Abstract
Background
Prader-Willi syndrome (PWS) is a complex genetic disorder characterized by hyperphagia and morbid obesity with increased cardiopulmonary and hyperphagia-related mortality. Survival trends in PWS were evaluated to assess the impact of modern interventions on mortality risk.
Methods
The PWSA (USA) 40-year mortality syndrome-specific database of 486 death reports was utilized to examine survival trends in PWS and cohort effects for recent deaths (years 2000–2015, N=331) relative to deaths prior to 2000 (N=94). Cox Proportional Hazards regression modeling was applied to generate log rank statistics and Kaplan-Meier curves examining sex, cause of death and cohort.
Results
Risk for all-cause mortality in PWS was 1.5 (95%CI=1.2–1.9) times higher for the Early than the Recent era cohort reflected in female cardiac failure (HR=1.8; 95%CI=1.3–2.6), pulmonary embolism (HR=6.1; 95%CI=1.7–22), and GI-related (HR=3.2; 95%CI=1.1–7.4) causes. Accidental deaths in males increased in the Recent era cohort (HR=5.7; 95%CI=1.2–27.1) possibly due to enhanced weight management and mobility. Risk of death from respiratory failure was unchanged.
Conclusions
We report measurable increases in survival effecting cardiovascular and GI-related causes in PWS most likely attributable to earlier diagnosis and proactive interventions to prevent morbid obesity. More research is needed to address underlying vulnerability to respiratory failure, an unchanged mortality risk in PWS.
Keywords: Prader-Willi Syndrome, Survival, Mortality, Obesity, Cardiac and Respiratory Failure, Thromboembolism, GI-related Problems
INTRODUCTION
Prader-Willi syndrome (PWS) is a complex neurodevelopmental genetic disorder with multiple cognitive, behavioral and endocrine abnormalities caused by errors in genomic imprinting generally due to lack of paternally expressed genes from the 15q11-q13 region.1–3 First identified in 1956, PWS is considered the most common known genetic cause of life-threatening obesity in humans with a prevalence of 1 in 10,000 to 30,000 live births.1–5 A shortened life expectancy is found in relationship to the level of intellectual disability primarily from complications of hyperphagia and obesity-related comorbidities.5,6
PWS is characterized by severe central hypotonia, a poor suck and feeding difficulties, hypogonadism and hypogenitalism observed in both males and females beginning in infancy7. Growth and other hormone deficiencies lead to growth failure, short stature, small hands and feet, infertility and endocrine disturbances.1,2,8 Hypothalamic dysfunction produces temperature instability, high pain threshold, sleep disturbances and multiple endocrine problems.8–10 Infants with PWS are at risk for respiratory problems due to a narrow upper airway, possible aspiration, central hypotonia, hypoventilation, a dry mouth with sticky saliva and swallowing difficulties with gastroenteritis which complicate medical care and response to treatment requiring close monitoring throughout life.1–3,7,10–12
Hyperphagia or the unrelenting pathologic urge to consume food with unremitting hunger in PWS typically develops in childhood leading to excessive food-seeking behavior and life-threatening obesity, if not controlled.1–3,7,13 The risk for obesity is exacerbated by a low basal metabolic rate that is approximately 60% of normal, decreased lean muscle mass and increased fat mass related to endocrine abnormalities.1,2,14 Intractable hyperphagia in PWS continues throughout adulthood with limited options for treatment and significantly diminishes the quality of life for those affected and in family members. Developmental delays and mild intellectual disability are noted with behavioral problems recognized in childhood including tantrums, stubbornness, obsessive compulsions and self-injury (skin picking) that continue into adolescence and adulthood.1–4 Morbid obesity is a significant contributor to mortality in PWS through cardiorespiratory failure,10 pulmonary thromboembolism and associated diabetes with multi-organ failure. Hyperphagia-related behaviors also increase the risk for gastrointestinal perforation and necrosis, aspiration, choking and swallowing difficulties due to a combination of factors from central hypotonia, reduced GI motility and rapid food consumption.11–13 Additionally, excoriation can create severe wounds and precipitate life-threatening infections.
