Abstract
Importance
Hand-foot syndrome (HFS) is a common adverse effect of capecitabine treatment.
Objective
To compare the incidence and time to onset of grade 2 or greater HFS in patients receiving pyridoxine vs placebo and to identify biomarkers predictive of HFS.
Design, Setting, and Participants
This single-center, randomized double-blind, placebo-controlled phase 3 trial conducted at National Cancer Centre Singapore assessed whether oral pyridoxine could prevent the onset of grade 2 or higher HFS in 210 patients scheduled to receive single-agent capecitabine chemotherapy for breast, colorectal, and other cancers.
Interventions
Patients were randomized to receive concurrent pyridoxine (200 mg) or placebo daily for a maximum of 8 cycles of capecitabine, with stratification by sex and use in adjuvant or neoadjuvant vs palliative setting. Patients were withdrawn from the study on development of grade 2 or higher HFS or cessation of capecitabine.
Main Outcomes and Measures
Primary end point was the incidence of grade 2 or higher HFS in patients receiving pyridoxine. Secondary end points included the time to onset (days) of grade 2 or higher HFS and identification of biomarkers predictive of HFS, including baseline folate and vitamin B12 levels, as well as genetic polymorphisms with genome-wide arrays.
Results
In this cohort of 210 patients (median [range] age, 58 [26-82] years; 162 women) grade 2 or higher HFS occurred in 33 patients (31.4%) in the pyridoxine arm vs 39 patients (37.1%) in the placebo arm (P = .38). The median time to onset of grade 2 or higher HFS was not reached in both arms. In univariate analysis, the starting dose of capecitabine (odds ratio [OR], 1.99; 95% CI, 1.32-3.00; P = .001), serum folate levels (OR, 1.27; 95% CI, 1.10-1.47; P = .001), and red blood cell folate levels (OR, 1.25; 95% CI, 1.08-1.44; P = .003) were associated with increased risk of grade 2 or higher HFS. In multivariate analyses, serum folate (OR, 1.30; 95% CI, 1.12-1.52; P < .001) and red blood cell folate (OR, 1.28; 95% CI, 1.10-1.49; P = .001) were the only significant predictors of grade 2 or higher HFS. Grade 2 or higher HFS was associated with 300 DNA variants at genome-wide significance (P < 5 × 10−8), including a novel DPYD variant (rs75267292; P = 1.57 × 10−10), and variants in the MACF1 (rs183324967, P = 4.80 × 10−11; rs148221738, P = 5.73 × 10−10) and SPRY2 (rs117876855, P < 1.01 × 10−8; rs139544515, P = 1.30 × 10−8) genes involved in wound healing.
Conclusions and Relevance
Pyridoxine did not significantly prevent or delay the onset of grade 2 or higher HFS. Serum and red blood cell folate levels are independent predictors of HFS.
Trial Registration
clinicaltrials.gov Identifier: NCT00486213
This randomized clinical trial compares the incidence and time to onset of grade 2 or higher hand-foot syndrome in patients receiving pyridoxine vs placebo and identifies biomarkers predictive of hand-foot syndrome.
Key Points
Questions
Can pyridoxine prevent capecitabine-induced hand-foot syndrome (HFS), and what are the predictors of HFS?
Findings
The incidence of grade 2 or higher HFS and median time to onset of grade 2 or higher HFS were not significantly different with prophylactic pyridoxine compared with placebo. Increase in serum folate and red blood cell folate levels, as well as genomic variants linked to wound healing and cytoskeletal anchoring, were associated with increased risk of HFS.
Meaning
Pyridoxine is not effective in preventing or delaying the onset of grade 2 or higher HFS; serum and red blood cell folate levels are independent determinants of capecitabine-induced HFS.
Introduction
Hand-foot syndrome (HFS), also known as palmoplantar erythrodysesthesia, is a common adverse effect of the fluoropyrimidine chemotherapy agent capecitabine. Hand-foot syndrome of any grade is reported to affect 43% to 71% of patients treated with single-agent capecitabine chemotherapy. Although not life-threatening, it can have adverse effects on the quality of life (QoL) and daily living activities of a patient. The dose interruptions and reductions required after observation of HFS can also impact on dose intensity and treatment outcomes.
Despite its common occurrence, the pathophysiology of HFS is not well understood. At the time of conception of this study, pyridoxine (vitamin B6) was commonly used by clinicians to prevent and/or treat HFS with capecitabine. This was based on similarity to acrodynia caused by pyridoxine deficiency in rats, anecdotal reports, and retrospective series on the effectiveness of pyridoxine in ameliorating HFS associated with fluorouracil and capecitabine. Although a trial on patients with gastrointestinal tract malignancies receiving capecitabine either as single agent or in combination with cisplatin with or without docetaxel has since reported the ineffectiveness of pyridoxine 200 mg daily in preventing HFS, pathophysiologic biomarkers predictive of HFS were not evaluated.
Accumulating data suggests that there are regional differences in the tolerability of fluoropyrimidines, with many reports indicating that East Asian patients experience a lower incidence of serious toxic effects when treated with fluorouracil-based or capecitabine-based regimens compared with white patients. Dietary folate intake and pharmacogenomics have been highlighted as possible contributors to these disparities.
