Abstract
Background
Clear cell renal cell carcinoma (RCC) continues to be the most commonly diagnosed subtype and is associated with more aggressive behavior than papillary and chromophobe RCC. Predicting disease recurrence after surgical extirpation is important for counseling and targeting those at high risk for adjuvant therapy clinical trials.
Objective
To validate a postoperative nomogram predicting 5-yr recurrence-free probability (RFP) for clinically localized clear cell RCC.
Design, setting, and participants
We identified all patients who underwent nephrectomy for clinically localized RCC from 1990 to 2009 at Memorial Sloan Kettering Cancer Center. After excluding patients with bilateral renal masses, familial RCC syndromes, and T3c or T4 tumors due to the limited number, 1642 participants were available for analysis.
Interventions
Partial or radical nephrectomy.
Outcome measurements and statistical analysis
Disease recurrence was defined as any new tumor after nephrectomy or kidney cancer–specific mortality, whichever occurred first. A postoperative nomogram was used to calculate the predicted 5-yr RFP, and these values were compared with the actual 5-yr RFP. Nomogram performance was evaluated by concordance index and calibration plot.
Results and limitations
Median follow-up was 39 mo (interquartile range: 14–79 mo), and disease recurrence was observed in 50 patients. The nomogram concordance index was 0.81. The calibration curve showed that the nomogram underestimated the actual 5-yr RFP. We updated the nomogram by including the entire patient population, which maintained performance and significantly improved calibration.
Conclusions
The updated clear cell RCC postoperative nomogram performed well in the combined cohort. Underestimation of actual 5-yr RFP by the original nomogram may be due to increased surgeon experience and other unknown variables.
Patient summary
We updated a valuable prediction tool used for assessing the disease recurrence probability after nephrectomy for clear cell renal cell carcinoma.
Keywords: Renal cell carcinoma, Clear cell, Nomogram, Recurrence
1. Introduction
Kidney cancer continues to be one of the most commonly diagnosed genitourinary malignancies in Europe [1] and the United States [2]. Historically, 20–40% of patients experience disease recurrence after nephrectomy for clinically localized renal cell carcinoma (RCC) [3]. However, an analysis from the National Cancer Data Base shows a significant trend toward diagnosing RCC at earlier stages [4]. This stage migration, driven mainly by the increased use of abdominal imaging for nonspecific abdominal or musculoskeletal indications, has created new opportunities for surgical cure of tumors with aggressive biology. Nonetheless, the behavior of these cancers is heterogeneous, prompting analysis of clinicopathologic factors that can help counsel patients regarding their prognosis and target those at high risk of recurrence for adjuvant therapy clinical trials that may include targeted agents and/or immunotherapy.
RCC is subdivided into several histologic subtypes [5,6]. Across multiple studies, conventional clear cell RCC was not only the most common subtype diagnosed, accounting for 60–90% of all RCCs [7], but it also showed more aggressive behavior including higher rates of recurrence, metastasis, and death when compared with papillary and chromophobe RCC [8–11]. For this reason, we focused our efforts on clear cell RCC and developed a postoperative nomogram based on a cohort of 701 patients that predicts recurrence at 5 yr following surgery [12]. In the present study, we performed validation of this nomogram by including a contemporary cohort of patients who underwent surgery for clear cell RCC at Memorial Sloan Kettering Cancer Center (MSKCC).
2. Materials and methods
2.1. Patient selection
Institutional review board approval was obtained, and we identified all patients who underwent partial or radical nephrectomy for clinically localized clear cell RCC between 1990 and 2009 from a prospectively maintained kidney cancer database at MSKCC. Following the criteria from our published postoperative nomogram, patients with bilateral renal masses and familial RCC syndromes such as von Hippel-Lindau disease were excluded from the analysis [12]. Due to limited numbers, patients with T3c and T4 tumors as well as those with sarcomatoid elements were also excluded from the analysis. The cohort that underwent analysis contained 1642 patients.
2.2. Disease characterization
Presurgical staging consisted of abdominopelvic cross-sectional imaging to characterize the kidney tumor and detect metastatic disease below the diaphragm as well as chest radiograph to rule out lung metastasis. If findings suspicious for metastatic disease were discovered on these studies and/or the patient reported symptoms suggestive of systemic disease, additional imaging such as computed tomography (CT) chest, CT brain, or bone scan were obtained to rule out metastatic disease prior to nephrectomy.
