Abstract
Hepatitis B virus (HBV) infects hepatocytes specifically and causes immune mediated liver damage. How HBV interacts with the innate immunity at the early phase of infection, either with the hepatocytes or other cells in the liver remains controversial. To address this question, we utilized various cell culture models and humanized Alb-uPA/SCID mice. All these models were unable to mount an interferon (IFN) response despite robust HBV replication. To elucidate the mechanisms involved in the lack of IFN response, we examined whether HBV actively inhibits innate immune functions of hepatocytes. By treating HBV infected cells with known inducers of IFN signaling pathway, we observed no alteration of either sensing or downstream IFN response by HBV. We showed that the DNA innate sensing pathways are poorly active in hepatocytes, consistent with the muted innate immune recognition of HBV. Upon exposure to high-level HBV, macrophages could be activated with increased inflammatory cytokine expressions. Conclusion: HBV behaves like a “stealth” virus and is not sensed by nor actively interferes with the intrinsic innate immunity of the infected hepatocytes. Macrophages are capable of sensing HBV but require exposure to high HBV titers, potentially explaining the long “window period” during acute infection and HBV’s propensity to chronic infection.
Keywords: HBV, Innate immune responses, Interferon signaling, Pattern recognition receptors
Hepatitis B virus (HBV) infection remains a public health burden worldwide. Despite the success of HBV vaccination, the chronically infected population remained high at 250 million worldwide (1) with an annual death rate from HBV of 800,000 (2). HBV has a double-stranded relaxed circular DNA (rcDNA) genome of 3.2 Kbp. During replication, rcDNA is converted to covalently closed circular DNA (cccDNA), which persists in the nucleus as minichromosome and serves as a template for viral RNA transcription. The core proteins package viral pre-genomic RNA (pgRNA) and form the nucleocapsid. Viral reverse transcriptase then converts the pgRNA to the rcDNA genome and the matured nucleocapsid is either enveloped and secreted, or recycled to cell nucleus to replenish the cccDNA pool.
Unlike other viruses, such as hepatitis C virus (HCV) or HIV, which enter a logarithmic phase of propagation right after infection (3), acute HBV infection is typically preceded by low HBV-DNA and antigens in serum and liver for many weeks before the subsequent amplification and spreading phase of HBV infection, which is characterized by high quantities of viral particles and antigens in the circulation with little or no type I and III interferon (IFNs) in most HBV-infected patients (3, 4). Consistent with these clinical observations, genomic analysis of HBV-infected chimpanzees or woodchucks showed neither type I IFN or interferon-stimulated gene (ISG) production in the expansion phase of HBV infection (5) nor type I IFN-associated ISG expression in persistently infected animals (6). Collectively, these observations underpin the “stealth” nature of HBV. In contrast, several reports challenged the “stealth” notion and described that HBV infection can activate innate immunity of hepatocytes and trigger production of type I or III IFNs (7, 8).
HBV sensing in hepatocyte has been attributed to a variety of pattern recognition receptors (PRRs), like retinoic acid inducible protein I (RIG-I) (8), melanoma differentiation-associated protein 5 (MDA5) (9), or stimulator of IFN genes (STING) dependent DNA sensing pathway (10). Meanwhile, numerous studies have proposed HBV-mediated mechanisms to interfere with immune detection. Such proposed mechanisms involve HBV polymerase, HBx protein, e antigen (HBeAg) and surface antigen (HBsAg) (11–13), HBV virion (14) or virus-induced host proteins (15) that interfere with innate immune signaling pathways of hepatocytes and lead to inhibition of IFN production or suppression of IFN function (16). In theory, immune evasion, activation and inhibition may co-exist; however, the published data to-date has been largely contradictory. Much of the data were generated in non-infectious or non-primary HBV model systems, raising doubt about their biological relevance. In this study, we utilized a variety of HBV infection model systems to clarify the interplay between HBV and the innate immunity of hepatocytes and macrophages.
Materials and Methods
CELL CULTURE
Culture of HepG2-NTCP, differentiated-HepaRG (dHepaRG), stem cell-derived hepatocyte-like cells (HLC) was performed as described (17). Cryopreserved primary human hepatocytes (PHHs) from different donors (Hu1574, Hu1663, Hu1832, Hu8196) were purchased from Life Technologies Corporation (New York, USA). THP-1 monocytes were cultured in RPMI1640 medium supplemented with 10%FBS, 2 mM L-gutamine and 1% penicillin/streptomycin. For macrophage differentiation, medium was supplemented with 100 ng/mL PMA (SIGMA, USA) for 48 h. Human monocytes isolated from peripheral blood mononuclear cells were differentiated to macrophages via 5 day treatment with 20 ng/mL recombinant human M-CSF (Peprotech, Rocky Hill, NJ). Further details are provided in the Supporting Information.
HBV INFECTION IN CULTURED CELLS
Cell culture derived HBV (HBVcc) stock was prepared from HepG2.2.15 culture supernatant. Patient sera are from two treatment-naïve hepatitis B patients. For infection, cells were incubated with 400 genomes/cell of HBV in medium containing 5% PEG 8000 (Sigma Aldrich, St. Louis, USA) for 16 h. Infection efficiency on day 7 was 50%–70% for HepG2-NTCP, 20% for dHepaRG and 60%–80% for HLC and PHH based on HBV nucleic acid in situ hybridization as previously published (17).
STATISTICAL ANALYSIS
Student’s unpaired two-tailed t tests or Mann-Whitney test were performed (GraphPad Software, CA, USA) as indicated. Two-sided p values <0.05 were considered significant. *P<0.05, **P <0.01, ***P <0.001, ****P<0.0001.