This randomized, double-blind phase 3 trial was designed to evaluate the efficacy of pyridoxine at preventing HFS in patients receiving capecitabine single-agent chemotherapy as a primary objective, given the potential for combination treatment to confound the assessment of HFS. To elucidate the predictors of HFS further, biomarker analyses including folate and vitamin B12 levels and genome-wide analyses were performed to explore predictors of capecitabine-induced HFS. To our knowledge, this is the first genome-wide association study (GWAS) of capecitabine-induced HFS conducted in an Asian population.
Methods
Study Design
This single-center, randomized double-blind, placebo-controlled phase 3 trial conducted at National Cancer Centre Singapore assessed whether oral pyridoxine could prevent the onset of grade 2 or higher HFS in patients scheduled to receive single-agent capecitabine chemotherapy for any malignancy, either in the neoadjuvant, adjuvant, or palliative setting. Exclusion criteria included the intake of concomitant drugs known to induce HFS or neuropathy, drugs known to interact with pyridoxine, concurrent pyridoxine-containing preparations, use of urea-containing or lactic acid-containing topical creams or lotions, previous treatment with capecitabine, and/or known hypersensitivity to pyridoxine. Each patient provided written informed consent prior to study entry. The study protocol was approved by the Singapore Health Services (SingHealth) institutional review board (Supplement 1).
The primary objective was incidence of grade 2 or higher HFS; secondary objectives included time from study commencement to first occurrence of grade 2 or higher HFS, health-related QoL and analysis of biomarkers predictive of HFS. Based on literature review, the overall incidence of grade 2 or higher HFS for capecitabine monotherapy was estimated to be 36% to 40%. To demonstrate a reduction in incidence of grade 2 or higher HFS from 36% to 20% with α = .05 and power of 80%, a requirement of 123 patients per group, or 246 patients in total, was determined. Up to 20% of patients were anticipated to discontinue capecitabine before the fourth cycle of therapy and affect the evaluation of HFS, hence target accrual was set at 296 patients, corresponding to an increase in sample size of 25 patients (approximately 20%) per group. However, the trial was terminated before reaching the original target of 296 patients due to slow accrual associated with the declining use of single-agent capecitabine, especially for gastrointestinal cancers.
Treatment Administration
Patients were stratified by sex and treatment setting (adjuvant or neoadjuvant vs palliative), and randomized to receive either pyridoxine (200 mg/d) or placebo in a 1:1 ratio for a maximum of 8 cycles of capecitabine. The minimum starting dose for the first 59 patients was 800 mg/m2 or more twice daily for the first 2 weeks (rounded up or down to the closest dose comprising 150 mg and 500 mg tablets) on a 3-weekly cycle. The protocol was subsequently amended to stipulate a minimum starting dose of 1000 mg/m2 or more twice daily due to increasing evidence of better tolerability in Asian patients, in addition to the inclusion of baseline folate and vitamin B12 levels for biomarker analyses. Patients were withdrawn from the study on occurrence of grade 2 or higher HFS or capecitabine discontinuation, for example, owing to unacceptable toxic effects or evidence of progressive disease, whichever occurred earlier.
Assessment and Management of Toxic Effects
Intensity of adverse events was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 at the end of each cycle. Hand-foot syndrome was classified as grade 1 if there were minimal skin changes or dermatitis without pain; grade 2, if skin changes (eg, bleeding, blisters, peeling, edema) or pain not interfering with function was present; and grade 3, if there was ulcerative dermatitis or skin changes with pain interfering with function. While patients were given a diary for symptoms of HFS to help the physician with documenting the onset and severity of HFS, the toxic effects were graded by the treating physician for assessment of the primary end point, as well as other adverse events.
Patients who developed HFS of grade 2 or greater severity would be withdrawn from the study, and institution of daily pyridoxine with or without dose reduction of capecitabine could subsequently occur at the clinician’s discretion. Patients who developed grade 1 HFS could be treated with topical emollients if deemed necessary by the treating oncologist, but dose interruption or reduction for grade 1 HFS was not permitted. Interruption, delay, or modification of dose of capecitabine for other toxic effects was permitted based on the discretion of the treating clinician.
Assessment of QoL
Quality of life was assessed using the EuroQol EQ-5D-3L questionnaire (version 5.0) at baseline, the start of cycles 2, 4, 6, and 8, as well as at the end of the study or after treatment discontinuation.
Assessment of Biomarkers
Blood samples were collected at baseline from patients who consented for biomarker analyses. In addition to testing of red blood cell folate, serum folate, and vitamin B12, DNA was extracted using the EZ1 Advanced automated nucleic acid extractor (Qiagen). Genotyping of the DNA for more than 890 000 markers within East Asia populations was performed using HumanOmniZhongHua-8 BeadChips (Illumina). Phasing was performed using SHAPEIT, while imputation was performed with IMPUTE2 using the cosmopolitan 1000 Genomes Phase 1 integrated haplotypes panel (December 2013 release) as a reference. Variants with a call rate less than 0.95 and those that deviated from Hardy-Weinberg equilibrium (HWE) (P < 10−5) were excluded from analysis. Checks were also performed for population outliers against the HapMap3 database, and cohort outliers using smartpca in EIGENSOFT.
SNPTEST was used for logistic regression analysis based on an additive genetic model. To control for potential population stratification and other confounders, analyses were adjusted for race, age, sex, and capecitabine dose. Statistical significance was inferred at the genome-wide threshold of P < 5 × 10−8.