Modern genomic technology has facilitated the detailed genetic characterization of PWS and its subtypes and enabled diagnosis of PWS in infancy.14–17 Earlier diagnosis has increased the opportunity for treatment and intervention to control access to food, reduce risks and prevent the onset of obesity. Growth hormone was first approved to treat short stature in PWS in the 1980’s and had limited use in patients with PWS until approved by the US Food and Drug Administration in the year 2000 to increase stature and lean muscle mass and reduce risk of obesity.18–20 Growth hormone is now widely utilized to treat PWS in the United States beginning at birth through adulthood.18–20 We anticipate that increased diagnosis and earlier interventions associated with this modern era have improved the long term outcomes for individuals with PWS; however, recent longitudinal analyses of mortality rates in PWS still indicate a significantly shortened lifespan.19–21 The present study examines the impact of cohort era prior to and after the year 2000 on causes of death and mortality in PWS. We report survival trends and analysis of the largest collection of causes of death in PWS utilizing the PWSA (USA) syndrome-specific mortality survey database spanning the past 40 years.
SUBJECTS AND METHODS
The Prader-Willi Syndrome Association [PWSA (USA)] is a non-profit parent support organization founded by PWS families to supply information and assist or support families and others caring for those affected with this disorder. Data on causes of death in PWS were collected and recorded by PWSA (USA) since 2001 through a supportive bereavement program for PWS families22. The data includes descriptive information collected through a brief survey administered by the bereavement coordinator. An additional detailed questionnaire with a release of medical records including autopsy reports was disseminated to families known to experience the death of a relative with PWS in 2005. Genetic confirmation of PWS diagnosis was obtained when available; however, the PWS deletion seen in about 70% of those with PWS was not discovered until 1981 and genetic confirmation using DNA methylation testing which is 99% accurate was not available until 1992.22 Thus, deaths occurring prior to 1992 and many older adults may not have been genetically confirmed. The collected data were assembled into a database, reviewed by PWS experts and cleaned to correct errors. The reported causes and contributors to each death and autopsy data were evaluated clinically by a licensed cardiologist, parent and expert in the care of PWS who assessed the primary cause of death for each individual. The defined causes of death were then classified into 13 major categories as described by Butler et al. (2016).22
Statistical Analysis
The dataset included 486 (N=263 males, N=217 females, N=6 sex unknown) individuals with PWS.22 A precise cause of death was available for 311 (N=173 males, N=137 females, N=1 unknown sex) and a precise age of death was known for 425 (N=224 males, N=199 females, N=2 sex unknown). Both the age and cause of death were available for 302 (N=166 males, N=135 females, N=2 sex unknown) individuals.22 Kaplan-Meier plots and Logrank statistics were examined and Cox proportional hazards regression was applied to assess the risk of all-cause mortality as a function of each reason of cause, gender, and their interactions. Time-to-event outcomes were analyzed with hazard ratios as estimates of cumulative hazard or risk of death at time “t” as a measure of the relative death rate. Analyses were carried out using the total data set with the data available for individual variables under study to maximize statistical power.
The impact of historical time frame on mortality risk was evaluated by dividing the sample into two categories: Recent deaths - occurring from 2000 to 2015 with N=331 (N=171 male, N=159 females, N=1 sex unknown) cases having a known age of death. Of these, N=254 (N= 140 males, N= 113 females, N=1 sex unknown) individuals had both a known age and cause of death; and Early deaths - occurring prior to the year 2000 beginning in 1975 with N=94 (N=53 male, N=40 females, N=1 sex unknown) cases having a known age of death. Of these, N=57 (N= 33 males, N= 24 females) individuals had both a known age and cause of death. Chi Squared tests, Kaplan-Meier plots and Logrank statistics were examined and Cox proportional hazards regression was applied to assess the risk of all-cause mortality as a function of cohort era, gender, and their interactions. All statistical comparisons were performed using SAS Statistical Software version 9.4 (Cary, NC).