Results
HBV INFECTION DOES NOT ACTIVATE IFN RESPONSE IN HEPATOCYTES
Although IFN can inhibit HBV replication, it is controversial whether HBV can indeed induce an IFN response in hepatocytes. To answer this question, we tested HBV from different sources in various hepatic cell culture systems. We first used HBVcc (genotype D) to infect HepG2-NTCP, dHepaRG, HLC or PHH and compared with Poly(I:C), Sendai virus (SeV) or HCV as they are known IFN inducers. Unlike the positive controls, HBV infection did not induce type I and III IFNs, and the downstream ISGs in cultured hepatocytes (Fig. 1A). Considering a recent study that suggests HBV-induced IFN response is genotype-dependent (8), we transfected plasmids containing HBV genome of genotype A or D or empty vector into HepG2-NTCP cells. Both HBV constructs resulted in HBV genome replication as shown by intracellular HBV-RNA levels. In contrast to the previous report, neither genotype A nor D HBV plasmid induced higher levels of IFNs or ISGs above the empty vector control (Fig. 1B).
FIG. 1.
In vitro and in vivo analysis of IFN response in hepatocyte against HBV. (A) In vitro cultured hepatocyte (HepG2-NTCP, dHepaRG, HLC, PHH) were either mock infected or infected with HBVcc (400 genomes/cell) for 7 days. Poly (I:C) transfection (2 µg/ml), SeV infection (1 HAU/ml) or HCV infection (0.5 TCID50/cell) were performed as positive control. 24 hours later, target genes expression were measured by qPCR. (B) HepG2-NTCP cells were either transfected with empty plasmid vector or plasmids containing 1.3-fold HBV genome of genotype A or genotype D. 48 hours later, target gene expression were determined by qPCR. Cells transfected with poly (I:C) (2 µg/ml) for 24 hours were served as positive control. Student unpaired two-tailed t tests, **P<0.01, ***P<0.001, n.s. = not significant. (C) Patient serum containing HBV (genotype A or C) or HBVcc (genotype D) were used to infect HLC for 7 days. HBV non-infected cells were transfected with poly (I:C) (1 µg/ml) for 24 hours to serve as positive control. (D and E) Alb-uPA/SCID mice were transplanted with PHHs from the same donor (noninfected mice: n = 3) and infected with HBV (genotype C, 105 genomes) or HCV (genotype 1b, 105 genomes). After virus infection, blood was sampled at indicated time points and mice were sacrificed either in the early stage of infection (10 d.p.i.) (HBV-infected: n = 5; HCV-infected: n = 5) or after the viremia reach the plateau (8 w.p.i.) (HBV-infected: n = 5; HCV-infected: n = 5). Viral titers in the blood were determined by q-PCR and hepatic IFNs expression were quantified by RT-qPCR with human specific Taqman probes and the detection limit were set as one. d.p.i. = day post infection. w.p.i. = week post infection. Mann-Whitney test, *P<0.05. For (A to C), means ± SD are shown. For (D and E), means ± SEM are shown.
Previous studies on HBV-induced type I or III IFN production utilized HBV patient sera for infection (7, 8). To investigate the possible difference between virus of different origin, HLCs were inoculated with either HBV-infected patient sera (genotype A or C) or HBVcc. Successful infections were confirmed by intracellular HBV-RNA quantification (Fig. 1C). Similar to HBVcc, patient-derived viruses of both genotype A and C did not induce IFN response in contrast to poly (I:C) control (Fig. 1C).
To closely simulate the physiological situation, we also tested PHH engrafted in vivo using the Alb-uPA/SCID mice (18). Following inoculation with HBV patient sera, intrahepatic IFN induction was not observed at any stage of HBV propagation (Fig. 1D). This contrasts with HCV infection, where predominant type III IFN induction was detected as previously reported (19) (Fig. 1E). These data further support the inability of HBV to induce IFN response.
HBV INFECTION DOES NOT SUPPRESS INNATE IMMUNE FUNCTION OF HEPATOCYTES
Our data support a lack of IFN response in HBV-infected hepatocytes, which contrast with other viral pathogens. However, the underlining mechanism(s) is not defined. One explanation is that HBV actively suppresses the hepatocytes’ innate immune responses (11–13). To clarify this question, we infected HepG2-NTCP for 7 days and stimulated the cells with various pathogen-associated molecular patterns (PAMPs). If HBV indeed elicited suppression of innate immunity, we would expect a difference of IFN response between HBV-infected cell and non-infected cells. 16 hours after poly (I:C) transfection, the induced type I/III IFN response and the downstream ISGs did not differ between HBV-infected and mock-infected cells at various doses of poly (I:C) stimulation (Fig. 2A). As an alternative approach to IFN induction, SeV was used. Different doses of SeV were applied to HBV-infected cells for 24 hours. As immunofluorescence staining illustrated super-infection of SeV and HBV in many cells (Supporting Fig. S1), the IFN response against SeV in either HBV-infected or non-infected cells was not different (Fig. 2B). We also tested poly(dA:dT), a repetitive double-stranded DNA sequence of poly(dA-dT)•poly(dA-dT), which in general can be detected by several cytosolic DNA sensors (20, 21) or by RIG-I after being transcribed by RNA polymerase III (Pol III) into dsRNA (22). Again, results showed no effect of HBV infection on IFN response (Fig. 2C).
FIG. 2.
