Abstract
Proper development of a multicellular organism relies on well-coordinated regulation of cell fate specification, cell proliferation and cell differentiation. The C. elegans postembryonic mesoderm provides a useful system for uncovering factors involved in these processes and for further dissecting their regulatory relationships. The single Spalt-like zinc finger containing protein SEM-4/SALL is known to be involved in specifying the proliferative sex myoblast (SM) fate. We have found that SEM-4/SALL is sufficient to promote the SM fate and that it does so in a cell autonomous manner. We further showed that SEM-4/SALL acts through the SoxC transcription factor SEM-2 to promote the SM fate. SEM-2 is known to promote the SM fate by inhibiting the expression of two BWM-specifying transcription factors. In light of recent findings in mammals showing that Sall4, one of the mammalian homologs of SEM-4, contributes to pluripotency regulation by inhibiting differentiation, our work suggests that the function of SEM-4/SALL proteins in regulating pluripotency versus differentiation appears to be evolutionarily conserved.
Keywords: SEM-4, SALL, SEM-2, SoxC, pluripotent, mesoderm, cell fate specification, M lineage, sex myoblast, coelomocyte, bodywall muscle, proliferation, differentiation
INTRODUCTION
During the development of a multicellular organism, cell fate specification, cell proliferation and cell differentiation need to be tightly regulated and precisely coordinated. The C. elegans postembryonic mesoderm provides a unique system for dissecting the regulatory network underlying these developmental processes. In C. elegans, all the postembryonic non-gonadal mesoderm cells arise from the M lineage. The M lineage is derived from a single pluripotent precursor cell, the M mesoblast. In a hermaphrodite animal, the M cell undergoes two waves of proliferation to produce thirty-two differentiated cells, which include fourteen striated body wall muscles (BWMs), two non-muscle coelomocytes (CCs), and sixteen non-striated egg-laying muscles of four different types (four type I and four type II vulval muscles and four type I and four type II uterine muscles) (Sulston and Horvitz, 1977). The sixteen egg-laying muscles are derived from two pluripotent cells, called the sex myoblasts (SMs). The BWMs, CCs and SMs are products of the first wave of M cell proliferation during the L1 larval stage, while all the egg-laying muscles are products of the SM cells during the second wave of M lineage proliferation. Importantly, the sister cells of the two SMs are differentiated BWMs (Figure 1A).
Figure 1. SEM-4 is required for specifying M lineage-derived coelomocytes (CCs) and sex myoblasts (SMs).
(A, B) Diagrams showing the postembryonic M lineage in wild-type (A) and sem-4(0) mutant (B) animals. a, anterior; p, posterior; d, dorsal; v, ventral; l, left; r, right. (C–D) Composite GFP/DIC images of sem-4(h769)/+ (C) and sem-4(h769) (D) worms with their CCs labeled with a secreted CC::GFP and type I vulval muscles (VM1s) labeled with egl-15::GFP. The sem-4(h769)/+ worm in panel C expresses myo-2p::GFP from the “+” balancer chromosome hT2[qIs48], which is absent in sem-4(h769) homozygous worms. * points to the vulva region, and arrows point to M lineage-derived CCs. (E) Diagram of the SEM-4 protein structure marked with locations of the different sem-4 mutations. ZF: zinc finger motif. (F) Summary of the M lineage phenotypes of various sem-4 alleles. (G) Alignment between ZF2-4 and ZF5-7 in the SEM-4 protein, showing high degree of similarity between ZF2-3 and ZF5-6.
Multiple transcription factors and signaling pathways are known to play a role in the proper proliferation and subsequent specification and differentiation of the various cell types that are derived from the M lineage (Krause and Liu, 2012). In particular, we have previously shown that the SoxC transcription factor SEM-2 is required for the binary fate decision between the SMs and BWMs, and that SEM-2 is both necessary and sufficient to promote the proliferative SM fate and inhibit the differentiated BWM fate (Tian et al., 2011). In sem-2 mutants, two redundant transcription factors required for specifying the BWM fate, HLH-1/MRF and FOZI-1 (Amin et al., 2007; Harfe et al., 1998), are de-repressed in the two presumptive SMs, and these two cells adopt the fate of their sister cells, the differentiated BWMs (Tian et al., 2011).
Another transcription factor, a zinc finger containing protein SEM-4 has also been shown to be required for specifying the SM fate (Basson and Horvitz, 1996). SEM-4 contains seven C2H2-type zinc finger motifs and is homologous to the Spalt-like (SALL) proteins in flies and mammals. SEM-4 is known to function in multiple cell types in C. elegans. It acts to restrict the touch neuron fate by directly binding to the promoters of the Hox factor EGL-5 and the LIM homeodomain protein MEC-3 to repress their expression (Toker et al., 2003). In the developing vulva, SEM-4 regulates the expression of the Hox factor LIN-39 to promote vulval cell fate determination (Grant et al., 2000). SEM-4 is also required for Y-to-PDA trans-differentiation, a natural epithelial-neuronal reprogramming event in C. elegans (Jarriault et al., 2008). It has been shown that the function of SEM-4 in this reprogramming process involves its association with the transcriptional repressor EGL-27/MTA1 in a NODE (Nanog and Oct4-associated deacetylase)-like complex (Jarriault et al., 2008; Kagias et al., 2012).