RESULTS
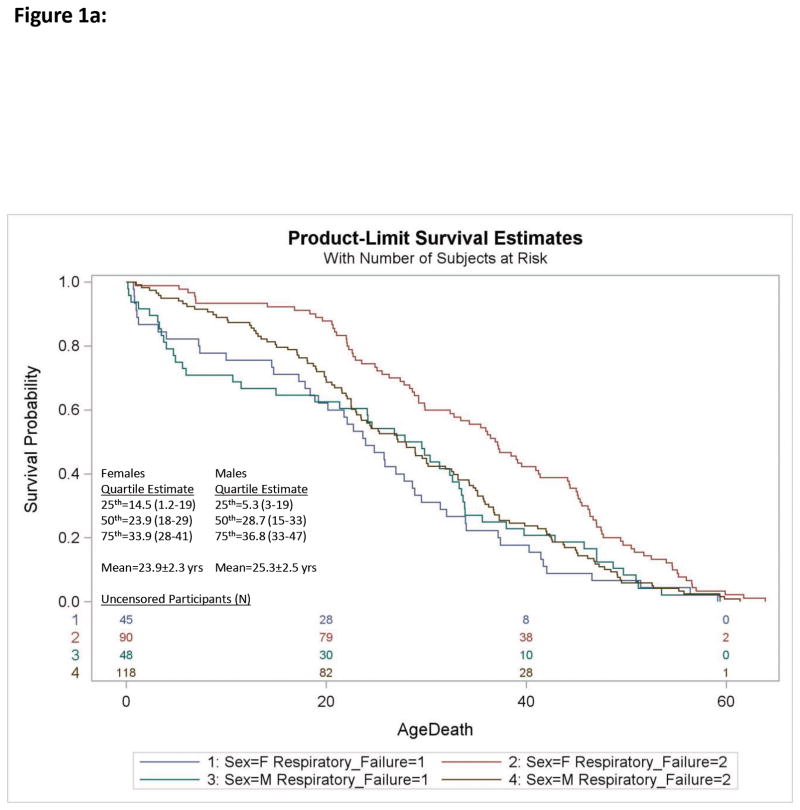
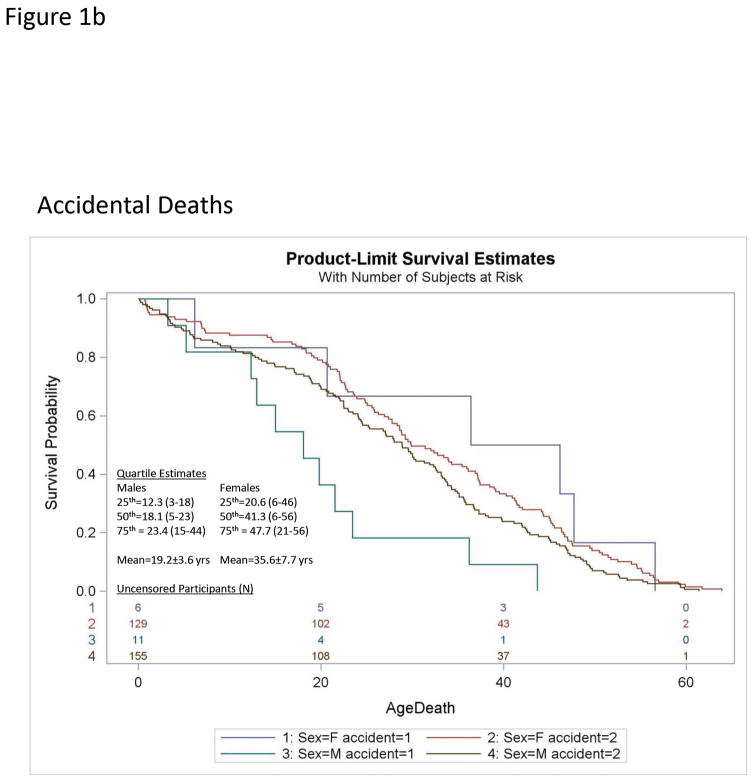
Cox Proportional Hazards regression analysis of individual causes of death relative to all-cause mortality for N=224 males and N=199 females with PWS found the risk of death due to respiratory failure was significantly increased over all-causes [Log-Rank χ2 = 8.2, df=1; p<0.004; Hazard Ratio (HR)=1.4; 95%CI= 1.1–1.8] which was attributable to a sex specific increase in risk of respiratory failure in females (HR=2.0; 95%CI=1.4–2.8 females; HR=1.1; 95%CI= 0.76–1.5 Males; see Figure 1a, Table 1) A sex specific increase in risk of accidental death was also observed in males (HR=2.2; 95%CI=1.2–4.0 males; HR=0.8; 95%CI=0.34–1.8 females; see Figure 1b, Table 1). No other differences were observed for individual causes of death relative to all-cause rates or by gender.
Figure 1.
Figure 1a. Kaplan-Meier plots of survival probability for deaths due to respiratory failure [Respiratory Failure (1)] relative to all other causes [Respiratory Failure (2)] in Prader-Willi syndrome are shown for females [F] and males [M]. The number of uncensored participants are listed by group at the bottom of each figure. Quartile estimates for age of death and mean age of mortality due to respiratory failure are indicated.
Figure 1b. Kaplan-Meier plots of survival probability for accidental deaths [accident (1)] relative to all other causes [accident (2)] in Prader-Willi syndrome for females (F) and males (M). The number of uncensored male and female participants are listed at the bottom of each figure. Quartile estimates for age of death and mean age of mortality are indicated for males and females.
Table 1.
