Abstract
Background
Clinicians may be reluctant to transplant small pediatric kidneys that have prolonged cold ischemia time (CIT) for fear of an additional deleterious effect because pediatric grafts are thought to be more sensitive to ischemia. We aimed to assess the risks associated with transplantation of small pediatric kidneys with prolonged CIT.
Methods
We performed a retrospective cohort study examining US registry data between 1998 and 2013 of adult first-time kidney-only recipients of small pediatric kidneys from donors weighing 10 to 20 kg, stratified by CIT levels of 0 to 18 (n = 1413), 19 to 30 (n = 1116), and longer than 30 (n = 338) hours.
Results
All-cause graft survival by CIT groups at 1-year was 92%, 88%, and 89%, respectively. 1-year risk-adjusted graft survival hazard ratios were significantly higher with CIT of 19 to 30 hours (adjusted hazard ratios, 1.37; 95% confidence interval, 1.04-1.81) and somewhat higher with CIT greater than 30 hours (adjusted hazard ratios, 1.24; 95% confidence interval, 0.82-1.88) relative to recipients with CIT 0 to 18 hours. There was little variation in the effect of CIT on graft survival when restricted to single kidney transplants only and no significant interaction of CIT category and single kidney transplantation (P = 0.93).
Conclusions
Although prolonged CIT is associated with lower early graft survival in small pediatric donor kidney transplants, absolute decreases in 1-year graft survival rates were 3% to 4%.
As the imbalance between kidney need and supply continues to widen, optimal utilization of the current donor pool is critically important. This has led clinicians to consider small pediatric donors (defined as < 21 kg); however, utilization has historically been in select centers and current estimates indicate that 42% of active transplant centers do not perform any transplants from these types of donors.1 The concentration of these transplants in select centers may be an optimal strategy because large volume centers have been found to provide a mitigating effect on the risk of graft loss1; however, because roughly 45% of transplanted small pediatric donor kidneys are imported1 from outside of the local donor service area, cold ischemia time (CIT) is prolonged due to the time required to find an accepting center and the time to transport the organ. Additionally, increases in CIT due to attempts at placement may contribute to the ultimate discard of the kidney. In fact, 33% of recovered kidneys from donors less than 21 kg are discarded in the United States.1 The maximum limitation of CIT for pediatric grafts is unknown. Pediatric grafts are thought to be more sensitive to ischemia2-4 than adult grafts. Already high parenchymal resistance augmented by prolonged CIT may diminish blood flow.5 It has been recommended that they should be transplanted as soon as possible2 or within 24 hours after recovery.3
Another strategy to optimize the current donor pool is by transplanting small pediatric kidneys as singles rather than en bloc. Although splitting small pediatric kidneys is not recommended when the donor weight is less than 10 kg due to excessive graft loss,1,6 splitting of kidneys from donors 10 to 20 kg is preferred because the overall outcomes of single kidney transplant (SKT) from this weight group is similar to that of unideal standard criteria donor kidney transplants6 and the net gain of additional functioning grafts is estimated to outweigh the “cost” of performing single kidney transplantation, as reflected by the increased numbers of grafts that would be lost.1 Recent data suggest that exclusive SKT for all kidneys from greater than 10-kg donors would result in 300 additional recipients with functioning grafts 1 year after transplantation.1 Although recent data indicate that small pediatric donor kidneys suffer a 2% increased hazard of 1-year graft loss per hour of cold time, it is unknown whether the magnitude of the risk increases at specific thresholds of CIT. Also because kidneys from donors 10 to 20 kg have the greatest potential to increase the number of transplants, it is important to determine whether the effect of CIT is greater when small pediatric kidneys are split.
To analyze the risks associated with transplantation of small pediatric kidneys with prolonged CIT, we examined national registry data for outcomes of adult transplant recipients of small pediatric kidneys from donors weighing 10 to 20 kg. We additionally examined the associations in the subset of recipients who received single kidney recipients.
MATERIALS AND METHODS
Sources of Data
We used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the Organ Procurement and Transplantation Network and SRTR contractors. This study was approved by the Institutional Review Board of the Albert Einstein College of Medicine. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Study Population
SRTR data of all adult, first-time, deceased-donor kidney-only recipients between January 1998 and October 2014 from donors weighing 10 to 21 kg were examined. Recipients were stratified by CIT groups of 0 to 18, 19 to 30, and more than 30 hours. Exclusions were previous organ transplant, hepatitis C or human immunodeficiency virus positivity, and missing information on CIT, and donor weight.