Despite the initial finding by Basson and Horvitz (Basson and Horvitz, 1996) that SEM-4 is required for specifying the SM fate, how SEM-4 functions in this process is not known. In this study, we dissected the role of SEM-4 and its relationship with SEM-2 in SM fate specification. We found that SEM-4 is both necessary and sufficient to promote the SM fate, and that it does so by acting through SEM-2.
MATERIALS AND METHODS
C. elegans strains
All strains were maintained at 20°C unless otherwise noted. The following strains were used in this study: sem-2(n1343), sem-4(n1971)/hT2[qIs48], sem-4(n2477)/hT2[qIs48], sem-4(n1378)/hT2[qIs48], sem-4(h769)/hT2[qIs48], sem-4(tm547)/hT2[qIs48], sem-4(n2087)/hT2[qIs48]. Additional integrated transgenic lines used are: LW0001: hT2[qIs48]/qC1; ayIs2(egl-15::GFP) IV; arIs39(secreted CC::GFP) X. LW0081: ccIs4438(intrinsic CC::GFP) III; ayIs2(egl-15::GFP) IV; ayIs6(hlh-8::GFP) X. LW1380: jjIs1380(egl-15::mRFP + intrinsic CC::mRFP + unc-119(+)). LW1479: jjIs1479(myo-3::NLS::mRFP::lacZ ) + unc-119(+)) V. LW3900: jjIs3900(hlh-8p::NLS::mCherry::lacZ + myo-2p::mCherry) IV. LW4191: jjIs4191(hlh-8p::sem-4 cDNA::sem-4 3’UTR + myo-2p::mCherry); ccIs4438(intrinsic CC::GFP) III; ayIs2(egl-15::GFP) IV; ayIs6(hlh-8::GFP) X. PD4251: ccIs4251(myo-3p::NLS::GFP::lacZ + myo-3p::mit::GFP + dpy-20(+)). MH1337: kuIs34(sem-4::GFP +unc-119(+)) and MH1346: kuIs35(sem-4::GFP+unc-119(+)) (Grant et al., 2000).
Plasmid constructs, transgenic lines and RNAi
The sem-4 cDNA clone yk1509a11 (gift from Yuji Kohara, National Institute of Genetics, Japan), which contains the coding region and the 3’UTR of sem-4, was used to generate pSQF2 (hlh-8p::sem-4cDNA::sem-4 3’UTR).
The following sem-4 transcriptional fusion reporter plasmids are gifts from Michael Koelle (Yale University): pMK326 (SphI-SacII 11kb sem-4 genomic DNA including 25bp sem-4 ORF::GFP), pMK329 (MluI-SacII 4.5kb sem-4 genomic DNA::GFP), pMK330 (StuI-SacII 3.7kb sem-4 genomic DNA::GFP), pMK331 (SmaISacII 2.1kb sem-4 genomic DNA::GFP), pMK332 (NsiI-SacII, 0.4kb sem-4 genomic DNA::GFP). pSQF14 (4.7kb first intron::pes-10p::4xNLS::GFP::unc-54 3’UTR) was used to assay for enhancer activity in the first intron of sem-4.
All sem-2 containing constructs are described in (Tian et al., 2011), which include pJKL776 (hlh-8p::sem-2cDNA::unc-54 3’UTR), the GFP::sem-2 translational fusion-containing fosmid, and pCXT33 (598bp sem-2 M lineage enhancer::pes-10p::NLS::GFP::lacZ::unc-54 3’UTR).
Transgenic lines were generated using the plasmid pCFJ90 (myo-2p::mCherry::unc-54 3’UTR) (Frokjaer-Jensen et al., 2008) and pJKL449 (myo-2p::GFP::unc-54 3’UTR) as markers.
sem-2 dsRNA was prepared using yk404e6 as a template, and JKL-1585 and JKL-1586 as primers. In vitro transcription and RNAi by soaking were carried out as described in (Tian et al., 2011). Water was used as a soaking control.
Microscopy
Animals were visualized under a Leica DMRA2 compound microscope equipped with a Hamamatsu Orca-ER camera using the iVision software (Biovision technology, Inc.). Subsequent image processing was performed using Fiji (Schindelin et al., 2012).
