Abstract
The global adoption of artemisinin-based combination therapies (ACTs) in the early 2000s heralded a new era in effectively treating drug-resistant Plasmodium falciparum malaria. However, several Southeast Asian countries have now reported the emergence of parasites that have decreased susceptibility to artemisinin (ART) derivatives and ACT partner drugs, resulting in increasing rates of treatment failures. Here we review recent advances in understanding how antimalarials act and how resistance develops, and discuss new strategies for effectively combatting resistance, optimizing treatment and advancing the global campaign to eliminate malaria.
The World Health Organization (WHO) estimates that in 2015 malaria caused 212 million clinical episodes and 429,000 deaths, mostly in Africa in children under five years of age1. The majority of malarial deaths are caused by the intracellular protozoan parasite Plasmodium falciparum. Its infection of the human host begins in hepatocytes and proceeds to the disease-causing and potentially fatal intra-erythrocytic asexual blood stages (ABSs) before developing into sexual stages (gametocytes) that are transmissible to Anopheles mosquito vectors (Fig. 1). Although less virulent than P. falciparum, Plasmodium vivax is geographically widespread and is characterized by symptomatic relapses2.
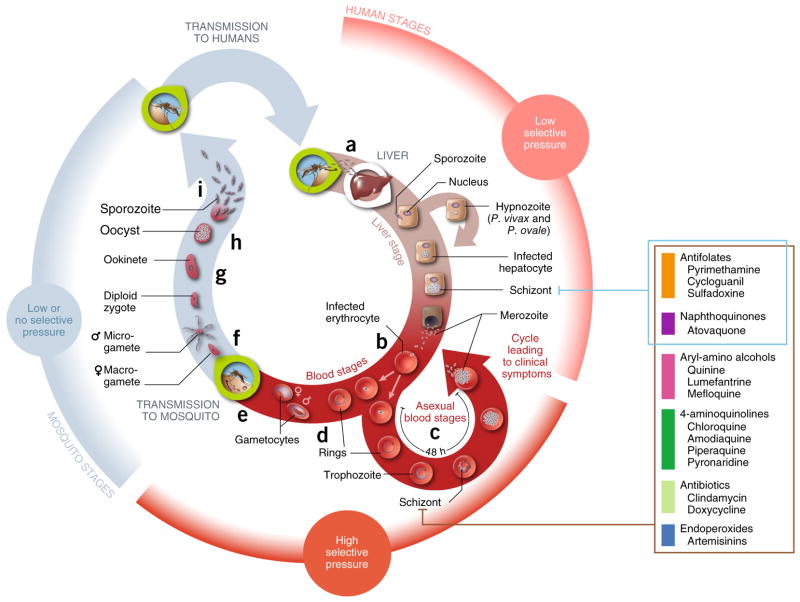
Figure 1.
Plasmodium’s life cycle and its relationship to drug resistance. (a) Human infection begins when infective female Anopheles mosquitoes deliver fewer than 100 sporozoites into the dermis during blood feeding147. These motile forms migrate rapidly into the bloodstream and to the liver, where they invade hepatocytes. A prodigious phase of replication over a week results in an estimated 10,000–30,000 merozoite progeny per intracellular parasite. (b) Liberated merozoites (~105–106 in total) then invade human mature red blood cells (RBCs), developing inside a parasitophorous vacuole and initiating ~48-h cycles of asexual blood stage (ABS) parasite growth, egress and reinvasion. (c) ABS parasites, typically 108–1012, are responsible for disease manifestations. (d) About 1–2% of intra-erythrocytic parasites enter an alternative program of sexual development, a process that over ~10–12 d produces mature male and female gametocytes that are transmissible to Anopheles mosquitoes. (e) An estimated 103–104 mature gametocytes are taken up during a blood meal. (f–i) These gametocytes then form male and female gametes (~102–103) that undergo sexual recombination (f), forming ookinetes (<100; g) and then oocysts (typically 1–2; h) before completing their life cycle by forming 103 to 104 sporozoites that migrate to salivary glands (i), ready for further human infection. In acute cases, ABS parasites can infect up to 10–20% of all erythrocytes (i.e., >1012). Primary causes of death include severe malaria anemia, or cerebral malaria that causes brain herniation and respiratory arrest148. Immunity is acquired slowly and is nonsterilizing; its maintenance is dependent on continued infection149. Selective forces that drive the emergence and spread of drug resistance differ throughout the life cycle. Important factors include the parasite numbers and drug pressure at different stages, stage specificity of drug action, the essentiality of the targeted pathways in the mosquito vector and vertebrate host, host immunity, multiplicity of infection, and local factors that affect therapeutics use and compliance. The pathogenic ABS reproduction cycle experiences the highest parasitemias and drug pressure, whereas the lower numbers of clinically silent liver-stage parasites provide much less fertile ground for the emergence of resistance150. Human-to-mosquito transmission is possible only if sufficient densities of mutant gametocytes are produced, which can be triggered in some instances by drug treatment151. Parasite number estimates were derived from refs. 2,152–154. Stages targeted by current and former first-line drugs used to treat P. falciparum are shown.
The success of malaria prevention, control and cure is contingent on the sustained clinical efficacy of first-line ACTs, for which the emergence and spread of drug resistance poses a constant threat. Modeling the scenario of widespread ACT resistance in malaria-endemic countries predicts an impact of >100,000 additional deaths per year3. Here we review recent advances in understanding the genetic and molecular basis of antimalarial resistance, gleaned from studies with patient-derived parasite isolates or in vitro culture-adapted parasite lines. Our discussion extends to how changes in parasite fitness and transmissibility to mosquito vectors can affect the spread of resistance. This review also examines the in vitro resistance profiles of new chemical entities that have entered human clinical trials or that show promise as advanced candidates, and discusses current approaches to overcoming multidrug resistance.
Targeting asexual blood-stage parasite development
A key requirement for curative antimalarials is their ability to eliminate ABS parasites. In P. falciparum, these intra-erythrocytic parasites cycle every ~48 h from the post-invasion rings to the mature trophozoites and then to the schizonts and daughter merozoites that reinitiate a new cycle of red blood cell (RBC) infection (Fig. 1). Ring-stage infected RBCs are present in the peripheral circulation, whereas the more mature trophozoite- and schizont-infected RBCs display parasite-encoded adhesins at the RBC surface that mediate binding to endothelial cell surface ligands and result in the sequestration of the infected RBCs in the microvasculature. Recent studies into the biology of ABS parasites have provided important insights into the modes of action of antimalarial drugs and the mechanisms by which resistance is acquired.
An important process targeted by multiple antimalarials in ABS parasites is the degradation and detoxification of host hemoglobin (Hb). ABS parasites, having established themselves within a parasitophorous vacuole, import Hb into their acidic digestive vacuole (Fig. 2). Hb is then digested by parasite proteases, liberating its constituent α and β chains, which are further cleaved into peptides and then into amino acids required for parasite protein synthesis4. This process releases Fe2+ iron-containing reactive heme moieties that are oxidized inside the digestive vacuole into Fe3+-protoporphyrin (FPIX), triggering oxidative stress. Inside this vacuole, FPIX is detoxified through its incorporation into chemically inert hemozoin—a crystal lattice of heme dimers5. Several 4-aminoquinolines, notably chloroquine (CQ) and amodiaquine (AQ), are thought to act primarily by binding hemozoin crystals at their actively growing surfaces and thus preventing further incorporation and detoxification of free heme. Other parasite-specific biological processes targeted by clinically used antimalarial drugs include folate synthesis in the cytosol, the mitochondrial electron transport chain that is required for pyrimidine biosynthesis, and protein synthesis in the apicoplast (Fig. 2). Antimalarial drug discovery and development efforts have recently uncovered multiple new targets, as discussed below in the context of resistance.
Figure 2.

