Abstract
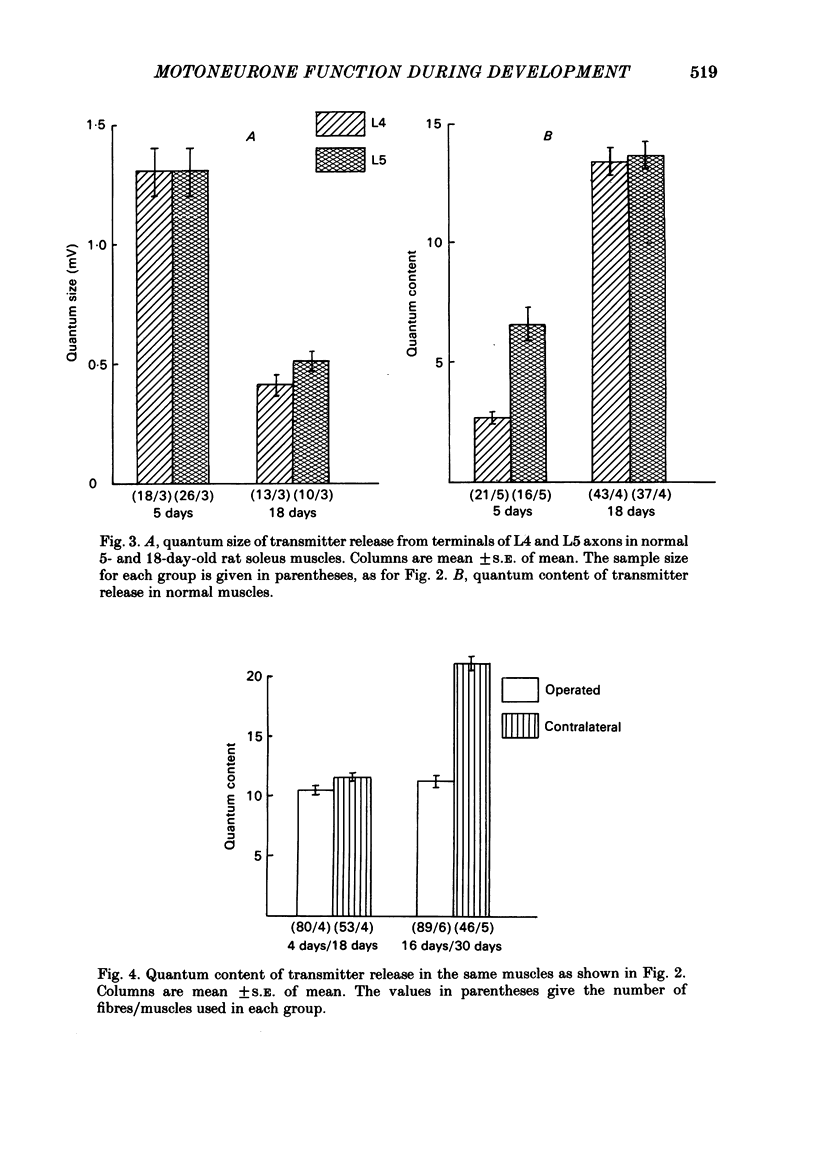
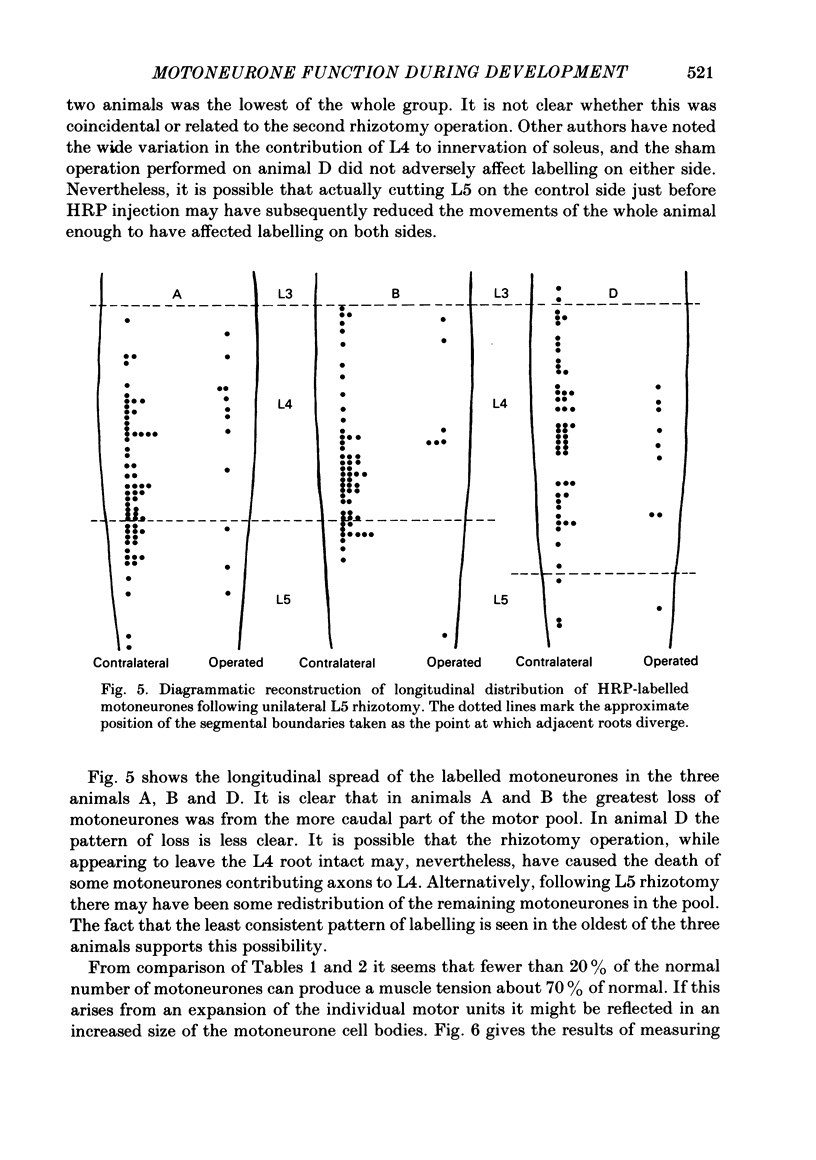
In soleus muscles of 4- to 5-day-old rats the quantum content of axon terminals from L4 spinal roots is less than half that from L5. With development the size of L4 motor units decreases and the quantum content of L4 nerves increases to become similar to that of L5 axons. During this time the overlap of territories of L4 and L5 axons is reduced from 46% at 4-6 days to 2% at 18-20 days. This reduction occurs entirely at the expense of L4 territory. Removal of the L5 ventral ramus (v.r.) at 4-6 days prevents the reduction of L4 territory so that at 18 days L4 motor units are about 4 X normal size. In spite of this enlarged peripheral field of L4 axons the quantum content of their terminals increases to normal levels. When L5 v.r. was removed at 16-18 days, i.e. when the reduction of the L4 peripheral field was complete, expansion of L4 motor units was also seen, but in this case the quantum content of L4 terminals was less than normal. Thus it appears that during early stages of development, before synaptic reorganization within the muscle is complete, motoneurones are able to adapt their function to increased peripheral demands more effectively than at later stages of post-natal development. Retrograde labelling of soleus motor pool with horseradish peroxidase (HRP) showed that removal of L5 v.r. either at 4 or 15 days of age reduced the number of motoneurones supplying soleus muscle to less than 20%. No change in size of the remaining motoneurones was seen, indicating that the adjustment of transmitter output at the neuromuscular junctions in the younger group had no effect on the size of the cell.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. R. Development of neuromuscular synapses. Physiol Rev. 1983 Jul;63(3):915–1048. doi: 10.1152/physrev.1983.63.3.915. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A., Uchitel O. D. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980 Feb;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D. Transmitter release from frog motor nerve terminals depends on motor unit size. Nature. 1980 Oct 16;287(5783):649–651. doi: 10.1038/287649a0. [DOI] [PubMed] [Google Scholar]
- Kelly S. S. The effect of age on neuromuscular transmission. J Physiol. 1978 Jan;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y., Yoshioka K. Selective elimination of motor nerve terminals in the rat soleus muscle during development. J Physiol. 1980 Dec;309:631–646. doi: 10.1113/jphysiol.1980.sp013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. A., Vrbová G. Acetylcholine synthesis in nerve endings to slow and fast muscles of developing chicks: effect of muscle activity. Neuroscience. 1978;3(12):1227–1230. doi: 10.1016/0306-4522(78)90142-2. [DOI] [PubMed] [Google Scholar]
- Thompson W. J. Lack of segmental selectivity in elimination of synapses from soleus muscle of new-born rats. J Physiol. 1983 Feb;335:343–352. doi: 10.1113/jphysiol.1983.sp014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W., Jansen J. K. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2(4):523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]