Outcomes
The primary outcome was time to (a) all-cause graft failure (defined as return to chronic dialysis, allograft nephrectomy, retransplantation, or death). Secondary outcomes were (a) delayed graft function (DGF), defined as dialysis within 7 days posttransplantation, (b) patient survival, and (c) 1-year acute rejection (defined as presence of rejection regardless of treatment as coded in follow up forms at 3, 6, or 12 months). The odds of DGF was assessed in cases with at least 7 days graft survival based on their status as being at risk for this event and absence of primary nonfunction.
Covariates
Recipient covariates included in the multivariable models were donor and recipient: age (cutoffs at 20th and 80th percentile), sex, race (black, other), and recipient diabetes mellitus (as primary diagnosis of end-stage renal disease or presence of diabetes), duration of pretransplant maintenance dialysis (none, <3, ≥3 years, missing), number of HLA-A, -B, and -DR mismatches (human leukocyte antigen mismatch; ≤3, >3), panel-reactive antibody (PRA) level (>30%, ≤30%, missing), body mass index (BMI) (kg/m2, continuous), transplant year, and transplant procedure type (single vs en bloc). BMI was calculated as weight (kg)/height (m)2. In cases where recipient height was not available, candidate height was used instead. BMI outliers (<10 and >70) were coded as missing. The appropriate functional form of model covariates was determined by exploratory data analysis in unadjusted models and perceived impact on clinical meaningfulness. Backwards selection was used with a P value of 0.10 or less as the retention criterion to select model covariates that significantly predict outcome. Additional analyses were conducted to evaluate the robustness of the primary results including repeat analysis with missing data excluded, assessment for changes in outcomes over time, and examination of death-censored graft survival as an end-point. The odds 1-year acute rejection was assessed between patients who had at least 1 year of graft survival.
Statistical Analysis
Discrete variables were expressed as percentages and continuous variables, whose distributions approximated normality, were expressed as means and standard deviations. Survival distributions were depicted with Kaplan-Meier curves and compared using the log-rank test. Cox proportional hazards models were fit to adjusted hazard ratios (aHR) and 95% confidence intervals (95% CI) for exposure groups for time to outcome data and logistic regression k models were fit to adjusted odds ratios (aOR) and 95% CIs after accounting for potential confounders with a retention (P < 0.10). All multivariate models were additionally fit with an interaction term of transplant type (single vs en bloc) with CIT category. CIT was also evaluated as a continuous variable (instead of CIT category) in separate models. Exposure groups and covariates were examined for adherence to the proportional hazard assumption using log-log plots for categorical data and martingale plots for continuous data. No important departures from proportionality were observed. Ties in the failure time were handled using the EXACT method. Time to outcome were defined as time from the transplant date until date of outcome (death or graft failure), censored for loss to follow-up and end of study period (October 31, 2013).
All data were analyzed using SAS software, version 9.4. Two-sided P values of less than 0.05 were considered statistically significant, and P value approximating 0.1 was considered a trend.
RESULTS
The study populations for the CIT groups 0 to 18, 19 to 30, and greater than 30 hours consisted of 1413, 1116, and 338 kidney transplant recipients, respectively. Although differences were small, the longer CIT recipients were more likely to be male and receive single kidneys, kidneys from donation after circulatory determination of death, female donors, and better HLA matched donors (Table 1).
TABLE 1.
Recipient and donor characteristics by CIT group

All cause graft survival by CIT 0 to 18, 19 to 30, and greater than 30-hour groups at 1-year was 92%, 88%, and 89%, respectively. On univariate analysis, overall graft survival between patients receiving kidneys with CIT 0 to 18, 19 to 30, and greater than 30 hours was significantly different with lower CIT associated with better graft survival (Figure 1). One-year risk-adjusted graft survival hazard ratios were significantly higher with CIT 19 to 30 hours (aHR, 1.37; 95% CI, 1.04-1.81) and somewhat higher with CIT greater than 30 hours (aHR, 1.24; 95% CI, 0.82-1.88) relative to recipients with CIT 0 to 18 hours.