Analysis of IFN response of hepatocytes in the late stage of HBV infection. HepG2-NTCP cells were mock (dashed line) or HBV infected (400 genomes/cell, solid line) for 7 days and followed by poly (I:C) transfection (A), SeV infection (B), or poly (dA:dT) transfection (C) at dose indicated. 16 hours after transfection or 24 hours after SeV infection, cells were lysed and RT-qPCR was used to measure target genes expression. Similarly, 7 days post-HBV infection in HLC (D) and PHH (E), HBV-infected and mock-infected cells were treated in parallel with indicated dose of SeV or 2 µg/ml poly (dA:dT) as above. 7 days post-HBV infection in HepG2-NTCP (F) or PHH (G), cells were treated with IFN-α at indicated dose for 6 hours before gene expression analysis. Results are presented as fold induction. Means ± SD are shown. d.p.i. = day post infection.
HepG2-NTCP is thought to have an impaired innate immune function because of its transformed nature (23). To validate the above findings, we performed same experiments in HLCs and PHHs, which were more sensitive to SeV infection with a high IFN induction. Similar to findings in HepG2-NTCP, both HLCs and PHHs responded to various PAMPs in the same magnitude regardless of concurrent HBV infection (Fig. 2D&E).
Previous studies have suggested that HBV might actively suppress interferon’s antiviral effects by interfering with various IFN-induced downstream events (16, 24). We tested this possibility by treating HBV-infected HepG2-NTCP or PHH with different doses of IFN-α, then measuring the ISG expressions. We consistently could not observe any suppressive effect of HBV on ISG expression (Fig. 2F&G).
A recent study suggested that HBV’s inhibition of hepatocyte’s interferon response might occur early after HBV inoculation through virus-cell interaction prior to viral replication (14). We tested this hypothesis in HepG2-NTCP cells after overnight inoculation with HBV. After removing the inoculum, cells were treated with poly(I:C) for 6 hours or infected with a fixed dose of SeV for different durations or with different doses of SeV for 24 hours. In accordance with our previous results, we did not observe any differences in IFN or ISG expression between HBV-infected or mock-infected cells (Supporting Fig. S2). Thus, we conclude there is no inhibition of cellular innate response at the early time of HBV infection. Overall, our data support that either in the early or late stage of HBV replication, HBV does not actively suppress the innate sensing or downstream IFN signaling pathways of the infected cells.
SINGLE CELL ANALYSIS OF INTERFERON RESPONSE IN HBV-INFECTED HEPATOCYTES
During infection, cells may be unevenly infected by HBV. Thus, it is important to examine the immune response at the single-cell level. For this purpose, HepG2-NTCP cells were infected with HBV for 7 days and then super-infected with SeV for 24 hours. Immunofluorescence staining was performed with antibody against HBsAg and IFN regulatory factor (IRF) 3, based on the rationale that nuclear translocation of IRF3 is indicative of PRR sensing activation. Mono-infection with SeV, but not HBV, induced IRF3 nuclear translocation, proving the inability of HBV to induce IFN response in contrast to SeV. In SeV and HBV co-infected cells, HBsAg-positive cells with positive IRF3 nuclear staining were readily observed (Fig. 3A). Image quantification revealed that HBV infection efficiency was around 50% (Supporting Fig. S3A). SeV mono-infection led to ~30% of nuclei being positive with IRF3, and this ratio was similar to SeV and HBV co-infected cells (Supporting Fig. S3B). In the co-infection sample, positive HBsAg-stained cells showed similar percentage (30%) of IRF3 translocation as HBsAg-negative cells (Supporting Fig. S3C). These data confirm the notion that HBV infection does not impair cellular sensing of SeV infection.
FIG. 3.
IRF3 and STAT1 nuclear translocation in HBV-infected HepG2-NTCP. HepG2-NTCP was either mock infected or HBV (400 genomes/cell) infected. On day 7 postinfection, cells were superinfected with SeV (100 HAU/ml) for 24 hours and followed by immune staining with antibodies against HBsAg in red and IRF3 (A) or STAT1 (B) in green. Yellow boxed areas are enlarged and shown below. White arrows indicate cells being double positive for HBsAg and nuclear translocation of IRF3 or STAT1. Scale bar = 20 µm.
Using a similar approach, signal transducer and activator of transcription 1 (STAT1) nuclear translocation, which is an activation marker for IFN signaling, was studied. As expected, HBV mono-infection did not induce any nuclear translocation of STAT1. SeV increased both cellular expression and nuclear translocation of STAT1, which was not affected by HBV (Fig. 3B).
To further validate our findings, we expanded the single-cell analysis to HLC and PHH. Both models are highly sensitive to the cytotoxic effect of SeV infection. We therefore used a lower dose (1 HAU/mL) of SeV for 24 hours infection. Under this condition, only a limited number of HLCs were stained positive for IRF3 in the nuclei. Regardless, HBV-infected cells still exhibited the capacity for IRF3 translocation in response to SeV co-infection (Supporting Fig. S4A). On the other hand, a low dose of SeV efficiently induced STAT1 increase and nuclear translocation, which was again readily observable in both HBV-infected and non-infected cells (Supporting Fig. S4B). Similarly, we tested poly(I:C) and stained for IRF3 staining in HBV-infected PHHs. HBsAg-positive PHHs also supported IRF3 nuclear translocation by poly(I:C) (Supporting Fig. S5A) as well as STAT1 nuclear translocation induced by SeV infection (Supporting Fig. S5B).
