Regional heterogeneity in the mechanisms of pressure-induced myogenic tone implies that resistance vessels may be able to alter myogenic signaling pathways to adapt to their environment. A better understanding of the spectrum of myogenic mechanisms could provide new targets to treat diseases that affect resistance artery and arteriolar function.
Keywords: hamster, microcirculation, arterioles, myogenic tone
Abstract
Myogenic tone is an important feature of arterioles and resistance arteries, but the mechanisms responsible for this hallmark characteristic remain unclear. We used pharmacological inhibitors to compare the roles played by phospholipase C (PLC; 10 μM U73122), inositol 1,4,5-trisphosphate receptors (IP3Rs; 100 μM 2-aminoethoxydiphenylborane), protein kinase C (10 μM bisindolylmaleimide I), angiotensin II type 1 receptors (1 μM losartan), Rho kinase (10 nM−30 μM Y27632 or 300 nM H1152), stretch-activated ion channels (10 nM−1 μM Gd3+ or 5 μM spider venom toxin GsMTx-4) and L-type voltage-gated Ca2+ channels (0.3–100 μM diltiazem) in myogenic tone of cannulated, pressurized (80 cmH2O), second-order hamster cremaster or cheek pouch arterioles. Effective inhibition of either PLC or IP3Rs dilated cremaster arterioles, inhibited Ca2+ waves, and reduced global Ca2+ levels. In contrast, cheek pouch arterioles did not display Ca2+ waves and inhibition of PLC or IP3Rs had no effect on myogenic tone or intracellular Ca2+ levels. Inhibition of Rho kinase dilated both cheek pouch and cremaster arterioles with equal efficacy and potency but also reduced intracellular Ca2+ signals in both arterioles. Similarly, inhibition of mechanosensitive ion channels with Gd2+ or GsMTx-4 produced comparable dilation in both arterioles. Inhibition of L-type Ca2+ channels with diltiazem was more effective in dilating cremaster (86 ± 5% dilation, n = 4) than cheek pouch arterioles (54 ± 4% dilation, n = 6, P < 0.05). Thus, there are substantial differences in the mechanisms underlying myogenic tone in hamster cremaster and cheek pouch arterioles. Regional heterogeneity in myogenic mechanisms could provide new targets for drug development to improve regional blood flow in a tissue-specific manner.
NEW & NOTEWORTHY Regional heterogeneity in the mechanisms of pressure-induced myogenic tone implies that resistance vessels may be able to alter myogenic signaling pathways to adapt to their environment. A better understanding of the spectrum of myogenic mechanisms could provide new targets to treat diseases that affect resistance artery and arteriolar function.
myogenic tone is a hallmark feature of resistance arteries and arterioles in the microcirculation (10). However, the mechanisms responsible for this pressure-dependent, steady-state contractile activity of vascular smooth muscle cells in the wall of these microvessels remain unclear (10, 20). Many studies have supported a major role for phospholipase C (PLC) in the signaling pathway leading to myogenic tone in a variety of blood vessels (3, 9, 24, 28–30, 40, 41, 44, 45, 49, 50, 58, 62). However, the pathways downstream from PLC remain uncertain. For example, inositol 1,4,5-trisphosphate (IP3) and IP3 receptors (IP3Rs) contribute to myogenic tone in rat cerebral arteries (16, 18) and hamster and mouse cremaster arterioles (61, 62). However, IP3 and IP3Rs do not appear to contribute to myogenic tone in murine mesenteric resistance arteries (39). Similarly, protein kinase C (PKC), presumably activated by diacylglycerol (DAG) released from the actions of PLC on membrane phospholipids, has been proposed to significantly contribute to myogenic tone in cerebral arteries (13, 48, 55, 63), coronary arteries (42), subcutaneous resistance arteries (9), small arteries from rat gracilis muscle (38), rabbit facial veins (33), murine mesenteric resistance arteries (39), and ferret aortas (51). However, an early microcirculatory study (19) found no effect of PKC inhibition on myogenic tone in rat cremaster arterioles, although the myogenic response (response to a step increase in pressure) appeared inhibited. A more recent study (24) of rat ophthalmic arteries also have failed to detect a significant role for PKC in myogenic tone within the physiological pressure range (60–120 mmHg) despite effective inhibition of this enzyme. Taken together, these data suggest that there may be regional differences in the mechanisms responsible for myogenic tone. Consistent with this hypothesis, studies have shown that myogenic tone in rat renal afferent arterioles is abolished by the lanthanide Gd3+ (10 μM) (57). However, in first-order rat cremaster arterioles, 1–100 μM Gd3+ had no effect on myogenic tone (3). Similar results have been reported in the rabbit basilar artery (63). These data support the hypothesis that there may be regional differences in the mechanisms underlying myogenic tone. However, methodological, animal strain, and species differences may also contribute to these examples of apparent mechanism heterogeneities.
The purpose of the present study was to directly test the hypothesis that there are regional differences in the mechanisms underlying myogenic tone by comparing the effects of several inhibitors on myogenic tone in hamster cremaster and cheek pouch arterioles using identical methods. Our results support this hypothesis and suggest that 1) the PLC/IP3R pathway, while essential for myogenic tone in hamster cremaster arterioles, is not involved in the myogenic tone of hamster cheek pouch arterioles; 2) PKC isoforms inhibited by bisindolemaleimide I (10 μM) do not contribute to myogenic tone in either class of arteriole; and 3) L-type voltage-gated Ca2+ channels contribute to 86% of myogenic tone in cremaster arterioles but only 54% in cheek pouch arterioles.
METHODS
Animals and arteriole preparations.