FIGURE 1.

All cause graft survival of small pediatric kidneys from donors 10 to 20 kg single and en bloc transplants by CIT group.
Overall graft survival until the follow-up of the study was comparable between recipients of pediatric kidneys with CIT 19 to 30 hours (aHR, 1.12; 95% CI, 0.96-1.31) and CIT greater than 30 hours (aHR, 1.14; 95% CI, 0.91-1.43) relative to recipients with CIT 0 to 18 hours (Table 2). A separate model with CIT as a continuous variable demonstrated a modest increase in hazard ratio per hour of CIT both for 1-year graft survival (aHR, 1.02; 95% CI, 1.00-1.03) and overall graft survival (aHR, 1.01; 95% CI, 1.00-1.02).
TABLE 2.
Multivariate model for all-cause graft survival of single and en bloc pediatric kidney transplants

Among 1420 cases, a single kidney only was transplanted and all cause graft survival by CIT groups at 1 year was 91%, 87%, and 87%, respectively. There was little variation in the effect of CIT on graft survival when restricted to SKTs only and no significant interaction of CIT category and single kidney transplantation (P = 0.93, Figure 2).
FIGURE 2.
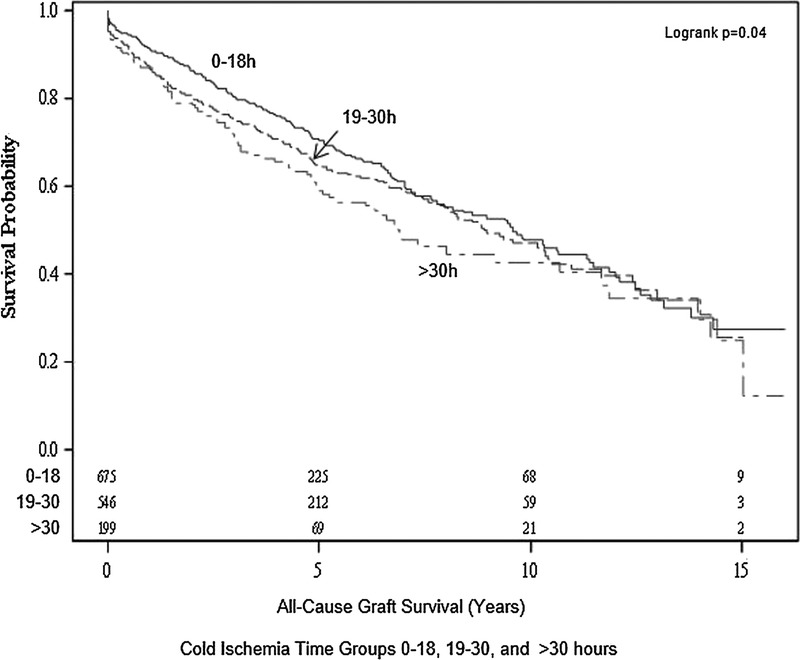
All cause graft survival of small pediatric kidneys from donors 10 to 20 kg single transplants.
Unadjusted patient survival between recipients with higher CIT relative to recipients with lower CIT were not significantly different between the 3 groups of CIT (Figure 3). On multivariate analysis, the absence of significant difference remained for CIT 19 to 30 hours (aHR, 1.03; 95% CI, 0.85-1.25) and CIT greater than 30 hours (aHR, 1.23; 95% CI, 0.94-1.62) relative to CIT 0 to 18 hours.
FIGURE 3.

Patient survival of small pediatric kidneys from donors 10 to 20 kg single and en bloc transplants.
Among 2658 transplants, the cumulative incidence of DGF by CIT group is 9.7%, 13.6%, and 19.6% (P < 0.01), respectively. On multivariate analysis, there was a significant association between DGF and CIT 19 to 30 hours (aOR, 1.4; 95% CI, 1.07-1.85) and CIT greater than 30 hours (aOR, 1.98; 95% CI, 1.37-2.86) relative to CIT 0 to 18 hours (Table 3). Additionally, single kidney transplantation was independently associated with a twofold odds of DGF (aOR, 2.30; 95% CI, 1.74-3.05).
TABLE 3.
