Key Points
Question
Is iron polysaccharide complex more effective than ferrous sulfate in improving the hemoglobin concentration in infants and young children with nutritional iron-deficiency anemia?
Findings
In this double-blind, randomized clinical trial that included 80 patients, those who received ferrous sulfate for 12 weeks had a 1.0 g/dL greater increase in hemoglobin concentration than those receiving iron polysaccharide complex.
Meaning
Among infants and young children with nutritional iron-deficiency anemia, ferrous sulfate compared with iron polysaccharide complex resulted in a greater increase in hemoglobin concentration at 12 weeks.
Abstract
Importance
Iron-deficiency anemia (IDA) affects millions of persons worldwide, and is associated with impaired neurodevelopment in infants and children. Ferrous sulfate is the most commonly prescribed oral iron despite iron polysaccharide complex possibly being better tolerated.
Objective
To compare the effect of ferrous sulfate with iron polysaccharide complex on hemoglobin concentration in infants and children with nutritional IDA.
Design, Setting, and Participants
Double-blind, superiority randomized clinical trial of infants and children aged 9 to 48 months with nutritional IDA (assessed by history and laboratory criteria) that was conducted in an outpatient hematology clinic at a US tertiary care hospital from September 2013 through November 2015; 12-week follow-up ended in January 2016.
Interventions
Three mg/kg of elemental iron once daily as either ferrous sulfate drops or iron polysaccharide complex drops for 12 weeks.
Main Outcomes and Measures
Primary outcome was change in hemoglobin over 12 weeks. Secondary outcomes included complete resolution of IDA (defined as hemoglobin concentration >11 g/dL, mean corpuscular volume >70 fL, reticulocyte hemoglobin equivalent >25 pg, serum ferritin level >15 ng/mL, and total iron-binding capacity <425 μg/dL at the 12-week visit), changes in serum ferritin level and total iron-binding capacity, adverse effects.
Results
Of 80 randomized infants and children (median age, 22 months; 55% male; 61% Hispanic white; 40 per group), 59 completed the trial (28 [70%] in ferrous sulfate group; 31 [78%] in iron polysaccharide complex group). From baseline to 12 weeks, mean hemoglobin increased from 7.9 to 11.9 g/dL (ferrous sulfate group) vs 7.7 to 11.1 g/dL (iron complex group), a greater difference of 1.0 g/dL (95% CI, 0.4 to 1.6 g/dL; P < .001) with ferrous sulfate (based on a linear mixed model). Proportion with a complete resolution of IDA was higher in the ferrous sulfate group (29% vs 6%; P = .04). Median serum ferritin level increased from 3.0 to 15.6 ng/mL (ferrous sulfate) vs 2.0 to 7.5 ng/mL (iron complex) over 12 weeks, a greater difference of 10.2 ng/mL (95% CI, 6.2 to 14.1 ng/mL; P < .001) with ferrous sulfate. Mean total iron-binding capacity decreased from 501 to 389 μg/dL (ferrous sulfate) vs 506 to 417 μg/dL (iron complex) (a greater difference of −50 μg/dL [95% CI, −86 to −14 μg/dL] with ferrous sulfate; P < .001). There were more reports of diarrhea in the iron complex group than in the ferrous sulfate group (58% vs 35%, respectively; P = .04).
Conclusions and Relevance
Among infants and children aged 9 to 48 months with nutritional iron-deficiency anemia, ferrous sulfate compared with iron polysaccharide complex resulted in a greater increase in hemoglobin concentration at 12 weeks. Once daily, low-dose ferrous sulfate should be considered for children with nutritional iron-deficiency anemia.
Trial Registration
clinicaltrials.gov Identifier: NCT01904864
This randomized clinical trial compares the effects of ferrous sulfate vs iron polysaccharide complex on nutritional iron-deficiency anemia among infants and children aged 9 to 48 months receiving care at an outpatient hematology clinic at a US tertiary care hospital.
Introduction
Iron-deficiency anemia (IDA) affected more than 1 billion persons worldwide in 2010, including up to 3% of children aged 1 to 2 years in the United States. The most common cause of IDA in infants and young children is inadequate dietary iron intake resulting from excessive cow milk consumption, prolonged breastfeeding without appropriate iron supplementation, or both. It typically occurs in infants and young children when rapid growth outstrips availability of dietary iron. Consequences include irritability, malaise, pica, and both short- and long-term neurodevelopmental impairment.
Successful treatment of IDA requires recognition and correction of the underlying etiology accompanied by iron replacement therapy to normalize the hemoglobin concentration and replenish iron stores. Treatment failure is common due to medication nonadherence, adverse effects related to excessive dosing, and lack of evidence-based management guidelines. Few randomized clinical trials inform the selection of iron preparation, dosage schedule, and duration of therapy, irrespective of the underlying etiology, age, or sex of affected individuals. In infants and young children, standard dosing recommendations are 2 to 6 mg/kg/d of elemental iron given from 1 to 3 times daily for a duration of 3 to 6 months. The literature on adults suggests that lower therapeutic dosing strategies may be sufficient for successful treatment.