Molecular targets of and mechanisms of resistance to major antimalarial drugs. Frequently targeted biological pathways include heme detoxification in the digestive vacuole, folate and pyrimidine biosynthesis in the cytosol, and electron transport in the mitochondrion. The 4- aminoquinolines, including CQ and AQ, as well as PPQ, the Mannich base pyronaridine (PND), and to some degree the aryl-amino alcohol quinine (QN), are all thought to concentrate in the digestive vacuole, where they bind reactive heme and interfere with its detoxification through incorporation into chemically inert hemozoin. Ferrous (Fe2+) iron-heme—liberated after parasite protease-mediated degradation of imported host hemoglobin (Hb)—can cleave and thereby activate the endoperoxide bridge of ART derivatives (star symbol). Point mutations (pink circles) in the transporters PfCRT and PfMDR1 are important determinants of resistance to 4-aminoquinolines. Resistance to PPQ is associated with increased expression of the hemoglobinases plasmepsin 2 and 3 (PM2/PM3, in the digestive vacuole), and might in some instances involve mutant PfCRT. Copy-number changes in pfmdr1, as well as PfCRT and PfMDR1 sequence variants, also affect the parasite’s susceptibility to the aryl-amino alcohols quinine (QN), lumefantrine (LMF) and mefloquine (MFQ) and can modulate ART potency. Variant forms of K13, thought to localize at the ER and in vesicular structures, are the primary mediator of ring-stage parasite resistance to ART. Mutations in two key enzymes of the folate biosynthesis pathway, dihydropteroate synthetase (DHPS) and dihydrofolate reductase (DHFR), can confer resistance respectively to the antifolates sulfadoxine (SDX) and both pyrimethamine (PYR) and cycloguanil (CYC). Atovaquone (ATQ) inhibits mitochondrial electron transport, and a single point mutation in the cytochrome b subunit (CYTb) of the bc1 complex can confer resistance to this drug. The ETC is important in ABS parasites because of its role in providing electrons for the ubiquinone-dependent dihydroorotate dehydrogenase (DHODH), an enzyme essential for de novo pyrimidine biosynthesis. Antibiotics such as clindamycin (CLD) and doxycycline (DOX) inhibit protein translation inside the apicoplast. CLD resistance is mediated by a point mutation in the apicoplast-encoded 23S rRNA.
Artemisinin resistance
Mode of ART action
ART, derived from the Chinese sweet wormwood Artemisia annua, and its derivatives are central to all current first-line antimalarial combination therapies. Inside ABS parasites, ARTs undergo reductive scission of their endoperoxide bridge by Fe2+-heme. In this activated state, these drugs are lethal to parasites, presumably as a result of their alkylation of biomolecules, including heme, proteins and lipids, thereby causing oxidative stress and cellular damage6. The highly potent activity of ART derivatives against Plasmodium ABS parasites might be primarily due to the abundance of Fe2+-heme that becomes accessible upon Hb degradation (Fig. 2). ART is highly active against trophozoites, in which Hb catabolism reaches its peak7. Unlike most other antimalarials, ART is also active against early ring-stage parasites8. Recent data suggest that parasite-mediated endocytosis and proteolysis of host Hb begin in very early ring stages (within several hours of RBC invasion), which could provide a potential source of activation for ART7,8.
ACTs all contain ART derivatives with improved pharmacological properties, namely artemether, artesunate or dihydroartemisinin (DHA). When used against ART-sensitive parasites, these derivatives can reduce the parasite biomass by up to 10,000-fold every 48 h, providing exceptionally rapid clearance rates9. However, these compounds are rapidly metabolized, with half-lives in the range of 1–2 h, and as such cannot eliminate infections without the added contribution of longer-lasting, albeit slower-acting, partner drugs2.
Origins and mechanisms of ART resistance
Emerging resistance to ART was reported nearly a decade ago in Cambodia (Fig. 3), where some individuals infected with P. falciparum displayed prolonged parasite clearance rates following artesunate or ACT treatment9,10. The parasite clearance rate can be quantified as the time required to reduce the parasite biomass by twofold, which for sensitive parasites is typically 1–3 h, and increases to >5 h for resistant parasites11. Mathematical modeling predicted that ART resistance was a result of ring stages becoming refractory to drug action12. This prediction was consistent with results from in vitro ‘ring-stage survival assays’ (RSA0–3 h), in which cultures of slow-clearing patient isolates exposed as very early ring stages (0–3 h postinvasion) to a 6-h pulse of 700-nM DHA survived at much higher rates than did drug-sensitive parasites (as assessed 3 d after the drug pulse)13.
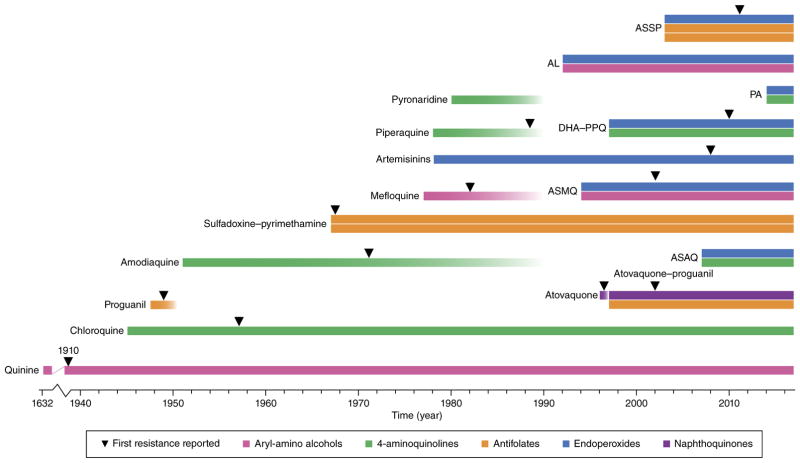
Figure 3.
History of the introduction of the principal antimalarials and of the first emergence of resistance in the field. Single bars refer to monotherapies; double- and triple-bar boxes denote combination therapies. Colors refer to the chemical classes to which the antimalarials belong. Quinine, first imported into Europe in the 1630s to treat malaria, encountered partial resistance in the early twentieth century, and later, was replaced by chloroquine (CQ), a former gold standard used massively until resistance appeared a decade later. Resistance to proguanil was detected within a year of clinical use. The replacement drug sulfadoxine–pyrimethamine (SP) quickly encountered resistance, and today is used primarily for intermittent preventive treatment during pregnancy and for seasonal malaria chemoprevention in combination with AQ (SPAQ, not shown) and as first-line treatment in combination with artesunate (ASSP) in some SP-sensitive areas155. Artemisinins (ARTs) were first used in monotherapy (and injectable artesunate is still used for severe malaria), although their short half-life in plasma and issues of resistance led to the development of artemisinin-based combination therapies (ACTs) for uncomplicated malaria. Several ACT partner drugs (such as amodiaquine and mefloquine) had been used as monotherapies and remained in use as single agents long after resistance was first found. Piperaquine and pyronaridine were introduced in China as a replacement of CQ ~40 years ago47,156,157. Resistance to piperaquine monotherapy was reported there in the late 1980s157. ART resistance (as manifested by delayed parasite clearance following treatment with an artesunate monotherapy or with an ACT) was first reported in 2008 (ref. 10) but was already present several years earlier in western Cambodia23. Treatment failure owing to resistance to one or both components of an ACT has been documented first for ASMQ and, more recently, for DHA-PPQ36,39,40,47,158. The WHO has recently reported AL treatment failures in Laos. Atovaquone -proguanil (Malarone) is currently prescribed as a prophylactic agent for travelers to malaria-endemic areas. AL, artemether + lumefantrine; ASMQ, artesunate + mefloquine; ASAQ, artesunate + amodiaquine; DHA-PPQ, dihydroartemisinin + piperaquine; PA, artesunate + pyronaridine; ASSP, artesunate + SP.
An important breakthrough in understanding the genetic basis of ART resistance came with whole-genome sequence analysis of in vitro–derived ART-resistant parasites, combined with candidate-gene studies of resistant and sensitive parasites, which implicated mutations in the Kelch-like protein K13 (ref. 14) (Supplementary Table 1). Subsequent gene-editing studies, which used a panel of culture-adapted isolates and either introduced K13 mutations into wild-type alleles or removed mutations from mutant alleles, confirmed a primary role for K13 mutations in ART resistance15,16. In support of this, a large clinical study found a strong association between slowly clearing infections (with parasite clearance half-lives of >5 h) and single point mutations in the K13 propeller domain17. Epidemiological studies have since observed multiple events of independent emergence of mutations in K13 resulting in drug resistance, which have spurred a drug-selective sweep across parasites in Southeast Asia18–24.