Statistical Analyses
Primary efficacy analysis was based on the intent-to-treat population, which included all randomly assigned patients. The incidence of grade 2 or higher HFS was compared using a χ2 test; time to onset was estimated using the Kaplan-Meier method and compared using a log-rank test, and QoL was analyzed using ANOVA for the EQ-5D OHS and χ2 tests for each of the 5 dimensions of the EQ descriptive system.
Logistic regression was used to estimate the odds of HFS (grade ≥2 vs <2) with respect to clinical characteristics. The exact procedure was used when quasiseparation was detected. All covariates were included in multivariate analysis with a stepwise selection process using significance levels of 0.1. Interaction between treatment and covariates suggested by the stepwise procedure was explored. Alternative models were assessed in terms of discrimination and calibration respectively using the c-index and the Akaike Information criterion (AIC). The Statistical Analysis System version 9.4 (SAS Institute Inc) was used for analysis. A P value of less than .05 was considered statistically significant.
Results
Patients
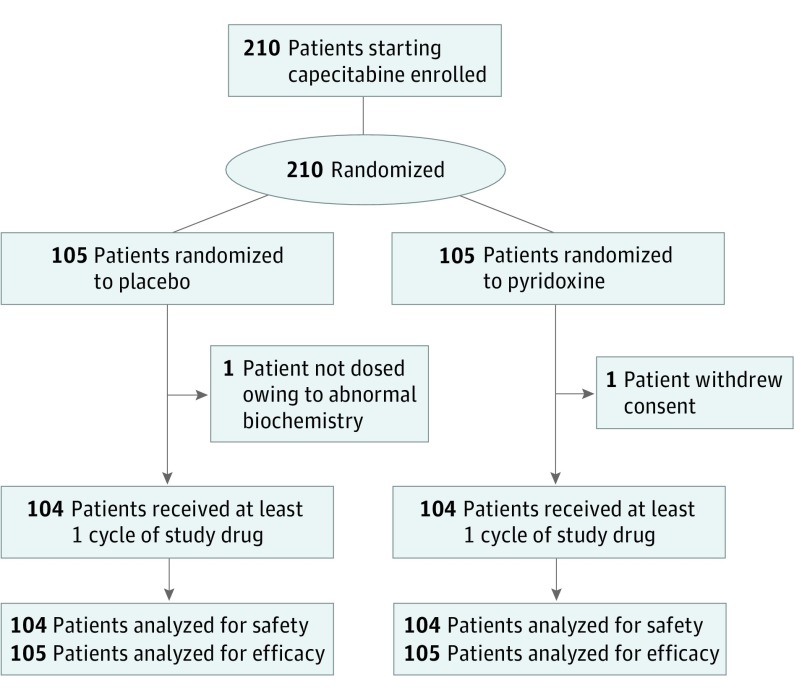
Between June 2007 and May 2014, 210 patients starting capecitabine were enrolled and randomized to pyridoxine or placebo (Figure). Baseline demographic and clinical characteristics were well balanced between the 2 arms (Table 1). Overall, 44 patients (21.0%) received treatment in the neoadjuvant or adjuvant setting, while 166 patients (79.0%) received treatment on a palliative basis. The majority of the patients had metastatic breast cancer (n = 138 [66%]); 144 patients (67.6%) received prior chemotherapy, with a median (range) of 2 (0-9) regimens.
Figure. CONSORT Diagram.
Between June 2007 and May 2014, 210 patients starting capecitabine were enrolled and randomized to pyridoxine or placebo. Baseline demographic and clinical characteristics were well balanced between the 2 arms.
Table 1. Baseline Characteristics of the Study Cohort According to Treatment Arms.
| Characteristic | All | Placebo | Pyridoxine | P Value |
|---|---|---|---|---|
| Total patients, No. | 210 | 105 | 105 | >.99 |
| Age at entry, median (range), y | 58 (26-82) | 57 (26-79) | 58 (31-82) | .51 |
| Sex, No. (%) | >.99 | |||
| Female | 162 (77) | 81 (77) | 81 (77) | |
| Male | 48 (23) | 24 (23) | 24 (23) | |
| Race, No. (%) | .29 | |||
| Chinese | 170 (81) | 82 (78) | 88 (84) | |
| Malay | 24 (11) | 12 (11) | 12 (11) | |
| Other | 16 (8) | 11 (11) | 5 (5) | |
| Primary site, No. (%) | .83 | |||
| Breast | 138 (66) | 67 (64) | 71 (68) | |
| Colorectal | 60 (29) | 32 (31) | 28 (27) | |
| Others | 12 (6) | 6 (6) | 6 (6) | |
| ECOG, No. (%) | .56 | |||
| 0 | 123 (59) | 66 (63) | 57 (54) | |
| 1 | 66 (31) | 30 (29) | 36 (34) | |
| 2 | 9 (4) | 5 (5) | 4 (4) | |
| 3 | 2 (1) | 1 (1) | 1 (1) | |
| Not assessed | 10 (5) | 3 (3) | 7 (7) | |
| Treatment intent, No. (%) | >.99 | |||
| Neoadjuvant or adjuvant | 44 (21) | 22 (21) | 22 (21) | |
| Palliative | 166 (79) | 83 (79) | 83 (79) | |
| Prior chemotherapy regimens, median (range) | 2 (0-9) | 2 (0-8) | 2 (0-9) | .78 |
| Starting capecitabine dose, median (range), mg/m2 twice daily | 1007 (793-1250) | 1010 (845-1250) | 1000 (793-1250) | .75 |
| Serum folate, median (range), nmol/La | 16 (5-56) | 16 (5-56) | 16 (6-56) | .92 |
| Red blood cell folate, median (range), nmol/Lb | 880 (233-2849) | 853 (359-2849) | 894 (233-1921) | .99 |
| Serum vitamin B12, median (range), pmol/Lc | 301 (79-1107) | 306 (79-1107) | 299 (107-1107) | .66 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Serum folate results were available for 75 patients in the placebo arm and 74 patients in the pyridoxine arm.