Dozens of oral iron preparations (most of them over-the-counter supplements) are available for IDA treatment. Ferrous sulfate, an iron salt, is the standard and most commonly used agent to treat nutritional IDA. Alternatively, iron polysaccharide complex preparations containing ferric iron may be prescribed due to their potentially improved tolerability and better taste. Given the high prevalence of IDA in infants and young children, this double-blind, randomized clinical trial was designed to investigate whether an iron polysaccharide complex agent was more efficacious than ferrous sulfate in increasing hemoglobin concentration in infants and children aged 9 to 48 months with nutritional IDA.
Methods
The BESTIRON study was a randomized, double-blind single-center superiority trial designed to compare the efficacy of an iron polysaccharide complex (NovaFerrum, Gensavis Pharmaceuticals LLC) vs ferrous sulfate for the treatment of nutritional IDA in infants and young children who were referred for care at the Children’s Medical Center in Dallas, Texas.
The institutional review board at the University of Texas Southwestern Medical Center approved the study. Written informed consent was obtained from the parents of each participant prior to enrollment. The study was performed and analyzed in accordance with the Consolidated Standards of Reporting Trials. The trial start date was September 3, 2013, and it continued through November 5, 2015. The final follow-up date was January 28, 2016. A single protocol modification regarding inclusion of laboratory criteria was made prior to the enrollment of any patients and formal institutional review board approval was received the day after the first patient was enrolled. The study protocol appears in Supplement 1.
Eligible patients were infants and children aged 9 to 48 months with a diagnosis of nutritional IDA resulting from excessive cow milk intake of greater than 720 mL per day, breastfeeding without iron supplementation, or both. Given that racial and ethnic minority groups are disproportionately affected by IDA, this information was collected and reported for all patients. Data were obtained from the electronic medical record, which contains self-reported racial and ethnic identification based on fixed categories. Iron-deficiency anemia was confirmed by the following hematologic indices: hemoglobin concentration of 10 g/dL or less, mean corpuscular volume of 70 fL or less, reticulocyte hemoglobin equivalent of 25 pg or less, and either serum ferritin level of 15 ng/mL or less or total iron-binding capacity of 425 μg/dL or greater.
Patients were excluded from enrollment if they had clinical or laboratory evidence of other causes of anemia (entire list of eligibility criteria appears in eTable 1 in Supplement 2). Receipt of a packed red blood cell transfusion for severe symptomatic anemia at presentation did not exclude enrollment. All patients who received a transfusion had repeat hematologic indices assessed after the transfusion to ensure eligibility.
Enrolled patients were randomized with a 1:1 allocation to receive either oral ferrous sulfate drops (15 mg/mL) or oral iron polysaccharide complex drops (15 mg/mL). Randomization was stratified by degree of anemia (hemoglobin concentration ≥8.0 g/dL vs <8.0 g/dL). Within each stratum, patients were randomly assigned to either experimental group with a 1:1 allocation by a computer-generated randomization schedule using permuted blocks of 4.
Allocation concealment occurred after each participant was formally enrolled in the trial, and all baseline measurements (history, physical examination, laboratory assessment) were completed. Implementation of the sequence generation and allocation concealment mechanism was performed by an investigational pharmacist who had no direct contact with study patients. Trial investigators performed enrollment and all study procedures after randomization. Except for the investigational pharmacist, the members of the study team were blinded to treatment allocation.
Patients were prescribed a single daily dose of 3 mg/kg of elemental iron administered via oral syringe by the parents or caregiver at bedtime. Daily doses were rounded up to the nearest 0.5 mL. Parents or caregivers were asked not to administer other iron-containing preparations and instructed not to mix the study medication with any food or drink and specifically to avoid milk intake within 1 hour of administering the study medication. Nutritional counseling about the need to reduce cow milk intake to a maximum of 600 mL per day was provided. A daily diary with the dosing instructions was given to parents or caregivers at each visit to record medication administration and adverse effects.
Subsequent outpatient study visits were scheduled at weeks 4, 8, and 12 after enrollment to review between-interval history and perform laboratory testing (complete blood count, reticulocyte count, reticulocyte hemoglobin equivalent, serum ferritin level, serum iron level, total iron-binding capacity, and blood lead level [the latter performed during week 4 only]). Treatment failure was defined as a hemoglobin increment of less than 0.5 g/dL above the baseline concentration at week 8. The final visit at week 12 involved a comprehensive history, including whether the study drug had been mixed with other liquids or foods. Adverse effects were assessed every 2 weeks via telephone contact and at clinic visits. At each follow-up visit, returned medication bottles were submitted to the investigational pharmacy and the volume of unused study medication was measured. Further details regarding adverse effect assessment appear in the eMethods in Supplement 2.