RESULTS
sem-4 mutants exhibit a fate transformation of the proliferative SMs to differentiated BWMs
sem-4 mutations have been previously reported to affect the specification of M lineage-derived SMs and CCs (Basson and Horvitz, 1996), in which the SMs are transformed to their BWM siblings, and the CCs are transformed to their ventral counterparts, the SM mothers, which subsequently give rise to two BWMs each (Figure 1B, 1D). To further dissect the function of SEM-4 in M lineage development, we examined the M lineage phenotypes of six sem-4 mutant alleles (Figure 1E) by introducing into each mutant three cell type specific GFP markers: hlh-8p::GFP that labels all undifferentiated cells in the M lineage, egl-15p::GFP that labels the SM-derived type I vulval muscles (VM1s), and a secreted CC::GFP that labels all CCs, including the two M-derived CCs (see materials and methods). We also introduced into n1378 and n1971 the myo-3p::GFP marker to label the BWMs. Examining the M lineage phenotypes of the sem-4 mutants using these markers showed that different alleles exhibited different penetrance in their SM or CC mis-specification defects (Figure 1F). While all six alleles exhibited a high penetrance of the SM-to-BWM phenotype, the penetrance of the CC mis-specification phenotype in n1971, tm547 and n1378 are 75%, 69% and 59%, respectively. Previous work has shown that different sem-4 mutant alleles exhibit different severity of their neuronal versus mesoderm defect (Basson and Horvitz, 1996; Toker et al., 2003). Our observations showed that even in the mesoderm, different sem-4 mutations affect the CCs and the SMs differently. sem-4 encodes a predicted protein that contains seven C2H2 type zinc finger (ZF) domains (Figure 1E). tm547 contains an in-frame deletion that deletes ZF2 and ZF3, while n1378 is a nonsense mutation that results in a truncated protein lacking ZF5, ZF6 and ZF7. The amino acid sequences for ZF2 and ZF3 closely resemble those of ZF5 and ZF6, particularly between ZF3 and ZF6 ((Basson and Horvitz, 1996); Figure 1G). It is possible that ZF2-3 and ZF5-6 share redundant functions in specifying M-derived CCs. The n1971 allele is a splice site mutation that would result in a truncation of the SEM-4 protein before the first ZF, and it has been previously considered to be a null allele of sem-4 (Basson and Horvitz, 1996). The incomplete penetrance of the CC defect of n1971 mutants suggests that it may not be a null allele. It is possible that cryptic splice site(s) are used in n1971 mutants to generate partially functional SEM-4 proteins for CC specification.
The high penetrance of the SM-to-BWM fate transformation phenotype shared by all six sem-4 alleles suggests that SEM-4 is required for specifying the proliferative SM fate. We therefore focused our subsequent analysis on the role of SEM-4 in SM fate specification. We used the allele h769 for our studies because it has a nonsense mutation in residue 275 (W275Stop) and exhibits 100% penetrance for both the SM-to-BWM and the CC-to-BWM phenotypes (Figure 1D, 1F).
sem-4 is expressed throughout the M lineage
To determine how SEM-4 functions to specify M-derived SMs, we examined the expression pattern of a sem-4::GFP partial translational fusion reporter (kuIs34 and kuIs35 [sem-4::GFP+unc119(+)] (Grant et al., 2000)) relative to M lineage development. As shown in Figure 2A–I, sem-4::GFP is expressed throughout the M lineage from the 1-M stage to the 16-SM stage, and in the SM sub-lineage from the 2-SM stage to the 16-SM stage, before the cells differentiate. When these cells terminally differentiate, sem-4::GFP becomes undetectable in the differentiated CCs and vulval muscles (Figure 2JO), but remains expressed in the differentiated BWMs (Figure 2G–I).
Figure 2. SEM-4 is expressed in all the undifferentiated cells in the M lineage.
(A–I) sem-4::GFP (A, D, G) expression in the undifferentiated M lineage cells marked by hlh-8::NLS::mCherry::lacZ (B, E, H) at the 1-M stage (A–C), 8-M stage (D–F) and the 16-SM stage (G–I). C, F, I show the corresponding merged images. Images are shown with anterior to the left and dorsal up. Only images of the left side of the animal are shown in panels D-F (showing 4 M-derived cells) and in panels G-I (showing 8 SM-derived cells). Expression is also seen in the equivalent cells on the right side (not shown). Open arrowheads in panels A, D and G point to M lineage cells expressing sem-4::GFP. Arrows in panel I point to the sem-4::GFP-expressing BWMs derived from the M lineage, which also express the hlh-8::NLS::mCherry::lacZ reporter due to its perdurance. (J–O) sem-4::GFP expression (J, M) is not detected in the VM1s labeled by egl-15::mRFP (K) or M-derived CCs labeled by an intrinsic CC::mRFP (N). L and O are the corresponding merged images. (P) Summary of sem-4::GFP expression in the M lineage. sem-4::GFP-expressing cells are in green.