Red blood cell blood folate results were available for 76 patients in the placebo arm and 74 patients in the pyridoxine arm.
Serum vitamin B12 results were available for 76 patients in the placebo arm and 75 patients in the pyridoxine arm.
Efficacy of Pyridoxine for HFS Prophylaxis
The incidence of grade 2 or higher HFS did not differ (P = .38) between the placebo group (39 of 105 [37.1%]; 95% CI, 27.9%-46.4%) and the pyridoxine group (33 of 105 [31.4%]; 95%CI 22.6%-40.3%) (Table 2). There were no significant differences in the severity and incidence of other drug-related adverse events (eTable 1 in Supplement 2). Capecitabine was well tolerated overall, with less than 2% of patients experiencing grade 3 diarrhea. The maximum severity of vomiting and stomatitis was grade 2, reported in 6 patients (2.9%) and 2 patients (1.0%), respectively. The median time until grade 2 or higher HFS was not reached in both arms, with no difference between the 2 arms (P = .73; eFigure 1 in Supplement 2). The incidence of grade 2 or higher HFS was 15.4% (95% CI, 8.2%-22.7%) and 13.7% (95% CI, 6.7%-20.6%) for the placebo and pyridoxine group, respectively, at 60 days, and 41.0% (95% CI, 30.3%-51.8%) and 36.9% (95% CI, 25.7%-48.1%), respectively, at 120 days.
Table 2. Incidence and Onset of HFS.
| Variable | All | Placebo | Pyridoxine | P Value |
|---|---|---|---|---|
| HFS Grade, No. (%)a | ||||
| 0 | 76 (36) | 35 (33) | 41 (39) | |
| 1 | 60 (29) | 30 (29) | 30 (29) | |
| 2 | 66 (31) | 37 (35) | 29 (28) | |
| 3 | 6 (3) | 2 (2) | 4 (4) | |
| Missing | 2 (1) | 1 (1) | 1 (1) | |
| Grade ≥2 HFS | ||||
| Incidence, frequency, % (95% CI) | 34 (28-41) | 37 (28-46) | 31 (23-40) | .38 |
| No. of cycles, median (range) | 3 (2-8) | 3 (2-8) | 3 (2-7) | |
| Median time | Not reached | Not reached | Not reached | .73 |
Abbreviation: HFS, hand-foot syndrome.
Based on the worst grade recorded by each patient through the entire treatment cycle.
Efficacy of Pyridoxine for QoL Outcomes
Analysis of the EuroQol EQ-5D-3L questionnaire revealed no significant differences between each of the 5 dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) or the overall health status at any time point (eFigure 2 in Supplement 2).
Association of HFS With Clinical Variables
In univariate analysis, serum folate, red blood cell folate, and starting capecitabine dosage were significant predictors of grade 2 or higher HFS (Table 3). For multivariate analysis, it was noted that serum folate and red blood cell folate were significantly associated (Pearson correlation coefficient, 0.7245; P < .001). Hence, 2 alternative models containing either serum folate (Model 1) or red blood cell folate (Model 2) and all other variables were explored. The factors independently associated with grade 2 or higher HFS in Model 1 were serum folate (odds ratio [OR], 1.30; 95% CI, 1.12-1.52 with every increase of 5 nmol/L; P < .001), and in Model 2, red blood cell folate (OR, 1.28; 95% CI, 1.10-1.49 with every increase of 200 nmol/L; P = .001). Although every increase of 100 mg/m2 twice daily in starting capecitabine dose increased the odds of grade 2 or higher HFS by 71% (OR, 1.71; 95% CI, 0.93-3.17; P = .09) in Model 1 and by 81% (OR, 1.81; 95% CI, 0.97-3.35; P = .06) in Model 2, it did not reach statistical significance.
Table 3. Association Between Demographic and Biochemistry Characteristics With Grade 2 or Higher HFSa.
| Variable | Reference or Unit | Odds Ratio (95%CI) | P Value |
|---|---|---|---|
| Age at diagnosis | Increase of 10 y | 0.90 (0.72-1.14) | .39 |
| Sex | |||
| Male | Female | 0.95 (0.48-1.87) | .88 |
| Race | .16 | ||
| Malay | Chinese | 0.84 (0.33-2.14) | |
| Others | Chinese | 2.62 (0.93-7.39) | |
| Primary cancer site | .03b | ||
| Colorectal | Breast | 0.83 (0.41-1.63) | .68 |
| Others | Breast | 0.10 (0.00-0.49)c | .009 |
| ECOG | .23 | ||
| 1 | 0 | 0.65 (0.34-1.25) | |
| 2/3 | 0 | 0.36 (0.07-1.74) | |
| Prior chemotherapy regimens | Increase of 1 regimen | 1.07 (0.95-1.20) | .26 |
| Treatment Intent | |||
| Palliative | Neoadjuvant or adjuvant | 1.44 (0.73-2.84) | .30 |
| Starting capecitabine dose | Increase of 100 mg/m2 twice daily | 1.99 (1.32-3.00) | .001 |
| Treatment arm | |||
| Pyridoxine | Placebo | 0.78 (0.44-1.37) | .38 |
| Serum folate | Increase of 5 nmol/L | 1.27 (1.10-1.47) | .001 |
| Red blood cell folate | Increase of 200 nmol/L | 1.25 (1.08-1.44) | .003 |
| Vitamin B12 | Increase of 50 pmol/L | 1.03 (0.96-1.10) | .35 |
Abbreviation: HFS, hand-foot syndrome.