The primary outcome was the change in hemoglobin concentration during the 12 weeks after initiation of oral iron therapy. Secondary outcomes included the proportion of patients with complete resolution of IDA (defined as hemoglobin concentration >11 g/dL, mean corpuscular volume >70 fL, reticulocyte hemoglobin equivalent >25 pg, serum ferritin level >15 ng/mL, and total iron-binding capacity <425 μg/dL at the 12-week visit), changes in other iron laboratory measures, adverse effects, lost to follow-up rates, resolution of IDA signs and symptoms, and medication adherence as measured by patient diary entries and returned medication volume.
Estimates for baseline mean hemoglobin concentration of 7.8 g/dL (SD, 1.5 g/dL) were derived from data on new patients with IDA seen at the Children’s Medical Center outpatient hematology clinic during the preceding 4½-year period. We hypothesized that at the time of study completion there would be a 1-g/dL between-group difference in the mean hemoglobin concentration values (ferrous sulfate: 11 g/dL; iron polysaccharide complex: 12 g/dL). The effect size of 1 g/dL was chosen as a clinically significant difference based on an analysis of studies evaluating IDA and neurocognitive outcomes by the World Health Organization and work examining the relationship between serum ferritin level and hemoglobin concentration in young children. We assumed a within-patient correlation of 0.25. Using a linear mixed model and assuming a lost to follow-up rate of 25%, the accrual goal was 40 patients per group (80 total) to allow for a 2-sided type I error of .05 at 80% power.
We hypothesized that iron polysaccharide complex would result in a greater hematologic response compared with ferrous sulfate as measured by change in hemoglobin concentration over 12 weeks. The primary analysis consisted of a linear mixed regression model in an intention-to-treat population. It included treatment and time as covariates and patient random effects to account for correlation among longitudinal measurements from the same patients. The linear mixed model readily accommodates missing data so imputation for missing data was not performed. Changes in other continuous measures were modeled with a similar linear mixed regression method. The generalized estimation equation approach was used to estimate model parameters, which accommodates incomplete data and is robust against deviation from the normality assumption.
The χ2 test was used to compare categorical outcomes, including the proportion of patients with a complete response, dropouts, adverse effects, and adherence measures. The percentage volume of unused study medication returned at each visit was compared using the Wilcoxon rank sum test. All tests were 2-sided with a significance level of .05. Adjustment for multiple comparisons of secondary end points was not performed. Inference about secondary outcomes should be interpreted as exploratory. A post hoc between-group comparison of the proportion of patients who complained of abdominal pain, constipation, vomiting, and diarrhea (combined gastrointestinal adverse effect profile) also was conducted using the χ2 test. All analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
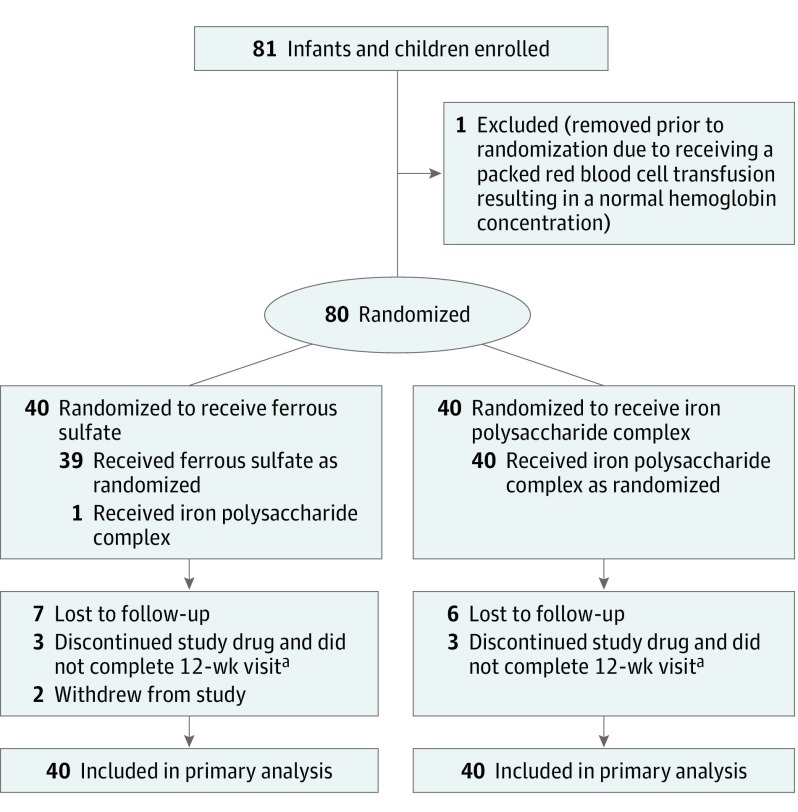
The trial enrolled 81 eligible study patients, of whom 80 were randomized (40 in the ferrous sulfate group and 40 in the iron polysaccharide complex group; Figure 1). The baseline demographic characteristics, laboratory values, and the percentage who received a packed red blood cell transfusion prior to enrollment were similar for both groups (Table 1). The mean age was 23 months and 55% were male. Sixty-one percent were Hispanic white, 9% were non-Hispanic white, and 11% were black.