K13 mutations are also found in Africa, but only at very low prevalence (and the mutations associated with ART resistance in Asia are virtually absent). There is currently no detectable impact of K13 mutations on ART efficacy in Africa, except for one recent report of a migrant worker with a P. falciparum K13-variant infection from western Africa who showed delayed parasite clearance following ACT treatment20,21,25. Explanations for the current lack of impact of K13 mutations in Africa could include the greater degree of acquired immunity there, resulting from repeated exposure to P. falciparum, which builds host immunity to help control drug-resistant infections. There is also a much greater incidence of polyclonal infections in Africa that would select against drug-resistant parasites if they were to cause a growth-rate disadvantage, in addition to a greater incidence of chronic asymptomatic infections that are not exposed to drug pressure, and potentially, a lack of favorable parasite genetic backgrounds. Of note, K13 mutations do not seem to protect trophozoites, for which the increased potency of ART (resulting from higher levels of the heme activator) could potentially overwhelm mutant K13-mediated parasite defenses.
The function of K13 and the mechanisms by which its mutations can protect ring stages remain elusive. Evidence suggests an enhanced capacity of ART-resistant rings to regulate the cellular stress response to ART, potentially involving the unfolded protein response or the ubiquitin/proteasome system26,27. Another study has evoked a role for mutant K13 in reducing ART interactions with P. falciparum phosphatidylinositol-3-kinase (PfPI3K), potentially resulting in less PfPI3K polyubiquitination and lowered production of the phospholipid signaling molecule PI3P28. Prior studies have implicated important roles for PI3P in intracellular trafficking events, including protein export and Hb endocytosis29.
Fitness and transmissibility of K13 mutant parasites
Recent studies show that the initial ‘soft sweeps’ of multiple mutant K13 variants, which emerged and spread on several genetic backgrounds in the Greater Mekong subregion, have transitioned into a ‘hard sweep’ across the region of a small number of parasite genotypes that predominantly harbor the K13 C580Y mutation19,23,24. In a recent in vitro study, Asian parasites genetically engineered to express the K13 C580Y mutation outproliferated their isogenic counterparts expressing K13 R539T or I543T30. Earlier in vitro studies had nonetheless found that the two latter mutations resulted in the highest levels of ART resistance16. These data suggest that the relatively improved fitness seen with C580Y relative to parasites with the other mutations might be a dominant factor driving its apparent success in endemic areas, rather than the degree of resistance.
The C580Y mutation also conferred essentially no fitness cost in recently adapted Cambodian parasite lines. This was in contrast to parasite lines acquired decades earlier in which the K13 C580Y mutation imparted a more substantial fitness cost, suggesting that additional genetic determinants in current Cambodian isolates can mitigate mutant K13 fitness defects30. Several such candidates, encoding transporters or the iron-sulfur protein ferredoxin, were recently identified in large-scale genome-wide association studies18. Separately, a multisite clinical study reported higher numbers of gametocytes pre- and post-treatment in patients with slow parasite clearance, which suggests that ART-resistant P. falciparum infections might have a transmission advantage that could help to drive the spread of resistance17. A recent study showed that mutant K13 parasites have no apparent barrier to transmission by different Anopheles species, suggesting that mosquito-vector differences between Asia and Africa would not pose a hurdle for successful dissemination of mutated K13 alleles of Asian origin across Africa31.
Multidrug resistance (MDR)
Genetic determinants of P. falciparum resistance to ACT partner drugs
Six ACTs have now been developed for clinical use, listed in Table 1 (see also Fig. 3). Whereas in Africa ACTs remain effective, in Asia the prior combination of artesunate plus the partner drug mefloquine (ASMQ) has been largely replaced by dihydroartemisinin plus the partner drug piperaquine (DHA-PPQ) as a result of the high proportion of Asian parasites expressing multiple copies of the P. falciparum multidrug resistance-1 transporter gene (pfmdr1). This gene encodes a digestive vacuole membrane-bound ABC transporter. pfmdr1 over-expression or sequence variants constitute a major determinant of parasite resistance to mefloquine and also reduce susceptibility to the related aryl-amino alcohol lumefantrine, as observed in clinical studies and laboratory investigations with genetically modified parasites (Box 1)32,33. Since 2008, DHA-PPQ has been the official first-line ACT in Cambodia. Structurally, PPQ comprises two CQ-like 4-aminoquinoline moieties with a central linker. This drug is generally effective against parasites that evolved resistance to CQ through specific sets of point mutations in the P. falciparum CQ resistance transporter (PfCRT), which is also present on the digestive vacuole membrane34,35 (Fig. 2 and Box 1).
Table 1.
Artemisinin-based combination therapies (ACTs) deployed for clinical use
| Combination* | Abbreviation | Geographic use |
|---|---|---|
| Artemether + lumefantrine | AL | Most widely used ACT in Africa |
| Artesunate + amodiaquine | ASAQ | Used mostly in western Africa |
| Dihydroartemisinin + piperaquine | DHA–PPQ | First-line in several Southeast Asian countries# |
| Artesunate + mefloquine | ASMQ | Preceded DHA-PPQ in Southeast Asia |
| Artesunate + sulfadoxine– pyrimethamine | ASSP | Used in India and some Middle Eastern and eastern African countries |
| Artesunate + pyronaridine | PA | Recently received a positive scientific opinion under Article 58 by the EMA, currently being registered in both Africa and Asia |
Asterisk (*) notes that these combinations are all therapeutically indicated for the treatment of uncomplicated P. falciparum or P. vivax malaria. Artesunate is recommended for the treatment of severe malaria2. Pound sign (#) indicates that DHA–PPQ is also being widely tested in Africa for mass drug administration and for intermittent preventive treatment of malaria in pregnancy.
Box 1. PfCRT and PfMDR1: determinants of resistance to ACT partner drugs and other heme-binding agents.
The central importance of Hb catabolism in P. falciparum as both a source of amino acids for protein synthesis and as a toxic liability make this process a prime target for multiple first-line antimalarial drugs. The digestive vacuole membrane proteins PfCRT and PfMDR1 are primary determinants of resistance to several heme-interacting drugs (Fig. 2). The earlier massive worldwide use of CQ selected for a few mutant pfcrt alleles (harboring from four to nine point mutations) that emerged independently in Southeast Asia and South America, and that later spread from Asia to Africa159,165 (Fig. 4). These mutations include K76T, ubiquitous to all CQ-resistant alleles irrespective of origin and a sensitive marker of in vivo CQ treatment failure (Supplementary Table 1). In addition, several novel PfCRT mutations have been observed that can revert parasites to a CQ-sensitive status despite the presence of K76T, including C101F—which arose under pip-eraquine (PPQ) or amantadine pressure in vitro42,166—or C350R, which has recently spread throughout French Guiana167. Some PfCRT variants also mediate resistance to the active metabolite of AQ (monodesethyl-AQ), which might have been an important driving force for selection168,169. Importantly, all mutant CQ-resistant pfcrt alleles known so far increase P. falciparum susceptibility to lumefantrine, both in vitro and in field settings, and often increase sensitivity to ART derivatives, benefiting the clinical efficacy of the first-line ACT AL170–172.
Mechanistically, mutant PfCRT is thought to mediate CQ resistance through active, H+-dependent drug efflux out of the digestive vacuole, thereby preventing CQ from binding its heme receptor173,174. PfCRT’s pleiotropic drug-transport properties are thought to impact other antimalarials by altering their local concentration at their site of action159. Drugs might also be able to bind PfCRT isoforms, resulting in impaired function and parasite hyper-susceptibility175,176.
Most mutant pfcrt alleles come with a fitness cost, indicated in the field by a decrease in their allelic prevalence in the absence of CQ pressure177, and in vitro as reduced growth rates when compared to recombinant isogenic parasites expressing the wild-type allele35,169,178. Reduced fitness might involve less efficient Hb catabolism and peptide transport in PfCRT mutants, as well as other digestive-vacuole-related physiological processes178,179. Interestingly, parasites from Cambodia harbor a uniquely polymorphic pfcrt allele (Cam734, with nine point mutations) that mediates moderate CQ resistance without compromising parasite fitness35,179.