Univariate analysis.
Result based on exact test.
Median unbiased estimate provided.
Association of HFS With DNA Variants
A total of 7 614 942 variants from 202 patients (161 Chinese, 24 Malay, 8 Indian, 1 white, and 6 patients of other race/ethnicities) passed quality control and were used for analysis. Two patients with a high amount of missing data (>3%) and heterozygosity beyond 3 standard deviations of the mean were excluded. No duplicated or related patients were found during variant quality control procedures.
Three hundred variants were associated with grade 2 or higher HFS at the genome-wide significance level (P < 5 × 10−8) (Supplement 3), of which 21 had a minor allele frequency of at least 1% in East Asians or South Asians in the 1000 Genomes Project (Table 4). Of note, 2 variants in SPRY2 rs117876855 and rs139544515 were identified and found to be in complete linkage disequilibrium with each other, as well as 3 other variant positions (r2 = 1.00). Together these variants were predicted to alter 35 regulatory motifs according to HaploReg. SPRY2 acts as a negative feedback regulator of fibroblast growth factor signaling in dermal tissue and may perform a role in wound healing. DAVID pathway analysis of genes in closest proximity to the 300 variants highlighted an enrichment of genes involved in cytoskeletal anchoring, cell wall catabolism and N-linked glycosylation (Supplement 3). Using RegulomeDB, 14 variants had a score of 3a or lower, indicating potential to exert gene regulatory function. These included rs148221738 in MACF1, also known as ACF7, a gene which has been shown to coordinate the upward migration of bulge cells for epidermal wound healing.
Table 4. DNA Variants Associated With Grade 2 or Higher HFS With P < 5 × 10−8 and East Asian or South Asian Population Allele Frequency Greater Than 0.01.
| SNP | Chromosome | Position, bp | Nearest Gene(s)a | Feature | MAF | Risk Allele/Control Allele | P Valueb | Heterozygote, OR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| rs185346775 | 6 | 120 513 720 | MAN1A1 | 0.0102375 | A/G | 1.65 × 10−14 | 11.01 (0.75-161.44) | |
| rs146898897 | 1 | 1 517 816 | SSU72n | 0.01999 | C/T | 4.13 × 10−13 | 4.30 (0.90-20.48) | |
| rs187805828 | 12 | 9 073 251 | PHC1n,nmd | INT | 0.0117825 | C/T | 5.63 × 10−13 | 8.06 (0.83-77.99) |
| rs144470777 | 3 | 93 843 385 | NSUN3n | INT | 0.013475 | C/T | 8.95 × 10−13 | 7.55 (0.94-60.49) |
| rs143414470 | 10 | 62 296 000 | ANK3n,nmd | INT | 0.0110125 | T/C | 3.30 × 10−10 | 8.40 (0.75-94.39) |
| rs141531882 | 11 | 78 920 425 | ODZ4n | INT | 0.0102176 | C/A | 4.50 × 10−10 | 15.49 (0.66-364.10) |
| rs116134453 | 4 | 187 567 469 | FAT1n | INT | 0.0118874 | T/C | 5.85 × 10−10 | 5.73 (0.72-45.77) |
| rs117484357 | 10 | 81 033 980 | ZMIZ1rv,n | INT | 0.0105375 | C/T | 1.64 × 10−9 | 15.02 (0.78-288.37) |
| rs77475703 | 3 | 111 110 173 | CD96 | 0.0175876 | C/G | 3.33 × 10−9 | 5.23 (0.99-27.80) | |
| rs117308378 | 3 | 100 396 227 | GPR128n | INT | 0.02119 | T/C | 3.59 × 10−9 | 32.24 (1.95-533.75) |
| rs141213385 | 12 | 537 613 | CCDC77n | INT | 0.0109201 | G/C | 4.03 × 10−9 | 3.51 (0.49-25.25) |
| rs138385713 | 1 | 12 434 138 | VPS13Dn | INT | 0.0184202 | G/A | 9.30 × 10−9 | 9.01 (1.36-59.64) |
| rs117876855 | 13 | 82 439 359 | SPRY2 | 0.0123225 | A/G | 1.01 × 10−8 | 4.17 (0.62-28.09) | |
| rs10024471 | 4 | 126 494 937 | MIR2054 | 0.0107325 | A/G | 1.20 × 10−8 | NA | |
| rs185217050 | 6 | 126 234 837 | NCOA7n,sv,rv | INT | 0.014255 | T/C | 1.27 × 10−8 | 16.30 (1.20-220.69) |
| rs139544515 | 13 | 82 397 461 | SPRY2 | 0.0134376 | A/G | 1.30 × 10−8 | 4.09 (0.66-25.26) | |
| rs191934521 | 20 | 1 889 316 | SIRPAn, KIAA0922n | INT | 0.0100751 | G/T | 1.87 × 10−8 | 2.97 (0.40-22.27) |
| rs117412990 | 4 | 154 553 692 | (LOC100419170n) | INT | 0.01552 | G/A | 2.36 × 10−8 | 9.80 (1.21-79.28) |
| rs139368788 | 13 | 52 444 464 | CCDC70n | 0.01209 | A/G | 3.70 × 10−8 | 7.24 (0.82-63.68) | |
| rs146644707 | 12 | 25 872 709 | IFLTD1rv | 0.010995 | T/C | 4.43 × 10−8 | 9.71 (0.81-116.70) | |
| rs72765700 | 1 | 234 646 727 | LOC100506795n | 0.0141777 | T/A | 4.59 × 10−8 | 11.11 (1.02-121.31) |
Abbreviations: HFS, hand-foot syndrome; INT, intronic; MAF, minor alelle frequency; NA, not applicable; OR, odds ratio; SNP, single-nucleotide polymorphisms.