Figure 1. Enrollment, Randomization, and Follow-up of the Study Patients.
The total number of patients screened and the reasons for exclusion are not available.
aConsidered a treatment failure (predefined in the study protocol as a hemoglobin increment <0.5 g/dL above the baseline concentration at week 8, which resulted in the discontinuation of the study drug by the study team).
Table 1. Baseline Characteristics.
| Ferrous Sulfate Group (n = 40)a |
Iron Polysaccharide Complex Group (n = 40)a |
|
|---|---|---|
| Age at enrollment, median (range), mo | 22 (10-37) | 23 (11-34) |
| Female sex | 18 (45) | 18 (45) |
| Race/ethnicity | ||
| Hispanic white | 23 (58) | 26 (65) |
| Non-Hispanic white | 4 (10) | 3 (8) |
| Black | 5 (13) | 4 (10) |
| Asian | 2 (5) | 2 (5) |
| Native Hawaiian/Pacific Islander | 0 | 1 (3) |
| >1 Race/ethnicity | 6 (15) | 4 (10) |
| Etiology of nutritional iron-deficiency anemia | ||
| Breast milk without iron supplementation | 11 (27) | 8 (20) |
| Excessive cow milk | 29 (73) | 32 (80) |
| Daily amount of cow milk, mean (SD), mL | 1230 (330) | 1260 (330) |
| Signs and symptoms of iron-deficiency anemiab | ||
| Pallor | 18 (45) | 18 (45) |
| Decreased energy | 13 (33) | 16 (40) |
| Pica | 18 (45) | 21 (53) |
| Baseline values | ||
| Hemoglobin concentration, mean (SD), g/dL | 7.9 (1.5) | 7.7 (1.6) |
| Mean corpuscular volume, mean (SD), fL | 60.2 (5.2) | 59.7 (5.4) |
| Serum ferritin, median (interquartile range), ng/mL | 3.0 (1.7-6.0) | 2.0 (1.0-4.3) |
| Total iron-binding capacity, mean (SD), μg/dL | 501 (100) | 506 (85) |
| Reticulocyte hemoglobin equivalent, mean (SD), pg | 16.8 (3.8)c | 17.1 (3.8)c |
| Receipt of a packed red blood cell transfusiond | 10 (25) | 8 (20) |
SI conversion factors: To convert ferritin to pmol/L, multiply by 2.247; hemoglobin to g/L, multiply by 10.0; total iron-binding capacity to μmol/L, multiply by 0.179.
Data are expressed as No. (%) unless otherwise indicated. Percentages may not sum to 100% due to rounding.
Reported by parents or caregiver.
There were 26 infants and children for this variable in each group.
Occurred prior to study enrollment.
One child in the ferrous sulfate group erroneously received iron polysaccharide complex. This patient experienced treatment failure at week 8, was removed from study at that time, and was analyzed within the ferrous sulfate group. The other 79 patients received the intended treatment.
Primary Outcome
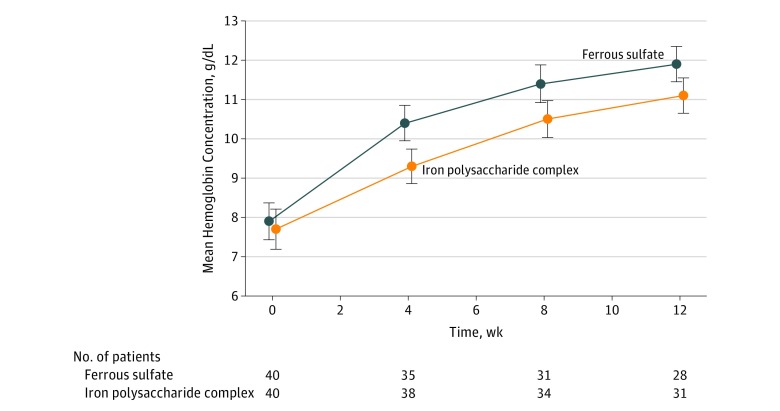
The mean hemoglobin concentration increased from 7.9 g/dL to 11.9 g/dL in the ferrous sulfate group compared with an increase from 7.7 g/dL to 11.1 g/dL in the iron polysaccharide complex group over 12 weeks (Figure 2). The primary outcome (using a linear mixed model) demonstrated a significant difference in the change in hemoglobin concentration of 1.0 g/dL (95% CI, 0.4-1.6; P < .001) between the 2 groups, favoring ferrous sulfate.
Figure 2. Hemoglobin Concentration Over Time.
The error bars indicate 95% CIs. Using a linear mixed model, there was a significant difference in the change in hemoglobin concentration over time (1.0 g/dL [95% CI, 0.4-1.6 g/dL]; P < .001) between the 2 groups, favoring ferrous sulfate.