The ABC transporter PfMDR1 is another key mediator of parasite susceptibility to multiple antimalarials180. In Southeast Asia, mefloquine or lumefantrine treatment both select for parasites with increased pfmdr1 copy number, a finding corroborated in vitro with genetically modified parasites33,172. This phenomenon, however, does not extend to Africa, where pfmdr1 copy-number variants are exceedingly rare181. A potential explanation is that the combination of lower drug pressure at the population level and more frequent mixed infections exacerbates the fitness cost resulting from pfmdr1 overexpression, and results in a predominance of single-copy pfmdr1 parasites. Distinct PfMDR1 haplotypes can modulate the efficacy of several antimalarials. For example, the N86Y mutation contributes to partial resistance to monodesethyl-AQ and can augment the level of CQ resistance imparted by mutant PfCRT (Supplementary Table 1). This mutation is also counterselected by lumefantrine, artemisinins and mefloquine, showing the interconnectedness of many of these drugs in terms of cross-resistance or inverse susceptibility patterns180,182. Importantly, multiple new mutations in PfMDR1 as well as in PfCRT have recently been documented in a survey of 2,500 P. falciparum genomes sampled across Asia and Africa20, which suggests that the recent switch from CQ to various ACTs is selecting for novel variants of these multidrug-resistant transporters. Interestingly, a set of novel PfCRT mutations has appeared in Cambodian PPQ-resistant isolates, suggesting that they might contribute to resistance to this CQ-like drug37.
Both PfCRT and PfMDR1 also are known to contribute to quinine resistance, which so far remains partial and which might also involve an enzyme involved in protein ubiquitination183 (Supplementary Table 1). PfCRT- or PfMDR1-mediated changes in P. falciparum susceptibility to CQ, AQ or mefloquine are often accompanied by altered parasite susceptibility to quinine184, but in ways that can be unpredictable (i.e., leading to cross-resistance, or, inversely, to hypersensitivity). Results imply a complex and enigmatic mode of quinine action, which partially involves the inhibition of heme detoxification (Fig. 2).
The rapid increase in recent years in the prevalence of mutant K13 strains in Cambodia has resulted in greater numbers of parasites being exposed to the ACT partner drugs as monotherapy agents, once the short-lived and less-effective ART component has dropped to sub-therapeutic levels. Subsequently to this increased selection pressure, resistance to the partner drug PPQ has now emerged and is spreading quickly in Cambodia, rendering DHA-PPQ treatment increasingly inefficacious36–38. Genome-wide association studies with a set of PPQ-resistant or PPQ-sensitive Cambodian isolates have identified an amplification of the plasmepsin 2 and 3 genes as molecular markers of PPQ resistance39,40 (Supplementary Table 1). These genes encode aspartic proteases that are present in their active form in the digestive vacuole, where they act as hemoglobinases. One proposed hypothesis is that plasmepsin amplification results in faster rates of Hb digestion, leading to increased globin-derived peptide and subsequent amino acid availability for protein synthesis40. This could help to counteract the ability of PPQ to inhibit Hb catabolism and heme detoxification41. Results of transfection-based studies that alter the plasmepsin 2 and 3 copy numbers and definitively test this association are keenly awaited, as are studies that biochemically quantify the products of Hb catabolism in PPQ-resistant Cambodian parasites.
Recently, in vitro evidence has suggested that PfCRT might act as another determinant of PPQ resistance. Introducing the PfCRT C101F mutation (previously identified in drug selection studies42) into P. falciparum parasites harboring the CQ-resistant Dd2 allele resulted in resistance to PPQ, which also rendered parasites CQ sensitive41. Hb fractionation data showed that PPQ acts similarly to CQ in inhibiting hemozoin production and producing increased levels of free heme. Close concordance was observed between the concentrations that inhibited parasite growth or hemozoin formation by 50%41. At slightly higher concentrations, PPQ also inhibited Hb proteolysis. A potential involvement of PfCRT in PPQ resistance was further evoked by the recent observation of novel PfCRT mutations (H97Y, M343L or G353V, each present in addition to the set of mutations typified by the Dd2 allele) in a subset of Cambodian PPQ-resistant isolates37.
The spread of MDR
In addition to recently being a source of emerging resistance to ART and PPQ, Cambodia is also the original source of Asian parasite resistance to the former first-line drugs CQ and sulfadoxine–pyrimethamine (SP; known as Fansidar) (Fig. 4). Resistance resulted from the acquisition of multiple mutations in PfCRT (for CQ) or the folate biosynthesis enzymes dihydropteroate synthase and dihydrofolate reductase (DHPS and DHFR; for sulfadoxine and pyrimethamine, respectively)43. From there, resistance spread to Africa, causing a dramatic worsening of the malaria situation until ACTs were deployed. In Senegal, the emergence of CQ resistance was estimated to have caused up to a six–fold increase in mortality rates in children with malaria44. There is thus a valid concern that resistance mechanisms originating in Southeast Asia could once again spread into Africa, with potentially devastating consequences45.
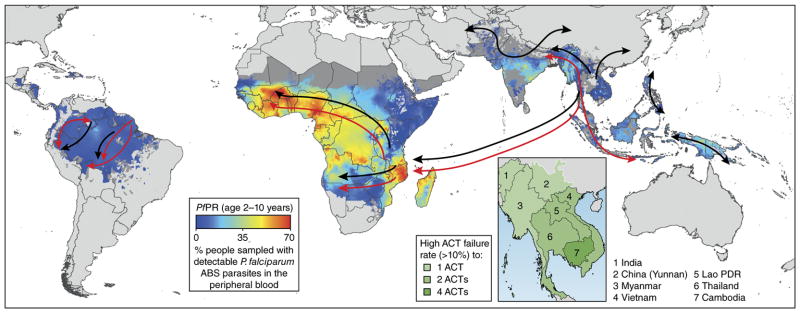
Figure 4.
Emergence and spread of P. falciparum resistance to CQ, pyrimethamine and ART derivatives. Resistance to CQ is thought to have arisen in multiple sites and spread globally (black arrows) owing to the selection pressure of CQ on mutant pfcrt alleles, resulting in a selective sweep at this locus. CQ-resistant parasites in Southeast Asia near the Thai–Cambodian border are thought to have seeded the introduction of CQ-resistant parasites into Africa, carried by individuals with the infection159. Resistance to pyrimethamine also emerged in Southeast Asia as well as in South America, and with triple-mutant dhfr Asian alleles, spread to Africa (red arrows)160,161. Pyrimethamine-resistantdhfr alleles also emerged independently in Africa162,163. Arrows are overlaid onto a 2010 map of P. falciparum endemicity on the basis of P. falciparum parasite rate (PfPR) surveys, in 2–10 year olds, using model-based geostatistics164. ART resistance was first detected in Cambodia (inset), driven by the emergence of mutant k13 alleles, and has since been detected in multiple countries in the region. Taken with permission from “Artemisinin and artemisinin-based combination therapy resistance,” October 2016 Status Report from the Global Malaria Programme of the World Health Organization, document WHO/HTM/GMP/2016.11; available at http://apps.who.int/iris/bitstream/10665/250294/1/WHO-HTM-GMP-2016.11-eng.pdf.
Why is Cambodia the favored region for the emergence of multi-drug resistance? One contributor could be frequent recourse to ACTs and issues of incomplete patient compliance and substandard drugs46. Another likely contributor is that the largely monoclonal nature of P. falciparum infections in Asia would lessen the detrimental impact of resistance-associated fitness costs there, allowing parasites to optimize resistance mechanisms and enable their successful dissemination. This contrasts with the frequently polyclonal infections in Africa, where vigorous parasite growth rates are essential for a mutant strain to remain competitive47. An additional factor could include host immunity, which was earlier shown in a therapeutic efficacy study in Mali to have an important role in the clearance of resistant P. falciparum infections48. Host immunity is typically lower in Asian settings because of the reduced frequency with which humans are exposed to malaria parasites, as compared to high-transmission settings in Mali and many other African countries. Thus, in Asia lower levels of antimalarial immunity would result in a weaker ability to clear drug-resistant infections naturally. Hemoglobinopathies (mainly HbAE or HbEE), and possibly, glucose-6-phosphate dehydrogenase (G6PD) deficiency, which are highly prevalent in Southeast Asian populations, might also have a role. These disorders alter the redox state of RBCs, helping to protect against severe forms of malaria, and possibly influencing the action of drugs such as ART derivatives that are suspected to act in part by increasing oxidative stress in a parasitized cell49–54.