Cox Proportional Hazard Regression Modeling
| Respiratory Failure vs All-Cause Mortality | |||
|---|---|---|---|
| Global Test | Chi-Square | DF | p-value |
| Likelihood Ratio | 18.1 | 3 | 0.0004 |
| Type 3 Test | |||
| Respiratory Failure | 0.16 | 1 | 0.69 |
| Female Sex | 9.7 | 1 | 0.002 |
| Females Sex*Respiratory Failure | 5.9 | 1 | 0.01 |
| Accidental Deaths vs All-Cause Mortality | |||
|---|---|---|---|
| Global Test | Chi-Square | DF | p-value |
| Likelihood Ratio | 10.9 | 3 | 0.01 |
| Type 3 Test | |||
| Accidental Deaths | 6.1 | 1 | 0.01 |
| Sex | 3.6 | 1 | 0.06 |
| Male Sex*Accidental Death | 3.8 | 1 | 0.05 |
| Risk of Death for Early vs Recent Cohorts | |||
|---|---|---|---|
| Global Test | Chi-Square | DF | p-value |
| Likelihood Ratio | 17.9 | 3 | 0.0005 |
| Type 3 Test | |||
| Early vs Recent Deaths | 2.9 | 1 | 0.08 |
| Female Sex | 5.6 | 1 | 0.02 |
| Females Sex*Early Deaths | 1.9 | 1 | 0.1 |
| Risk of Death from Cardiac Failure for Early vs Recent Cohorts | |||
|---|---|---|---|
| Global Test | Chi-Square | DF | p-value |
| Likelihood Ratio | 17.9 | 3 | 0.0005 |
| Type 3 Test | |||
| Early vs Recent Deaths | 2.9 | 1 | 0.08 |
| Female Sex | 5.6 | 1 | 0.02 |
| Females Sex*Early Deaths | 1.9 | 1 | 0.2 |
| Risk of Death from Accidents for Recent vs. Early Cohorts | |||
|---|---|---|---|
| Global Test | Chi-Square | DF | p-value |
| Likelihood Ratio | 23.8 | 3 | 0.0001 |
| Type 3 Test | |||
| Early vs Recent Deaths | 15.2 | 1 | 0.0001 |
| Accidental Death | 5.7 | 1 | 0.017 |
| Accidents*Early Deaths | 14.3 | 1 | 0.0002 |
Time Era Cohort Effects in PWS
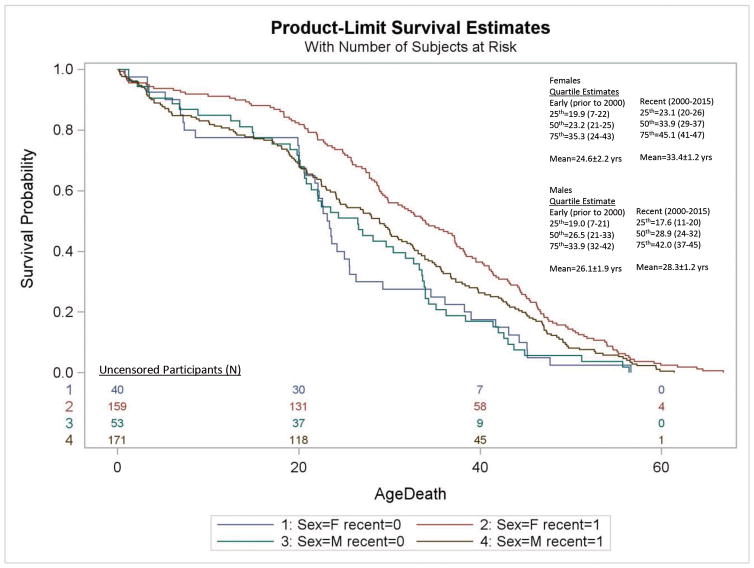
Examination of era cohort effects on survival outcomes identified a significantly greater risk of death due to all causes for the Early era cohort (deaths occurring prior to 2000) relative to the Recent era PWS cohort (years 2000–2015) with an overall hazard of Early death that is 1.5 times greater than that for Recent death (HR=1.5; 95%CI=1.2–1.9; Log-Rank χ2=13.2, df=1, p<0.0003). This corresponded with a gender-selective increase in risk of female deaths in the Early cohort (HR=1.8 Early death; 95%CI=1.3–2.6) not found for males (HR=1.3 Early death; 95%CI=0.96–1.8; see Figure 2; Table 1). This association paralleled an increased risk for cardiac deaths among females in the Early era cohort (HR=1.8 Early death; 95%CI=1.3–2.6; see Table 1). The overall frequency of cardiac deaths were significantly higher in the Early era cohort [15 of 57 (26%)] than the Recent era cohort 36 of 254 (14%) (OR=2.2; 95%CI=1.1–4.3; χ2=5.0, p<0.03) supporting a trend toward reduced overall cardiac death in the PWS population. Evaluation of cohort effects for individual causes of death are limited by complexity and small sample size particularly in the Early era cohort. No other differences were noted in the frequency of any individual cause of death or sex by cohort.
