC as a 1‐SD increase (WC—HR: 1.18 [95% CI, 1.11–1.26; P<0.001]; BMI—HR: 1.04 [95% CI, 0.99–1.10; P=0.121]).
Influence of Abdominal Obesity for New‐Onset AF in Different BMI Categories
Figure 2A shows the relationship between BMI and WC. WC was positively correlated with BMI. The patients who developed AF during the follow‐up period are marked with black points (Figure 2B). The ratio of new‐onset AF in participants with abdominal obesity was higher than that in those without abdominal obesity in the underweight (2.7% versus 0.6%, P<0.001), normal (1.1% versus 0.5%, P<0.001) and overweight groups (1.0% versus 0.6%, P<0.001) in contrast to the obese group (1.0% versus 1.0%, P=0.99).
Figure 2.
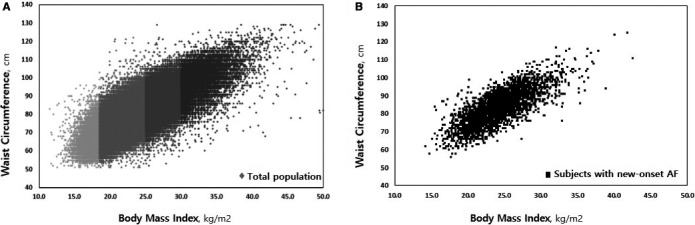
Relationship between body mass index and waist circumference in total population (A) and participants with new‐onset AF (B). AF indicates atrial fibrillation.
Figure 3 shows the Kaplan–Meier curves for new‐onset AF according to abdominal obesity in patients with different BMI categories. Abdominal obesity was associated with new‐onset AF in the underweight (P<0.001), normal (P<0.001), and overweight groups (P<0.001) but not in the obese group (P=0.894).
Figure 3.

Kaplan–Meier curve for developing AF in participants who were underweight (A), normal (B), overweight (C), and obese (D). AF indicates atrial fibrillation.
Influence of Abdominal Obesity for New‐Onset AF Risk in Patients With Different Comorbidities and Habits of Smoking and Alcohol
The HR of abdominal obesity for new‐onset AF in patients with different comorbidities and habits of smoking and alcohol are presented in Figure 4. Age‐adjusted HRs for new‐onset AF by abdominal obesity were 1.44 (95% CI, 1.32–1.58; P<0.001) in men and 1.23 (95% CI, 1.11–1.37; P<0.001) in women. After adjustment for age and sex, abdominal obesity by WC was a risk factor for new‐onset AF in nonobese participants (HR: 1.30; 95% CI, 1.21–1.40; P<0.001) but not in obese individuals (HR: 0.74; 95% CI, 0.35–1.60; P=0.446).
Figure 4.

Age‐ and sex‐adjusted HRs for the effect of abdominal obesity on incident atrial fibrillation in subgroups. Heavy alcohol use was defined as >1 drink per day for women and >2 drinks per day for men. BMI indicates body mass index; CKD, chronic kidney disease; ESRD, end‐stage renal disease; HR, hazard ratio; MI, myocardial infarction.
Participants with abdominal obesity showed an increased risk of new‐onset AF regardless of diabetes mellitus, smoking history, or heavy alcohol use (all P<0.001). However, age‐ and sex‐adjusted HRs for new‐onset AF significantly increased in patients without hypertension (HR: 1.43; 95% CI, 1.28–1.61; P<0.001), congestive heart failure (HR: 1.36; 95% CI, 1.17–1.47; P<0.001), previous MI (HR: 1.35; 95% CI, 1.25–1.45; P<0.001), and chronic kidney disease or end‐stage renal disease (HR: 1.41; 95% CI, 1.31–1.53; P<0.001) compared with their respective counterparts with these conditions.
Relationship of LA Size to BMI and WC
Additional analysis was performed in 1037 consecutive AF patients (mean age: 58±11 years; 248 [23.9%] women) who were admitted to Severance Hospital from 2009 to 2015 to evaluate the relationship of LA size to BMI and WC. Baseline characteristics and echocardiographic findings are shown in Table S2. In linear regressions, both BMI and WC demonstrated significantly positive correlations with LA size (β=2.40, P<0.001 for BMI; β=2.19, P<0.001 for WC). Our hospital data showed that WC tended to increase the risk of LA enlargement after age, sex, and BMI adjustment, but it did not reach statistical significance (odds ratio: 1.24; 95% CI, 0.97–1.59; P=0.081).