A group of experienced uropathologists determined the histologic features of the tumors that included subtype according to the Heidelberg classification [5], Fuhrman grade [13], presence of microvascular invasion, and tumor necrosis. Tumor staging was performed according to the American Joint Committee on Cancer Staging Manual, 6th ed., published in 2002 [14]. Clinical presentation was classified as incidental, locally symptomatic, or systemically symptomatic. Incidental lesions were defined as tumors detected on abdominal imaging for an unrelated condition. Local symptomatic lesions were defined as tumors that presented as an abdominal mass or were associated with ipsilateral flank pain or hematuria. Systemic symptomatic lesions were defined as tumors causing paraneoplastic signs and symptoms such as fever, night sweats, weight loss, extreme fatigue, anemia, hypercalcemia, and hepatic dysfunction.
2.3. Definition of recurrence
Patients were followed with chest radiograph and renal/retroperitoneal ultrasound or cross-sectional imaging every 3–6 mo, depending on pathologic stage and grade. In general, patients with greater than pathologic T2 disease or Fuhrman grade 3–4 underwent more intense follow-up. For patients who chose not to obtain follow-up studies at our institution, we reviewed outside imaging when available. Disease recurrence was defined as the appearance of any new tumor of RCC origin after nephrectomy or kidney cancer–specific mortality, whichever event occurred first. Patterns of disease recurrence included local, metastatic, or a metachronous tumor in the contralateral kidney, but this information was not used in the analysis.
2.4. Statistical analysis
Chi-square and Fisher exact tests were used to compare clinicopathologic data between the present cohort and the cohort from Sorbellini et al [12]. Using clinicopathologic data gathered from each patient, the conventional clear cell RCC postoperative nomogram [12] was used to calculate the 5-yr predicted recurrence-free probability (RFP). Model validation was conducted in two ways. First, the discrimination ability was evaluated with the Harrell concordance index (c-index) [15], which is equivalent to the area under the receiver operating characteristic curve but tailored to the censored outcomes. C-index values range from 0.5, indicating no discrimination ability, to 1.0, indicating perfect discrimination. Calibration accuracy was evaluated by plotting predicted versus actual 5-yr RFP. The 5-yr RFP was estimated using the Kaplan-Meier method. The Sorbellini cohort and present cohort were combined to generate an updated nomogram. Statistical analyses were performed using the open source R statistical software v.3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) with packages utils, base, ClevClinicQHS, rms, and Hmisc.
3. Results
Table 1 details the clinicopathologic characteristics of the patient cohort examined. A total of 1642 patients were available for analysis. One patient did not have information for presentation type and was excluded, so the final cohort consisted of 1641 patients. Overall, 829 (50%) underwent partial nephrectomy, and the remainder underwent radical nephrectomy. Most of the patients presented with a pT1a (49.8%) or pT1b (21.3%) tumor. The median tumor size was 3.9 cm (interquartile range [IQR]: 2.6–5.8 cm). A total of 195 (11.9%) of the patients had a Fuhrman grade I tumor, 913 (55.6%) had Fuhrman grade II, 427 (26.0%) had Fuhrman grade III, and 107 (6.5%) had Fuhrman grade IV. Necrosis was present in 122 patients (7.4%), and vascular invasion was present in 127 (7.7%). With respect to clinical presentation, 1266 patients (77.1%) had an incidentally detected renal mass, 334 (20.4%) presented with local symptoms, and 41 (2.5%) presented with systemic symptoms.