Male Golden Syrian hamsters (6–10 wk old, 75–150 g) were obtained from Harlan Laboratories (Indianapolis, IN). Animals were euthanized by CO2 asphyxiation in conformance with the American Veterinary Medicine Association Guidelines for Euthanasia (2013). Animal use was approved by the Institutional Animal Care and Use Committee of Michigan State University in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (46). Cheek pouches and cremaster muscles were rapidly removed and placed in Ca2+-free physiological salt solution [PSS; composed of (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.4]. Tissues were pinned onto Sylgard pads in a cooled (4°C) dissection chamber filled with Ca2+-free PSS containing 0.1% BSA (USB, Cleveland, OH), sodium nitroprusside (10 μM), and diltiazem (10 μM). Sodium nitroprusside and diltiazem were included in the dissection solutions to maintain the arteriolar smooth muscle cells in a relaxed state during dissection, as previously reported (8, 25, 27, 61, 62). Second-order cheek pouch (26) or cremaster arterioles (8, 25, 62) were hand dissected from the tissues, transferred to a cannulation chamber, cannulated onto glass micropipettes, and secured to the pipettes using 11-0 ophthalmic suture (Ashaway Line and Twine, Ashaway, RI) as previously described (8, 25, 62). The chamber was mounted on the stage of a microscope (Nikon Diaphot, Melville, NY, or Leica DMIL, Wetzlar, Germany), where the vessels were superfused with PSS containing CaCl2 (1.8 mM), visualized with charge-coupled device cameras, and warmed to 37°C (cheek pouch arterioles) or 34°C (cremaster arterioles). Luminal pressure then was stepped from 20 to 80 cmH2O as previously described (8, 25, 62). All vessels studied displayed myogenic reactivity in response to this pressure step and in steady state had at least 20% myogenic tone when compared with the maximum diameter of the vessel obtained in Ca2+-free PSS. Vessels were continually superfused with PSS [composed of (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.4] alone or containing a drug.
Ca2+ imaging.
Smooth muscle cells of cannulated vessels were loaded with the intensiometric Ca2+ indicator fluo-4 by bath incubation with solutions containing 5 μM fluo 4-AM dye (Invitrogen, Carlsbad, CA) in 0.5% DMSO and 0.1% BSA (USB) in PSS for 2 h at room temperature. Vessels then were superfused with PSS for 30 min to wash fluo-4 from the bath and to allow for dye deesterification and gradual temperature increase. Arterioles were imaged with a ×40 water-immersion objective (numerical aperture: 0.8, working distance: 3 mm, Leica). Illumination was provided by a 488-nm diode laser coupled to a CSU-10B Yokagawa spinning disk confocal head (Solamere, Salt Lake City, UT). Emitted 526-nm fluorescence was acquired at 30 frames/s (500 images: 16.67-s recording time) with an intensified charge-coupled device camera (XR Mega-10, Stanford Photonics, Palo Alto, CA) using Piper software (Stanford Photonics, Palo Alto, CA). Images were analyzed using SparkAn (courtesy of M. T. Nelson and A. D. Bonev, University of Vermont) and ImageJ (1). The occurrence of Ca2+ waves (number of cells displaying Ca2+ events/total number of cells imaged per recording) was counted manually by visualizing each smooth muscle cell within a vessel separately using a masking procedure and scoring whether any Ca2+ waves appeared during the recording period. These occurrences were then verified using SparkAn by placing a 10 × 10-pixel region of interest (ROI) at the peak of each event and assessing increases in fluorescence that were at least 15% above basal levels for each cell. As an estimate of global Ca2+, a Z projection of the average intensity of each image stack was made using ImageJ, and the average intensity of an ROI that encompassed the entire vessel image section was obtained from the projection. Identical ROIs were used for all measurements within a single vessel with results expressed relative to the average intensity within the ROI during the control period.
Diameter measurements.
In most experiments, internal arteriolar diameter was recorded using DiamTrak software (47) as previously described (8, 25, 62). In Ca2+ imaging experiments, diameter was measured asynchronously immediately after acquisition of a flou-4 image stack by focusing at the midplane of the vessel and acquiring an image sequence with dim, 564-nm transillumination. The average diameter during the recording period then was measured using ImageJ calibrated with a stage micrometer. All vessels were exposed to 0 Ca2+ PSS at the end of experiments to define the maximal diameter of arterioles. All diameter measurements were accurate to ±1 μm. Myogenic tone was calculated as (Dmax – Dtone)/Dmax × 100%, where Dmax is the maximal diameter of an arteriole after exposure to 0 Ca2+ PSS at the end of an experiment and Dtone is the steady-state diameter of an arteriole.
Materials.
Fluo-4 was purchased from Invitrogen, whereas 2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide HCl [bisindolylmaleimide I (BIM I)], and phorbol-12-myristate-13-acetate (PMA) were purchased from EMD Chemicals (Gibbstown, NJ). All other drugs and chemicals were acquired from Sigma Chemical (St. Louis, MO).
Statistics.
Except for the occurrence of Ca2+ waves, all data are expressed as means ± SE, with n as the number of vessels studied from at least three animals with no more than two arterioles from a given animal. Means were compared by paired t-tests or repeated-measures ANOVA followed by Tukey’s test when three or more means were compared. Concentration-response data were analyzed using GraphPad Prism 5.0 software (La Jolla, CA) to estimate the concentration required to produce 50% of the maximal response (EC50) and maximum effect (Emax). All statistical comparisons were performed at the 95% confidence level.
RESULTS
Arteriolar diameters and myogenic tone.
Second-order arterioles from cremaster muscles had resting diameters of 46 ± 2 μm (n = 38) with maximal diameters of 68 ± 2 μm. Second-order cheek pouch arterioles were slightly larger with resting diameters of 55 ± 2 μm (n = 38) and maximal diameters of 80 ± 3 μm (P < 0.05 vs. cremaster muscles). However, both vessel types developed similar levels of myogenic tone (cremaster: 33 ± 1% vs. cheek pouch: 30 ± 1%, P > 0.05).
PLC contributes to myogenic tone in hamster cremaster arterioles but not in hamster cheek pouch arterioles.
We have previously shown that the PLC antagonist U73122 (10 μM) inhibits myogenic tone in second-order hamster cremaster arterioles (62). Confirming these data, we found a similar effect in the present study (Fig. 1A): U73122 (10 μM) dilated cremaster arterioles 79 ± 6% of the maximum obtained in 0 Ca2+ PSS (P < 0.05).
Fig. 1.
Phospholipase C (PLC) and inositol 1,4,5-trisphosphate receptors (IP3Rs) contribute to myogenic tone in cremaster arterioles. Both U73122 and 2-aminoethoxydiphenylborane (2-APB) inhibited myogenic tone in second-order hamster cremaster arterioles. Data are mean diameters ± SE for arterioles before (control), during exposure to U73122 (A; 10 μM, n = 7) or 2-APB (B; 100 μM, n = 5), or during exposure to 0 Ca2+ physiological salt solution (PSS) as indicated. *P < 0.05, significantly different from control.