Discussion
Main Findings
The main findings of this study are as follows. First, the nationwide community‐based data indicate that general obesity and abdominal obesity are risk factors for AF. The association between obesity and subsequent AF development was still observed after accounting for concomitant conditions, such as hypertension, diabetes mellitus, congestive heart failure, and MI. Second, abdominal obesity is a risk factor for AF in nonobese, but not in obese, Asian populations. Finally, WC was related to the increased risk of AF in persons without comorbidities, such as hypertension, congestive heart failure, previous MI, and renal disease. These data increase the possibility that interventions to decrease abdominal obesity may reduce the population burden of AF in Asian populations.
Effect of General Obesity as Quantified by BMI on New‐Onset AF Risk
In a range of community‐based cohorts, general obesity and increased BMI are associated with increased AF risk (HR 1.65–2.35) at follow‐up.5, 7, 30, 31, 32, 33 In the Women's Health Study (mean age: 56±7 years), each 1‐kg/m2 increase in BMI is associated with a 5% increased risk of AF over a 13‐year follow‐up. Similarly, each 1‐kg/m2 increase in BMI adjusted for age and sex was associated with a 5% increased risk of AF in the present study. Overweight status was identified as a risk factor for AF, contributing a 10‐year population‐attributable risk of 12% in the Women's Health Initiative study (mean age: 63±7 years).28 Black participants had a lower AF incidence rate by 41% compared with white participants in the Women's Health Initiative study. BMI >35 is also associated with increased AF risk by an HR of 3.50 in young (30.6±4.7 years), otherwise healthy women in a nationwide cohort study from Denmark.34
Multiple plausible biological pathways link body fat and lean body mass to AF occurrence. Height, weight, and body fat predispose to LA enlargement, which in turn predisposes to AF.35, 36 Higher BMI is associated with inflammation, which is reflected in higher concentrations of C‐reactive protein.37 In addition, general obesity is a major risk factor for obstructive sleep apnea; sleep apnea predisposes to AF.30 Finally, general obesity predisposes to the intermediate occurrence of hypertension, diabetes mellitus, acute MI, heart failure, and heart valve disease, which in turn increase the risk of AF.38
Influence of Abdominal Obesity for New‐Onset AF in Different BMI Categories
Assessments of the associations between different types of obesity (visceral versus subcutaneous) and AF are limited. WC and hip circumference and waist‐to‐hip circumference ratio are associated with AF risk. In community‐dwelling cohorts, increased WC and waist‐to‐hip circumference ratio are associated with 11% to 13% higher risk of AF over a 13‐ to 15‐year follow‐up period.4, 8, 39 Elevated body fat mass has been recently associated with a 29% increased risk of AF during a 13.5‐year follow‐up period.4 In this study, abdominal obesity assessed by WC was positively associated with 35% increase risk of AF after adjustment for age and sex during follow‐up. Findings from the current study suggest that abdominal obesity is positively associated with AF risk in Asian persons; however, a nonlinear J‐shaped association was found between continuous BMI variables and AF risk—AF risk positively increased with the increase of WC. Aronis et al reported that BMI, abdominal circumference, and total fat mass are associated with risk of AF among white and black older adults.40 The LEGACY study demonstrated the beneficial effects of long‐term sustained weight loss and participation in a tailored exercise program on reducing AF recurrence in obese persons.41 Obesity reduction can modify the incidence of AF.40 Nevertheless, there are limited data assessing the association between risk of AF and abdominal obesity in Asian populations.
In the present study, compared with participants without abdominal obesity as quantified by WC, those with abdominal obesity showed increased AF risk in all BMI categories except those in the obese group. The Asian population currently has a lower prevalence of obese persons than the Western population.17 Obese participants also had the lowest number among the other groups in our cohort. The World Health Organization expert consultation suggests that Asian populations have different associations among BMI, percentage of body fat, and disease risks than European populations.17 Asians tend to have higher percentages of abdominal fat at any given BMI18; therefore, BMI does not fully capture information on abdominal obesity. Thus, WC measurement may have additional associations with prospective risk of AF, particularly in Asian populations. The observation that the HRs of WC for obese Asian population that had cardiovascular risk factors did not reach statistical significance may reflect either a threshold effect or reduced statistical power to detect an effect among this population.
The 1000 person‐year incidence of new‐onset AF was higher in patients with abdominal obesity than in those without abdominal obesity in the underweight (8.50 versus 1.55, P<0.001), normal (2.75 versus 1.39, P<0.001) and overweight groups (2.71 versus 1.47, P<0.001). This finding suggests that abdominal obesity may have a role in increasing AF risk in nonobese Asian persons. Given that BMI is generally lower in Asian populations than in Western populations, abdominal obesity may be a more important, potentially modifiable risk factor for AF in nonobese Asian patients.