An earlier hypothesis for the emergence of drug resistance in Cambodia was that Cambodian parasites had an increased rate of DNA mutation, termed “accelerated resistance to multiple drugs”55. This hypothesis, however, is not supported by recent findings56–58. DNA mismatch-repair mutations are nonetheless present at a high frequency in Cambodian parasites, which display an unusual population structure with highly distinct genetic lineages18,59. Possibly, this situation favors the suppression of sexual recombination events between gametes in the mosquito host (Fig. 1), which would preserve the inheritance of complex genetic traits. This hypothesis remains to be tested.
It is important to note that whereas high-grade resistance to PPQ has emerged in Cambodia, the other partner drugs AQ and mefloquine have encountered only partial resistance in certain regions, dependent on the status of PfCRT and PfMDR1 (Box 1). There is as yet little evidence of high-grade resistance to lumefantrine (pfmdr1 amplification provides low-grade resistance), in part because it has been used only in combination therapy (with artesunate). So far, no resistance has been reported to pyronaridine (Fig. 3).
Resistance to antifolates
Earlier, the loss of CQ efficacy, driven by the spread of mutant pfcrt (Box 1), heralded the adoption of the antifolate combination SP as a first-line treatment for malaria. Resistance, however, was detected within the first year of clinical use (Fig. 3)43. Pyrimethamine resistance results primarily from mutations in DHFR, including S108N, as observed in drug-pressured mutant lines, the progeny of a genetic cross between resistant and sensitive parasites, and field isolates60 (Fig. 2 and Supplementary Table 1). Point mutations in DHPS constitute the primary cause of resistance to sulfadoxine. Field studies in Malawi have identified a strong association between SP treatment failures and a quintuple set of mutations (DHFR N51I/C59R/S108N + DHPS A437G/K540E) in parasites60, providing a useful set of molecular markers (Supplementary Table 1). In some parts of Asia and South America, P. falciparum parasites can carry a fourth amino acid substitution—DHFR I164L—that affords high-grade resistance to pyrimethamine. Fitness costs arising from the impaired function of this highly mutated enzyme seem to be compensated in strains from Asia by amplification of the gene encoding GTP cyclohydrolase 1 (GCH1), which is located upstream of DHFR in the folate bio-synthetic pathway61,62. The I164L mutation and gch1 amplification, however, are rare in Africa, presumably because the fitness costs in Africa are too noncompetitive to permit widespread dissemination. Field-based data also suggest that the antifolate combination of tri-methoprim and sulfamethoxazole (also known as co-trimoxazole), used for the treatment and prophylaxis of bacterial infections in individuals with HIV infection, helps to sustain SP-resistant P. falciparum parasites63. Currently, SP is largely restricted to use as an intermittent preventive treatment during pregnancy (IPTp), although other alternatives, including AL or DHA-PPQ, are being explored in areas with a high prevalence of SP resistance64,65.
Seasonal malaria chemoprevention (SMC) with SP plus AQ (SPAQ) is also being scaled up in the Sahel subregion, as part of ongoing efforts to reduce the malaria burden through targeted intervention and prevention campaigns. Here resistance remains a major concern, and studies are examining the impact of SMC campaigns on the prevalence of SP resistance in local parasite populations66. Recently, a next-generation DHFR inhibitor, P218, has been designed that has pyrimidine side-chain flexibility and additional binding features that render it equally effective at inhibiting DHFR isoforms that are wild type or quadruple mutants. This study shows the benefit of leveraging knowledge about the genetic and structural aspects of resistance as a path to developing new and effective antifolates67.
Resistance to atovaquone–proguanil
Atovaquone–proguanil (Malarone) is a fixed-dose antimalarial combination that is prescribed as a prophylactic agent for travelers to malaria-endemic areas. This synergistic combination is also efficacious at treating children and adults who have uncomplicated malaria, but the treatment’s high cost has limited its general use in malaria-endemic countries. Atovaquone–proguanil was used recently as part of a plan to contain ART resistance in the Greater Mekong subregion; however, its suitability as a second-line therapy has been debated because of the ease with which atovaquone resistance arises68–70. Atovaquone, a naphthoquinone, inhibits the mitochondrial electron transport chain (ETC) through inhibition of the malarial cytochrome bc1 complex71. In ABS parasites, the ETC serves mainly as an electron provider for the ubiquinone-dependent mitochondrial enzyme dihydroorotate dehydrogenase (DHODH), whose activity is essential for pyrimidine biosynthesis72,73 (Fig. 2). The expression of yeast DHODH in P. falciparum ABS parasites rescues them from otherwise lethal concentrations of atovaquone or other bc1 inhibitors. Parasites with this metabolic bypass nonetheless remain susceptible to the biguanide proguanil73. Alone, proguanil has minimal intrinsic activity; when combined with atovaquone, however, this prodrug synergistically enhances the collapse of the mitochondrial membrane potential. Synergy is ablated in parasites carrying cytochrome b (cytb) mutations that confer atovaquone resistance74. Proguanil is also metabolized into cycloguanil, which is a potent inhibitor of wild-type parasite DHFR. In patients who are infected with DHFR-mutated parasites or who are poor CYP2C19 metabolizers of proguanil, the efficacy of atovaquone–proguanil relies almost exclusively on atovaquone action75,76.
Resistance to atovaquone alone in P. falciparum develops rapidly in vitro and in vivo, and is predominantly conferred by single point mutations in the multicopy mitochondrial cytb gene. Clinical failures of atovaquone–proguanil therapy have been associated primarily with point mutations at cytb codon 268 (Y268S, or less frequently, Y268C or Y268N) that typically induce a >1,000-fold increase in the atovaquone IC50 (the inhibitory concentration at which growth is reduced by 50%)77–79. Biochemical data suggest that the Y268S mutation results in decreased stability and impaired catalytic activity of the CYTb enzyme, thereby incurring a fitness penalty that in ABS parasites can be compensated for by an increased expression of bc1 complex genes80. Although low in prevalence, CYTb mutations have also been detected in African parasite isolates without a history of atovaquone exposure81. Consistent with this, patient-based stochastic modeling suggests that in the absence of atovaquone pressure, cytb mutations might pre-exist in one or more copies of mitochondrial DNA (termed heteroplasmy) as a result of spontaneous, random mitochondrial DNA mutations occurring during ABS-parasite replication. Although not abundant enough to be detected using conventional sequencing techniques, these pre-existing mutated alleles within heteroplasmic-mutant populations might contribute to the rapid selection of atovaquone-resistant cytb alleles during atovaquone–proguanil treatment82,83. Of note, Y286S/C/N mutations were also described in cytb genes of atovaquone-selected rodent parasites in mice72,84, but were not identified among CYTb point mutations (predominantly M133I) that have been reported from atovaquone in vitro selection experiments85–87 (Supplementary Table 1).
Recent data show that a number of common CYTb point mutants (albeit lacking Y268S) were severely impaired in their mosquito infectivity and in the number of oocysts produced when infection occurred. These findings suggest that atovaquone resistance, although arising relatively readily during the ABS phase of human infection, is often, if not always, nontransmissible87. This high transmission cost of resistance implies that mutations would have to emerge repeatedly de novo. Such a scenario would reduce the likelihood that resistance could spread rapidly, and it favors the prospects of atovaquone–proguanil remaining effective at a population level for antimalarial prophylaxis83.
Next-generation antimalarials
The emergence of resistance to many of the drugs in the antimalarial armamentarium has highlighted the urgency of developing future medicines with modes of action distinct to the current therapies. The development of high-throughput screening platforms that measure efficacy against liver and ABS parasites as well as gametocytes has facilitated the identification of multiple new chemotypes that are entering clinical development. The Medicines for Malaria Venture (MMV) has taken a key role in supporting many such projects, spanning the spectrum from drug-discovery research to clinical efficacy trials and drug registration (https://www.mmv.org/research-development/mmv-supported-projects). One major focus has been on chemotypes that can surmount ART resistance.