Secondary Outcomes
The 59 patients who completed all study visits (28 in the ferrous sulfate group and 31 in the iron polysaccharide complex group) were analyzed for the predefined complete resolution of IDA (Table 2), which occurred in a larger proportion of patients receiving ferrous sulfate (29%, n = 8) compared with those receiving iron polysaccharide complex (6%, n = 2) (P = .04). There were no significant between-group differences in iron measurements at baseline.
Table 2. Secondary Outcomes.
| No./Total (%)a | Between-Group Difference, % (95% CI) |
P Value |
||
|---|---|---|---|---|
| Ferrous Sulfate Group |
Iron Polysaccharide Complex Group |
|||
| Complete resolution of iron-deficiency anemiab | 8/28 (29) | 2/31 (6) | 22 (3 to 41) | .04 |
| Lost to follow-up | 7/40 (18) | 6/40 (15) | 3 (−14 to 19) | .76 |
| Signs and symptoms of iron-deficiency anemia at 12 wk | ||||
| Resolution of pallor | 21/28 (75) | 26/31 (84) | −9 (29 to 12) | .39 |
| Increase in energy | 25/28 (89) | 27/31 (87) | 2 (−14 to 18) | >.99 |
| Persistence of pica | 5/27 (19) | 6/31 (19) | −1 (−21 to 19) | .93 |
| Medication adherence | ||||
| Successful administrationc | 27/33 (82) | 34/36 (94) | −13 (−25 to −1) | .009 |
| Mixed medication with juice or food | 5/28 (18) | 4/31 (13) | 5 (−13 to 23) | .73 |
| Doses taken, %d | ||||
| Wk 0-4e | 93 | 99 | −6 (−15 to 4) | .25 |
| Wk 4-8f | 94 | 100 | −6 (−15 to 3) | .18 |
| Wk 8-12g | 93 | 98 | −6 (−17 to 7) | .37 |
Unless otherwise indicated.
Defined as hemoglobin concentration greater than 11 g/dL, mean corpuscular volume greater than 70 fL, reticulocyte hemoglobin equivalent greater than 25 pg, serum ferritin level greater than 15 ng/mL, and total iron-binding capacity less than 425 μg/dL at the 12-week visit.
Infant or child did not spit out medication.
Measured by returned medication volume.
Data were available for 32 in the ferrous sulfate group and 37 in the iron polysaccharide complex group.
Data were available for 30 in the ferrous sulfate group and 32 in the iron polysaccharide complex group.
Data were available for 28 in the ferrous sulfate group and 30 in the iron polysaccharide complex group.
The median serum ferritin level increased from 3.0 ng/mL to 15.6 ng/mL in the ferrous sulfate group compared with an increase from 2.0 ng/mL to 7.5 ng/mL in the iron polysaccharide complex group over 12 weeks (a greater difference of 10.2 ng/mL [95% CI, 6.2 to 14.1 ng/mL] with ferrous sulfate, P < .001; Table 3). The mean total iron-binding capacity decreased from 501 μg/dL to 389 μg/dL in the ferrous sulfate group compared with a decrease from 506 μg/dL to 417 μg/dL in the iron polysaccharide complex group over 12 weeks (a greater difference of −50 μg/dL [95% CI, −86 to −14 μg/dL] with ferrous sulfate, P < .001; Table 3).
Table 3. Secondary Outcomes of Serum Ferritin Level and Total Iron-Binding Capacity.
| Ferrous Sulfate Group | Iron Polysaccharide Complex Group | |||
|---|---|---|---|---|
| No. of Patients |
Outcome | No. of Patients |
Outcome | |
| Serum Ferritin Level, Median (Interquartile Range), ng/mLa | ||||
| Wk 0 | 40 | 3.0 (1.7-6.0) | 40 | 2.0 (1.0-4.3) |
| Wk 4 | 35 | 17.8 (9.9-24.8) | 38 | 4.7 (3.5-12.9) |
| Wk 8 | 31 | 17.7 (12.2-25.0) | 34 | 6.6 (3.5-12.9) |
| Wk 12 | 28 | 15.6 (8.8-27.7) | 31 | 7.5 (5.0-11.4) |
| Total Iron-Binding Capacity, Mean (95% CI), μg/dLb | ||||
| Wk 0 | 40 | 501 (469-533) | 40 | 506 (479-533) |
| Wk 4 | 35 | 413 (380-448) | 38 | 473 (447-499) |
| Wk 8 | 31 | 400 (375-425) | 34 | 445 (418-473) |
| Wk 12 | 28 | 389 (360-418) | 31 | 417 (391-444) |
SI conversion factors: To convert ferritin to pmol/L, multiply by 2.247; total iron-binding capacity to μmol/L, multiply by 0.179.
Based on a linear mixed model, treatment with ferrous sulfate resulted in a greater difference of 10.2 ng/mL (95% CI, 6.2 to 14.1 ng/mL) over 12 weeks vs iron polysaccharide complex (P < .001).
Based on a linear mixed model, treatment with ferrous sulfate resulted in a greater difference of −50 μg/dL (95% CI, −86 to −14 μg/dL) over 12 weeks vs iron polysaccharide complex (P < .001).