In stark contrast to our present (Fig. 1A) and previous findings in cremaster arterioles (62), this PLC inhibitor had no significant effect on the diameter of second-order hamster cheek pouch arterioles (P > 0.05; Fig. 2A), despite the presence of an equivalent degree of myogenic tone. The lack of effect of U73122 in cheek pouch arterioles was not due to a lack of efficacy, because this PLC antagonist abolished constrictions induced by bolus application of the α1-adrenergic agonist phenylephrine (20 nmol), as shown in Fig. 2C. U73343, the nonactive analog of U73122, was without effect on resting diameter or the response to phenylephrine (Fig. 2C). The inhibition of phenylephrine-induced constriction by U73122 was not simply due to a nonspecific effect because constriction induced by the L-type Ca2+ channel agonist BayK 8644 (5 nM) was unaffected by the PLC inhibitor. Before application of U73122, BayK 8644 (5 nM) constricted hamster cheek pouch arterioles from 47 ± 1.8 to 22 ± 3.1 μm (n = 3, P < 0.05), whereas in the presence of U73122, BayK 8644 (5 nM) constricted arterioles from 48 ± 2.4 to 17 ± 1.8 μm (n = 3, P < 0.05).
Fig. 2.
PLC and IP3Rs do not contribute to myogenic tone in cheek pouch arterioles. Neither U73122 nor 2-APB affected myogenic tone in second-order hamster cheek pouch arterioles, but they effectively inhibited phenylephrine-induced constriction. In A and B, data are mean diameters ± SE (n = 6) for arterioles before (control), during exposure to the PLC inhibitor U73122 (A; 10 μM) or 2-APB (B; 100 μM), or during exposure to 0 Ca2+ PSS as indicated. C and D: mean diameters ± SE (n = 3) before (PSS) or at the peak of constriction induced by bolus application of 20 nmol phenylephrine as indicated before (control) or during exposure to U73122 (10 μM; C), its nonactive analog U73343 (10 μM; C), or the IP3R antagonist 2-APB (100 μM; D). *P < 0.05, significantly different from control.
IP3Rs contribute to myogenic tone in hamster cremaster arterioles but not in hamster cheek pouch arterioles.
As we have previously reported (62), the putative IP3R antagonist 2-aminoethoxydiphenylborane (2-APB; 100 μM) dilated hamster cremaster arterioles (Fig. 1B). However, consistent with the lack of effect of the PLC inhibitor in cheek pouch vessels (Fig. 2A), 2-APB (100 μM) also was without effect on hamster cheek pouch arterioles (Fig. 2B). Again, this concentration of 2-APB effectively inhibited constrictions induced by phenylephrine (Fig. 2D), suggesting effective inhibition of IP3R signaling.
Lack of Ca2+ waves in cheek pouch arterioles.
Smooth muscle cells in hamster cremaster arterioles display Ca2+ waves that result from periodic Ca2+ release from internal stores in a process that involves both PLC and IP3Rs, which appear to contribute to Ca2+-dependent myogenic tone in these vessels (62). Confirming these findings, Ca2+ waves were observed in fluo-4-loaded cremaster arterioles in the present study (Fig. 3A): under control conditions, 41 ± 10% of the cells observed in confocal slices displayed asynchronous Ca2+ waves, with amplitudes of 1.87 ± 0.10 F/Fo and frequencies of 0.20 ± 0.03 Hz (104 cells from 5 arterioles).
Fig. 3.
Smooth muscle cells in hamster cremaster arterioles display Ca2+ waves, whereas cheek pouch arterioles do not. A, inset: image of a tangential, confocal slice through a cremaster arteriole loaded with fluo-4 along with nine regions of interest (ROIs) that were placed at the peak of Ca2+ waves. The data plot shows relative fluorescence (F/Fo) for the nine ROIs showing the amplitude and duration of these Ca2+ events. Application of SparkAn to these data with a threshold of 15% above baseline revealed 19 Ca2+ waves with a mean amplitude of 1.52 ± 0.07 F/Fo (range: 1.2–2.6) during the 16-s recording period shown for this vessel. Similar results were obtained in four additional cremaster arterioles, confirming a previous study (62). B: data similar to those shown in A for a cheek pouch arteriole. In the nine ROIs shown, no events with an amplitude of >15% above baseline were detected during the 16-s recording period shown. Similar results were obtained in five additional cheek pouch arterioles. The scale bars shown in A and B = 20 μm. See text for more details.
The lack of effect of U73122 and 2-APB on myogenic tone in cheek pouch arterioles suggested that smooth muscle cells in these vessels might not display Ca2+ waves. Therefore, we loaded smooth muscle cells in cheek pouch arterioles with fluo-4 and imaged these cells using parameters identical to those used to image Ca2+ waves in cremaster arterioles (Fig. 3A). Consistent with the lack of effect of U73122 and 2-APB on myogenic tone in cheek pouch arterioles, we found that smooth muscle cells in these vessels did not display Ca2+ events with amplitudes of >15% above baseline (Fig. 3B): in 112 cells analyzed from 6 arterioles, no events were detected that exceeded threshold. Furthermore, consistent with the lack of effect of these agents on myogenic tone, and in contrast to the effects of these drugs on Ca2+ signals in cremaster arterioles (62), neither U73122 (10 μM) nor 2-APB (100 μM) had a significant effect on global Ca2+ levels in cheek pouch arterioles (P > 0.05 for both drugs; Fig. 4).
Fig. 4.
Lack of effect of U73122 or 2-APB on global Ca2+ levels in hamster cheek pouch arterioles. A and B: projected average images of 500 image stacks in the absence (A) and presence (B) of the PLC inhibitor U73122 (10 μM) showing that U73122 does not cause a significant fall in global fluo-4 fluorescence. Scale bars in A and B = 25 μm. C and D: mean fluo-4 intensities ± SE (n = 6) expressed relative to the average fluo-4 intensity in control image stacks before (control) and in the presence of U73122 (10 μM; C) or the IP3R antagonist 2-APB (100 μM; D). No significant differences were observed (P > 0.05).