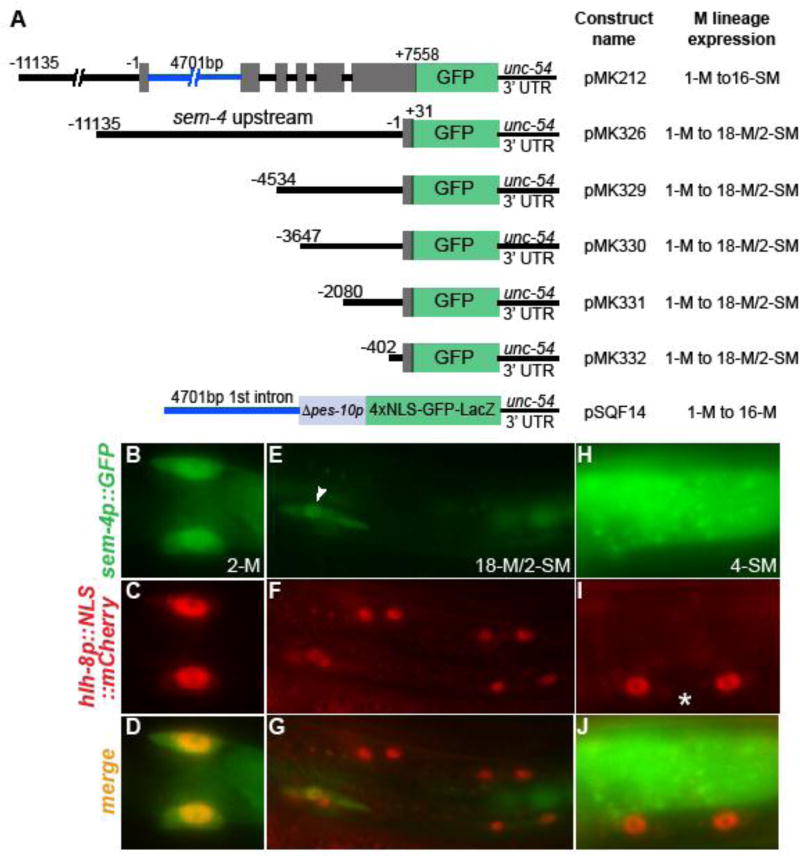
We attempted to identify the cis-regulatory element(s) involved in regulating the expression of sem-4 in the M lineage. As described above, the integrated sem-4::GFP partial translational fusion reporter (kuIs34 and kuIs35) contains about 11kb upstream and 7.5kb exon/intron sequences of sem-4, and is expressed throughout the M lineage (Figure 3A). Using a series of promoter deletion constructs (the pMK plasmid series, Figure 3A) and an enhancer assay construct (pQFS14), we found that sem-4 has at least two cis-regulatory elements mediating its expression in the early M lineage. One element is located within the 402bp region immediately upstream of the ATG (pMK332 in Figure 3A), and is sufficient to drive GFP expression from the 1-M stage till the stage when the 2 SMs are migrating (we have referred to as the 18-M/2-SM stage) (Figure 3A–G). Another element is located in the 4.7kb first intron (pSQF14 in Figure 3A), and is sufficient to drive GFP expression from the 1-M stage till the 16-M stage. Once the 2-SMs have migrated to the presumptive vulva region, none of these reporters showed GFP expression in the SM lineage (Figure 3H–J). While the lack of reporter expression in the SM lineage is simply due to the drop of GFP expression level below the threshold for detection, it is also possible that additional cis-regulatory element(s) is required for sem-4 expression in the SM lineage.
Figure 3. sem-4 expression in the M lineage requires multiple cis-regulatory elements.
(A) Diagrams of tested sem-4::GFP reporter constructs and their M lineage expression patterns. 18-M/2-SM refers to the stage when 18 M lineage descendants are born and the 2 SMs are migrating. At least two independent transgenic lines were examined for each construct. (B–J) Examples of sem-4::GFP reporter expression in the M lineage using transgenic animals carrying pMK332 as an example. Images are shown with anterior to the left and dorsal up. Only images of the left side of the animal are shown. Arrowhead in panel E points to the migrating SM cell that expresses the sem-4::GFP reporter. Asterisk denotes the vulva.
sem-4 functions within the M lineage to specify the SMs
The expression pattern of sem-4::GFP in the M lineage suggests that it may function autonomously within the M lineage to specify M-derived SMs. We tested this hypothesis by expressing sem-4 cDNA exclusively in the M lineage in the null sem-4(h769) mutants using the M lineage-specific hlh-8 promoter. As shown in Table 1, forced expression of sem-4 in the M lineage can partially rescue the M lineage phenotype of sem-4(h769) mutants: 12% of the transgenic animals (n=102) re-gained the normal number of SMs. Moreover, 11% of the transgenic animals had extra SM-like cells. Taken together, forced expression of sem-4 cDNA exclusively in the M lineage could restore the SM fate in 23% of sem-4(h769) transgenic animals, indicating that SEM-4 functions autonomously within the M lineage to specify M-derived SMs. The low penetrance of rescue (23%) might be due to the mosaic nature of the extra chromosomal array carrying the hlh-8p::sem-4 cDNA transgene. The observed 11% penetrance of the extra SM-phenotype could be a result of sem-4 overexpression (see below).