WC increased the risk of AF in all subgroups except in patients with hypertension, congestive heart failure, previous MI, and chronic kidney disease or end‐stage renal disease. These comorbidities are important factors affecting increased AF risk.26, 42 Concomitant comorbidities in these subgroups may, in part, account for the attenuation of the effect of abdominal obesity on incident AF from these factors in the present study. Previous studies have observed that overweight status, obesity, and high WC may be associated with favorable prognosis—referred to as the “obesity paradox”—in patients with cardiovascular disease.43, 44, 45, 46, 47 In populations with severe comorbidities (heart failure, renal disease), obesity may also have protective effects against AF (the obesity paradox).
Study Limitations
This study has several limitations. First, this study includes a potential selection bias. The NHIS recommends but does not obligate all health insurance subscribers to undergo at least 1 biennial health examination. Second, we could not analyze initial, paroxysmal, persistent, and permanent AF and atrial flutter separately. Finally, the possible variations in their relationships to anthropometric features could not be confirmed.
Conclusion
Although AF incidence gradually increased as BMI or WC increased, abdominal obesity as quantified by WC is associated with AF incidence, even after adjustments of other risk factors in participants without general obesity. This finding suggests that AF risk may be more affected by WC, and decreasing abdominal obesity may reduce the population burden of AF in nonobese Asian persons.
Sources of Funding
This study was supported by research grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF‐2012R1A2A2A02045367), and grants from the Korean Healthcare Technology R&D project funded by the Ministry of Health & Welfare (HI12C1552, HI16C0058, and HI15C1200).
Disclosures
None.
Supporting information
Table S1. Definition of Diagnoses, Comorbidities, and Outcomes From NHIS‐NSC/Yonsei University
Table S2. Baseline Characteristics and Echocardiographic Findings of Severance Hospital Data
Figure S1. Spline curves for body mass index and waist circumference and the hazard ratio of new‐onset AF.
Acknowledgments
The authors would like to express appreciation to the following individuals for their contribution to statistical analysis and editorial assistance with the manuscript: Yun‐Ho Roh, (Biostatistics Collaboration Unit, Yonsei University College of Medicine) and Michael Sukhyun Kim (Duke University).
(J Am Heart Assoc. 2017;6:e004705 DOI: 10.1161/JAHA.116.004705.)28588091
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 3. Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Horton R, Burkhardt JD, Lakkireddy D, Reddy YM, Casella M, Dello Russo A, Tondo C, Natale A. Atrial fibrillation and the risk of incident dementia: a meta‐analysis. Heart Rhythm. 2012;9:1761–1768. [DOI] [PubMed] [Google Scholar]
- 4. Frost L, Benjamin EJ, Fenger‐Gron M, Pedersen A, Tjonneland A, Overvad K. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity (Silver Spring). 2014;22:1546–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women's Health Study). J Am Coll Cardiol. 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 7. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 8. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 10. Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy‐Dicey A, Harris TB, Pencina MJ, D'Agostino RB Sr, Levy D, Kannel WB, Wang TJ, Kronmal RA, Wolf PA, Burke GL, Launer LJ, Vasan RS, Psaty BM, Benjamin EJ, Gudnason V, Heckbert SR. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, Keaney JF Jr, Wang TJ, Vasan RS, Benjamin EJ. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, Verrengia M, Rea A, Fratta F, Castaldi B, Gala S, Coppola F, Russo MG, Caso P, Perrone L, Calabro R. Atrial myocardial deformation properties in obese nonhypertensive children. J Am Soc Echocardiogr. 2008;21:151–156. [DOI] [PubMed] [Google Scholar]
- 13. Girerd N, Pibarot P, Fournier D, Daleau P, Voisine P, O'Hara G, Despres JP, Mathieu P. Middle‐aged men with increased waist circumference and elevated C‐reactive protein level are at higher risk for postoperative atrial fibrillation following coronary artery bypass grafting surgery. Eur Heart J. 2009;30:1270–1278. [DOI] [PubMed] [Google Scholar]
- 14. Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verdecchia P, Dagenais G, Healey J, Gao P, Dans AL, Chazova I, Binbrek AS, Iacobellis G, Ferreira R, Holwerda N, Karatzas N, Keltai M, Mancia G, Sleight P, Teo K, Yusuf S; Ongoing Telmisartan A, in Combination With Ramipril Global Endpoint TrialTelmisartan Randomized AssessmeNt Study in ACEiswcDI . Blood pressure and other determinants of new‐onset atrial fibrillation in patients at high cardiovascular risk in the ongoing telmisartan alone and in combination with ramipril global endpoint trial/telmisartan randomized assessment study in ACE intolerant subjects with cardiovascular disease studies. J Hypertens. 2012;30:1004–1014. [DOI] [PubMed] [Google Scholar]
- 16. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all‐cause mortality. J Am Coll Cardiol. 2013;62:921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Consultation WHOE . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 18. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. [DOI] [PubMed] [Google Scholar]