PKC does not contribute to myogenic tone in hamster cremaster or cheek pouch arterioles.
In addition to forming IP3, PLC also produces DAG, which activates a number of isoforms of PKC (11) and appears to contribute to myogenic tone in some (9, 13, 21, 33, 38, 39, 42, 48, 51, 55, 63) but not all (19, 24) blood vessels. Therefore, we assessed the effects of BIM I (10 μM), which effectively inhibits most isoforms of PKC (37, 59). We first verified the efficacy of BIM I using the phorbol ester PMA (10 nM) to activate PKC. In the absence of the inhibitor, we found that hamster cremaster arterioles were quite sensitive to the PKC activator PMA (10 nM): from a resting diameter of 38 ± 1.8 μm, PMA (10 nM) constricted cremaster arterioles by 12.4 ± 0.5 μm (P < 0.05, n = 5). Superfusion of cremaster arterioles with 10 μM BIM I had no significant effect on resting arteriolar diameter (Fig. 5); however, constriction induced by PMA (10 nM) was abolished (1 ± 0.8 μm, n = 5, P > 0.05), demonstrating effective inhibition of the PKC isoforms activated by PMA and likely involved in the modulation of myogenic tone. Unsurprisingly, BIM I (10 μM) also did not inhibit myogenic tone in cheek pouch arterioles (control diameter: 60 ± 4 μm and diameter after 10 μM BIM I: 49 ± 6 μm, n = 3, P > 0.05). These data suggest that conventional and novel isoforms of PKC are not involved in myogenic tone in hamster cremaster or cheek pouch arterioles.
Fig. 5.
Lack of effect of the PKC inhibitor bisindolylmaleimide I (BIM I) on the diameter of hamster cremaster arterioles. Data are mean diameters ± SE (n = 5) before (rest) and during exposure to phorbolmysterate acetate (PMA), in the absence (control) and presence of BIM I, or after superfusion with 0 Ca2+ PSS as indicated. *P < 0.05, different from control; **P < 0.05, greater than all other groups.
Lack of a role for angiotensin II type 1 receptors or α1-adrenergic receptors in the myogenic tone of cremaster arterioles.
Recent studies have provided compelling evidence that angiotensin II type 1 receptors (AT1Rs) are involved in the signaling pathway of myogenic tone in several types of arteries and are upstream from PLC and IP3Rs (18, 21, 41, 52, 54). Given the prominent roles that PLC and IP3Rs play in the myogenic tone of cremaster arterioles, we assessed the effects of the AT1R antagonist losartan (1 μM) on myogenic tone in cremaster arterioles as a test of the hypothesis that AT1Rs also contribute to myogenic tone in these vessels. As shown in Fig. 6, losartan effectively inhibited vasoconstriction induced by bolus application of 20 pmol angiotensin II, but had no effect on myogenic tone. We also tested the effect of the α1-adrenergic receptor antagonist prazosin (30 nM) on resting diameter as these G protein-coupled receptors are highly expressed in cremaster arterioles (25). Prazosin effectively inhibited constriction induced by bolus application of phenylephrine, but this antagonist also had no effect on myogenic tone (Fig. 6).
Fig. 6.
Angiotensin II (ANG II) receptors do not contribute to myogenic tone in hamster cremaster arterioles. Effective blockade of ANG II receptors with losartan or α1-adrenergic receptors with prazosin has no effect on myogenic tone in cremaster arterioles. Data are mean diameters ± SE (n = 5) before (rest) or during exposure to ANG II or phenylephrine (PHE) in the absence (Control) or presence of the ANG II receptor blocker losartan, the α1-adrenergic receptor blocker prazosin, or exposure to 0 Ca2+ PSS as indicated. *P < 0.05, significantly different from rest; **P < 0.05, significantly greater than all other groups.
Rho kinase contributes to myogenic tone and Ca2+ signaling in both hamster cremaster and cheek pouch arterioles.
Ca2+ sensitization of the contractile machinery induced by the activity of Rho kinase has been proposed to contribute to myogenic tone in several preparations (4, 31, 53, 63). Therefore, we examined if there might be differences in the role played by Rho kinase in hamster cremaster and cheek pouch arterioles. We found that the Rho kinase inhibitor Y27632 produced similar, concentration-dependent vasodilation of both hamster cremaster and cheek pouch arterioles (P > 0.05; Fig. 7). The EC50 value obtained was 1.79 μM (95% confidence interval: 0.58−5.58 μM).
Fig. 7.
Role of Rho kinase in myogenic tone of cremaster and cheek pouch arterioles. The Rho kinase inhibitor Y27632 causes concentration-dependent dilation of hamster arterioles. Data are mean diameters ± SE (n = 4) in response to cumulative addition of Y27632 to cannulated cremaster or cheek pouch arterioles as indicated. Concentration-dependent dilation was observed in both vessels (P < 0.05). Resting and maximal diameters were 42 ± 2 and 59 ± 1 μm for cremaster arterioles and 40 ± 2 and 58 ± 4 μm for cheek pouch arterioles, respectively. See text for more information.
Surprisingly, we found that this Rho kinase inhibitor also significantly inhibited Ca2+ signaling in both preparations. In cremaster arterioles, Y27632 (10 μM) effectively abolished the occurrence of Ca2+ waves and significantly reduced the amplitude and frequency of what few waves remained (Fig. 8A). Global fluo-4 intensity levels also were diminished by Y27632 (Fig. 8A), suggesting a fall in global Ca2+. Confirming the data shown in Fig. 7, Y27632 also dilated fluo-4-loaded cremaster arterioles (Fig. 8A). Application of this Rho kinase inhibitor to hamster cheek pouch arterioles produced a similar reduction in the global fluo-4 signal to 67.3 ± 4.8% of the control value (n = 4, P < 0.05), indicating a significant fall in global Ca2+ levels associated with the dilation shown in Fig. 7.
Fig. 8.