Multivariate model for DGF of single and en bloc pediatric kidney transplants

Among 2337 transplants, the cumulative incidence of acute rejection at 1 year by CIT group is 8%, 9%, and 6%, respectively. On multivariate analysis, there was no significant association of acute rejection and CIT 19 to 30 hours (aOR, 1.08; 95% CI, 0.76-1.51) or CIT greater than 30 hours (aOR, 0.53; 95% CI, 0.25-1.01) relative to CIT 0 to 18 hours.
There was little impact on the magnitude of the hazard ratio or the significance of the findings of the primary outcome after exclusion of cases with missing data, and there was little temporal variation in the primary outcome during the study period. Inferences did not change with the end-point of death-censored graft survival (1 year: 19-30 CIT; aHR, 1.42; 95% CI, 1.03-1.97; > 30 CIT; aHR, 1.21; 95% CI, 0.74-1.96; overall: 19–30 CIT; aHR, 1.21; 95% CI, 0.99-1.48, >30 CIT; aHR, 1.18; 95% CI, 0.88-1.59).
DISCUSSION
Uncertainties exist regarding the extent and reversibility of renal injury that occurs when small pediatric kidneys are subjected to prolonged CITs. Using SRTR data of kidney transplants between 1998 and 2013, we found significantly lower early graft survival associated with CIT greater than 18 hours. The absolute decrease in the 1-year graft survival rate was 3 to 4 percentage points. Also, there was no interaction effect with CIT and number of kidneys transplanted (single vs en bloc). Our results suggest that despite the vulnerability of small pediatric kidneys to ischemia, the magnitude of the decrement in graft survival associated with prolonged CIT is modest and may offer acceptable outcomes to some recipients. Additionally, in those cases where splitting the kidneys is deemed preferable, there does not appear to be an additive effect of CIT on outcomes of single kidneys.
We also found no significant association of CIT with longer-term outcomes; however, these results are limited by small sample sizes at follow-up and therefore were not robustly analyzable. Nevertheless, our finding of a lack of an adverse effect of CIT on the long-term graft survival of small pediatric kidneys with a prior ischemic event is in keeping with other investigations on the impact of CIT on graft outcomes which also suggest an absence of an effect of CIT on graft survival, at least to the extent that CIT thresholds are practiced.7-9 Additionally, animal models have shown that kidneys from young donors have a greater stress tolerance and ability to withstand injury engendered by transplantation because of more vigorous proliferative and repair mechanisms.10
We focused on the outcomes of SKTs from donors 10 to 20 kg because the risk-benefit consideration favors single kidney transplantation within this weight range.1,6 Conflicting results have been reported from centers examining outcomes of small pediatric donor kidneys with prolonged CIT.1,2,5,11-16 Bretan and colleagues11 found similar rates of immediate graft function but significantly lower overall graft survival among adults receiving pediatric donor (2-14 kg) kidneys with greater than 35 hours CIT (n = 12) relative to less than 35 hours CIT (n = 10). Yagisawa and others2 found no differences in 44 kidney transplant recipients of single (n = 35) or en bloc (n = 9) kidneys from donors < 11 years of age with 5 months to 8 years of follow-up between 3 groups of CIT of 12, 12 to 24, and 24 to 36 hours. Satterthwaite et al12 found a 2.6-fold higher relative risk of delayed function with CIT > 36 hours among 91 kidney transplant recipients of donors < 4 years of age transplanted as single (n = 59) or en bloc (n = 22) and no differences in graft survival between groups of more and less than 36 hours of CIT. Other reports also suggest faster function and improved graft survival in transplants with low CIT.13-16 Recently, Maluf and others1 examined SRTR registry kidney transplant outcomes from small (≤20 kg) pediatric donors and found a 2.4% (aHR, 1.024; P = 0.003) increase hazard of 1-year graft failure per hour of increasing cold time. These findings are like ours (aHR, 1.02); however, we additionally examined the clinical magnitude of the finding by examining CIT thresholds and investigating potential interaction effects with splitting kidneys.