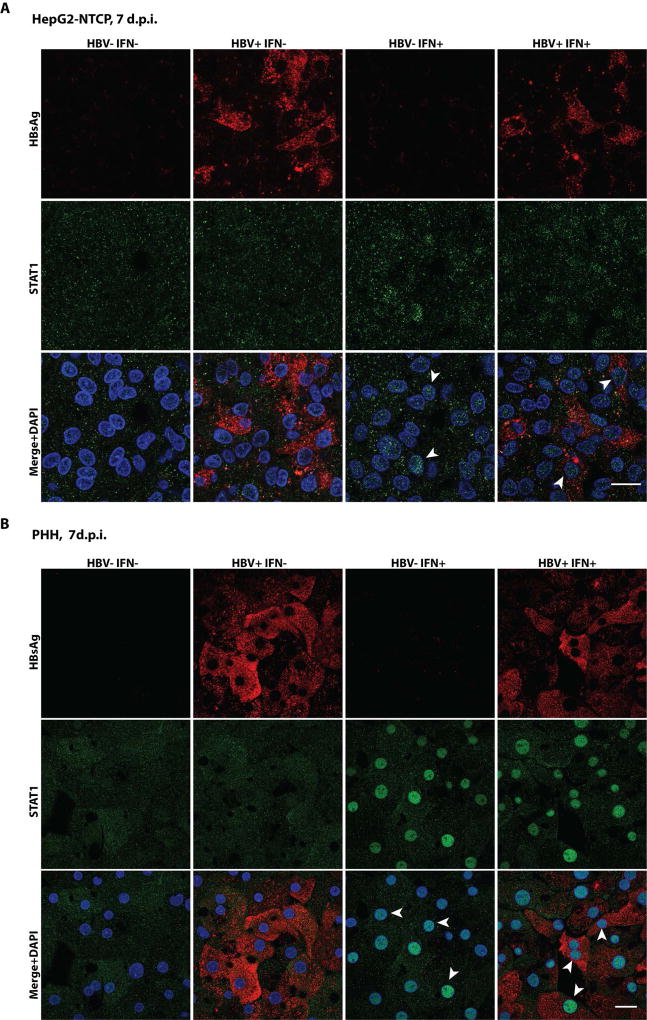
We further performed single-cell analysis of IFN-α treated cells. HBV-infected HepG2-NTCP cells or PHHs were treated with IFN-α and then stained for HBsAg and STAT1. The HepG2-NTCP cells were not as efficient in STAT1 nuclear translocation as the PHHs in response to IFN-α treatment. Nevertheless, in both systems, the STAT1 nucleus staining was similar between HBV-infected and non-infected cells (Fig. 4).
FIG. 4.
STAT1 nuclear translocation in IFN-α treated and HBV-infected hepatocytes. HepG2-NTCP (A) or PHH (B) were mock or HBV infected (400 genomes/cell) for 7 days and then treated with IFN-α (1000 IU/ml) for 1.5 hours. Cells were then fixed and stained with antibody against HBsAg (red) and STAT1 (green). Example cells showing STAT1 nuclear import are marked by white arrows. Scale bar = 20 µm.
DEFICIENT DNA SENSING MACHINERY IN HEPATOCYTE CONTRIBUTE TO LACK OF HBV-SPECIFIC INTERFERON RESPONSE
As has been demonstrated above, the apparent lack of IFN response is not due to a suppression of innate immune functions of host cell by the virus, raising the question of alternative mechanisms. HBV infection generates both viral DNA and RNA during propagation, which could be detected by various PRRs. We thus characterized PRR expression and function in different hepatic cell models. PRR transcript levels were first quantified and compared to those in the differentiated THP-macrophage line, which is known to express high levels of various PRRs. The PRRs that mainly sense RNA ligands are expressed higher in PHHs than in THP-macrophages (Fig. 5A). Conversely, the molecules involved in DNA sensing are expressed in PHHs at levels less than 10% of those in the THP-macrophages (Fig. 5B), with certain sensor, like DNA-dependent activator of IFN-regulatory factor (DAI) and absent in melanoma 2 (AIM2), barely detectable (data not shown). HLC differentiated from either induced pluripotent cells (SC3D-HLC) or human embryonic cells (H9-HLC) showed similar levels of PRR expression as the PHHs, except toll-like receptor (TLR)-3 (Fig. 5A&B).
FIG. 5.
PRRs expression and functionality in cultured hepatocytes. Relative expression of RNA sensors (A) and DNA sensors (B) were determined in different hepatocyte models (relative to TBP) and the levels were compared to their expression in THP-macrophages, which were set as 100%. Experiment was performed in triplicates and means ± SD are shown. (C) Cultured cells were transfected with 2 µg/ml PRR specific ligands (described in Supporting Information) or mock transfected. IFNB, IFNL1 and ISG15 expression were determined by qPCR 16 hours posttreatment and results are presented as fold induction. Means ± SD are shown.
As stem cell differentiation to HLCs in vitro follows the same genetic program as organ development, the PRR expression kinetics during the HLC differentiation process was also examined. Concomitant with the up-regulation of hepatic specific markers (Supporting Fig. S6A), RNA sensors expression increased with hepatic differentiation (Supporting Fig. S6B). In contrast, DNA sensors expression remained low or decreasing (Supporting Fig. S6C). Hepatocyte dedifferentiation is the reverse process of differentiation and occurs to PHHs cultured under sub-optimal condition in vitro, as reflected by a down-regulation of hepatic specific gene expression (Supporting Fig. S6D). While the RNA sensor expression showed a decreasing tendency with dedifferentiation (Supporting Fig. S6E), the levels of DNA sensors were increasing (Supporting Fig. S6F). Finally, although transformed hepatocytes, dHepaRG and HepG2-NTCP had slightly different expression profile compared to PHH or HLC, they exhibited a similar pattern: higher levels of RNA sensors and lower levels of DNA sensors in comparison to THP-macrophages (Fig. 5AB). Additionally, in all tested models, HBV-infected cells showed similar levels of PRRs expression as uninfected cells, indicating that HBV infection does not affect these PRRs expression (Supporting Fig. S7).