Figure 2.
Kaplan-Meier plot of survival probability for deaths due to all causes for Early era [recent (0)] and Recent era [recent (1)] cohorts with Prader-Willi syndrome are shown for females (F) and males (M). The numbers of uncensored Recent and Early era participants are listed at the bottom of the figure. Quartile estimates for age of death and mean age of mortality are indicated for Recent and Early era groups.
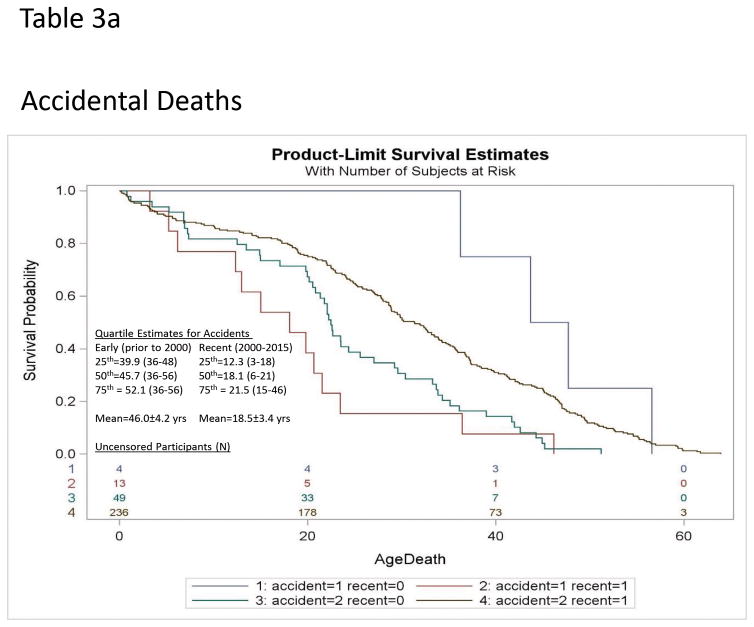
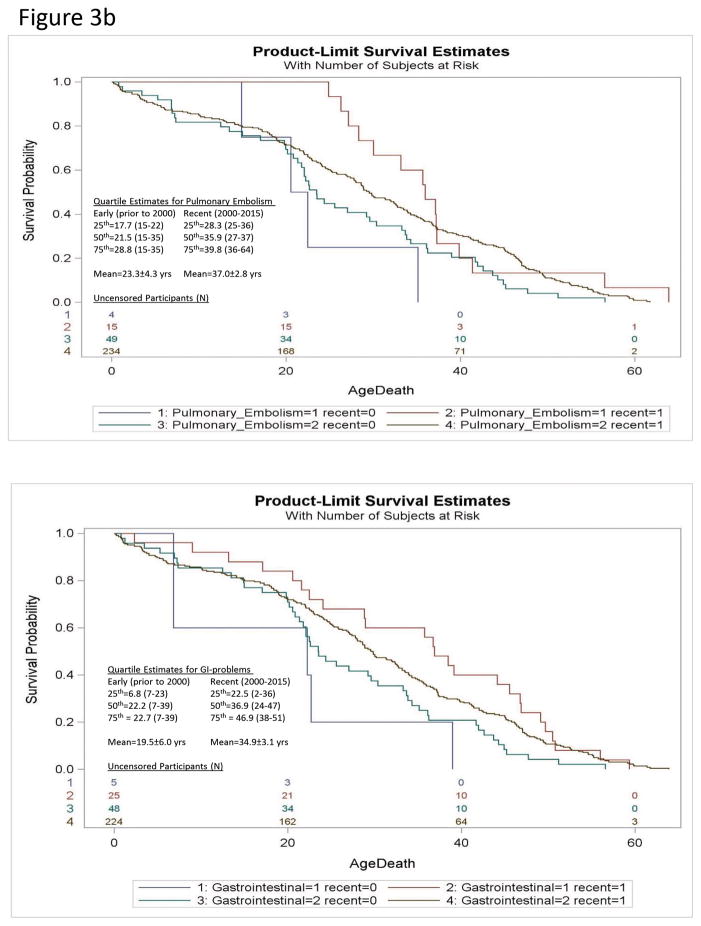
We identified an unexpected increase in the risk of early mortality due to accidental death among the Recent era cohort (HR = 5.2; 95%CI=1.7–16.0 for Recent era relative to Early era cohorts; see Table 1; Figure 3a) driven by an effect in males (HR=5.7 Recent death; 95%CI=1.2–27.1). This unexpected increase in risk for accidental deaths among males may have offset decreases in mortality risk associated with improvements in other hemodynamic factors (e.g., cardiac failure, thromboembolism). The specific risk of accidental death among females could not be analyzed due to the small number of deaths (N=6). Disease-specific examination without consideration of gender or competing risk from other causes of death found the risk of death due to pulmonary embolism (HR=6.1 Early death; 95%CI=1.7–22; χ2=7.5; p<0.006) and gastrointestinal (GI)-complications (HR=3.2 Early death; 95%CI=1.1–7.4; χ2=4.5; p<0.03) were significantly higher in the early era cohort than the Recent era cohort but the risk of death due to respiratory failure and choking were not different. Controlled regression modeling with consideration of competing effects of other causes of death did not achieve significance (see Figure 3b).