Rho kinase antagonists inhibit myogenic tone and Ca2+ signaling in hamster cremaster arterioles. Data are the occurrence of Ca2+ waves (Occ; number of cells showing waves/total number of cells studied) ± 95% confidence interval or means ± SE (n = 5) for the amplitude (Amp; F/Fo) or frequency (Freq; Hz) of Ca2+ waves and global fluo-4 levels (an index of global Ca2+) and arteriolar diameter in the absence (control) or presence of the Rho kinase inhibitors Y27632 (10 μM, n = 5; A) or H-1152 (300 nM, n = 4; B). * P < 0.05, significantly different from control value.
We also tested the effects of the more potent Rho kinase inhibitor H-1152 (300 nM) in hamster cremaster arterioles. Similar results to those obtained with Y27632 were obtained (Fig. 8B): H-1152 inhibited the occurrence, amplitude, and frequency of Ca2+ waves, produced a fall in global Ca2+ (as assessed by the global fluo-4 fluorescence), and dilated the arterioles.
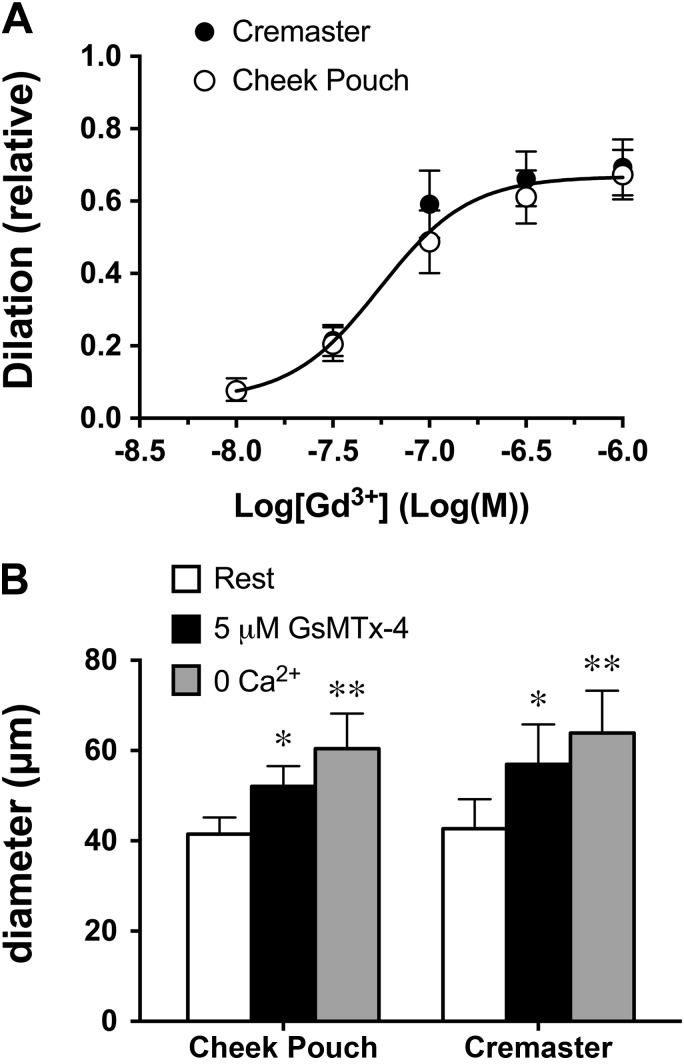
Gd3+ and GsMTx-4 similarly inhibit myogenic tone in cremaster and cheek pouch arterioles.
Transient receptor potential (TRP) channels appear to participate in the mechanisms underlying myogenic tone in cerebral arteries (2, 7, 13, 14, 16, 18, 41, 60). Therefore, we assessed the effects of the lanthanide Gd3+ (10 nM−1 μM) and the Chilean rose tarantula venom toxin GsMTx-4 (5 μM), known blockers of TRP channels, on myogenic tone in both cheek pouch and cremaster arterioles. We found that Gd3+ dilated both vessel types with similar potency (logEC50: −7.26 ± 0.1) and efficacy (Emax: 67 ± 4%; Fig. 9A). Similarly, exposure of cheek pouch or cremaster arterioles to GsMTx-4 (5 μM) produced comparable dilation in both vessel types (Fig. 9B).
Fig. 9.
Role of mechanosensitive transient receptor potential (TRP) channels in myogenic tone of cremaster and cheek pouch arterioles. Gd3+ and the spider venom toxin GsMTx-4 inhibited myogenic tone in hamster arterioles. A: data are mean dilation relative to 0 Ca2+ diameter ± SE (n = 4 for cremaster and n = 9 for cheek pouch). The solid curve represents the best fit for a sigmoid (logEC50: −7.26 ± 0.10 and Emax: 0.67 ± 0.04) to both data sets. B: data are mean diameters ± SE (n = 4 for each vessel type) before (rest), in the presence of GsMTx-4, and upon exposure to 0 Ca2+ PSS as indicated. *P < 0.05, significantly different from rest; **P < 0.05, significantly greater than all other groups.
Diltiazem is more effective in dilating cremaster arterioles than cheek pouch arterioles.
Ca2+ influx through L-type voltage-gated Ca2+ channels is essential for myogenic tone (10, 20). Therefore, we also assessed the effects of the L-type Ca2+ channel blocker diltiazem on myogenic tone. In contrast to the effects of Gd2+ and GsMTx-4, we found that diltiazem was more effective in dilating cremaster arterioles (Emax: 86 ± 5%) compared with cheek pouch arterioles (Emax: 54 ± 4%, P < 0.05 vs. cremaster arterioles; Fig. 10). The potency of diltiazem was not significantly different between the two vessel types (cremaster logEC50: −6.3 ± 0.3 vs. cheek pouch log EC50: −5.4 ± 0.3, P > 0.05; Fig. 10).
Fig. 10.
Diltiazem is more effective in cremaster than cheek pouch arterioles. Data are mean dilation relative to 0 Ca2+ PSS diameter ± SE (n = 4 for cremaster and n = 6 for cheek pouch) during cumulative addition of the L-type Ca2+ channel blocker diltiazem to the superfusate. Solid curves are the best fit sigmoids. Ditiazem had similar potency (logEC50: −5.99 ± 0.21) in both vessels. However, this Ca2+ channel blocker was more effective in cremaster arterioles (Emax: 0.86 ± 0.05) than cheek pouch arterioles (Emax: 0.54 ± 0.04) as indicated. *P < 0.05 for Emax.