Adverse Effects and Events
The adverse effect profiles demonstrated significantly more parent reports of diarrhea with iron polysaccharide complex (Table 4). More parent reports of vomiting were made in the ferrous sulfate group; however, this difference was not statistically significant. A combined post hoc gastrointestinal adverse effect profile (abdominal pain, constipation, vomiting, and diarrhea) showed no significant between-group differences. One patient receiving ferrous sulfate experienced a transient episode of methemoglobinemia of unknown cause during week 4. The patient was successfully treated with methylene blue and continued in the study without recurrence.
Table 4. Adverse Effects.
| No./Total (%) Reporting Adverse Effect |
P Value |
||
|---|---|---|---|
| Ferrous Sulfate Group |
Iron Polysaccharide Complex Group |
||
| Difficulty in Giving Medication | |||
| Ever reported | 26/40 (65) | 20/40 (50) | .17 |
| Wk 2 | 13/30 (43) | 12/30 (40) | .88 |
| Wk 4 | 14/35 (40) | 11/38 (29) | .32 |
| Wk 6 | 11/22 (50) | 3/22 (14) | .01 |
| Wk 8 | 13/31 (42) | 3/34 (9) | .002 |
| Wk 10 | 8/18 (44) | 2/20 (10) | .03 |
| Wk 12 | 8/28 (29) | 4/31 (13) | .14 |
| Abdominal Pain | |||
| Ever reported | 9/40 (23) | 13/40 (33) | .32 |
| Wk 2 | 4/30 (13) | 2/30 (7) | .67 |
| Wk 4 | 5/35 (14) | 5/38 (13) | >.99 |
| Wk 6 | 4/22 (18) | 2/22 (9) | .66 |
| Wk 8 | 4/31 (13) | 4/34 (12) | >.99 |
| Wk 10 | 1/18 (6) | 2/20 (10) | >.99 |
| Wk 12 | 4/28 (14) | 5/31 (16) | >.99 |
| Vomiting | |||
| Ever reported | 23/40 (58) | 18/40 (45) | .26 |
| Wk 2 | 10/30 (33) | 5/30 (17) | .14 |
| Wk 4 | 12/35 (34) | 7/38 (18) | .13 |
| Wk 6 | 6/22 (27) | 2/22 (9) | .24 |
| Wk 8 | 8/31 (26) | 2/34 (6) | .04 |
| Wk 10 | 3/18 (17) | 1/20 (5) | .33 |
| Wk 12 | 3/28 (11) | 5/31 (16) | .71 |
| Diarrhea | |||
| Ever reported | 14/40 (35) | 23/40 (58) | .04 |
| Wk 2 | 8/30 (27) | 9/30 (30) | .77 |
| Wk 4 | 7/35 (20) | 6/38 (16) | .64 |
| Wk 6 | 4/22 (18) | 5/22 (23) | >.99 |
| Wk 8 | 4/31 (13) | 5/34 (15) | >.99 |
| Wk 10 | 3/18 (17) | 2/20 (10) | .65 |
| Wk 12 | 2/28 (7) | 8/31 (26) | .08 |
| Gastrointestinala | |||
| Ever reported | 29/40 (73) | 32/40 (80) | .43 |
| Wk 2 | 16/30 (53) | 12/30 (40) | .30 |
| Wk 4 | 21/35 (60) | 16/38 (42) | .13 |
| Wk 6 | 12/22 (55) | 7/22 (32) | .13 |
| Wk 8 | 14/31 (45) | 10/34 (29) | .19 |
| Wk 10 | 6/18 (33) | 5/20 (25) | .57 |
| Wk 12 | 10/28 (36) | 13/31 (42) | .62 |
Abdominal pain, vomiting, diarrhea, or constipation.
Lost to Follow-up Rates
Sixteen percent of patients were lost to follow-up (7 [18%] in the ferrous sulfate group vs 6 [15%] in the iron polysaccharide complex group; P = .72). There were no significant differences in baseline characteristics (eTable 2 in Supplement 2) or adverse effects (eTable 3 in Supplement 2) between those patients who completed the study vs those who were lost to follow-up.
Signs and Symptoms of IDA
Both groups had improvements in patient-centered outcomes such as increased energy level, decreased pica, and resolution of pallor that were not significantly different (Table 2). None of the patients had an elevated blood lead level at the week 4 study visit.
Medication Adherence
Parents reported more successful administration of all individual iron doses (ie, the child not spitting out the medication) with iron polysaccharide complex (94% vs 82% in the ferrous sulfate group; P = .009). There were no other significant between-group measures for medication adherence. Via patient diaries, 78% of families reported missing no doses of iron polysaccharide complex compared with 53% of families in the ferrous sulfate group (P = .12).
Fifty percent of parents reported difficulty with administration of iron polysaccharide complex at any point in the study compared with 65% in the ferrous sulfate group (P = .17; Table 4). Returned medication volume indicated no significant difference in adherence with iron polysaccharide complex compared with ferrous sulfate. As a marker of difficulty with medication administration, there was no significant difference in the proportion of parents who reported mixing the iron preparation with juice or food (Table 2).
