Abstract
Rhesus macaques experimentally infected with Simian Immunodeficiency Virus (SIV) experience immunosuppression and often opportunistic infection. Among the most common opportunistic infections is rhesus cytomegalovirus (RhCMV), a ubiquitous betaherpesvirus that undergoes continuous low-level replication in immunocompetent monkeys. Upon SIV-mediated immunodeficiency, RhCMV reactivates and results in lesions in numerous organ systems including the nervous and reproductive systems. We report the first case of cytomegaloviral hypophysitis in a SIV-immunocompromised rhesus macaque.
Keywords: non-human primate, CMV, cytomegalovirus, pituitary, model, viral, inclusions, intra-nuclear, intra-cytoplasmic
Introduction
Cytomegaloviruses (CMV) are betaherpesviruses that infect humans and a wide range of non-human primate species including Macaca mulatta, Saguinus fuscicollis, Saimiri sciureus, Mandrillus lecophaeus, and Papio spp.1-4 Animals shed CMV through saliva, urine, semen, cervical secretions, and breast milk; most group-housed animals are exposed to CMV within the first year of life.5-7 Rhesus cytomegalovirus (RhCMV) has a reported seroprevalence of 100% in animals older than one year at the California National Primate Research Center, and 95% within wild Indian populations.5,8 Despite the high seroprevalence of CMV in rhesus macaques, clinical manifestation is infrequent in immunocompetent animals. Unlike other herpesviruses that undergo periods of latency, CMV replicates continuously in infected tissues.9 The balance between viral replication and immune response results in low-level viremia and a lack of clinical signs.8
Human cytomegalovirus (hCMV) is epidemiologically similar to its simian counterpart. Approximately 10% of human infants are infected with hCMV by six months of age.10 Up to 40% of infants that nurse for longer than one month from seropositive mothers become infected.11 Increased hCMV seropositivity is seen in populations where individuals live in close proximity.12 The widespread distribution of viral infection in these communities mirrors that of rhesus macaque colonies, making macaques a good model for studying hCMV.
Similar to hCMV in immunosuppressed humans, RhCMV may result in clinical disease in rhesus macaques immunocompromised by simian immunodeficiency virus (SIV) infection. In SIV infected macaques, the severity of clinical disease correlates with SIV-induced immune suppression and an increase in plasma RhCMV DNA.13-16
RhCMV lesions in SIV infected animals are widespread and reported in the central and peripheral nervous systems, gastrointestinal tract, reproductive tract, and respiratory system. RhCMV has a predilection for neurologic and reproductive tissues.17,18 Within the peripheral nervous system, lesions have been demonstrated in the pulmonary, hepatic, mesenteric, penile, facial, sciatic, periocular, and trigeminal nerves.17, 19-21 In a review of SIV-RhCMV co-infected rhesus macaque lesions, the meninges were the most consistently and severely affected tissue.17 However, hypophyseal infection with RhCMV has not been previously reported. We report the first case of RhCMV infection of the pituitary gland in a SIV-infected rhesus macaque.
Case report
Clinical presentation
A 4.6kg, six-year-old SIV infected female rhesus macaque (Macaca mulatta) was admitted for weakness, ataxia, epistaxis, and intermittently lying down in her cage. The animal had received multiple intravaginal doses of SIVmac239 at 1000 TCID50/1ml/week for 5 weeks, then 5000 TCID50/1ml/week for 5 weeks, and then10,000 TCID50/1ml/week until the animal was confirmed SIV-infected (plasma viral load > 500 copies/ml for 2 consecutive weeks). The animal was confirmed infected after 6 inoculations at 10,000 TCID50/ml.. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the SNPRC. The SNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International. There was no clinical history prior to study enrollment. She was specific pathogen free for simian T-lymphotrophic virus 1 (STLV1) and 2 (STLV2), simian retrovirus 2 (SRV), SIV, and macacine herpesvirus 1 before infection with SIV. A complete blood count (CBC), clinical chemistry, and cerebrospinal fluid (CSF) analysis were performed. The CBC revealed a mild hypochromic, normocytic anemia; lymphopenia; and monocytosis. The serum chemistry revealed hypoproteinemia, elevated BUN/creatinine ratio, and a metabolic alkalosis. The CSF analysis was within normal limits. Due to the patient's deteriorating clinical condition, established study end points, and poor prognosis, euthanasia was elected and the animal was submitted for necropsy. At the time of euthanasia, the animal was SIV-infected for only 14 weeks (3 months) with a plasma viral load of 126,526,833 copies/ml and CD4 count of 1810 CD4/mm (Calculated).