Figure 3.
Figure 3a. Kaplan-Meier plot of survival probability for deaths due to pulmonary embolism [Pulmonary_Embolism (1)] relative to all other causes [Pulmonary_Embolism (2)] in Prader-Willi syndrome for Recent era [recent (1)] vs Early era [recent (0)] cohorts are shown. The numbers of uncensored Recent and Early era participants are listed at the bottom of each figure. Quartile estimates for age of death and mean age of mortality are indicated for Recent and Early era groups with pulmonary embolism.
Figure 3b. Kaplan-Meier plot of survival probability for deaths due to Gastrointestinal-related problems [Gastrointestinal (1)](top) and accidents [accident (1)](bottom) relative to all other causes [Gastrointestinal (2); accident (2)] in Prader-Willi syndrome for Recent era [recent (1)] vs Early era [recent (0)] are shown. The numbers of uncensored Recent and Early era participants are listed at the bottom of each figure. Quartile estimates for age of death and mean age of mortality are indicated for Recent and Early era groups for gastrointestinal-problems or accidents respectively.
DISCUSSION
This detailed investigation of survival trends and risk factors extends our previous characterization of mortality age, causes and related factors in PWS and identifies gender-specific influences and causal risk factors leading to early mortality observed in PWS.22 Respiratory failure has emerged as a leading contributor to mortality in PWS manifesting very early in life for both males (mean age = 25.3±2 yrs) and females (mean age = 23.9±2 yrs). Reported cases of respiratory failure were not correlated with obesity, typically reported as unexpected (82%) contributors to death by family members and often associated with prior complaints of minor illness, respiratory distress or associated emergency room visits. More research is needed to characterize the underlying pathology for these sudden deaths. As previously shown, males are at increased risk for early mortality due to behavioral or activity-oriented causes (e.g., accidents, choking) which may reflect gender differences in activity level, behavioral activation or manifestation of hyperphagia.22
Previous regression modeling in this sample identified quartile point estimates for all-cause mortality with 50% mortality at 29 years of age [95%CI (27–32yrs)] and 75% mortality at 42 years of age [95%CI (39–44yrs)].22 Examination of Recent vs Early era mortality trends paints a more optimistic future for PWS revealing clear, gender and disease-specific increases in long-term survival trends since the year 2000. Major contributors to early mortality in PWS - cardiac failure and the recently identified risk of thromboembolism were significantly delayed and/or reduced in recent years along with gastrointestinal complications.6,23–29 Increased awareness, early diagnosis, dietary intervention with restricted caloric intake and significant controls on access to food (locking cabinets, refrigerators) with established exercise programs for each person with PWS have led to successful weight loss and BMI reduction. Earlier diagnosis is expected to correlate with increased physical activity and strength as well as exposure to growth hormone which was approved for use in PWS in 2000. The use of growth and other hormone replacements have helped to normalize body composition and stature in PWS with a positive impact on the control of obesity.18–21 Our data provides empirical evidence that active management of obesity and hyperphagia in PWS does prolong life.