Discussion
In this randomized clinical trial of infants and children aged 9 to 48 months with nutritional IDA treated at a single academic medical center, ferrous sulfate drops compared with iron polysaccharide complex drops resulted in a greater increase in hemoglobin concentration at 12 weeks. Studies of neurocognitive outcomes in children with IDA have demonstrated the clinical importance of the between-group difference in hemoglobin concentration of 1 g/dL found in this trial.
Among the secondary outcomes, the proportion of patients with a complete resolution of IDA (ie, no further iron therapy required) was higher in the ferrous sulfate group. Similar to the primary outcome, changes in iron indices favored the ferrous sulfate group. More parent reports of diarrhea were made in the iron polysaccharide complex group but no other significant differences in adverse effects were found. Patients were more likely to ingest the iron polysaccharide complex during administration compared with ferrous sulfate, but there were otherwise no significant between-group differences in the lost to follow-up rates, resolution of IDA symptoms, or medication adherence.
Iron salts have long been used for the treatment of IDA, with ferrous sulfate being the most common choice in persons of all ages. Reports of poor taste and gastrointestinal adverse effects often result in the recommendation of other iron salts (eg, ferrous gluconate and fumarate) or iron polysaccharide complex preparations. In the current trial, an iron polysaccharide complex agent developed specifically to have improved taste and tolerability was hypothesized to promote a more effective hematologic response. Although both preparations were effective and well-tolerated, ferrous sulfate resulted in a greater increase in hemoglobin concentration and improved diverse measures of iron homeostasis.
Previous radioisotope studies have demonstrated more effective use of iron when administered in the form of medicinal iron in contrast to iron-fortified foods. Small studies in adults suggest that bivalent salts such as ferrous sulfate are more effectively absorbed than trivalent ones. Limited data from prior studies suggest improved absorption with ferrous sulfate compared with iron polysaccharide complexes. A small case series by Diamond et al compared a palatable iron carbohydrate complex with ferrous sulfate, and the latter resulted in a more rapid hemoglobin concentration increase over 3 weeks.
Oral iron absorption tests were conducted and those patients receiving ferrous sulfate demonstrated a markedly higher increase in serum iron, supporting the notion of more effective absorption. Therefore, this trial failed to demonstrate the original hypothesis that iron polysaccharide complex would be more effective than ferrous sulfate, likely due to more effective absorption of the latter.
Recommended therapeutic dosing of elemental iron in children ranges widely from 2 to 6 mg/kg/d administered from 1 to 3 times daily. In this trial, a daily iron dose at the lower end of the recommended range (3 mg/kg of elemental iron) administered just once daily, at bedtime, and on an empty stomach was chosen. Two prior randomized clinical trials in children informed this minimalist strategy aimed to enhance adherence and limit adverse effects. One trial compared once daily ferrous sulfate (3 mg/kg of elemental iron) with placebo in infants aged 12 months and demonstrated no significant differences in adverse gastrointestinal effects. The other trial conducted in rural Ghana documented similar success rates in correcting anemia when ferrous sulfate was administered once or 3 times daily. Recommendations based primarily on expert opinion from the US Centers for Disease Control and Prevention and the World Health Organization have endorsed low-dose, once daily iron therapy.
Studies involving adults with IDA also support the use of low-dose oral iron treatment. In 1 trial, octogenarians with anemia were randomized to 15 mg, 50 mg, or 150 mg of ferrous salts (gluconate or citrate) administered once daily and had virtually identical hematologic responses, but there were far less gastrointestinal toxic effects in the group receiving 15 mg. A study in women with iron deficiency using stable iron isotopes demonstrated that a single dose of iron engendered a prompt increase in serum hepcidin level that resulted in markedly reduced absorption of subsequent iron doses 12 hours or even 24 hours later. Taken together, these findings suggest that once daily low-dose iron therapy might result in greater fractional absorption of iron in lieu of multiple doses of iron each day.
Unlike nearly all prior therapeutic studies of oral iron agents, this trial was designed as a double-blind, randomized clinical trial. In concert with other literature, these results should help stimulate the conduct of further clinical trials evaluating lower or less frequent dosing of oral iron. Anticipated outcomes might include improved patient adherence as well as enhanced iron absorption leading to a more favorable hematologic response.
Limitations
The trial has several limitations. First, it was conducted at a single tertiary care children’s hospital. Second, there were a disproportionate number of lower income and minority patients whose anemia was often severe, with approximately 23% requiring a blood transfusion prior to enrollment. Third, the trial had a lost to follow-up rate of 25% at the final 12-week visit. Fourth, these results may not be generalizable to the general pediatric population due to the strict monitoring involved in a clinical trial compared with standard community practice. Yet by choosing to use a simplified dosing regimen, the goal was to create a practical treatment strategy that could improve outcomes even when applied in a clinical setting.