Our analysis demonstrated incremental increases in DGF with increasing duration of CI; however, the magnitude of the effect of CIT on graft outcomes was low, suggesting that ischemic injury is likely to be a reversible lesion.17 DGF was 2 times more likely when kidneys were transplanted as singles in our analysis highlighting an increased difficulty of management. Other consequences of DGF noted in previous studies are substantial, including prolonged hospitalization, higher cost of transplantation, increased complexity of management of immunosuppressive drugs, and an adverse effect on the rehabilitation of potential transplant recipients.18-21
Acute rejection was not associated with prolonged CIT among recipients of small pediatric kidneys in our study. This finding is important because rejection may compromise the fine vasculature of the kidneys and ureters.22 Another vulnerability may be the inability to tolerate acute rejection during the initial growth phase.22 Whereas our results compare to a recent registry analysis that did not find an association of CIT with acute rejection,23 others have found positive associations including a 20% increase in adjusted rejection risk with CIT greater than 36 hours,24 a 4% increased risk of acute rejection for every hour of CIT,25 and higher unadjusted acute rejection rates for the second of transplanted mate kidneys (28.1% vs 22.3%, respectively, P < 0.01),26 supporting the hypothesis that prolonged cold storage results in increased allograft immunogenicity. However, our results might suggest, considering the conflicting results in the literature, that our understanding of the relationship between ischemic injury and acute rejection is unclear.
Our results are subject to the limitations inherent in observational data. Because recipients are often not randomly selected to receive kidneys with prolonged CIT, it is possible that they were in some unmeasured way systemically less healthy such that a decrease in risk could have prevented an increase in graft failure or death despite an increase in CIT. There is the possibility for residual confounding because of recipient- or center-related factors not captured in registry data. Our analyses included many but not all the factors that may confer risks at or after transplantation such as implantation technique, anastomosis time, machine perfusion, immunosuppression type and dosing, recipient anatomic abnormalities, and anticoagulation. Other important outcomes, such as proteinuria and hyperfiltration injury, could not be assessed. This study included adult recipients undergoing their first kidney transplant, and therefore, the results cannot be generalized to all kidney recipients. Potential issues relating to the determination of acute rejection include missing or incomplete data, reporting bias, sampling and technique errors, measures of quantification, and subjective interpretation.
There has been extensive focus in the field of transplantation on recovery and placement of all possible donor organs. This study suggests that prolonged CIT greater than 18 hours negatively impacts short-term graft survival of kidneys from small pediatric donors; however, differences in absolute graft survival rates are small. Additionally, there is no differential effect of CIT whether transplanted as singles or en bloc; this finding may suggest that the approach of increasing transplant opportunities by splitting pediatric kidneys remains warranted even in the setting of prolonged CIT.
Footnotes
Published online 27 June, 2017.
Dr. Kayler received support from Astellas, Inc. to conduct this study.
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the Scientific Registry of Transplant Recipients or the US Government. No other authors received support for this study and there are no other conflicts of interest.
L.K. participated in research design, the writing of the paper, the performance of the research and data analysis. X.Y. participated in writing of the paper and performance of the research. M.L. participated in writing of the paper and performance of the research. P.F. participated in writing of the paper and data analysis.
REFERENCES
- 1.Maluf DG, Carrico RJ, Rosendale JD, et al. Optimizing recovery, utilization and transplantation outcomes for kidneys from small, ≤20 kg, pediatric donors. Am J Transplant. 2013;13:2703–2712. [DOI] [PubMed] [Google Scholar]
- 2.Yagisawa T, Kam I, Chan L, et al. Limitations of pediatric donor kidneys for transplantation. Clin Transplant. 1998;12:557–562. [PubMed] [Google Scholar]