Table 1–
Clinicopathologic characteristics of the validation cohort
| Parameters | Present cohort | Sorbellini et al cohort [12] | p value |
|---|---|---|---|
| No. of cases | 1642 | 701 | |
| Presentation type, n (%) | |||
| Incidental | 1266 (77.1) | 488 (69.9) | 0.00031 |
| Local | 334 (20.4) | 178 (25.5) | |
| Systemic | 41 (2.5) | 32 (4.6) | |
| Not available | 1 (0.0) | 3 (0.0) | |
| Median tumor size, cm (IQR) | 3.9 (2.6–5.8) | 4.5 (2.6–6.8) | 0.00027 |
| AJCC 2002 stage, n (%) | |||
| T1a | 817 (49.8) | 281 (40.1) | 0.00044 |
| T1b | 349 (21.3) | 163 (23.3) | |
| T2 | 102 (6.2) | 58 (8.3) | |
| T3a | 235 (14.3) | 130 (18.6) | |
| T3b | 139 (8.5) | 68 (9.7) | |
| Not available | 0 (0.0) | 1 (0.0) | |
| Fuhrman grade, n (%) | |||
| I | 195 (11.9) | 66 (9.4) | 0.00451 |
| II | 913 (55.6) | 397 (56.6) | |
| III | 427 (26.0) | 139 (19.8) | |
| IV | 107 (6.5) | 25 (3.6) | |
| Not available | 0 (0.0) | 74 (10.6) | |
| Tumor necrosis, n (%) | 122 (7.4) | 23 (3.4) | 0.00021 |
| Vascular invasion, n (%) | 127 (7.7) | 33 (4.8) | 0.01101 |
AJCC = American Joint Committee on Cancer; IQR = interquartile range.
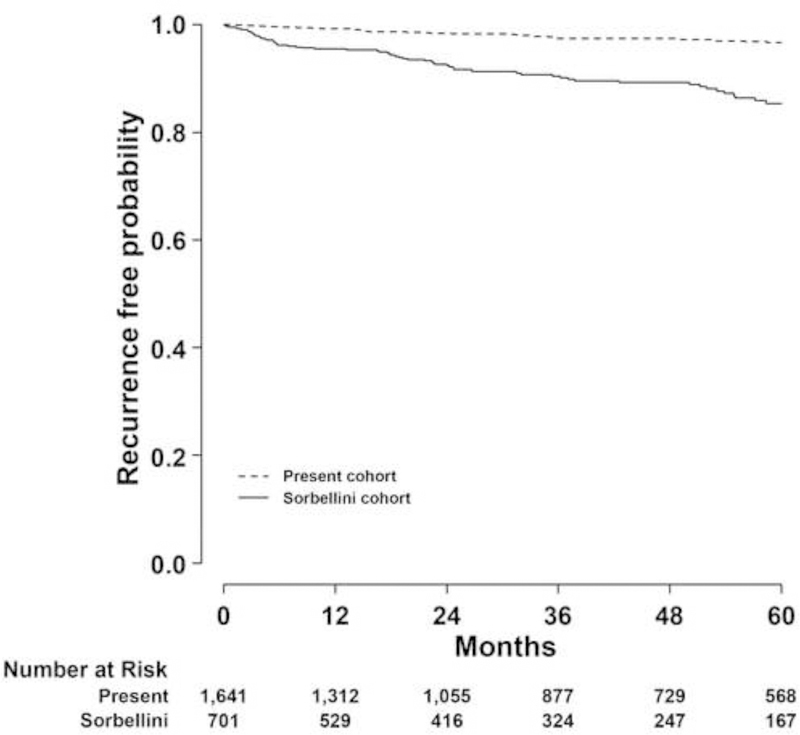
Median follow-up was 39 mo (IQR: 14–79 mo). Fifty patients had disease recurrence. The actual 5-yr RFP for this cohort was 96.7% (95% confidence interval [CI], 95.3– 97.6), which was significantly higher than the actual 5-yr RFP for the Sorbellini cohort (80.9%; 95% CI, 75.7–85.1) (Fig. 1).
Fig. 1 –
Kaplan-Meier plot of recurrence-free probability after surgery for clear cell renal cell carcinoma.
RFP = recurrence-free probability.
Compared with our previous study, patients presented with lower median tumor size (3.9 cm vs 4.5 cm; p < 0.01) and lower pathologic stage (T1 tumors: 71.1% vs 634%; p < 0.01) (Table 1). More patients presented incidentally (77.1% vs 69.6%; p < 0.01), and fewer had local (20.4% vs 25.4%) and systemic symptoms (2.5% vs 4.6%; p < 0.01). However, the tumors in this cohort had a higher proportion of Fuhrman grade III–IV (32.5% vs 23.4%; p < 0.01) and vascular invasion (7.7% vs 4.7%; p = 0.01).
The performance of the nomogram on the validation cohort was similar to its performance on the Sorbellini cohort with a c-index of 0.81. Nonetheless, the calibration curve showed that the nomogram underestimated the actual 5-yr RFP, especially in patients with predicted 5-yr RFP <0.8 (Fig. 2). Notably, 1339 patients (81.5%) had a predicted 5-yr RFP >0.8. We then combined both patient populations to generate an updated nomogram (Fig. 3a). The performance of the updated nomogram is comparable with the Sorbellini nomogram with a c-index of 0.81, and calibration is improved significantly (Fig. 3b).