Table 1.
SEM-4 functions through SEM-2 to specify the SM fate.
| Genotype | % with 1–2 SMs |
% with > 2 SMs |
% with 0 SMs | # of worms scored |
|||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1–2 M- CCs |
0 M- CCs |
1–2 M- CCs |
0 M- CCs |
1–2 M- CCs |
0 M- CCs |
||
| Wild-type | 100% | 0 | 0 | 0 | 0 | 0 | 133 |
| sem-4(h769) | 0 | 0 | 0 | 0 | 0 | 100% | 154 |
| sem-4(h769); jjEx3385[hlh-8p::sem-4 cDNA]* | 12% | 0 | 0 | 11% | 0 | 77% | 102 |
| sem-4(h769); jjEx4690[hlh-8p::sem-2 cDNA]* | 0 | 14% | 0 | 0 | 0 | 86% | 56 |
| N2; jjIs4191[hlh-8p::sem-4 cDNA] | 10% | 0 | 0 | 88% | 1% | 1% | 130 |
| sem-2(RNAi) | 1% | 0 | 0 | 0 | 99% | 0 | 1167 |
| sem-2(RNAi); jjIs4191[hlh-8p::sem-4 cDNA] | 1% | 0 | 0 | 0 | 0 | 99% | 1037 |
The SMs and their descendants were scored by using a combination of hlh-8p::gfp (labeling the SMs) and egl-15p::gfp (labeling the VM1s). The CCs were scored using either the secreted CC::gfp(arIs39) or the intrinsic CC::gfp(ccIs4438).
Two transgenic lines were examined. Data from one transgenic line are shown.
Overexpression of sem-4 in the M lineage is sufficient to convert most M-derived cells to SM-like cells
To directly determine the consequences of sem-4 overexpression in the M lineage, we introduced the hlh-8p::sem-4 cDNA transgene into wild-type worms that has normal endogenous sem-4 expression and generated stable lines with the transgene integrated into the genome. As shown in Figure 4 and Table 1, 88% of these transgenic worms (N=130) had extra number of SM-like cells and a concurrent loss of M-derived CCs. When we directly followed M lineage development in 30 of these transgenic animals from the 1-M stage to the 18-M stage, we found that in 26 of them both M-derived CCs and variable number of M-derived BWMs were transformed to SM-like cells. The loss of M-derived CCs and some M-derived BWMs in these animals were confirmed using the CC::GFP reporter and the myo-3p::NLS::mRFP::lacZ reporter, which labels the BWMs (Figure 4). Taken together, the above results indicate that high levels of SEM-4 in the M lineage is sufficient to drive most M lineage cells to the proliferative SM-like fate.
Figure 4. Overexpression of sem-4 is sufficient to convert M-derived CCs and BWMs to SM-like cells.
(A–B) Wild-type (A) or transgenic animals carrying hlh-8p::sem-4 cDNA that over-expresses sem-4 in the M lineage (B) expressing the VM1 marker egl-15::GFP and the CC::GFP marker. Note the extra VM1-like cells and the loss of M-derived CCs in the sem-4 over-expressing worm. (C–D) Wild-type (C) or sem-4 over-expressing (D) animals carrying the myo-3p::NLS::mFRP::lacZ that marks the BWMs. The sem-4 over-expressing worm, marked by the myo-2p::mRFP transgene, has fewer BWMs in the region posterior to the vulva, which is marked by an asterisk.
sem-4 is required for the M lineage specific expression of the SoxC transcription factor SEM-2
We have previously shown that SEM-2, the sole SoxC protein in C. elegans, is both necessary and sufficient to promote the proliferating SM fate (Tian et al., 2011). Because SEM-4 is also necessary and sufficient for specifying the SM fate, we dissected the regulatory relationship between SEM-2 and SEM-4. We first examined the expression of sem-4::GFP in the M lineage in sem-2(n1343) mutants. sem-2(n1343) is a transposon-induced mutation that specifically disrupts sem-2 expression and function in the M lineage (Tian et al., 2011). As shown in Figure 5A–C, sem-4::GFP expression in the M lineage is unaltered in sem-2(n1343) mutants compared to wild-type animals. In contrast, the M lineage expression of a functionally tagged GFP::SEM-2 is undetectable in sem-4(h769) mutants (Figure 5D–K). This loss of GFP::SEM-2 is specific to the M lineage because its expression outside of the M lineage remains unchanged (data not shown). These observations suggest that sem-4 is specifically required for the expression of sem-2 in the M lineage.
Figure 5. SEM-4 is required for sem-2 expression in the M lineage.