Overcoming ART resistance with improved endoperoxides
Data modeling suggests that ART-mediated parasite death is a function of the drug’s cumulative effective dose, and that a longer-lasting drug with the same mode of action could overcome K13-mediated ART resistance7. Considerable interest has thus been placed on the synthetic ozonide OZ439, which has a more stable peroxide bond that yields a longer half-life in plasma (23 h relative to 0.5 h for OZ439 and DHA, respectively, in rat studies)88. Recent reports on K13-modified isogenic lines have provided evidence that the C580Y mutation does not mediate cross-resistance to OZ439 (refs. 30,89), whereas the rare R539T mutation, in some studies, mediated partial cross-resistance30,89–91. Of note, a recent phase 2 clinical trial with OZ439 showed a nonstatistically significant trend toward slightly longer parasite clearance half-lives in individuals harboring K13-mutant infections, as compared to those with wild-type K13. However, this trend did not translate into increased failure rates measured at day 28 (ref. 92). Ongoing clinical studies should clarify whether OZ439 can be effective at treating K13-mutant infections, and whether specific K13 mutations such as R539T might reduce drug efficacy. Importantly, the earlier-generation ozonide OZ277, which is licensed for clinical use in India in combination with piperaquine (Synriam), was recently observed in several studies to lose potency against mutant K13 parasites30,89–91. These data argue for close pharmacovigilance of treatment outcomes of the OZ277-containing combination, especially given that K13 mutations have been identified in India or near its borders with Myanmar and Bangladesh93,94. Tetraoxanes have also been recently reported to serve as alternative endoperoxides that are effective against P. falciparum parasites harboring the K13 C580Y variant, and that have desirable fast-killing and long-lasting properties95.
Developing therapies with new modes of action
A separate approach to directly overcoming ART resistance has come from the discovery that inhibitors of the P. falciparum 20S proteasome can synergize with ART in drug-sensitive or drug-resistant parasites96,97. Encouragingly, one parasite-specific inhibitor showed antimalarial efficacy in a rodent in vivo model of malaria. Cryoelectron microscopy was used to solve the structure of the P. falciparum proteasome bound to a β2-subunit inhibitor, providing structural data to guide further compound design96.
Among other antimalarials with novel modes of action, one of the most advanced is KAE609 (Cipargamin), a spiroindolone compound that in patients shows the fastest clearance rates of any anti-malarial yet characterized98,99. This compound targets the P-type Na+ ATPase PfATP4, whose inhibition causes a rapid perturbation of Na+ homeostasis in the parasite that increases host cell membrane rigidity, thus blocking ABS development and transmission to mosquitoes100–104. However, in vitro resistance generation with this compound can select for point mutations in pfatp4 at multiple positions, often yielding an approximately ten-fold increase in IC50 value (although this remains in the low nanomolar level). Selection for resistance generally requires applying drug pressure to ~108 ABS parasites as a minimal population, making this a relatively frequent event58,98 (Supplementary Table 2).
SJ733, currently in phase 1 clinical trials, and PA21A092 also inhibit PfATP4. Resistance selection experiments, in which each agent is used against cultured P. falciparum ABS parasites, have generated point mutations in the pfatp4 gene not observed previously with KAE609, causing similar fold increases (range, 3–480; average, ~9 fold) in IC50 values100,101 (Supplementary Table 2). In vitro co-culture studies using isogenic lines expressing mutant or wild-type pfatp4 have provided evidence of a substantial fitness cost, associated with a higher resting concentration of cytosolic Na+ in the mutant line, an effect presumably caused by the PfATP4 mutation100. This finding suggests a possible obstacle to resistance in high-transmission, high-immunity settings with low levels of antimalarial drug use, in which sensitive strains could out-compete the less-fit PfATP4 variant, resistant parasites.
The mitochondrion-located DHODH enzyme is another clinically validated antimalarial drug target. The most advanced candidate is DSM265, a molecule that shows activity against both hepatic and ABS schizonts and that had promising efficacy with single-dose regimens in human trials105,106. Notably, the minimum inoculum for resistance (MIR), an in vitro measure of the frequency of resistance generation107, is similar to that of atovaquone (Supplementary Table 2). One approach to reducing the risk of resistance to DSM265 emerging would be to pair this compound with another agent that has a similar pharmacokinetic profile and against which resistance would require a separate set of parasite mutations, thus decreasing the probability of dual drug resistance108. A complementary approach could be to pre-emptively block DSM265-resistance pathways by choosing as a partner another DHODH inhibitor against which DSM265-resistant parasites would be hypersensitive109. A similar phenomenon, whereby resistance to one class of inhibitors causes the mutated target to become hypersensitive to another inhibitor, has also been described for some PfATP4 mutants110.
Targeting multiple life stages of P. falciparum
Targeting more than one life stage would be advantageous, and KAF156, an imidazolopiperazine, is another promising agent that is active against each of the three parasite stages (liver, ABS and gametocyte) present in the human host111,112. This compound recently showed encouraging clinical efficacy, with 14 of 21 patients cured of acute uncomplicated P. falciparum malaria following treatment with a single dose113. Resistance to KAF156 and related imidazolopiperazines in ABS can be mediated by point mutations in the P. falciparum cyclic amine resistance locus (pfcarl), which encodes a conserved protein that localizes in the cis-Golgi apparatus, where it might contribute to protein sorting or membrane trafficking111,114. Resistance to these compounds can also be mediated by mutations in an acetyl-CoA transporter (PfACT) or a UDP-galactose transporter (PfUGT), which are localized to the endoplasmic reticulum (ER)115 (Supplementary Table 2). The subcellular locations of these resistance mediators suggest that imidazolopiperazines might interfere with the transport of parasite biomolecules into the ER or Golgi115. Parasite resistance to different chemotypes has also been associated with mutations in pfcarl, suggesting evidence of its role as a multidrug-resistance mediator rather than the actual drug target114,116.
Another candidate with potent activity against multiple life-cycle stages is MMV390048 (ref. 117), an inhibitor of the lipid phosphatidylinositol 4-kinase (PI4K) that is required for correct membrane assembly around intracellular merozoites, just before their egress from a parasitized RBC (Fig. 1)118. Recently, a number of candidate drug targets central to mRNA processing or protein translation have also been identified (Supplementary Table 2 and see refs. 119–121). Among those, the eukaryotic elongation factor 2 (eEF2) and phenylalanine tRNA synthetase have been identified as targets of compounds active against all three parasite stages in humans122,123. With most of these targets, however, resistance has been flagged as a potential concern (Supplementary Table 2). Combination therapy will be necessary, although finding suitable partner drugs with matching pharmacokinetic profiles amplifies the complexity of the drug-development task124.
One compound of particular interest at present is ferroquine, a third-generation 4-aminoquinoline that is not compromised by existing mechanisms of resistance to the related drugs CQ and AQ, and that so far has resisted resistance selection in the laboratory125. This agent is currently undergoing evaluation in phase 2 trials126–128, including in combination with OZ439, and it has the potential to become an important first-line partner drug.
Can we design drugs that are ‘resistance proof’?
Although resistance has consistently plagued antimalarial chemotherapy, there are grounds for guarded optimism moving forward129. Clinical resistance to lumefantrine has been reported only once and has not been confirmed130, despite its massive clinical use. This observation implies that parasites cannot readily overcome lumefantrine’s complex mode of action (Supplementary Table 1). Resistance to ART is only partial, and it reflects a capacity of mutant parasites to survive short-lived drug pressure but not to replicate in the presence of drug. Longer-lasting drugs with similar modes of action might prove superior at reducing the risk of resistance. The rate of parasite killing is another important characteristic, with evidence showing that fast-acting antimalarials are less prone to yield resistance in vitro131. Resistance selection efforts have also proven unsuccessful with the potent antimalarial methylene blue that is active against the intra-erythrocytic asexual and gametocyte stages. This agent is thought to act broadly on redox processes in the parasite132,133 (also, D. Fidock, unpublished data). As mentioned above, resistance to components that act on the ETC is often elicited by mutations in CYTb, and evidence suggests that many of these mutations preclude successful parasite maturation in Anopheles, thus preventing their onward transmission87.
How then to design ‘resistance-proof ’ therapies? Some options would be to focus on drugs with a pleiotropic array of cellular targets, to target processes involving host proteins, such as parasite-mediated endocytosis of Hb, or to further leverage heme detoxification. Regarding the latter, an interesting approach has been the design of CQ-like compounds that couple targeting heme with a chemosensitizing component that counteracts mutant PfCRT-mediated CQ resistance134,135. Another strategy is to combine drugs that generate opposing selection pressures on the same target, as illustrated above with the example of DHODH, in which case a gain of resistance to one inhibitor can cause parasite hypersensitivity to another109. As mentioned in Box 1, detailed knowledge about PfCRT and PfMDR1 has shown that certain mutations conferring resistance to one drug can sensitize parasites to other agents, and has laid the theoretical groundwork for clinical trials (NCT02612545 and NCT02453308) with the triple ACTs AL plus AQ or DHA-PPQ plus mefloquine to test whether these combinations can successfully eliminate resistant and sensitive infections, and, potentially, block the emergence of parasites resistant to all three agents.