To validate the PRR transcription profile, the functionality of these PRR signaling pathways was tested by stimulating cells with various known PAMP ligands and quantifying the induced IFN response. THP-macrophages reacted to all the ligands by up-regulating type I/III IFN expression and the down-stream ISGs (Fig. 5C). Conversely, hepatic cells exhibited a more selective response to ligand stimulation. PHH responded to double-stranded RNA Poly(I:C) (Fig. 5C), which is recognized by RNA sensors like TLR-3, RIG-I and MDA-5 (25, 26). However, PHH did not react to HSV-60 or 3’3’-cGAMP (cyclic [G(3’,5’)pA(3’,5’)p]) stimulation (Fig. 5C), which activates the cytosolic DNA sensors (the STING-IRF3 axis) and induces IFN response (27, 28). PHHs responded to poly(dA:dT) (Fig. 5C), which is likely to be Pol III and RIG-I-dependent (22). Both HLC and HepG2-NTCP respond to the tested ligands in a similar manner as the PHH. Notably HLC closely mimics PHH’s response to these ligands (Fig. 5C).
Taken together, the PRR transcriptional profiles were similar across hepatocyte cell models and demonstrated a corresponding ligand-response bias toward RNA ligands in comparison to DNA ligands. Our data support the notion that hepatocytes are deficient in DNA sensing machinery, which likely contributes to the lack of IFN response in HBV infected hepatocytes.
To verify that deficient DNA sensing pathway is indeed responsible for inability of hepatocyte to mount IFN response against HBV infection, HepG2-NTCP cells were reconstituted with human cGAS and/or STING expression by lentivirus transduction. Immunofluorescence staining proves STING expression in majority of transduced cells but not un-transduced cells (Supporting Fig. S8A). Interestingly, cells with STING overexpression but not GFP control or cGAS expression (Supporting Fig. S8B), showed upregulated IFNB and IFNL expression as well as ISGs (Supporting Fig. S8C&D), indicating activation of interferon response. Additional treatment with 3’3’-cGAMP further enhanced IFN (mainly IFNL) and ISGs expression, demonstrating successfully reconstituted DNA sensing function (Supporting Fig. S8C&D). To avoid over-activation by STING, we reduced the dose of lentivirus expressing STING in the following experiment. As expected, a lower level of self-activation in cells overexpress STING was observed (Supporting Fig. S8E). Importantly, additional IFNL response was triggered after HBV infection (Supporting Fig. S8E&F), leading to reduced intracellular HBV RNA (Supporting Fig. S8G). These data confirm that deficiency of STING expression in hepatocyte is the reason for the lack of hepatic IFN response after HBV infection.
ACTIVATION OF INNATE IMMUNITY OF MACROPHAGES BY HBV
The lack of interplay between HBV infection and hepatocyte’s innate immunity raises the intriguing question of how HBV eventually activates adaptive immunity of infected host. To partly address this question, we studied the response of macrophages to HBV, as macrophages constitute more than 30% of hepatic non-parenchymal cells in the liver and can act as the initial mediators of innate immune responses to infection.
To control for potential contamination of innate immune activator(s) in the HBV inoculum, we prepared mock control inoculum from HepG2 cells. Using primary human monocyte-derived macrophages (from healthy donors) to mimic the liver Kupffer cells, we examined the mRNA levels of several inflammatory cytokines after stimulation with different doses of HBV. As a positive control, stimulation of macrophages with model PAMPs showed a marked IFN induction (Supporting Fig. S9). Only with a dose higher than 108 genomes/mL (equivalent to 102 genomes/cell) did we observe a substantial induction of various cytokines including IL1B, IL6, TNF-α (Fig. 6A) and CXCL10 (data not shown). Induction of IL10 (less than two-fold) was only detected after 24 hours of stimulation (data not shown). In one of three tested macrophage donors, we detected an up-regulation of IFN-β for about 5-fold; and in all the macrophages tested, there was no induction of type III IFN (data not shown). In contrast, IL18, which is important for NK cell activation, was specifically down-regulated (Fig. 6A). We also examined the kinetics of cytokine induction in response to HBV. Shortly after HBV exposure, we observed an induction of cytokines like IL1B, IL6 and TNF-α, which peaked around 3–6 hours (Fig. 6B). Using the differentiated THP-macrophage cell line, we also observed similar kinetics of cytokine induction when the cells were treated with media containing 108 genomes/mL of HBV (Supporting Fig. S10).
FIG. 6.