Pathologic Findings
No gross lesions were identified. Representative samples were fixed in 10% neutral buffered formalin and five micron H&E sections were examined. Histologic lesions were restricted to the pituitary gland with no other tissues affected. Areas of necrosis and neutrophilic inflammation surrounded the neurohypophysis and extended into the adenohypophysis. Degenerate cells within this area had large eosinophilic intranuclear inclusions and/or small eosinophilic cytoplasmic inclusions (Fig 1).
Figure 1.
The pituitary is largely replaced by necrosis and neutrophilic inflammation. Note eosinophilic intranuclear inclusions (N) in the adenohypophysis and the areas of necrosis. Note the small eosinophilic cytoplasmic inclusions (C) in the areas of necrosis. Bar = 50 um.
Immunohistochemistry
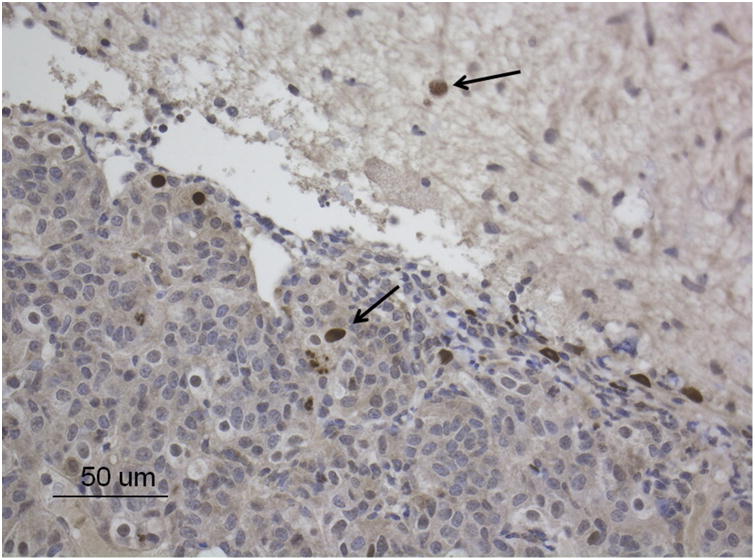
Immunohistochemistry was performed using a polyclonal antiserum for the RhCMV immediate-early protein (IE1) (Gift from Peter A. Barry, UC Davis) as previously described with modifications using the IHC automated Leica BondRx platform according to the manufacturer's recommendations.22 CMV positive cells were found within the pituitary gland and adjacent neuropil (Fig 2).
Figure 2.
Pituitary gland, rhesus macaque. RhCMV-induced hypophysitis characterized by the presence of numerous immunohistochemically positive cells (arrows) for CMV-IE1 (DAB; brown). Bar = 50 um.
Discussion
Hypophysitis in this report is consistent with RhCMV reactivation following SIV-induced immunosuppression and represents the first report of such lesion in rhesus macaques.16,17,19-21,23-28 Similar lesions have been seen in AIDS patients with hCMV, suggesting an additional parallel to human disease.29-31 Human cases of cytomegaloviral hypophysitis are most commonly identified as incidental findings in 4-5% of autopsies.32 Ferreiro reported three cases of hCMV infection in the anterior lobe of the pituitary in AIDS patients; none of which had any significant inflammation or necrosis of the gland.30 Pituitary necrosis was observed in 10 of 88 patients, however none of these were associated with CMV. 30 Sano et al. reported five cases of hypophyseal hCMV infections in AIDS patients; only one had a necrotic area that was associated with inflammatory infiltrate.29 All cases in both of these reports 29, 30 had evidence of systemic CMV infection; this differs from the findings in this macaque where lesions only involved the pituitary gland. The presence of CMV associated hypophyseal inflammation and necrosis observed in this animal also appears to be uncommon in the human cases. 29, 30 Clinical signs of hypophysitis in humans most commonly include headaches and visual disturbances,33 which are thought to relate to localized distention and inflammation. Other clinical signs are related to deficits in hormone production, mainly including adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), gonadotropins, and prolactin (PRL).33 The clinical signs in this case were non-specific and not definitively associated with the hypophysitis. The changes seen on bloodwork could be related to hormonal dysregulation secondary to hypophysitis, but without additional hormonal assays we cannot rule out SIV-associated comorbidities as the cause of the clinical presentation. Ultimately, further study of rhesus macaques and SIV-RhCMV coinfection is necessary to better understand the pathogenesis of CMV-associated neurologic disease in immunosuppressed individuals.6,8,14,15,23
Acknowledgments
This work was supported by NIAID grant R01 AI117862 and used resources that were supported by the Southwest National Primate Research Center grant P51 OD011133 from the Office of Research Infrastructure Programs, National Institutes of Health.