A separate strategy for preventing the emergence of resistance is to target host factors required for intra-erythrocytic parasite growth, for example, through the inhibition of human ferrochelatase, which is imported into parasites, where it seems to contribute to heme biosynthesis, or of a host tyrosine kinase that seems to be co-opted to assist with erythrocyte membrane destabilization and parasite egress136–139. High-throughput screens have also been developed to identify compounds that act exclusively on the nonreplicating mature gametocytes to prevent their transmission, without exerting selective pressure on ABS parasites that could generate resistance133,140–143. Reducing the transmissibility of drug-resistant parasites in low-transmission settings is also an important argument supporting the addition of a single low dose of primaquine to current therapeutic regimens144. The use of this liver-stage- and gametocyte-active 8-aminoquinoline drug, however, has been limited by the risk of toxicity to individuals with G6PD deficiency145. Accelerating the implementation of these multiple strategies could provide the drug tools needed to offset resistance while successfully driving down the parasite burden worldwide.
Conclusions
The tremendous progress made in recent years in reducing the malaria burden provides hope that malaria elimination is an achievable goal. This mission requires sustained efforts to bring together academic scientists, partners in industry and major global-health stakeholders, working together from the earliest steps of drug discovery through to community-based treatments and interventions. P. falciparum, whose dance of death with humans might date back to the early days of hominoid evolution146, has shown repeatedly its ability to develop and disseminate resistance to antimalarial remedies. An increased understanding of and exploitation to our own advantage of these mechanisms will provide unique opportunities to reduce the impact of resistance, and make a powerful contribution to improving the health of communities residing in malaria-endemic regions.
Supplementary Material
Acknowledgments
D.A.F. was supported by US National Institutes of Health (NIH) R01 grants AI50234, AI109023 and AI124678.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.World Health Organization. Fact Sheet: World Malaria Report 2016. WHO; 2016. available at http://www.who.int/malaria/media/world-malaria-report-2016/en/ [Google Scholar]
- 2.White NJ, et al. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 3.Lubell Y, et al. Artemisinin resistance--modelling the potential human and economic costs. Malar J. 2014;13:452. doi: 10.1186/1475-2875-13-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigala PA, Goldberg DE. The peculiarities and paradoxes of Plasmodium heme metabolism. Annu Rev Microbiol. 2014;68:259–278. doi: 10.1146/annurev-micro-091313-103537. [DOI] [PubMed] [Google Scholar]
- 5.Combrinck JM, et al. Insights into the role of heme in the mechanism of action of antimalarials. ACS Chem Biol. 2013;8:133–137. doi: 10.1021/cb300454t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilley L, Straimer J, Gnädig NF, Ralph SA, Fidock DA. Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol. 2016;32:682–696. doi: 10.1016/j.pt.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klonis N, et al. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc Natl Acad Sci USA. 2013;110:5157–5162. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie SC, et al. Haemoglobin degradation underpins the sensitivity of early ring stage Plasmodium falciparum to artemisinins. J Cell Sci. 2016;129:406–416. doi: 10.1242/jcs.178830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noedl H, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 11.White LJ, et al. Defining the in vivo phenotype of artemisinin-resistant falciparum malaria: a modelling approach. PLoS Med. 2015;12:e1001823. doi: 10.1371/journal.pmed.1001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saralamba S, et al. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 2011;108:397–402. doi: 10.1073/pnas.1006113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witkowski B, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariey F, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghorbal M, et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR–Cas9 system. Nat Biotechnol. 2014;32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 16.Straimer J, et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347:428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashley EA, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miotto O, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 2015;47:226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takala-Harrison S, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211:670–679. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaria, G.E.N.P.C.P. MalariaGEN Plasmodium falciparum Community Project. Genomic epidemiology of artemisinin resistant malaria. eLife. 2016;5:e08714. doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ménard D, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phyo AP, et al. Declining efficacy of artemisinin combination therapy against P falciparum malaria on the Thai-Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis. 2016;63:784–791. doi: 10.1093/cid/ciw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson TJ, et al. Population parameters underlying an ongoing soft sweep in Southeast Asian malaria parasites. Mol Biol Evol. 2017;34:131–144. doi: 10.1093/molbev/msw228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imwong M, et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis. 2017;17:491–497. doi: 10.1016/S1473-3099(17)30048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu F, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017;376:991–993. doi: 10.1056/NEJMc1612765. [DOI] [PubMed] [Google Scholar]
- 26.Mok S, et al. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2015;347:431–435. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dogovski C, et al. Targeting the cell stress response of Plasmodium falciparum to overcome artemisinin resistance. PLoS Biol. 2015;13:e1002132. doi: 10.1371/journal.pbio.1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbengue A, et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–687. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharjee S, Stahelin RV, Haldar K. Host targeting of virulence determinants and phosphoinositides in blood stage malaria parasites. Trends Parasitol. 2012;28:555–562. doi: 10.1016/j.pt.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straimer J, et al. Plasmodium falciparum K13 mutations differentially impact ozonide susceptibility and parasite fitness in vitro. mBio. 2017;8:e00172–17. doi: 10.1128/mBio.00172-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Laurent B, et al. Artemisinin-resistant Plasmodium falciparum clinical isolates can infect diverse mosquito vectors of Southeast Asia and Africa. Nat Commun. 2015;6:8614. doi: 10.1038/ncomms9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlemann AC, et al. Intrahost selection of Plasmodium falciparum pfmdr1 alleles after antimalarial treatment on the northwestern border of Thailand. J Infect Dis. 2007;195:134–141. doi: 10.1086/509809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidhu ABS, et al. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis. 2006;194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pascual A, et al. In vitro piperaquine susceptibility is not associated with the Plasmodium falciparum chloroquine resistance transporter gene. Malar J. 2013;12:431. doi: 10.1186/1475-2875-12-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen I, et al. Balancing drug resistance and growth rates via compensatory mutations in the Plasmodium falciparum chloroquine resistance transporter. Mol Microbiol. 2015;97:381–395. doi: 10.1111/mmi.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders DL, Vanachayangkul P, Lon C. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med. 2014;371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 37.Duru V, et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 2015;13:305. doi: 10.1186/s12916-015-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amaratunga C, et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis. 2016;16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amato R, et al. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis. 2017;17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witkowski B, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis. 2017;17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhingra SK, et al. A variant PfCRT isoform can contribute to Plasmodium falciparum resistance to the first-line partner drug piperaquine. MBio. 2017;8:e00303–17. doi: 10.1128/mBio.00303-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eastman RT, Dharia NV, Winzeler EA, Fidock DA. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob Agents Chemother. 2011;55:3908–3916. doi: 10.1128/AAC.01793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson TJ, Roper C. The origins and spread of antimalarial drug resistance: lessons for policy makers. Acta Trop. 2005;94:269–280. doi: 10.1016/j.actatropica.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64(Suppl 1–2):12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 45.Woodrow CJ, White NJ. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev. 2017;41:34–48. doi: 10.1093/femsre/fuw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Packard RM. The origins of antimalarial-drug resistance. N Engl J Med. 2014;371:397–399. doi: 10.1056/NEJMp1403340. [DOI] [PubMed] [Google Scholar]
- 47.Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Djimdé AA, et al. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:558–563. [PubMed] [Google Scholar]