The nearest RefSeq gene is annotated by the predicted consequence(s) of the variant: sv, splicing variant; n, nearby gene (within ±10 kb of SNP); nmd, nonsense-mediated decay transcript variant; rv, regulatory region variant.
Adjusted by race for the overall cohort; adjusted by age, sex, and capecitabine dose.
In addition, the data from a manually curated list of genes previously reported to be associated with fluoropyrimidine toxic effect (namely CDA, CES2, DPYD, MIR27A, MTHFR, TYMP, TYMS, and UMPS) were inspected for their association with grade 2 or higher HFS. Of these, an association with grade 2 or higher HFS was observed for a novel DPYD variant (rs75267292 [P < 1.57 × 10−10]).
Discussion
To date, there do not appear to be any effective therapies for the prevention of capecitabine-related HFS. In addition to Kang et al, a study by Corrie et al randomized 106 patients with breast or colorectal cancer receiving single-agent capecitabine to 50 mg pyridoxine 3 times daily or placebo. There was no statistically significant difference in the incidence of severe HFS-related adverse events and the need for capecitabine dose reduction. Moreover, Braik et al randomized 77 patients treated with capecitabine-containing chemotherapy regimens to supplemental pyridoxine 100 mg daily or placebo and observed no difference in the incidence of HFS (all grades). While urea- and lactic acid-based topical keratolytic agent was not superior to placebo cream for the prevention of capecitabine-associated HFS in one randomized trial, 10% urea cream was superior to Mapisal (Medac), a new ointment rich in antioxidants and nourishing oil extracts, in another study. Given these mixed results, topical urea is not routinely administered to patients receiving capecitabine for HFS prophylaxis. Although Zhang et al has reported that the addition of celecoxib led to significant reductions in HFS, the majority of the 150 patients with colorectal cancer in this randomized study received capecitabine with oxaliplatin, which can confound the assessment of HFS from capecitabine.
Differences in dietary folate intake has been suggested as a possible explanation for the lower incidence of toxic effects from fluoropyrimidines in patients of East Asian origin. After conversion of capecitabine to fluorouracil by the sequential actions of carboxylesterase, cytidine deaminase, and thymidine phosphorylase, binding of the active fluorouracil metabolite fluorodeoxyuridine monophosphate (FdUMP) to thymidylate synthase (TS) is stable only in the presence of folate. Increased levels of exogenous folate could hence potentially lead to enhanced TS inhibition and toxic effects. Clinical evidence of the association between dietary folate and toxic effects from fluoropyrimidines is limited to a phase 2 study of fixed-dose capecitabine in patients with advanced colorectal cancer that reported increased toxic effects with higher pretreatment serum folate in 36 patients. In support, the risk of grade 2 or higher HFS positively correlated with levels of serum folate, as well as red blood cell folate, in multivariate analysis in this study. Serum folate reflects the immediate folate pool and varies with dietary intake, while red blood cell folate is indicative of folate body stores and the folate turnover over the preceding 3 to 4 months and is not subject to short-term fluctuation. From these results, it is tempting to speculate that lower folate levels could be a factor in the better tolerance of East Asians to capecitabine. Nonetheless, only 1 in 150 patients in the current study had red blood cell folate levels less than 283 nmol/L, suggesting that true folate deficiency is uncommon in our population. Studies comparing folate levels of patients receiving capecitabine from different regions would help to investigate this further.
Although the variability in starting dose of capecitabine may be one of the limitations of this study, this allows us to study the correlation of HFS toxic effects with dose, and we found a nonsignificant trend on multivariate analysis. This is a reflection of real-world clinical practice, to which the findings of our study may be applied, and both our multivariate and GWAS analyses also corrected for the variability in dose. Although the US Food and Drug Administration approved dose of capecitabine is 1250 mg/m2 twice daily for 14 out of 21 days, lower doses are often prescribed, as postmarketing experience revealed significant toxic effects at the registered dose. Studies evaluating capecitabine at a starting dose of 1000 mg/m2 twice daily for 14 out of 21 days suggest similar efficacy to the higher approved dose. In fact, it is not uncommon for clinicians at some centers to prescribe even lower doses such as a fixed dose of 1000 mg twice daily for 14 out of 21 days.