Analysis of innate immune response in macrophages against HBV. (A) Primary human monocyte-derived macrophages were treated with indicated dose of HBV for 6 hours. Relative expression of target genes were measured by RT-qPCR and the results were expressed as fold change comparing to mock-treated cell except for IL6 which were compared to the detection limit. (B) HBV at 1 × 108 genomes/mL or mock media prepared in parallel was applied to macrophage cultures for different lengths of time as indicated. Gene expression analysis was done as described above. (C) Macrophages derived from four independent donors were treated with 1 × 108 copies/mL of HBV or similarly prepared mock or ETV-HBV (for ETV-HBV, data from one donor of the four are not available) for 6 hours. Gene expression analysis was performed similarly as mentioned above. (D) Macrophages were treated with 60 µM ETV or not for 6 hours, intracellular RNA were extracted to measure IL1B and TNFA expression. For (A), (B) and (D) means ± SD are shown. Student unpaired two-tailed t test is used. For (C) means ± SEM are shown and Mann-Whitney test is used. *P < 0.05, **P < 0.01, ****P < 0.0001, n.s. = not significant. n.d. = not detectable.
To test if macrophages can be activated by HBV produced de novo from infected cells, we set up a co-culture experiment in which primary human monocyte-differentiated macrophages were directly seeded onto the HBV-infected HepG2-NTCP cells. Cytokine gene expression was measured at multiple time points post co-culture. With exposure times ranging from 2–96 hours, we did not observe any significant up-regulation of these inflammatory cytokines (Supporting Fig. S11A&B). This is not entirely surprising because HBV secreted by the infected cell can only reach a maximal titer of about 106 genomes/mL after 48 hours of accumulation (Supporting Fig. S11C), which is around 100-fold below the concentration needed for cytokine induction by direct HBV stimulation as shown above.
To further explore the mechanism of macrophage activation by HBV, we prepared viral stock from entecavir (ETV)-treated cells (ETV-HBV) and compared it to the standard viral stock (HBV). Characterization of the two viral stocks showed ~103–fold lower HBV DNA content but 50% higher HBV RNA in the ETV-HBV stock than the HBV stock, while viral proteins levels (HBsAg and HBeAg) were comparable (Supporting Fig. S12). For macrophage stimulation, HBV was diluted to a final concentration of 108 genomes/mL, and the same dilution was prepared from the mock or ETV-HBV stock. With four different macrophage donors, IL1B and TNF-α mRNA expressions were all up-regulated by HBV (Fig. 6C). The induction by ETV-HBV was slightly weaker but still significantly higher than the mock treatment, suggesting that HBV DNA is not the major driver of macrophage activation. The weaker induction was not caused by the remaining ETV, because treatment of macrophages with 60 µM ETV (assuming no loss of ETV during concentrating the HBV stock) did not modify the cytokine gene expression (Fig. 6D). Collectively, high-titer HBV can activate macrophages to express inflammatory cytokines and this effect is unlikely to be the result of a single viral component like the HBV genome or HBsAg.
DISCUSSION
In the current study, we set out to elucidate the interactions between HBV and the innate immunity to better understand the mechanisms of immune activation in HBV infection. There are three key observations: 1) HBV does not trigger the interferon response of hepatocytes; 2) HBV does not interfere with the innate immune sensing functions of hepatocytes; 3) HBV does not inhibit the IFN-stimulated pathways of hepatocytes. On the other hand, macrophages can be activated by high-titer HBV to mount an immune response against HBV mainly through production of inflammatory cytokines like TNF-α and IL-6, which may play a role in limiting HBV infection and mediating viral clearance (29). Our data are consistent with previous studies performed in animal models including woodchucks (6) and chimpanzees (5), as well as a few limited studies in HBV-infected humans (3, 4). These observations are striking in the sense that HBV infection proceeds without activating the immune system until its replication peaks in the infected hosts.
HBV DNA exists intracellularly in the form of either rcDNA or cccDNA that are distinct from the host DNA and can potentially activate the DNA sensing mechanisms. In contrast, HBV mRNAs closely resemble host transcripts and do not contain the necessary components for recognition by the RNA PRRs like RIG-I, such as 5’ triphosphate and complicated secondary structures (30, 31). In addition, the viral polymerase-associated pgRNA are enclosed and shielded by the nucleocapsid. Thus, it is not surprising that HBV evades detection of RNA sensors in hepatocytes. Acute HBV infection in chimpanzees or humans is characterized by a quiescent phase of low viral activity, which is often interpreted as a sign of innate immune containment of virus replication. Based on our data, we favor the alternative explanation that the nature of HBV replication is slow and often takes time to reach a plateau. Data from in vitro HBV infectious models have indicated a slow ramping up of HBV replication after initial infection, reaching a steady level after 10–15 days (17). In the infected humanized immune deficient mouse model, HBV takes 6 to 10 weeks to reach peak viremia (18). In addition, the duration of the quiescent phase is closely correlated to the level of viral inoculum in HBV-infected patients or chimpanzees (32, 33), supporting the slow nature of HBV replication and propagation in vivo.
Based on the present study, we conclude that the lack of IFN response in HBV infection is not due to suppression of PRRs sensing pathway in hepatocytes. In contrast, several other studies have reported that HBV replication can inhibit those afore-mentioned PRR functions. One study showed HBeAg+ chronic hepatitis B patients have down-regulated TLR2 expression on the hepatocytes (34). The biological significance of this observation is unclear because TLR2 normally senses gram-positive infection by recognizing cell membrane components such as leipoteichoic acid, peptidoglycan, and various lipopeptides and lipoproteins (35). Other studies based on heterologous overexpression of viral proteins and/or host sensors have also addressed this issue but yielded conflicting results (9–12). Similarly, contradictory data exist regarding whether HBV infection suppresses cellular response to IFN treatment. One study demonstrated inhibition of STAT1 nuclear import by HBV polymerase (24), and another reported that the HBsAg and/or HBx protein interferes with the STAT1 signaling (16). The physiological relevance of these studies and the proposed inhibitory mechanisms need to be validated using HBV infection systems more closely resembling natural infection and with well-matched controls. In our study, we did not observe any interference of HBV on ISG induction or STAT1 nuclear translocation following PAMP or IFN-α treatment, emphasizing the importance of using physiologically relevant models. On the other hand, we cannot rule out the possibility that HBV may selectively suppress the antiviral function of certain ISG as a means to dampen the innate immune response against HBV.