- 3.Harmon WE, Stablein D, Alexander SR, et al. Graft thrombosis in pediatric renal transplant recipients. A report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation. 1991;51:406–412. [DOI] [PubMed] [Google Scholar]
- 4.Schneider JR, Sutherland DE, Simmons RL, et al. Long-term success with double pediatric cadaver donor renal transplants. Ann Surg. 1983;197:439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrows L, Knight R, Polokoff E, et al. Expanding the donor pool with the use of en bloc pediatric kidneys in adult recipients. Transplant Proc. 1996;28:173–174. [PubMed] [Google Scholar]
- 6.Mohanka R, Basu A, Shapiro R, et al. Single versus en bloc kidney transplantation from pediatric donors less than or equal to 15 kg. Transplantation. 2008;86:264–268. [DOI] [PubMed] [Google Scholar]
- 7.Lim WH, McDonald SP, Russ GR. Effect on graft and patient survival between shipped and locally transplanted well-matched cadaveric renal allografts in Australia over a 10-year period. Nephrology (Carlton). 2006;11:73–77. [DOI] [PubMed] [Google Scholar]
- 8.Stegall MD, Dean PG, McBride MA, et al. Survival of mandatorily shared cadaveric kidneys and their paybacks in the zero mismatch era. Transplantation. 2002;74:670–675. [DOI] [PubMed] [Google Scholar]
- 9.Watson CJ, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10:1991–1999. [DOI] [PubMed] [Google Scholar]
- 10.Melk A, Schmidt BM, Braun H, et al. Effects of donor age and cell senescence on kidney allograft survival. Am J Transplant. 2009;9:114–123. [DOI] [PubMed] [Google Scholar]
- 11.Bretan PN, Jr, Friese C, Goldstein RB, et al. Immunologic and patient selection strategies for successful utilization of less than 15 kg pediatric donor kidneys—long term experiences with 40 transplants. Transplantation. 1997;63:233–237. [DOI] [PubMed] [Google Scholar]
- 12.Satterthwaite R, Aswad S, Sunga V, et al. Outcome of en bloc and single kidney transplantation from very young cadaveric donors. Transplantation. 1997;63:1405–1410. [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, Cohen RA, Milford EL. In renal transplantation, one size may not fit all. J Am Soc Nephrol. 1992;3:162–169. [DOI] [PubMed] [Google Scholar]
- 14.Alexander JW, Bennett LE, Breen TJ. Effect of donor age on outcome of kidney transplantation. A two-year analysis of transplants reported to the United Network for Organ Sharing Registry. Transplantation. 1994;57:871–876. [PubMed] [Google Scholar]
- 15.Alexander JW, Vaughn WK, Carey MA. The use of marginal donors for organ transplantation: the older and younger donors. Transplant Proc. 1991;23:905–909. [PubMed] [Google Scholar]
- 16.Trevino G, dickerman RM, Coggins JT, et al. The optimal use of pediatric donors for renal transplantation. Transplant Proc. 1988;20:359–362. [PubMed] [Google Scholar]
- 17.Sudhindran S, Pettigrew GJ, Drain A, et al. Outcome of transplantation using kidneys from controlled (Maastricht category 3) non–heart-beating donors. Clin Transplant. 2003;17:93–100. [DOI] [PubMed] [Google Scholar]
- 18.Matas AJ, Gillingham KJ, Elick BA, et al. Risk factors for prolonged hospitalization after kidney transplants. Clin Transplant. 1997;11:259–264. [PubMed] [Google Scholar]
- 19.Freedland SJ, Shoskes DA. Economic impact of delayed graft function and suboptimal kidneys. Transplant Rev. 1999;13:23–30. [Google Scholar]
- 20.Rosenthal JT, Danovitch GM, Wilkinson A, et al. The high cost of delayed graft function in cadaveric renal transplantation. Transplantation. 1991;51:1115–1118. [PubMed] [Google Scholar]
- 21.Barama A, Kiberd BA, Belitsky P, et al. Financial impact of cold ischemia time in renal transplantation. Transplant Proc. 1997;29:1563–1564. [DOI] [PubMed] [Google Scholar]
- 22.Bretan PN, Koyle M, Singh K, et al. Improved survival of en bloc renal allografts from pediatric donors. J Urol. 1997;157:1592–1595. [PubMed] [Google Scholar]
- 23.Kayler LK, Magliocca J, Zendejas I, et al. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11:2647–2656. [DOI] [PubMed] [Google Scholar]
- 24.Halloran PF, Homik J, Goes N, et al. The “injury response”: a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc. 1997;29:79–81. [DOI] [PubMed] [Google Scholar]
- 25.Mikhalski D, Wissing KM, Ghisdal L, et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation. 2008;85:S3–S9. [DOI] [PubMed] [Google Scholar]
- 26.Giblin L, O’Kelly P, Little D, et al. A comparison of long-term graft survival rates between the first and second donor kidney transplanted—the effect of a longer cold ischaemic time for the second kidney. Am J Transplant. 2005;5:1071–1075. [DOI] [PubMed] [Google Scholar]