Recently, there has been increasing awareness of interethnic heterogeneity in the tolerability of various drugs. The risk of serious toxic effects from fluoropyrimidines is significantly lower among East Asians compared with white patients. In fact, Asian patients with the 3R/3R genotype of the thymidylate synthase gene enhancer region (TSER) were able to tolerate capecitabine up to 1500 mg/m2 on a twice daily 2-week on and 1-week off schedule in a recent phase 1 study. The genetic polymorphisms previously reported to be associated with fluoropyrimidine toxic effects (variants in DPYD, TYMS, DPYS, CDA, and MTHFR) are generally uncommon and exist at even lower frequencies in Asian populations (eTable 2 in Supplement 2). This may explain the very low frequency of grade 3 or 4 diarrhea, stomatitis, and neutropenia in our study, although grade 2 or higher HFS still occurred in more than 30% of patients. The etiology and predisposing factors for HFS may be distinct from other toxic effects with capecitabine.
A feature of this study was the GWAS performed using arrays enriched in East Asian genotypes to explore genetic factors associated with capecitabine-related HFS. Previous studies on capecitabine-related HFS have either examined a limited number of known candidates, and/or focused on white patient–based populations. These studies have found specific variants in DYPD, TYMS and ENOSF1, a gene adjacent to TYMS, to be associated with overall toxic effects from capecitabine. From our study, a number of variants were identified with plausible involvement in HFS; rs117876855, rs139544515, and 3 other variant positions of SPRY2 were in complete linkage disequilibrium, and potentially alter a total of 35 regulatory motifs. In murine models, SPRY2 acts as a negative feedback regulator of fibroblast growth factor signaling in dermal tissue and functions to down-regulate angiogenesis in injured skin. In addition, rs148221738 of MACF1 was assigned a RegulomeDB score of “3a,” suggesting that it has potential to affect protein binding and exert gene regulatory potential. MACF1 encodes the microtubule crosslinking protein ACF7 and has been demonstrated to have a physiologically-relevant role in vivo in skin wound healing. GWAS also identified a novel but rare variant in DPYD (rs75267292; P = 1.57 × 10−10), a gene that has been strongly implicated in fluoropyrimidine toxic effects. Future functional studies and independent validation will help to ascertain whether these variants will be useful predictors of HFS risk.
Limitations
Limitations include the varying doses of capecitabine used in the study as discussed earlier, as well as termination of the trial before reaching the original accrual target. Given the relatively small sample size for GWAS analysis, these results are hypothesis-generating and will benefit from future validation in larger cohorts.
Conclusions
Our randomized, controlled phase 3 trial does not support the administration of pyridoxine to prevent HFS. We confirmed the predictive value of serum and red blood cell folate for risk of developing HFS, and identified candidate genetic polymorphisms that potentially underlie the pharmacoethnicity of capecitabine-induced HFS in an exploratory GWAS. This study highlights the value of incorporating correlative biomarkers in supportive care studies in order to improve the understanding of the pathophysiology and the risk factors of treatment-related adverse events.
Trial Protocol
eTable 1. Worst grade of other toxicities during treatment by treatment arm
eTable 2. Allele frequencies of pharmacogenetic variants previously reported to be associated with fluoropyrimidine toxicity according to ethnic allele frequency from the 1000 Genomes project and grade of HFS
eFigure 1. Development of Grade 2/3 HFS
eFigure 2. A, Overall Health Status; B, Mobility; C, Self-Care; D, Usual Activities; E, Pain/Discomfort; F, Anxiety/Depression
Supplementary Table SNPs
References
- 1.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696-2704. [DOI] [PubMed] [Google Scholar]
- 2.Oshaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12(9):1247-1254. [DOI] [PubMed] [Google Scholar]
- 3.Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92(7):1759-1768. [DOI] [PubMed] [Google Scholar]
- 4.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40(4):536-542. [DOI] [PubMed] [Google Scholar]
- 5.Vukelja SJ, Lombardo FA, James WD, Weiss RB. Pyridoxine for the palmar-plantar erythrodysesthesia syndrome. Ann Intern Med. 1989;111(8):688-689. [DOI] [PubMed] [Google Scholar]
- 6.Fabian CJ, Molina R, Slavik M, Dahlberg S, Giri S, Stephens R. Pyridoxine therapy for palmar-plantar erythrodysesthesia associated with continuous 5-fluorouracil infusion. Invest New Drugs. 1990;8(1):57-63. [DOI] [PubMed] [Google Scholar]
- 7.Lauman M, Mortimer J. Effect of pyridoxine on the incidence of palmoplantar erythroderma (PPE) in patients receiving capecitabine. Proc Am Soc Clin Oncol. 2001;20;abstract 1565. [Google Scholar]
- 8.Kang YK, Lee SS, Yoon DH, et al. Pyridoxine is not effective to prevent hand-foot syndrome associated with capecitabine therapy: results of a randomized, double-blind, placebo-controlled study. J Clin Oncol. 2010;28(24):3824-3829. [DOI] [PubMed] [Google Scholar]
- 9.Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26(13):2118-2123. [DOI] [PubMed] [Google Scholar]
- 10.Soo RA, Syn N, Lee SC, et al. Pharmacogenetics-guided phase I study of capecitabine on an intermittent schedule in patients with advanced or metastatic solid tumours. Sci Rep. 2016;6:27826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh M, Chua D, Yao Y, et al. Can population differences in chemotherapy outcomes be inferred from differences in pharmacogenetic frequencies? Pharmacogenomics J. 2013;13(5):423-429. [DOI] [PubMed] [Google Scholar]