Mortality risk in the Recent era timeframe appears to reflect a shift away from obesity-related causes to other behavioral and/or biological factors not previously recognized or as closely monitored such as blood clotting. Furthermore, morbid obesity, more frequently observed in the early era cohort, often led to physical immobility which precipitated cardiopulmonary failure. Recent era changes in medical management including general medical care for obesity and cardiovascular disease, reduced rates of morbid obesity including tight caloric restrictions in combination with an improved ability to be active appears to have increased the likelihood of fatal accidents in males with PWS. Decreased weight coupled with the effects of growth hormone, increased muscle mass, activity level, and social engagement provides more avenues for behavioral, hyperphagia-related problems and other accidents. Males with PWS appear to be more likely to engage in risky behaviors. It is not clear whether the tight caloric restrictions employed in the management of PWS has impacted food drive or seeking behaviors to impact risk.
The results of our study support the need for increased awareness, close monitoring and supervision during meals, outings and near roadways to reduce the risk of traffic collision from food seeking (e.g., darting into the road to reach a restaurant). Strict oversight of food access and quantity are critical to prevent excessive unsupervised food consumption, choking and gastric rupture which can occur from eating quickly. Chewing and swallowing may be compromised in PWS by decreased salivary secretions, dental caries, a poor muscle tone and reduced sensation with lack of vomiting.1–5 Implementation of preventive measures, specialized training and better awareness of families and group home care providers of these risks are recommended for all individuals with PWS including training for use of the Heimlich maneuver, supervised meals, food security along with food preparation and diet modification. The necessity for lifelong weight control and vigilant food restrictions to prevent excessive eating, overweight and life-threatening obesity in PWS is now well-understood among most families and medical experts. Further, unrestricted access to food provides no relief from the insatiable food cravings experienced in PWS and only serves to exacerbate health risks and physical disability ultimately leading to the loss of the fundamental freedom afforded by good health, mobility and longevity that is associated with leanness. The present study findings support increases in longevity in PWS with appropriate management and care.
CONCLUSIONS
In summary, the present survival analysis extension study represents the largest and most extensive examination of survival trends in PWS to date and the results support current understanding of disease pathology and mortality in PWS. Current survival estimates for PWS are significantly increased from those prior to the year 2000 particularly for cardiac deaths among females as well as thrombotic and GI-related mortality. Males may be more likely to engage in aggressive or risky food seeking behaviors than females leading to greater risk of accidental deaths such as traffic collision, choking or GI-perforation. Our analyses and interpretation of data trends are limited by the reliability of death reporting based upon the availability and knowledge of family members and access to confirmed genetic status and autopsy reports. Infants and children with PWS from the Early era cohort may have escaped diagnosis prior to death and older individuals may have been underreported or misdiagnosed. Never-the-less, the results provide useful insight into risk factors, mortality trajectory over time and areas of need. Family members, care providers and health care professionals involved with the immediate and long term care of individuals with PWS should be made aware of these risk factors and causation of death to improve the longevity and quality of life for those with PWS (at all ages) and family members.
Acknowledgments
We acknowledge the support of the Prader-Willi Syndrome Association (USA) and families as well as the National Institute of Child Health and Human Development (NICHD) grant number HD02528. Partial funding was also received from an unrestricted grant from Zafgen, Inc.
Footnotes
The authors have no conflicts of interest relevant to this article to disclose.
CONFLICTS OF INTEREST
The authors have no conflicts to declare.
References
- 1.Butler MG. Prader-Willi syndrome. current understanding of cause and diagnosis. Am J Med Genet. 1990;35(3):319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler MG, Lee PDK, Whitman BY, editors. Management of Prader-Willi Syndrome. 3. New York: Springer; 2006. [Google Scholar]
- 3.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 4.Hoybye C, editor. Laboratory and Clinical Research. New York: Nova Science Publishers, Inc; 2013. Prader-Will Syndrome. Congenital Disorders. [Google Scholar]