We reason that the lack of IFN response in HBV infection is due to poorly active STING-dependent DNA sensing mechanisms in hepatocytes. This loss of DNA sensing seems to be developmentally conserved as the expression of DNA sensor genes showed a decreasing trend during hepatic differentiation of the human stem cells. Why STING-dependent DNA sensing is functional in many cells types but selectively deficient in mature hepatocytes is not clear. It has been speculated to be attributable to the high proliferation potential of hepatocytes (36), as proliferation can lead to DNA accumulation in the cytosol and subsequent DNA-driven inflammatory responses. Our data revealed a clear inverse correlation of hepatocyte differentiation status with DNA sensor activity. The reduced DNA sensing might represent a developmental adaptation to hepatocyte polyploidy, which is generally considered to indicate terminal differentiation of hepatocytes with decreased proliferative capacity. Turning off the ability to detect “abnormal” amount of DNA accumulation may assist the hepatocytes to tolerate the state of tetraploidy and octoploidy. Furthermore, hepatocytes are highly metabolically active and contain high level of mitochondria. Mitochondrial DNA has been implicated as an important activator of cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS)-STING axis (37), which may be another reason that the DNA sensing pathway is down-regulated in hepatocytes. Finally hepatocytes are constantly exposed to large quantities of DNA from either intestinal microbiota or digested food in the portal circulation, and may therefore evolve a genetic program to down-regulate the DNA sensing pathways to avoid potentially exaggerated inflammatory response. Regardless of the reasons, HBV clearly benefits from this evolutionary convergence of hepatic differentiation. As this study and others shown that with over-expression of DNA sensors in hepatocytes, HBV infection could be limited by the evoked immune responses (10, 36).
The liver comprises mainly of hepatocytes, but also contains professional innate immune cells like liver macrophages (Kupffer cells). In HBV mouse models, macrophages have been implicated in the activation of HBV-specific adaptive immunity (38). Depletion of macrophages also results in decreased recruitment of inflammatory cells and ameliorated liver injury (39). We showed that human macrophages or THP cells respond to HBV with a rapid induction of TNF-α and IL6, similar to a previous report of rapid IL6 secretion of macrophage-hepatocyte co-culture (40). In our study using HBV stock generated in cell culture, we noted that a concentration around 108 genomes/mL is needed for macrophage activation. This high viral concentration certainly exceeds a typical inoculation size of any acute HBV infection, but can easily be achieved during the ramping up phase of HBV replication, especially considering the production and compartmentalization of HBV in the liver resulting in a higher local exposure to the liver resident macrophages.
At this point, we do not know which viral component(s) is responsible for the activation of macrophages. Although a role of HBsAg has been implicated (41), it is difficult to attribute the effect to a single viral component based on our data. This complex question needs to be further investigated. Overall, the high-viremia requirement for macrophage activation is in line with the delayed pro-inflammatory cytokine production as well as the late onset of immune control observed after the peak of HBV viremia in acutely infected patients (3, 42).
In conclusion, the present study resolves many controversies concerning the interactions between HBV and hepatocytes. Using various models, our study demonstrates a lack of activation or interference of the innate immune function of hepatocytes during HBV infection and provides a plausible mechanism for the lack of immune recognition by hepatocytes. Thus, HBV is indeed a stealth virus for hepatocytes. On the other hand, HBV is capable of activating macrophages at high viral titers, which may mark the initial step of an HBV-specific immune response (3). HBV may also interact with and activate other innate immune cells, such as NK, NKT or dendritic cells. We previously showed that in HBV-infected cells, HBsAg can perturb the intracellular lipid milieu to activate NKT cells via a CD1-dependent pathway (43). The exact nature of the HBV PAMP(s) and host PRR(s) involved, as well as the resulting biological and virological consequences are all important topics for future investigation.
Supplementary Material
Acknowledgments
We thank Dr. Ulrike Protzer for providing the HepG2-NTCP cells and Zhensheng Zhang for technical support.
Financial support:
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Yuchen Xia is partly sponsored by The International Liver Cancer Association (ILCA)-Fellowship.