References
- 1.Rangan SR, Chaiban J. Isolation and characterization of a cytomegalovirus from the salivary gland of a squirrel monkey (Saimiri sciureus) Lab Anim Sci. 1980;30:532–540. [PubMed] [Google Scholar]
- 2.Blewett EL, White G, Saliki JT, Eberle R. Isolation and characterization of an endogenous cytomegalovirus (BaCMV) from baboons. Arch Virol. 2001;146:1723–1738. doi: 10.1007/s007050170059. [DOI] [PubMed] [Google Scholar]
- 3.Bennet TB, Abee CR, Henrickson R. Nonhuman Primates in Biomedical Research: Diseases. San Diego: Academic Press; 1998. [Google Scholar]
- 4.Nigida SM, Falk LA, Wolfe LG, Deinhardt F. Isolation of a cytomegalovirus from salivary glands of white-lipped marmosets (Saguinus fuscicollis) Lab Anim Sci. 1979;29:53–60. [PubMed] [Google Scholar]
- 5.Vogel P, Weigler BJ, Kerr H, Henricks AG, Barry PA. Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab Anim Sci. 1994;44:25–30. [PubMed] [Google Scholar]
- 6.Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. Complete Sequence and Genomic Analysis of Rhesus Cytomegalovirus. J Virol. 2003;77:6620–6636. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asher DM, Gibbs CJ, JR, Lang DJ, Gajdusek DC. Persistent Shedding of Cytomegalovirus in the Urine of Healthy Rhesus. Proc Soc Exp Biol Med. 1974;145:794–801. doi: 10.3181/00379727-145-37897. [DOI] [PubMed] [Google Scholar]
- 8.Powers C, Fruh K. Rhesus CMV: an emerging animal model for human CMV. Med Microbiol Immunol. 2008;197:109–115. doi: 10.1007/s00430-007-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cell dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landolfo S, Gariglio M, Gribaudo G, Lembo D. The Human Cytomegalovirus. Pharmacol Ther. 2003;98:269–297. doi: 10.1016/s0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 11.Dworsky M, Yow M, Stagno S, Pass RF, Alford C. Cytomegalovirus infection of Breast Milk and Transmission in Infancy. Pediatrics. 1983;72:295–299. [PubMed] [Google Scholar]
- 12.Griffiths PD. Cytomegalovirus. In: Zuckerman AJ, Banatvala JE, Pattison JR, editors. Principles and Practice of Clinical Virology. London: John Wiley and Sons; 2000. pp. 79–116. [Google Scholar]
- 13.Baroncelli S, Barry PA, Capitanio JP, Lerche NW, Otysula M, Mendoza SP. Cytomegalovirus and Simian Immunodeficiency Virus Coinfection: Longitudinal Study of Antibody Responses and Disease Progression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:5–15. doi: 10.1097/00042560-199705010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kaur A, Hale CL, Noren B, Kassis N, Simon MA, Johnson RP. Decreased Frequency of Cytomegalovirus (CMV)-Specific CD4+ T Lymphocytes in Simian Immunodeficiency Virus-Infected Rhesus Macaques: Inverse Relationship with CMV Viremia. J Virol. 2002;76:3646–3658. doi: 10.1128/JVI.76.8.3646-3658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur A, Kassis N, Hale CL, Simon M, Elliott M, Gomez-Yafal A, Lifson JD, Desrosiers RC, Wang F, Barry PA, Mach M, Johnson RP. Direct Relationship between Suppression of Virus-Specific Immunity and Emergence of Cytomegalovirus Disease in Simian AIDS. J Virol. 2003;77:5749–5758. doi: 10.1128/JVI.77.10.5749-5758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequar G, Britt WJ, Lakeman FD, Lockridge KM, Tarara RP, Canfield DR, Zhou S, Gardner MB, Barry PA. Experimental Coinfection of Rhesus Macaques with Rhesus Cytomegalovirus and Simian Immunodeficiency Virus: Pathogenesis. J Virol. 2002;76:7661–6671. doi: 10.1128/JVI.76.15.7661-7671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskin GB. Disseminated Cytomegalovirus Infection in Immunodeficient Rhesus Monkeys. Am J Pathol. 1987;129:345–352. [PMC free article] [PubMed] [Google Scholar]