Conclusions
Among infants and children aged 9 to 48 months with nutritional iron-deficiency anemia, ferrous sulfate compared with iron polysaccharide complex resulted in a greater increase in hemoglobin concentration at 12 weeks. Once daily, low-dose ferrous sulfate should be considered for children with nutritional iron-deficiency anemia.
Trial protocol
eTable 1. BESTIRON eligibility criteria
eTable 2. Baseline clinical characteristics based on study completion
eTable 3. Adverse effects based on study completion
eMethods
References
- 1.McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454. [DOI] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brotanek JM, Gosz J, Weitzman M, Flores G. Secular trends in the prevalence of iron deficiency among US toddlers, 1976-2002. Arch Pediatr Adolesc Med. 2008;162(4):374-381. [DOI] [PubMed] [Google Scholar]
- 4.McDonagh MS, Blazina I, Dana T, et al. Screening and routine supplementation for iron deficiency anemia. Pediatrics. 2015;135(4):723-733. [DOI] [PubMed] [Google Scholar]
- 5.Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients. 2016;8(6):E330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325(10):687-694. [DOI] [PubMed] [Google Scholar]
- 7.Lozoff B, Jimenez E, Hagen J, et al. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105(4):E51. [DOI] [PubMed] [Google Scholar]
- 8.Lukowski AF, Koss M, Burden MJ, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years. Nutr Neurosci. 2010;13(2):54-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers JM, Daniel CL, McCavit TL, Buchanan GR. Deficiencies in the management of iron deficiency anemia during childhood. Pediatr Blood Cancer. 2016;63(4):743-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves JD, Yip R. Lack of adverse side effects of oral ferrous sulfate therapy in 1-year-old infants. Pediatrics. 1985;75(2):352-355. [PubMed] [Google Scholar]
- 11.Zlotkin S, Arthur P, Antwi KY, Yeung G. Randomized, controlled trial of single versus 3-times-daily ferrous sulfate drops for treatment of anemia. Pediatrics. 2001;108(3):613-616. [DOI] [PubMed] [Google Scholar]
- 12.Powers JM, Buchanan GR. Diagnosis and management of iron deficiency anemia. Hematol Oncol Clin North Am. 2014;28(4):729-745, vi-vii. [DOI] [PubMed] [Google Scholar]
- 13.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989. [DOI] [PubMed] [Google Scholar]
- 14.Rimon E, Kagansky N, Kagansky M, et al. Are we giving too much iron? Am J Med. 2005;118(10):1142-1147. [DOI] [PubMed] [Google Scholar]
- 15.Smith NJ. Iron as a therapeutic agent in pediatric practice. J Pediatr. 1958;53(1):37-50. [DOI] [PubMed] [Google Scholar]
- 16.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832-1843. [DOI] [PubMed] [Google Scholar]
- 17.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anemia In: Ezzati M, Lopez AD, Rodgers A, Murral CJL, eds. Comparative Quantification of Health Risks. Vol 1 Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 18.Abdullah K, Birken CS, Maguire JL, et al. Re-evaluation of serum ferritin cut-off values for the diagnosis of iron deficiency in children aged 12-36 months [published online April 18, 2017]. J Pediatr. doi: 10.1016/j.jpeds.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. [Google Scholar]
- 20.Powers JM, McCavit TL, Buchanan GR. Management of iron deficiency anemia. Pediatr Blood Cancer. 2015;62(5):842-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard JA. Hemoglobin regeneration in severe iron-deficiency anemia. JAMA. 1966;195(9):717-720. [PubMed] [Google Scholar]
- 22.Kerr DN, Davidson S. Gastrointestinal intolerance to oral iron preparations. Lancet. 1958;2(7045):489-492. [DOI] [PubMed] [Google Scholar]
- 23.Schulz J, Smith NJ. A quantitative study of the absorption of food iron in infants and children. AMA J Dis Child. 1958;95(2):109-119. [DOI] [PubMed] [Google Scholar]
- 24.Moore CV, Arrowsmith WR, Welch J, Minnich V. Studies in iron transportation and metabolism, IV. J Clin Invest. 1939;18(5):553-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore CV, Dubach R, Minnich V, Roberts HK. Absorption of ferrous and ferric radioactive iron by human subjects and by dogs. J Clin Invest. 1944;23(5):755-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond LK, Naiman JL, Allen DM, Oski FA. The treatment of iron-deficiency anemia. Pediatrics. 1963;31:1041-1044. [PubMed] [Google Scholar]
- 27.From the Centers for Disease Control and Prevention Iron deficiency. JAMA. 2002;288(17):2114-2116. [PubMed] [Google Scholar]
- 28.World Health Organization Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide for Programme Managers. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 29.Schrier SL. So you know how to treat iron deficiency anemia. Blood. 2015;126(17):1971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. BESTIRON eligibility criteria
eTable 2. Baseline clinical characteristics based on study completion
eTable 3. Adverse effects based on study completion
eMethods