- 19. Chao TF, Huang YC, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Hsieh MH, Lip GY, Chen SA. Acute myocardial infarction in patients with atrial fibrillation with a CHA2DS2‐VASc score of 0 or 1: a nationwide cohort study. Heart Rhythm. 2014;11:1941–1947. [DOI] [PubMed] [Google Scholar]
- 20. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GY, Chen SA. Using the CHA2DS2‐VASc score for refining stroke risk stratification in ‘low‐risk’ Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. [DOI] [PubMed] [Google Scholar]
- 21. Chao TF, Wang KL, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chung FP, Liao JN, Chen TJ, Chiang CE, Lip GY, Chen SA. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66:1339–1347. [DOI] [PubMed] [Google Scholar]
- 22. Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311:1401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, Brodsky M, Barrell P, Greene HL. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the atrial fibrillation follow‐up investigation of rhythm management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026–2033. [DOI] [PubMed] [Google Scholar]
- 24. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. WMJ. 1998;97:20–21, 24–25, 27–37. [PubMed] [Google Scholar]
- 25. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world‐wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. [DOI] [PubMed] [Google Scholar]
- 26. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice G . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 27. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 28. Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, Connelly S, Hlatky M, Wassertheil‐Smoller S, Stefanick ML. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women's Health Initiative Observational Study. Heart. 2013;99:1173–1178. [DOI] [PubMed] [Google Scholar]
- 29. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community‐based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. [DOI] [PubMed] [Google Scholar]
- 30. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 31. Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long‐term cardiovascular consequences of obesity: 20‐year follow‐up of more than 15 000 middle‐aged men and women (the Renfrew‐Paisley study). Eur Heart J. 2006;27:96–106. [DOI] [PubMed] [Google Scholar]
- 32. Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250:382–389. [DOI] [PubMed] [Google Scholar]
- 33. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. [DOI] [PubMed] [Google Scholar]
- 34. Karasoy D, Bo Jensen T, Hansen ML, Schmiegelow M, Lamberts M, Gislason GH, Hansen J, Torp‐Pedersen C, Olesen JB. Obesity is a risk factor for atrial fibrillation among fertile young women: a nationwide cohort study. Europace. 2013;15:781–786. [DOI] [PubMed] [Google Scholar]
- 35. Gottdiener JS, Reda DJ, Williams DW, Materson BJ. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1997;29:651–658. [DOI] [PubMed] [Google Scholar]
- 36. Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Doring A, Keil U, Hense HW, Schunkert H; Investigators MK . The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. 2009;54:1982–1989. [DOI] [PubMed] [Google Scholar]
- 37. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. [DOI] [PubMed] [Google Scholar]
- 38. Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. [DOI] [PubMed] [Google Scholar]
- 39. Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities Study. Circ Arrhythm Electrophysiol. 2014;7:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aronis KN, Wang N, Phillips CL, Benjamin EJ, Marcus GM, Newman AB, Rodondi N, Satterfield S, Harris TB, Magnani JW; Health ABCs . Associations of obesity and body fat distribution with incident atrial fibrillation in the biracial health aging and body composition cohort of older adults. Am Heart J. 2015;170:498–505.e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long‐term effect of goal‐directed weight management in an atrial fibrillation cohort: a long‐term follow‐up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 42. Nelson SE, Shroff GR, Li S, Herzog CA. Impact of chronic kidney disease on risk of incident atrial fibrillation and subsequent survival in Medicare patients. J Am Heart Assoc. 2012;1:e002097 DOI: 10.1161/JAHA.112.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper‐Dehoff RM, Zhou Q, Pepine CJ. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. [DOI] [PubMed] [Google Scholar]
- 44. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 45. De Schutter A, Lavie CJ, Milani RV. The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease‐the obesity paradox. Prog Cardiovasc Dis. 2014;56:401–408. [DOI] [PubMed] [Google Scholar]
- 46. Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta‐analysis. Heart. 2015;101:1631–1638. [DOI] [PubMed] [Google Scholar]
- 47. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD, Wallentin L. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J. 2016;37:2869–2878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of Diagnoses, Comorbidities, and Outcomes From NHIS‐NSC/Yonsei University
Table S2. Baseline Characteristics and Echocardiographic Findings of Severance Hospital Data
Figure S1. Spline curves for body mass index and waist circumference and the hazard ratio of new‐onset AF.