Fig. 2.
Calibration curve for clear cell renal cell carcinoma postoperative nomogram. Solid line with slope of 1 indicates an ideal reference where predicted 5-yr recurrence-free probability (RFP) would match the actual 5-yr RFP.
RFP = recurrence-free probability.
Fig. 3 –
(a) Updated nomogram generated using the combined patient population; (b) Calibration curve for updated clear cell renal cell carcinoma postoperative nomogram. Solid line with slope of 1 indicates an ideal reference where predicted 5-yr recurrence-free probability (RFP) would match the actual 5-yr RFP.
RFP = recurrence-free probability.
4. Discussion
Since the development of the Kattan postoperative nomogram for RCC [16], other groups have generated prediction tools based on regression analysis of clinicopathologic variables known to have prognostic value [17–20]. These efforts have been valuable in aiding the counseling of patients after surgery, determining an appropriate risk-based follow-up schedule as recommended by professional associations including the European Association of Urology, American Urological Association, and National Comprehensive Cancer Network, and targeting those with a high risk of recurrence for clinical trials assessing the benefit of adjuvant therapy [21,22]. In 2005, we published a postoperative nomogram that specifically focused on predicting recurrence after nephrectomy for conventional clear cell RCC. Our rationale for concentrating on this histologic subtype comes from its higher prevalence and malignant potential when compared with other subtypes. In this study, we present external validation of this nomogram on a larger cohort of patients from our center.
The data demonstrate that the nomogram maintained discriminative ability in this patient cohort with a c-index of 0.81. But the calibration curve showed that the nomogram underestimated the actual 5-yr RFP in patients who had a predicted 5-yr RFP <0.8. One factor that could contribute to this underestimation is the evolving presentation of RCC over the past two decades. We observed a clear stage migration with a pronounced shift toward those who underwent surgery for T1a tumors (49.8% vs 40.1%) that may contribute to the observed nomogram miscalibration. When renal cortical tumors are small and low stage, the opportunity for surgical cure remains high even for clear cell RCC with aggressive features. Although Fuhrman grade III–IV and microvascular invasion clearly remain very strong predictors of kidney cancer–specific mortality [23– 27], they may not have the same level of significance when a nomogram is applied to a population with tumors that are smaller and lower stage and have yet to metastasize. The result of these factors is reflected in the significantly higher actual 5-yr RFP of this cohort when compared with the original cohort (96.7% vs 80.9%). To account for the changing presentation and management of clear cell RCC, we updated the Sorbellini nomogram by combining both patient populations while using the same clinicopathologic predictors. Doing so maintained performance and significantly improved the nomogram’s calibration.
Several limitations to our study should be mentioned. Since the time span of the study spanned approximately two decades, there is potentially some variability with respect to pathologic assessment of the nephrectomy specimens. We had the same dedicated team of expert genitourinary pathologists examine these specimens over the course of the study period that mitigated this source of variability. Another limitation is heterogeneity in follow-up. Some patients who had scheduled imaging studies performed locally may only have had reports available for review, thus limiting our ability to assess uniformly for disease recurrence. Finally, the presence of sarcomatoid elements was not included in this nomogram because few of our patients had this adverse pathologic feature. Nonetheless, these patients should be considered at high risk for recurrence.
One of the main disadvantages of nomograms is that they are static. Our findings here underscore the need for updating predictive tools so they remain properly calibrated as patient demographics, tumor characteristics, and surgeon experience change over time. In this study, we showed that the lower recurrence risk in a contemporary validation cohort caused a miscalibration of the original nomogram, leading to underestimation of actual 5-yr RFP. The development of dynamic predictive tools can potentially solve this problem by using a computer-intensive algorithm so physicians and their patients are given the most accurate prognostic information [28]. As the molecular changes in clear cell RCC described by The Cancer Genome Atlas [29] are systematically evaluated in carefully curated data sets containing outcome information, there will undoubtedly be a number of new biomarkers that correlate with clinical end points. However, they should only be included in predictive tools if they add important information to existing factors and can change clinical decision making [30]. Until molecular data are validated and testing becomes commonplace and cost effective, updated nomograms using only clinicopathologic characteristics will remain necessary in predicting recurrence in patients with clear cell RCC who underwent nephrectomy.