DISCUSSION
Our data support the hypothesis that there are significant regional differences in the apparent mechanisms of myogenic tone in the hamster. We found that an inhibitor of PLC [U73122 (10 μM)] and an inhibitor of IP3Rs [2-APB (100 μM)] dilated hamster cremaster arterioles (Fig. 1), confirming prior studies in these vessels (62). These data support a role for PLC and IP3Rs in the mechanisms underlying myogenic tone in cremaster arterioles. In contrast, these same antagonists at the same concentrations had no significant effect on myogenic tone (Fig. 2) or intracellular Ca2+ (Fig. 4) in hamster cheek pouch arterioles studied by identical methods. We do not think that the lack of effect of U73122 and 2-APB in cheek pouch arterioles can be explained by a lack of efficacy of the drugs in this preparation, because we were able to effectively inhibit phenylephrine-induced vasoconstriction of the arterioles with both U73122 and 2-APB (Fig. 2). Furthermore, in contrast to what we have found in cremaster arterioles in both hamster [present study (Fig. 3A) and Ref. 62] and mouse (61), smooth muscle cells in hamster cheek pouch arterioles did not display Ca2+ waves. Thus, in cheek pouch arterioles, PLC and IP3Rs do not appear to contribute to myogenic tone or the Ca2+ signaling events that underlie this important function of microvascular smooth muscle cells. A lack of a role for IP3Rs in myogenic tone also has been reported in mouse mesenteric resistance arteries (39, 43).
We also observed that effective inhibition of PKC had no significant effect on myogenic tone in either cremaster or cheek pouch arterioles. These data confirm prior studies in both rat cremaster arterioles (19) and rat ophthalmic arteries (24). However, they are in contrast to studies in cerebral arteries (13, 48, 55, 63), coronary arteries (42), subcutaneous resistance arteries (9), small arteries from rat gracilis muscle (38), rabbit facial veins (33), murine mesenteric resistance arteries (39), and ferret aortas (51), where PKC appeared to significantly contribute to myogenic tone. Other than invoking regional, species, and methodological differences to account for a role (or lack thereof) for PKC in myogenic tone, we cannot explain these differences. However, these findings do support our central hypothesis that there are multiple mechanisms responsible for myogenic tone and that no one, single mechanism can explain all of the published data.
Given the stark difference in reliance on the PLC-IP3R signaling cascade between cremaster and cheek pouch arteries, we were surprised to find that the sensitivity of myogenic tone to the Rho kinase inhibitor Y27632 was identical in both vessels. Nonetheless, this observation suggests similar contributions of Rho kinase to myogenic tone in the two vessels. However, we cannot extrapolate to the role played by Rho kinase-mediated Ca2+ sensitization from our findings, because the Rho kinase inhibitor Y27632 substantially suppressed Ca2+ signaling in both vessels. This effect did not appear to be unique to Y27632, because another Rho kinase inhibitor, H1152, also inhibited Ca2+ waves and reduced global Ca2+ levels in smooth muscle cells of cremaster arterioles. These data suggest either that Rho kinase participates in the regulation of Ca2+ signaling in arteriolar smooth muscle cells or that Y27632 and H1152 have substantial off-target effects that limit their utility. A previous study (15) has suggested that Rho kinase is involved in the regulation of Ca2+ entry in rat aorta and mesenteric arteries. In addition, Rho kinase has been proposed to regulate the activity of voltage-activated K+ channels (35) and TRPM4 channels (34) in cerebral arteries, which, in turn, can influence Ca2+ signaling by impacting membrane potential. Thus, there is precedence for Rho kinase-mediated influences on smooth muscle Ca2+ regulation.
We found that the lanthanide Gd3+ (23) and GsMTx-4 (56), which are known to inhibit currents through a number of TRP channels thought to be involved in myogenic tone (2, 7, 13, 14, 17, 18, 41, 60), dilated both cremaster and cheek pouch arterioles. These data suggest that TRP channels contribute to the mechanisms underlying myogenic tone in both classes of arterioles. Gd3+ has been shown to inhibit currents through voltage-gated Ca2+ channels in cardiac myocytes with IC50 = 1.4 μM (32), much higher than we observed (~55 nM; Fig. 9). Thus, the effects of Gd3+ are unlikely to be mediated completely via nonselective effects on voltage-gated Ca2+ channels. In addition, we found that GsMTx-4, which is relatively selective for mechanosensitive TRP channels at micromolar concentrations (6), had similar nonmaximal effects on myogenic tone in both vessels. Therefore, the data shown in Fig. 9 support roles for TRP channels in both preparations. However, the similarity in the sensitivity of myogenic tone to Gd3+ and GsMTx-4 does not necessarily imply that identical channels are active and contribute to myogenic tone in cremaster and cheek pouch arterioles. While Ca2+ can permeate many TRP channels, they are primarily nonselective cation channels that participate in the regulation of smooth muscle cell membrane potential; activation of these channels leads to membrane depolarization and activation of voltage-gated Ca2+ channels (12). Thus, the inhibition of tone by Gd3+ and GsMTx-4 likely involves both direct inhibition of Ca2+ entry through TRP channels but also indirect inhibition of Ca2+ entry through voltage-gated Ca2+ channels. We observed that the L-type voltage-sensitive Ca2+ channel blocker diltiazem inhibited 86% of the Ca2+-dependent myogenic tone in cremaster arterioles but only 54% of the Ca2+-dependent tone in cheek pouch arterioles under similar experimental conditions, with similar EC50 values (~1 μM; Fig. 10). These findings suggest that the contribution of Ca2+ influx through nonvoltage-dependent pathways differs between cremaster (14% of total) and cheek pouch arterioles (46% of total). Therefore, despite the similarity of effects of Gd3+ and GsMTx-4 between the two vessel types, it seems likely that there are also differences in the contribution of TRP channels to myogenic tone in the two arterioles. Additional research will be required to define which TRP channels contribute to myogenic tone and their relative contribution(s) to myogenic tone in these hamster arterioles.
Limitations.