Abbreviations
- HBV
hepatitis B virus
- rcDNA
relaxed circular DNA
- cccDNA
covalently closed circular DNA
- pgRNA
pre-genomic RNA
- HCV
hepatitis C virus
- IFN
interferon
- ISG
interferon-stimulated gene
- PRR
pattern recognition receptor
- RIG-I
retinoic acid inducible protein I
- MDA5
melanoma differentiation-associated protein 5
- cGAS
cyclic guanosine monophosphate–adenosine monophosphate synthase
- STING
stimulator of IFN genes
- HBeAg
HBV e antigen
- HBsAg
HBV surface antigen
- HBVcc
cell culture derived HBV
- dHepaRG
differentiated-HepaRG
- HLC
hepatocyte like cell
- PHH
primary human hepatocyte
- PAMP
pathogen-associated molecular pattern
- IRF
IFN regulatory factor
- TLR
Toll-like receptor
- STAT1
signal transducer and activator of transcription 1
- SeV
sendai virus
- ETV
entecavir
- IFI16
IFN-inducible protein 16
References
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher SP, Chin DJ, Ji Y, Iniguez AL, Taillon B, Swinney DC, Ravindran P, et al. Transcriptomic analysis of the woodchuck model of chronic hepatitis B. Hepatology. 2012;56:820–830. doi: 10.1002/hep.25730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shlomai A, Schwartz RE, Ramanan V, Bhatta A, de Jong YP, Bhatia SN, Rice CM. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc Natl Acad Sci U S A. 2014;111:12193–12198. doi: 10.1073/pnas.1412631111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123–132. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Lu HL, Liao F. Melanoma differentiation-associated gene 5 senses hepatitis B virus and activates innate immune signaling to suppress virus replication. J Immunol. 2013;191:3264–3276. doi: 10.4049/jimmunol.1300512. [DOI] [PubMed] [Google Scholar]
- 10.Dansako H, Ueda Y, Okumura N, Satoh S, Sugiyama M, Mizokami M, Ikeda M, et al. The cyclic GMP-AMP synthetase-STING signaling pathway is required for both the innate immune response against HBV and the suppression of HBV assembly. FEBS J. 2016;283:144–156. doi: 10.1111/febs.13563. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Li J, Chen J, Li Y, Wang W, Du X, Song W, et al. Hepatitis B virus polymerase disrupts K63-linked ubiquitination of STING to block innate cytosolic DNA-sensing pathways. J Virol. 2015;89:2287–2300. doi: 10.1128/JVI.02760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar M, Jung SY, Hodgson AJ, Madden CR, Qin J, Slagle BL. Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. J Virol. 2011;85:987–995. doi: 10.1128/JVI.01825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 14.Luangsay S, Gruffaz M, Isorce N, Testoni B, Michelet M, Faure-Dupuy S, Maadadi S, et al. Early inhibition of hepatocyte innate responses by hepatitis B virus. J Hepatol. 2015;63:1314–1322. doi: 10.1016/j.jhep.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Khan M, Syed GH, Kim SJ, Siddiqui A. Hepatitis B Virus-Induced Parkin-Dependent Recruitment of Linear Ubiquitin Assembly Complex (LUBAC) to Mitochondria and Attenuation of Innate Immunity. PLoS Pathog. 2016;12:e1005693. doi: 10.1371/journal.ppat.1005693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christen V, Duong F, Bernsmeier C, Sun D, Nassal M, Heim MH. Inhibition of alpha interferon signaling by hepatitis B virus. J Virol. 2007;81:159–165. doi: 10.1128/JVI.01292-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia Y, Carpentier A, Cheng X, Block PD, Zhao Y, Zhang Z, Protzer U, et al. Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions. J Hepatol. 2017;66:494–503. doi: 10.1016/j.jhep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraga N, Imamura M, Hatakeyama T, Kitamura S, Mitsui F, Tanaka S, Tsuge M, et al. Absence of viral interference and different susceptibility to interferon between hepatitis B virus and hepatitis C virus in human hepatocyte chimeric mice. J Hepatol. 2009;51:1046–1054. doi: 10.1016/j.jhep.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, et al. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142:978–988. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 22.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keskinen P, Nyqvist M, Sareneva T, Pirhonen J, Melen K, Julkunen I. Impaired antiviral response in human hepatoma cells. Virology. 1999;263:364–375. doi: 10.1006/viro.1999.9983. [DOI] [PubMed] [Google Scholar]
- 24.Wu M, Xu Y, Lin S, Zhang X, Xiang L, Yuan Z. Hepatitis B virus polymerase inhibits the interferon-inducible MyD88 promoter by blocking nuclear translocation of Stat1. J Gen Virol. 2007;88:3260–3269. doi: 10.1099/vir.0.82959-0. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hosel M, Michler T, et al. Interferon-gamma and Tumor Necrosis Factor-alpha Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016;150:194–205. doi: 10.1053/j.gastro.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Decroly E, Ferron F, Lescar J, Canard B. Conventional and unconventional mechanisms for capping viral mRNA. Nat Rev Microbiol. 2011;10:51–65. doi: 10.1038/nrmicro2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollack JR, Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993;67:3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol. 2009;83:9652–9662. doi: 10.1128/JVI.00867-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker LF, Murray R. Relationship of virus dose to incubation time of clinical hepatitis and time of appearance of hepatitis--associated antigen. Am J Med Sci. 1972;263:27–33. doi: 10.1097/00000441-197201000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, Rodgers S, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45:102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira-Nascimento L, Massari P, Wetzler LM. The Role of TLR2 in Infection and Immunity. Front Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomsen MK, Nandakumar R, Stadler D, Malo A, Valls RM, Wang F, Reinert LS, et al. Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatology. 2016;64:746–759. doi: 10.1002/hep.28685. [DOI] [PubMed] [Google Scholar]
- 37.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, Zhang L, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. doi: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, et al. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 41.Boltjes A, van Montfoort N, Biesta PJ, Op den Brouw ML, Kwekkeboom J, van der Laan LJ, Janssen HL, et al. Kupffer cells interact with hepatitis B surface antigen in vivo and in vitro, leading to proinflammatory cytokine production and natural killer cell function. J Infect Dis. 2015;211:1268–1278. doi: 10.1093/infdis/jiu599. [DOI] [PubMed] [Google Scholar]
- 42.Bertoletti A, Ferrari C. Kinetics of the immune response during HBV and HCV infection. Hepatology. 2003;38:4–13. doi: 10.1053/jhep.2003.50310. [DOI] [PubMed] [Google Scholar]
- 43.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.