5. Conclusions
Our updated postoperative nomogram predicting 5-yr RFP after surgery for localized clear cell RCC performed well in a contemporary cohort. Future endeavors will focus on the generation of dynamic prediction tools for clear cell RCC to overcome the static nature of nomograms.
Acknowledgments
Funding/Support and role of the sponsor: Michael A. Feuerstein was supported by the National Cancer Institute/National Institutes of Health (NIH) under Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant T32 CA082088. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Financial disclosures: Paul Russo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [3].Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843–52. [DOI] [PubMed] [Google Scholar]
- [4].Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer 2008;113:78–83. [DOI] [PubMed] [Google Scholar]
- [5].Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol 1997;183:131–3. [DOI] [PubMed] [Google Scholar]
- [6].Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997;80:987–9. [DOI] [PubMed] [Google Scholar]
- [7].Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 2003;27:612–24. [DOI] [PubMed] [Google Scholar]
- [9].Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, Einarsson GV. Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: a retrospective nation-wide study of 629 patients. Eur Urol 2005;48:593–600. [DOI] [PubMed] [Google Scholar]
- [10].Teloken PE, Thompson RH, Tickoo SK, et al. Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol 2009;182:2132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Keegan KA, Schupp CW, Chamie K, Hellenthal NJ, Evans CP, Koppie TM. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol 2012;188:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol 2005;173:48–51. [DOI] [PubMed] [Google Scholar]
- [13].Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982;6:655–63. [DOI] [PubMed] [Google Scholar]
- [14].Greene F, Balch C, Haller D, Morrow M. AJCC Cancer Staging Manual, ed 6 New York, NY: Springer; 2002. [Google Scholar]
- [15].Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–6. [PubMed] [Google Scholar]
- [16].Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol 2001;166:63–7. [PubMed] [Google Scholar]
- [17].Zisman A, Pantuck AJ, Dorey F, et al. Mathematical model to predict individual survival for patients with renal cell carcinoma. J Clin Oncol 2002;20:1368–74. [DOI] [PubMed] [Google Scholar]
- [18].Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 2007;25:1316–22. [DOI] [PubMed] [Google Scholar]
- [19].Thompson RH, Leibovich BC, Lohse CM, et al. Dynamic outcome prediction in patients with clear cell renal cell carcinoma treated with radical nephrectomy: the DSSIGN score. J Urol 2007;177:477–80. [DOI] [PubMed] [Google Scholar]
- [20].Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002;168:2395–400. [DOI] [PubMed] [Google Scholar]
- [21].Donat SM, Diaz M, Bishoff JT, et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 2013;190:407–16. [DOI] [PubMed] [Google Scholar]
- [22].Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913–24. [DOI] [PubMed] [Google Scholar]
- [23].Brookman-May S, May M, Zigeuner R, et al. Collecting system invasion and Fuhrman grade but not tumor size facilitate prognostic stratification of patients with pT2 renal cell carcinoma. J Urol 2011;186:2175–81. [DOI] [PubMed] [Google Scholar]
- [24].Kroeger N, Rampersaud EN, Patard JJ, et al. Prognostic value of microvascular invasion in predicting the cancer specific survival and risk of metastatic disease in renal cell carcinoma: a multicenter investigation. J Urol 2012;187:418–23. [DOI] [PubMed] [Google Scholar]
- [25].Brookman-May SD, May M, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma--results from a comprehensive multi-centre database (CORONA/SATURN-Project). BJU Int 2013;112:909–16. [DOI] [PubMed] [Google Scholar]
- [26].Bianchi M, Gandaglia G, Trinh QD, et al. A population-based competing-risks analysis of survival after nephrectomy for renal cell carcinoma. Urol Oncol 2014;32:46e1–7. [DOI] [PubMed] [Google Scholar]
- [27].Bigot P, Hetet JF, Bernhard JC, et al. Nephron-sparing surgery for renal tumors measuring more than 7 cm: morbidity, and functional and oncological outcomes. Clin Genitourin Cancer 2014;12:e19–27. [DOI] [PubMed] [Google Scholar]
- [28].Vickers AJ, Fearn P, Scardino PT, Kattan MW. Why can’t nomograms be more like Netflix? Urology 2010;75:511–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst 2003;95:634–5. [DOI] [PubMed] [Google Scholar]