Our interpretation that PLC and IP3Rs participate in the Ca2+ signaling and myogenic tone in cremaster arterioles is based on the effects of U73122 and 2-APB on Ca2+ signals and tone. However, both drugs have been reported to have additional, off-target effects that complicate interpretation of the data (5, 22, 36). We do not think that our observations using these drugs can be dismissed as totally nonspecific effects, because we found that both U73122 and 2-APB were without effect on global Ca2+ levels and myogenic tone in cheek pouch arterioles. It seems unlikely that off-target effects would be vessel specific. Thus, given the presence of Ca2+ waves in hamster cremaster arteriolar smooth muscle cells, their inhibition by both U73122 and 2-APB, and the inhibition of myogenic tone in cremaster arterioles by these drugs, we think that a role for PLC and IP3Rs in myogenic tone in cremaster arterioles is likely as we have previously concluded (62). Nonetheless, we cannot exclude off-target effects of U73122 and 2-APB. Additional research using more selective approaches will be required to resolve these issues.
Conclusions.
Our findings of differences in the pharmacology of myogenic tone between arterioles that originate from different regions of the peripheral microcirculation indicate that the mechanisms responsible for this hallmark characteristic of arterioles and resistance arteries can arise through multiple, independent signaling pathways. This observation infers that it may be possible to develop drugs that target specific regions of the vasculature to selectively alter local vascular resistance and regional tissue blood flow. That multiple mechanisms exist to generate myogenic tone also suggests that arterioles and resistance arteries have alternative pathways to develop myogenic tone that are determined by their local environment and that could be modified during vascular pathology. A better understanding of the spectrum of mechanisms available and the control of expression and function of the ion channels responsible for these mechanisms could provide us with new drug targets to treat diseases that affect resistance artery and arteriolar function such as obesity, hypertension, and diabetes.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-32469 and P01-HL-070687 (to W. F. Jackson) and American Heart Association Fellowship 0815778G (to E. M. Boerman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.F.J. and E.M.B. conceived and designed research; W.F.J. and E.M.B. performed experiments; W.F.J. and E.M.B. analyzed data; W.F.J. and E.M.B. interpreted results of experiments; W.F.J. and E.M.B. prepared figures; W.F.J. drafted manuscript; W.F.J. and E.M.B. edited and revised manuscript; W.F.J. and E.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
REFERENCES
- 1.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int 11: 36–42, 2004. [Google Scholar]
- 2.Anfinogenova Y, Brett SE, Walsh MP, Harraz OF, Welsh DG. Do TRPC-like currents and G protein-coupled receptors interact to facilitate myogenic tone development? Am J Physiol Heart Circ Physiol 301: H1378–H1388, 2011. doi: 10.1152/ajpheart.00460.2011. [DOI] [PubMed] [Google Scholar]
- 3.Bakker EN, Kerkhof CJ, Sipkema P. Signal transduction in spontaneous myogenic tone in isolated arterioles from rat skeletal muscle. Cardiovasc Res 41: 229–236, 1999. doi: 10.1016/S0008-6363(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 4.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation 108: 342–347, 2003. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 5.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150, 2002. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 6.Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49: 249–270, 2007. doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brayden JE, Li Y, Tavares MJ. Purinergic receptors regulate myogenic tone in cerebral parenchymal arterioles. J Cereb Blood Flow Metab 33: 293–299, 2013. doi: 10.1038/jcbfm.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Signalling mechanisms underlying the myogenic response in human subcutaneous resistance arteries. Cardiovasc Res 49: 828–837, 2001. doi: 10.1016/S0008-6363(00)00314-X. [DOI] [PubMed] [Google Scholar]
- 10.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 279: L429–L438, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292: H2613–H2622, 2007. doi: 10.1152/ajpheart.01286.2006. [DOI] [PubMed] [Google Scholar]
- 14.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 15.Ghisdal P, Vandenberg G, Morel N. Rho-dependent kinase is involved in agonist-activated calcium entry in rat arteries. J Physiol 551: 855–867, 2003. doi: 10.1113/jphysiol.2003.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 299: C279–C288, 2010. doi: 10.1152/ajpcell.00550.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCγ1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 7: ra49, 2014. doi: 10.1126/scisignal.2004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MA, Falcone JC, Meininger GA. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol Heart Circ Physiol 259: H1586–H1594, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca(2+) signaling pathways underlying myogenic reactivity. J Appl Physiol (1985) 91: 973–983, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Hong K, Zhao G, Hong Z, Sun Z, Yang Y, Clifford PS, Davis MJ, Meininger GA, Hill MA. Mechanical activation of angiotensin II type 1 receptors causes actin remodelling and myogenic responsiveness in skeletal muscle arterioles. J Physiol 594: 7027–7047, 2016. doi: 10.1113/JP272834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol 126: 243–262, 2005. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001. doi: 10.1161/01.RES.88.3.325. [DOI] [PubMed] [Google Scholar]
- 24.Ito I, Jarajapu YP, Grant MB, Knot HJ. Characteristics of myogenic tone in the rat ophthalmic artery. Am J Physiol Heart Circ Physiol 292: H360–H368, 2007. doi: 10.1152/ajpheart.00630.2006. [DOI] [PubMed] [Google Scholar]
- 25.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol 155: 514–524, 2008. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson WF, Duling BR. The oxygen sensitivity of hamster cheek pouch arterioles. In vitro and in situ studies. Circ Res 53: 515–525, 1983. doi: 10.1161/01.RES.53.4.515. [DOI] [PubMed] [Google Scholar]
- 27.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation 4: 35–50, 1997. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 28.Jarajapu YP, Grant MB, Knot HJ. Myogenic tone and reactivity of the rat ophthalmic artery. Invest Ophthalmol Vis Sci 45: 253–259, 2004. doi: 10.1167/iovs.03-0546. [DOI] [PubMed] [Google Scholar]