(A–C) A sem-2(n1343) mutant carrying sem-4::GFP, showing GFP expression in the M lineage (marked by hlh-8p::NLS::mCherry) at the 18-M stage. (D–K) GFP::SEM-2 expression in wild-type (D–G) and in sem-4(h769) (H–K) animals. Notice the loss of GFP::SEM-2 expression in the SM mother cell (M.vlpa, arrowhead) in sem-4(h769) mutant. Images are shown with anterior to the left and dorsal up. Only images of the left side of the animal are shown in panels A-F and H-J. (G, K) Summary of GFP::SEM-2 expression in the M lineage of wild-type (G) and sem-4(h769) mutant (K). GFP::SEM-2-expressing cells are in green.
Expression of sem-2 in the M lineage is mediated by an M lineage enhancer located in the first large intron of sem-2 (Tian et al., 2011). We tested if SEM-4 acts through this enhancer element to regulate the M lineage expression of sem-2. We introduced a GFP reporter driven by this enhancer, pCXT33 (Tian et al., 2011), into the sem-4(h769) mutants. The level of M lineage expression of pCXT33 was not altered in h769 animals compared to their heterozygous h769/hT2[qIs48] siblings (data not shown), but the percentage of transgenic worms showing M lineage expression of the pCXT33 reporter is slightly reduced in h769 animals compared to their heterozygous h769/hT2[qIs48] siblings (Table 2). These observations suggest that this specific enhancer element contributes to, but is not essential for, the regulation of sem-2 expression by SEM-4. At present, it is unclear how SEM-4 may regulate sem-2 expression through this element, because we did not find any potential SEM-4 binding site(s) (Toker et al., 2003) within this element.
Table 2.
SEM-4 may partially act through the sem-2 M lineage enhancer to regulate the M lineage expression of sem-2.
| Genotype | % of transgenic animals expressing pCXT33* in the M lineage
|
|
|---|---|---|
| Transgenic line 1 | Transgenic line 2 | |
| sem-4(h769)/hT2[qIs48] | 37% (N=267) | 32% (N=508) |
| sem-4(h769) | 23% (N=137) | 21% (N=197) |
pCXT33(598bp sem-2 M lineage enhancer::pes-10p::NLS::gfp::lacZ::unc-54 3’UTR) plasmid was injected into sem-4(h769)/hT2[qIs48] worms. Transgenic sem-4(h769)/hT2[qIs48] and sem-4(h769) siblings from stable transgenic lines were scored for M lineage expression of the transgene.
SEM-4 functions through SEM-2 to specify M-derived SMs
The loss of sem-2 expression in the M lineage in sem-4(h769) mutants suggested that the role of SEM-4 in promoting SM fate specification may be mediated by SEM-2. To test this hypothesis, we first asked if forced expression of sem-2 in sem-4(h769) mutants can restore the formation of M-derived SMs. As shown in Table 1, forced expression of sem-2 specifically in the M lineage using the hlh-8p::sem-2 cDNA construct can partially restore the formation of M-derived SMs in sem-4(h769) null mutants (14%, n=56; Table 1). We further found that the formation of SMs and the ectopic SM-like cells in transgenic worms overexpressing sem-4 requires the presence of sem-2, because depleting sem-2 by RNAi soaking (see Materials and Methods) in transgenic animals carrying the hlh-8p::sem-4 cDNA transgene led to the loss of both the normal SMs and all the ectopic SM-like cells (n=1037, Table 1). These findings indicate that SEM-4 functions through SEM-2 to promote the proliferating SM fate in the M lineage.
DISCUSSION
In this study, we dissected the function of the SALL factor SEM-4 in specifying the SM fate in the C. elegans postembryonic mesoderm. The SM cells are pluripotent precursor cells that can further divide to produce four types of non-striated egg-laying muscles in C. elegans hermaphrodites (Figure 1). We have shown that in the binary cell fate decision between this pluripotent SM fate and a differentiated BWM fate, SEM-4 is both necessary and sufficient to promote the pluripotent SM fate. Furthermore, SEM-4 acts through the SoxC transcription factor SEM-2 to promote the SM fate. SEM-2 is known to promote the SM fate by inhibiting the expression of two redundant BWM-specifying transcription factors, FOZI-1 and HLH-1 (Tian et al., 2011). Together, these findings led us to the following model: in the cells fated to become the SMs, SEM-4, either directly or indirectly, activates the expression of SEM-2, which inhibits the expression of FOZI-1 and HLH-1, leading to the specification of SMs (Figure 6). Notably, the partial translational SEM-4::GFP fusion is also detectable in M-lineage cells that are fated to become BWMs (Figure 2). This could be due to perdurance of the GFP protein. Alternatively, it is possible that in cells fated to become BWMs, there is either an inhibitory factor Y that prevents SEM-4 from activating sem-2 expression, or there is a lack of a factor X that promotes sem-2 expression (Figure 6). The identification of additional factors involved in SM fate specification will help distinguish between these possibilities. Nevertheless, our work adds another missing piece to the regulatory network involved in the diversification and fate specification of the C. elegans postembryonic mesoderm (Krause and Liu, 2012). Photos and colleagues (Photos et al., 2006) showed that SEM-4 is required for specifying the SM fate in the nematode Pristionchus pacificus, which diverged from C. elegans about 200–300 million years ago. Thus the role of SEM-4 in specifying the pluripotent SM fate is conserved in other nematodes as well.