- 12.Krabbe PF, Peerenboom L, Langenhoff BS, Ruers TJ. Responsiveness of the generic EQ-5D summary measure compared to the disease-specific EORTC QLQ C-30. Qual Life Res. 2004;13(7):1247-1253. [DOI] [PubMed] [Google Scholar]
- 13.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(database issue):D930-D934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glienke J, Fenten G, Seemann M, Sturz A, Thierauch KH. Human SPRY2 inhibits FGF2 signalling by a secreted factor. Mech Dev. 2000;96(1):91-99. [DOI] [PubMed] [Google Scholar]
- 15.Wietecha MS, Chen L, Ranzer MJ, et al. Sprouty2 downregulates angiogenesis during mouse skin wound healing. Am J Physiol Heart Circ Physiol. 2011;300(2):H459-H467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44-57. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle AP, Hong EL, Hariharan M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135(1):137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, Shen QT, Oristian DS, et al. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3β. Cell. 2011;144(3):341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loganayagam A, Arenas Hernandez M, Corrigan A, et al. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br J Cancer. 2013;108(12):2505-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corrie PG, Bulusu R, Wilson CB, et al. A randomised study evaluating the use of pyridoxine to avoid capecitabine dose modifications. Br J Cancer. 2012;107(4):585-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braik T, Yim B, Evans AT, et al. Randomized trial of vitamin B6 for preventing hand-foot syndrome from capecitabine chemotherapy. J Community Support Oncol. 2014;12(2):65-70. [DOI] [PubMed] [Google Scholar]
- 24.Wolf SL, Qin R, Menon SP, et al. ; North Central Cancer Treatment Group Study N05C5 . Placebo-controlled trial to determine the effectiveness of a urea/lactic acid-based topical keratolytic agent for prevention of capecitabine-induced hand-foot syndrome: North Central Cancer Treatment Group Study N05C5. J Clin Oncol. 2010;28(35):5182-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofheinz RD, Gencer D, Schulz H, et al. Mapisal versus urea cream as prophylaxis for capecitabine-associated hand-foot syndrome: a randomized phase III trial of the AIO Quality of Life Working Group. J Clin Oncol. 2015;33(22):2444-2449. [DOI] [PubMed] [Google Scholar]
- 26.Zhang RX, Wu XJ, Wan DS, et al. Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial. Ann Oncol. 2012;23(5):1348-1353. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11(5):282-298. [DOI] [PubMed] [Google Scholar]
- 28.Sharma R, Rivory L, Beale P, Ong S, Horvath L, Clarke SJ. A phase II study of fixed-dose capecitabine and assessment of predictors of toxicity in patients with advanced/metastatic colorectal cancer. Br J Cancer. 2006;94(7):964-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielinski C, Gralow J, Martin M. Optimising the dose of capecitabine in metastatic breast cancer: confused, clarified or confirmed? Ann Oncol. 2010;21(11):2145-2152. [DOI] [PubMed] [Google Scholar]
- 30.Bajetta E, Procopio G, Celio L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol. 2005;23(10):2155-2161. [DOI] [PubMed] [Google Scholar]
- 31.Yap YS, Kendall A, Walsh G, et al. Clinical efficacy of capecitabine as first-line chemotherapy in metastatic breast cancer—how low can you go? Breast. 2007;16(4):420-424. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann M, Maass N, Costa SD, et al. First-line therapy with moderate dose capecitabine in metastatic breast cancer is safe and active: results of the MONICA trial. Eur J Cancer. 2010;46(18):3184-3191. [DOI] [PubMed] [Google Scholar]
- 33.Ambros T, Zeichner SB, Zaravinos J, et al. A retrospective study evaluating a fixed low dose capecitabine monotherapy in women with HER-2 negative metastatic breast cancer. Breast Cancer Res Treat. 2014;146(1):7-14. [DOI] [PubMed] [Google Scholar]
- 34.Midgley R, Kerr DJ. Capecitabine: have we got the dose right? Nat Clin Pract Oncol. 2009;6(1):17-24. [DOI] [PubMed] [Google Scholar]
- 35.Rosmarin D, Palles C, Church D, et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis. J Clin Oncol. 2014;32(10):1031-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosmarin D, Palles C, Pagnamenta A, et al. A candidate gene study of capecitabine-related toxicity in colorectal cancer identifies new toxicity variants at DPYD and a putative role for ENOSF1 rather than TYMS. Gut. 2015;64(1):111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Offer SM, Lee AM, Mattison LK, Fossum C, Wegner NJ, Diasio RB. A DPYD variant (Y186C) in individuals of african ancestry is associated with reduced DPD enzyme activity. Clin Pharmacol Ther. 2013;94(1):158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saif MW, Lee AM, Offer SM, McConnell K, Relias V, Diasio RB. A DPYD variant (Y186C) specific to individuals of African descent in a patient with life-threatening 5-FU toxic effects: potential for an individualized medicine approach. Mayo Clin Proc. 2014;89(1):131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Worst grade of other toxicities during treatment by treatment arm
eTable 2. Allele frequencies of pharmacogenetic variants previously reported to be associated with fluoropyrimidine toxicity according to ethnic allele frequency from the 1000 Genomes project and grade of HFS
eFigure 1. Development of Grade 2/3 HFS
eFigure 2. A, Overall Health Status; B, Mobility; C, Self-Care; D, Usual Activities; E, Pain/Discomfort; F, Anxiety/Depression
Supplementary Table SNPs