- 5.Butler JV, Whittington JE, Holland AJ, Boer H, Clarke D, Webb T. Prevalence of, and risk factors for, physical ill-health in people with Prader-Willi syndrome. a population-based study. Dev Med Child Neurol. 2002;44(4):248–255. doi: 10.1017/s001216220100202x. [DOI] [PubMed] [Google Scholar]
- 6.Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet. 2001;38(11):792–798. doi: 10.1136/jmg.38.11.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome. a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38(12):1249–1263. doi: 10.1007/s40618-015-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swaab DF. Prader-Willi syndrome and the hypothalamus. Acta Paediatr Suppl. 1997;423:50–4. doi: 10.1111/j.1651-2227.1997.tb18369.x. [DOI] [PubMed] [Google Scholar]
- 9.DiMario FJ, Jr, Burleson JA. Cutaneous blood flow and thermoregulation in Prader-Willi syndrome patients. Pediatr Neurol. 2002;26(2):130–133. doi: 10.1016/s0887-8994(01)00386-1. [DOI] [PubMed] [Google Scholar]
- 10.Hertz G, Cataletto M, Feinsilver SH, Angulo M. Sleep and breathing patterns in patients with Prader Willi syndrome (PWS): effects of age and gender. Sleep. 1993;16(4):366–371. doi: 10.1093/sleep/16.4.366. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson DA, Heinemann J, Angulo M, et al. Deaths due to choking in Prader-Willi syndrome. Am J Med Genet A. 2007;A.143A(5):484–487. doi: 10.1002/ajmg.a.31502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson DA, Heinemann J, Angulo M, et al. Gastric rupture and necrosis in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2007;45(2):272–274. doi: 10.1097/MPG.0b013e31805b82b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartley SL, Maclean WE, Jr, Butler MG, Zarcone J, Thompson T. Maladaptive behaviors and risk factors among the genetic subtypes of Prader-Willi syndrome. Am J Med Genet A. 2005;136(2):140–145. doi: 10.1002/ajmg.a.30771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill JO, Kaler M, Spetalnick B, Reed G, Butler MG. Resting metabolic rate in Prader-Willi syndrome. Dysmorphol Clin Genet. 1990;4(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- 15.Bittel DC, Butler MG. Prader-Willi syndrome. clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med. 2005;7:1–20. doi: 10.1017/S1462399405009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler MG. Prader-Willi syndrome. obesity due to genomic imprinting. Curr Genomics. 2011;12(3):204–15. doi: 10.2174/138920211795677877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Miller JL, Kuipers PJ, et al. Unique and atypical deletions in Prader-Willi syndrome reveal distinct phenotypes. Eur J Hum Genet. 2011;20(3):283–290. doi: 10.1038/ejhg.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwenk WF., 2nd Growth hormone therapy-established uses in short children. Acta Paediatr Suppl. 2006;95(452):6–8. doi: 10.1111/j.1651-2227.2006.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 19.Butler MG, Manzardo AM, Forster JL. Prader-Willi syndrome. Clinical genetics and diagnostic aspects with treatment approaches. Curr Pediatr Rev. 2016;12(2):136–66. doi: 10.2174/1573396312666151123115250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler MG. Single gene and syndromic causes of obesity. Illustrative examples. Prog Mol Biol Transl Sci. 2016;140:1–45. doi: 10.1016/bs.pmbts.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler MG, Lee J, Cox DM, et al. Growth charts for Prader-Willi syndrome during growth hormone treatment. Clin Pediatr. 2016;55(10):957–974. doi: 10.1177/0009922815617973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of Death in Prader-Willi Syndrome. Prader-Willi Syndrome Association (USA) 40-Year Mortality Survey. Genet Med. Nov 17; doi: 10.1038/gim.2016.178. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, Fryns JP. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A. 2004;124A(4):333–338. doi: 10.1002/ajmg.a.20371. [DOI] [PubMed] [Google Scholar]
- 24.Van Vliet G, Deal CL, Crock PA, Robitaille Y, Oligny LL. Sudden death in growth hormone-treated children with Prader-Willi syndrome. J Pediatr. 2004;144(1):129–131. doi: 10.1016/j.jpeds.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Eiholzer U. Deaths in children with Prader-Willi syndrome. Horm Res. 2005;63(1):33–39. doi: 10.1159/000082745. [DOI] [PubMed] [Google Scholar]
- 26.Nagai T, Obata K, Tonoki H, et al. Cause of sudden, unexpected death of Prader-Willi syndrome patients with or without growth hormone treatment. Am J Med Genet A. 2005;136A(1):45–48. doi: 10.1002/ajmg.a.30777. [DOI] [PubMed] [Google Scholar]
- 27.Tauber M, Diene G, Molinas C, Hébert M. Review of 64 cases of death in children with Prader-Willi syndrome (PWS) Am J Med Genet A. 2008;146A(7):881–887. doi: 10.1002/ajmg.a.32131. [DOI] [PubMed] [Google Scholar]
- 28.Lionti T, Reid SM, Rowell MM. Prader-Willi syndrome in Victoria. mortality and causes of death. J Paediatr Child Health. 2012;48(6):506–511. doi: 10.1111/j.1440-1754.2011.02225.x. [DOI] [PubMed] [Google Scholar]
- 29.Whittington JE, Holland AJ, Webb T. Ageing in people with Prader-Willi syndrome. mortality in the UK population cohort and morbidity in an older sample of adults. Psychol Med. 2015;45(3):615–21. doi: 10.1017/S0033291714001755. [DOI] [PubMed] [Google Scholar]