Figure 6. A proposed model for SEM-4 and SEM-2 function in SM fate specification.
Solid lines do not distinguish between direct and indirect regulatory relationships. Dashed lines represent hypothetical scenarios. Gray text indicates a lack of expression in the indicated cell.
SALL transcription factors are essential for normal development from humans to worms. In C. elegans, SEM-4 controls the development of multiple cell types, including neurons (Basson and Horvitz, 1996; Toker et al., 2003; Weinberg et al., 2013), the vulva (Eisenmann and Kim, 2000; Grant et al., 2000), as well as the postembryonic mesoderm (Basson and Horvitz, 1996, and this work). In particular, SEM-4 has been previously shown to act as a transcriptional repressor in the touch neurons in C. elegans (Toker et al., 2003). It has also been shown to associate with the transcriptional repressor EGL-27/MTA1 in a NODE (Nanog and Oct4-associated deacetylase)-like complex to regulate the Y-to-PDA natural reprogramming event (Jarriault et al., 2008; Kagias et al., 2012). Whether SEM-4 also functions as a repressor in the M lineage is not known. We have found that egl-27(0) mutants have extra SM-like cells (unpublished), a phenotype opposite to that of sem-4(0) mutants, suggesting that it is unlikely that SEM-4 acts together with EGL-27/MTA1 to specify the SM fate. This is consistent with the proposal that SEM-4 may exert its function differentially in different cell types, possibly through interacting with different cofactors.
The function of SEM-4 in specifying M-derived SMs and CCs appears paradoxical. On the one hand, the expression pattern and mutant phenotype of sem-4 suggest a permissive role for SEM-4 in SM specification. In this model, SEM-4 functions to maintain those cells that are fated to become SMs in a pluripotent-like state, permitting the expression of the SM-specifying factor SEM-2, which acts to repress HLH-1 and FOZI-1 expression, thus preventing these cells from adopting the differentiated BWM fate. Such a role for SEM-4 would be consistent with the role of Sall4, one of the mammalian homologs of SEM-4, in pluripotency regulation. Sall4 is known to be essential for early mammalian development (Elling et al., 2006; Sakaki-Yumoto et al., 2006; Warren et al., 2007) and is frequently overexpressed in various cancers (Zhang et al., 2015). Recent studies have shown that Sall4 contributes to pluripotency regulation by inhibiting differentiation (Miller et al., 2016; Yamaguchi et al., 2015). However, our ectopic expression results argue against a permissive role of SEM-4 in SM specification. We have shown that overexpression of sem-4 throughout the M lineage can convert most, if not all, M-derived cells (including the CCs and BWMs) into SM-like cells (Table 1 and Figure 4), suggesting that SEM-4 plays a positive role in promoting SM fate specification. Additionally, both loss of sem-4 (in sem-4 null mutants) and overexpression of sem-4 resulted in the loss of M-derived CCs. However, while loss of sem-4 caused a CC-to-BWM fate transformation, overexpression of sem-4 led to a CC-to-SM fate transformation. These observations suggest that the binary cell fate choices among BWMs, CCs and SMs in the M lineage can be influenced by different amount of SEM-4. Identifying additional factors that SEM-4 functionally interacts with will help uncover the molecular basis underlying these binary fate decisions.
HIGHLIGHTS.
SEM-4 is necessary and sufficient to specify the multipotent sex myoblast (SM) fate.
sem-4 functions autonomously to specify the SM fate.
SEM-4 functions through the SoxC transcription factor SEM-2 to specify the SM fate.