- 29.Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. Eur J Pharmacol 579: 298–307, 2008. doi: 10.1016/j.ejphar.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarajapu YP, Knot HJ. Role of phospholipase C in development of myogenic tone in rat posterior cerebral arteries. Am J Physiol Heart Circ Physiol 283: H2234–H2238, 2002. doi: 10.1152/ajpheart.00624.2002. [DOI] [PubMed] [Google Scholar]
- 31.Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol 587: 2537–2553, 2009. doi: 10.1113/jphysiol.2008.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacampagne A, Gannier F, Argibay J, Garnier D, Le Guennec JY. The stretch-activated ion channel blocker gadolinium also blocks L-type calcium channels in isolated ventricular myocytes of the guinea-pig. Biochim Biophys Acta 1191: 205–208, 1994. doi: 10.1016/0005-2736(94)90250-X. [DOI] [PubMed] [Google Scholar]
- 33.Laher I, Bevan JA. Staurosporine, a protein kinase C inhibitor, attenuates Ca2+-dependent stretch-induced vascular tone. Biochem Biophys Res Commun 158: 58–62, 1989. doi: 10.1016/S0006-291X(89)80176-7. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Brayden JE. Rho kinase activity governs arteriolar myogenic depolarization. J Cereb Blood Flow Metab 37: 140–152, 2017. doi: 10.1177/0271678X15621069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luykenaar KD, El-Rahman RA, Walsh MP, Welsh DG. Rho-kinase-mediated suppression of KDR current in cerebral arteries requires an intact actin cytoskeleton. Am J Physiol Heart Circ Physiol 296: H917–H926, 2009. doi: 10.1152/ajpheart.01206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macmillan D, McCarron JG. The phospholipase C inhibitor U-73122 inhibits Ca2+ release from the intracellular sarcoplasmic reticulum Ca2+ store by inhibiting Ca2+ pumps in smooth muscle. Br J Pharmacol 160: 1295–1301, 2010. doi: 10.1111/j.1476-5381.2010.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marmé D, Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem 268: 9194–9197, 1993. [PubMed] [Google Scholar]
- 38.Massett MP, Ungvari Z, Csiszar A, Kaley G, Koller A. Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am J Physiol Heart Circ Physiol 283: H2282–H2287, 2002. doi: 10.1152/ajpheart.00544.2002. [DOI] [PubMed] [Google Scholar]
- 39.Mauban JR, Zacharia J, Fairfax S, Wier WG. PC-PLC/sphingomyelin synthase activity plays a central role in the development of myogenic tone in murine resistance arteries. Am J Physiol Heart Circ Physiol 308: H1517–H1524, 2015. doi: 10.1152/ajpheart.00594.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauban JR, Zacharia J, Zhang J, Wier WG. Vascular tone and Ca2+ signaling in murine cremaster muscle arterioles in vivo. Microcirculation 20: 269–277, 2013. doi: 10.1111/micc.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27: 3092–3103, 2008. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller FJ Jr, Dellsperger KC, Gutterman DD. Myogenic constriction of human coronary arterioles. Am J Physiol Heart Circ Physiol 273: H257–H264, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol 518: 815–824, 1999. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama K, Tanaka Y. Stretch-induced contraction and Ca2+ mobilization in vascular smooth muscle. Biol Signals 2: 241–252, 1993. doi: 10.1159/000109505. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan J, Imig M, Roman RJ, Harder DR. Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol 266: H1840–H1845, 1994. [DOI] [PubMed] [Google Scholar]
- 46.National Research Council, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academies Press, 2011, p. xxv. [Google Scholar]
- 47.Neild TO. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels 26: 48–52, 1989. [PubMed] [Google Scholar]
- 48.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res 68: 359–367, 1991. doi: 10.1161/01.RES.68.2.359. [DOI] [PubMed] [Google Scholar]
- 49.Osol G, Laher I, Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol Heart Circ Physiol 265: H415–H420, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Park KS, Kim Y, Lee YH, Earm YE, Ho WK. Mechanosensitive cation channels in arterial smooth muscle cells are activated by diacylglycerol and inhibited by phospholipase C inhibitor. Circ Res 93: 557–564, 2003. doi: 10.1161/01.RES.0000093204.25499.83. [DOI] [PubMed] [Google Scholar]
- 51.Pawlowski J, Morgan KG. Mechanisms of intrinsic tone in ferret vascular smooth muscle. J Physiol 448: 121–132, 1992. doi: 10.1113/jphysiol.1992.sp019032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pires PW, Ko EA, Pritchard HAT, Rudokas M, Yamasaki E, Earley S. The angiotensin II receptor type 1b is the primary sensor of intraluminal pressure in cerebral artery smooth muscle cells. J Physiol 595: 4735–4753, 2017. doi: 10.1113/JP274310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poley RN, Dosier CR, Speich JE, Miner AS, Ratz PH. Stimulated calcium entry and constitutive RhoA kinase activity cause stretch-induced detrusor contraction. Eur J Pharmacol 599: 137–145, 2008. doi: 10.1016/j.ejphar.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schleifenbaum J, Kassmann M, Szijártó IA, Hercule HC, Tano JY, Weinert S, Heidenreich M, Pathan AR, Anistan YM, Alenina N, Rusch NJ, Bader M, Jentsch TJ, Gollasch M. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ Res 115: 263–272, 2014. doi: 10.1161/CIRCRESAHA.115.302882. [DOI] [PubMed] [Google Scholar]
- 55.Slish DF, Welsh DG, Brayden JE. Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol Heart Circ Physiol 283: H2196–H2201, 2002. doi: 10.1152/ajpheart.00605.2002. [DOI] [PubMed] [Google Scholar]
- 56.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takenaka T, Suzuki H, Okada H, Hayashi K, Kanno Y, Saruta T. Mechanosensitive cation channels mediate afferent arteriolar myogenic constriction in the isolated rat kidney. J Physiol 511: 245–253, 1998. doi: 10.1111/j.1469-7793.1998.245bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka Y, Hata S, Ishiro H, Ishii K, Nakayama K. Stretching releases Ca2+ from intracellular storage sites in canine cerebral arteries. Can J Physiol Pharmacol 72: 19–24, 1994. doi: 10.1139/y94-004. [DOI] [PubMed] [Google Scholar]
- 59.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991. [PubMed] [Google Scholar]
- 60.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 61.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol 590: 1849–1869, 2012. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol 300: H1616–H1630, 2011. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG, Lee YH. Role of protein kinase C- or RhoA-induced Ca2+ sensitization in stretch-induced myogenic tone. Cardiovasc Res 53: 431–438, 2002. doi: 10.1016/S0008-6363(01)00496-5. [DOI] [PubMed] [Google Scholar]