- 18.Assaf BT, Mansfield KG, Westmoreland SV, Kaur A, Oxford KL, Diamond DJ, Barry PA. Patterns of Acute Rhesus Cytomegalovirus (RhCMV) Infection Predict Long-Term RhCMV Infection. J Virol. 2012;86:6354–7. doi: 10.1128/JVI.00607-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assaf BT, Knight HL, Miller AD. Rhesus Cytomegalovirus (Macacine Herpesvirus 3)-Associated Facial Neuritis in Simian Immunodeficiency Virus-Infected Rhesus Macaques (Macaca mulatta) Vet Pathol. 2015;52:217–223. doi: 10.1177/0300985814529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutto E, Anderson DC, Mansfield KG. Cytomegalovirus-associated Discrete Gastrointestinal Masses in Macaques Infected with the Simian Immunodeficiency Virus. Vet Pathol. 2004;41:691–695. doi: 10.1354/vp.41-6-691. [DOI] [PubMed] [Google Scholar]
- 21.Clemmons EA, Gumber S, Strobert E, Bloomsmith MA, Jean SM. Self-Injurious Behavior Secondary to Cytomegalovirus-Induced Neuropathy in an SIV-Infected Rhesus Macaque (Macaca mulatta) Comp Med. 2015;65:266–270. [PMC free article] [PubMed] [Google Scholar]
- 22.Assaf BT, Mansfield KG, Strelow L, Westmoreland SV, Barry PA, Kaur A. Limited dissemination and shedding of the UL128 complex-intact, UL/bapos;-defective rhesus cytomegalovirus strain 180.92. J Virol. 2014;88:9310–20. doi: 10.1128/JVI.00162-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baskin GB, Murphey-Corb M, Watson EA, Martin LN. Necropsy Findings in Rhesus Monkeys Experimentally Infected with Cultured Simian Immunodeficiency Virus (SIV)/Delta. Vet Pathol. 1988;25:456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn EM, Matz-Rensing K, Stahl-Hennig C, Makoschey B, Hunsmann G, Kaup FJ. Intestinal Manifestations of Experimental SIV-Infection in Rhesus Monkeys (Macaca mulatta): A Histological and Ultrastructural Study. J Vet Med. 1997;44:501–512. doi: 10.1111/j.1439-0450.1997.tb01001.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn EM, Stolte N, Matz-Rensing K, Mach M, Stahl-Hennig C, Hunsmann G, Kaup FJ. Immunohistochemical Studies of Productive Rhesus Cytomegalovirus Infection in Rhesus Monkeys (Macaca mulatta) Infected with Simian Immunodeficiency Virus. Vet Pathol. 1999;36:51–56. doi: 10.1354/vp.36-1-51. [DOI] [PubMed] [Google Scholar]
- 26.Kaup FJ, Matz-Rensing K, Kuhn EM, Hunerbein P, Stahl-Hennig C, Hunsmann G. Gastrointestinal Pathology in Rhesus Monkeys with Experimental SIV Infection. Pathobiology. 1998;66:159–164. doi: 10.1159/000028015. [DOI] [PubMed] [Google Scholar]
- 27.King NW, Hunt RD, Letvin NL. Histopathologic Changes in Macaques With an Acquired Immunodeficiency Syndrome (AIDS) Am J Pathol. 1983;113:382–388. [PMC free article] [PubMed] [Google Scholar]
- 28.Yanai T, Lackner AA, Sakai H, Masegi T, Simon MA. Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta) J Med Primatol. 2006;35:106–112. doi: 10.1111/j.1600-0684.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 29.Sano T, Kovacs K, Scheithauer BW, Rosenblum MK, Petito CK, Greco CM. Pituitary Pathology in Acquired Immunodeficiency Syndrome. Pathol Lab Med. 1989;113:1066–1070. [PubMed] [Google Scholar]
- 30.Ferreiro J, Vinters HV. Pathology of the Pituitary Gland in Patients with the Acquired Immune Deficiency Syndrome (AIDS) Pathology. 1988;20:211–215. doi: 10.3109/00313028809059495. [DOI] [PubMed] [Google Scholar]
- 31.Myerson D, Hackman RC, Nelson JA, Ward DC, McDougall JK. Widespread Presence of Histologically Occult Cytomegalovirus. Hum Pathol. 1984;15:430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- 32.Vinters HV, Ferreiro JA. Pathology of Human Cytomeglovirus Infection. In: Becker Y, Darai G, editors. Molecular Aspects of Human Cytomegalovirus Diseases. Berlin: Springer-Verlag; 1993. pp. 46–67. [Google Scholar]
- 33.Caturegli P, Newshaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26:599–614. doi: 10.1210/er.2004-0011. [DOI] [PubMed] [Google Scholar]