Acknowledgments
We thank Min Han, Wendy Hanna-Rose, Bob Horvitz, Michael Koelle, Yuji Kohara, Shohei Mitani for strains or plasmids, Melisa DeGroot, Zhiyu Liu and two anonymous reviewers for critical comments on the manuscript. Some strains were obtained from the C. elegans Genetics Center, which is funded by NIH Office of 27 Research Infrastructure Programs (P40 OD010440). This work was supported by NYSTEM contract #C028110 and National Institutes of Health R01 GM066953 to J.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin NM, Hu K, Pruyne D, Terzic D, Bretscher A, Liu J. A Zn-finger/FH2-domain containing protein, FOZI-1, acts redundantly with CeMyoD to specify striated body wall muscle fates in the Caenorhabditis elegans postembryonic mesoderm. Development (Cambridge, England) 2007;134:19–29. doi: 10.1242/dev.02709. [DOI] [PubMed] [Google Scholar]
- Basson M, Horvitz HR. The Caenorhabditis elegans gene sem-4 controls neuronal and mesodermal cell development and encodes a zinc finger protein. Genes & development. 1996;10:1953–1965. doi: 10.1101/gad.10.15.1953. [DOI] [PubMed] [Google Scholar]
- Eisenmann DM, Kim SK. Protruding vulva mutants identify novel loci and Wnt signaling factors that function during Caenorhabditis elegans vulva development. Genetics. 2000;156:1097–1116. doi: 10.1093/genetics/156.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elling U, Klasen C, Eisenberger T, Anlag K, Treier M. Murine inner cell mass-derived lineages depend on Sall4 function. Proc Natl Acad Sci U S A. 2006;103:16319–16324. doi: 10.1073/pnas.0607884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nature genetics. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K, Hanna-Rose W, Han M. sem-4 promotes vulval cell-fate determination in Caenorhabditis elegans through regulation of lin-39 Hox. Developmental biology. 2000;224:496–506. doi: 10.1006/dbio.2000.9774. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Branda CS, Krause M, Stern MJ, Fire A. MyoD and the specification of muscle and non-muscle fates during postembryonic development of the elegans mesoderm. Development (Cambridge, England) 1998;125:2479–2488. doi: 10.1242/dev.125.13.2479. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Schwab Y, Greenwald I. A Caenorhabditis elegans model for epithelial-neuronal transdifferentiation. Proc Natl Acad Sci U S A. 2008;105:3790–3795. doi: 10.1073/pnas.0712159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagias K, Ahier A, Fischer N, Jarriault S. Members of the NODE (Nanog and Oct4-associated deacetylase) complex and SOX-2 promote the initiation of a natural cellular reprogramming event in vivo. Proc Natl Acad Sci U S A. 2012;109:6596–6601. doi: 10.1073/pnas.1117031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Liu J. Somatic muscle specification during embryonic and post-embryonic development in the nematode C. elegans. Wiley Interdiscip Rev Dev Biol. 2012;1:203–214. doi: 10.1002/wdev.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Ralser M, Kloet SL, Loos R, Nishinakamura R, Bertone P, Vermeulen M, Hendrich B. Sall4 controls differentiation of pluripotent cells independently of the Nucleosome Remodelling and Deacetylation (NuRD) complex. Development. 2016;143:3074–3084. doi: 10.1242/dev.139113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Photos A, Gutierrez A, Sommer RJ. sem-4/spalt and egl-17/FGF have a conserved role in sex myoblast specification and migration in P. pacificus and C. elegans. Developmental biology. 2006;293:142–153. doi: 10.1016/j.ydbio.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Sakaki-Yumoto M, Kobayashi C, Sato A, Fujimura S, Matsumoto Y, Takasato M, Kodama T, Aburatani H, Asashima M, Yoshida N, Nishinakamura R. The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development (Cambridge, England) 2006;133:3005–3013. doi: 10.1242/dev.02457. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Developmental biology. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Tian C, Shi H, Colledge C, Stern M, Waterston R, Liu J. The C. elegans SoxC protein SEM-2 opposes differentiation factors to promote a proliferative blast cell fate in the postembryonic mesoderm. Development (Cambridge, England) 2011;138:1033–1043. doi: 10.1242/dev.062240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker AS, Teng Y, Ferreira HB, Emmons SW, Chalfie M. The Caenorhabditis elegans spalt-like gene sem-4 restricts touch cell fate by repressing the selector Hox gene egl-5 and the effector gene mec-3. Development (Cambridge, England) 2003;130:3831–3840. doi: 10.1242/dev.00398. [DOI] [PubMed] [Google Scholar]
- Warren M, Wang W, Spiden S, Chen-Murchie D, Tannahill D, Steel KP, Bradley A. A Sall4 mutant mouse model useful for studying the role of Sall4 in early embryonic development and organogenesis. Genesis. 2007;45:51–58. doi: 10.1002/dvg.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg P, Flames N, Sawa H, Garriga G, Hobert O. The SWI/SNF chromatin remodeling complex selectively affects multiple aspects of serotonergic neuron differentiation. Genetics. 2013;194:189–198. doi: 10.1534/genetics.112.148742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi YL, Tanaka SS, Kumagai M, Fujimoto Y, Terabayashi T, Matsui Y, Nishinakamura R. Sall4 is essential for mouse primordial germ cell specification by suppressing somatic cell program genes. Stem Cells. 2015;33:289–300. doi: 10.1002/stem.1853. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan X, Zhu W, Qian H, Xu W. SALL4: an emerging cancer biomarker and target. Cancer Lett. 2015;357:55–62. doi: 10.1016/j.canlet.2014.11.037. [DOI] [PubMed] [Google Scholar]