- 49.Taylor SM, Cerami C, Fairhurst RM. Hemoglobinopathies: slicing the Gordian knot of Plasmodium falciparum malaria pathogenesis. PLoS Pathog. 2013;9:e1003327. doi: 10.1371/journal.ppat.1003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockett KA, et al. Reappraisal of known malaria resistance loci in a large multicenter study. Nat Genet. 2014;46:1197–1204. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antoine T, et al. Rapid kill of malaria parasites by artemisinin and semi-synthetic endoperoxides involves ROS-dependent depolarization of the membrane potential. J Antimicrob Chemother. 2014;69:1005–1016. doi: 10.1093/jac/dkt486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cyrklaff M, et al. Oxidative insult can induce malaria-protective trait of sickle and fetal erythrocytes. Nat Commun. 2016;7:13401. doi: 10.1038/ncomms13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kavishe RA, Koenderink JB, Alifrangis M. Oxidative stress in malaria and artemisinin combination therapy: pros and cons. FEBS J. 2017 doi: 10.1111/febs.14097. http://dx.doi.org/10.1111/febs.14097. [DOI] [PubMed]
- 54.Mbanefo EC, et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: a systematic review and meta-analysis. Sci Rep. 2017;7:45963. doi: 10.1038/srep45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bopp SE, et al. Mitotic evolution of Plasmodium falciparum shows a stable core genome but recombination in antigen families. PLoS Genet. 2013;9:e1003293. doi: 10.1371/journal.pgen.1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claessens A, et al. Generation of antigenic diversity in Plasmodium falciparum by structured rearrangement of Var genes during mitosis. PLoS Genet. 2014;10:e1004812. doi: 10.1371/journal.pgen.1004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee AH, Fidock DA. Evidence of a mild mutator phenotype in Cambodian Plasmodium falciparum malaria parasites. PLoS One. 2016;11:e0154166. doi: 10.1371/journal.pone.0154166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miotto O, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45:648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gregson A, Plowe CV. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev. 2005;57:117–145. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 61.Nair S, et al. Adaptive copy number evolution in malaria parasites. PLoS Genet. 2008;4:e1000243. doi: 10.1371/journal.pgen.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kümpornsin K, et al. Origin of robustness in generating drug-resistant malaria parasites. Mol Biol Evol. 2014;31:1649–1660. doi: 10.1093/molbev/msu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kateera F, et al. Molecular surveillance of Plasmodium falciparum drug resistance markers reveals partial recovery of chloroquine susceptibility but sustained sulfadoxine-pyrimethamine resistance at two sites of different malaria transmission intensities in Rwanda. Acta Trop. 2016;164:329–336. doi: 10.1016/j.actatropica.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandes S, et al. Cost effectiveness of intermittent screening followed by treatment versus intermittent preventive treatment during pregnancy in West Africa: analysis and modelling of results from a non-inferiority trial. Malar J. 2016;15:493. doi: 10.1186/s12936-016-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kakuru A, et al. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med. 2016;374:928–939. doi: 10.1056/NEJMoa1509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maiga H, et al. Seasonal malaria chemoprevention with sulphadoxine-pyrimethamine and amodiaquine selects pfdhfr-dhps quintuple mutant genotype in Mali. PLoS One. 2016;11:e0162718. doi: 10.1371/journal.pone.0162718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuthavong Y, et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci USA. 2012;109:16823–16828. doi: 10.1073/pnas.1204556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Looareesuwan S, et al. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 69.Maude RJ, Nguon C, Dondorp AM, White LJ, White NJ. The diminishing returns of atovaquone-proguanil for elimination of Plasmodium falciparum malaria: modelling mass drug administration and treatment. Malar J. 2014;13:380. doi: 10.1186/1475-2875-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saunders DL, et al. Atovaquone-proguanil remains a potential stopgap therapy for multidrug-resistant Plasmodium falciparum in areas along the Thai-Cambodian border. Antimicrob Agents Chemother. 2016;60:1896–1898. doi: 10.1128/AAC.02302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birth D, Kao WC, Hunte C. Structural analysis of atovaquone-inhibited cytochrome bc1 complex reveals the molecular basis of antimalarial drug action. Nat Commun. 2014;5:4029. doi: 10.1038/ncomms5029. [DOI] [PubMed] [Google Scholar]
- 72.Siregar JE, et al. Direct evidence for the atovaquone action on the Plasmodium cytochrome bc1 complex. Parasitol Int. 2015;64:295–300. doi: 10.1016/j.parint.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 74.Vaidya AB. Naphthoquinones: atovaquone, and other antimalarials targeting mitochondrial functions. In: Staines HM, Krishna S, editors. Treatment and Prevention of Malaria. Springer; Basel: 2012. pp. 127–139. [Google Scholar]
- 75.Kuhn S, Gill MJ, Kain KC. Emergence of atovaquone-proguanil resistance during treatment of Plasmodium falciparum malaria acquired by a non-immune north American traveller to west Africa. Am J Trop Med Hyg. 2005;72:407–409. [PubMed] [Google Scholar]
- 76.Krudsood S, et al. Efficacy of atovaquone-proguanil for treatment of acute multidrug-resistant Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg. 2007;76:655–658. [PubMed] [Google Scholar]
- 77.Musset L, Le Bras J, Clain J. Parallel evolution of adaptive mutations in Plasmodium falciparum mitochondrial DNA during atovaquone-proguanil treatment. Mol Biol Evol. 2007;24:1582–1585. doi: 10.1093/molbev/msm087. [DOI] [PubMed] [Google Scholar]
- 78.Sutherland CJ, et al. Mutations in the Plasmodium falciparum cytochrome b gene are associated with delayed parasite recrudescence in malaria patients treated with atovaquone-proguanil. Malar J. 2008;7:240. doi: 10.1186/1475-2875-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nixon GL, et al. Antimalarial pharmacology and therapeutics of atovaquone. J Antimicrob Chemother. 2013;68:977–985. doi: 10.1093/jac/dks504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fisher N, et al. Cytochrome b mutation Y268S conferring atovaquone resistance phenotype in malaria parasite results in reduced parasite bc1 catalytic turnover and protein expression. J Biol Chem. 2012;287:9731–9741. doi: 10.1074/jbc.M111.324319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ingasia LA, et al. Molecular characterization of the cytochrome b gene and in vitro atovaquone susceptibility of Plasmodium falciparum isolates from Kenya. Antimicrob Agents Chemother. 2015;59:1818–1821. doi: 10.1128/AAC.03956-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cottrell G, Musset L, Hubert V, Le Bras J, Clain J. Emergence of resistance to atovaquone-proguanil in malaria parasites: insights from computational modeling and clinical case reports. Antimicrob Agents Chemother. 2014;58:4504–4514. doi: 10.1128/AAC.02550-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goodman CD, Buchanan HD, McFadden GI. Is the mitochondrion a good malaria drug target? Trends Parasitol. 2017;33:185–193. doi: 10.1016/j.pt.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Srivastava IK, Morrisey JM, Darrouzet E, Daldal F, Vaidya AB. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol Microbiol. 1999;33:704–711. doi: 10.1046/j.1365-2958.1999.01515.x. [DOI] [PubMed] [Google Scholar]
- 85.Korsinczky M, et al. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother. 2000;44:2100–2108. doi: 10.1128/aac.44.8.2100-2108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwöbel B, Alifrangis M, Salanti A, Jelinek T. Different mutation patterns of atovaquone resistance to Plasmodium falciparum in vitro and in vivo: rapid detection of codon 268 polymorphisms in the cytochrome b as potential in vivo resistance marker. Malar J. 2003;2:5. doi: 10.1186/1475-2875-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goodman CD, et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science. 2016;352:349–353. doi: 10.1126/science.aad9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charman SA, et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc Natl Acad Sci USA. 2011;108:4400–4405. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siriwardana A, Iyengar K, Roepe PD. Endoperoxide drug cross-resistance patterns for Plasmodium falciparum exhibiting an artemisinin delayed-clearance phenotype. Antimicrob Agents Chemother. 2016;60:6952–6956. doi: 10.1128/AAC.00857-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang T, et al. Comparison of the exposure time dependence of the activities of synthetic ozonide antimalarials and dihydroartemisinin against K13 wild-type and mutant Plasmodium falciparum strains. Antimicrob Agents Chemother. 2016;60:4501–4510. doi: 10.1128/AAC.00574-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baumgärtner F, et al. In vitro activity of anti-malarial ozonides against an artemisinin-resistant isolate. Malar J. 2017;16:45. doi: 10.1186/s12936-017-1696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phyo AP, et al. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial. Lancet Infect Dis. 2016;16:61–69. doi: 10.1016/S1473-3099(15)00320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tun KM, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15:415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mishra N, et al. Emerging polymorphisms in falciparum Kelch 13 gene in Northeastern region of India. Malar J. 2016;15:583. doi: 10.1186/s12936-016-1636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Neill PM, et al. A tetraoxane-based antimalarial drug candidate that overcomes PfK13-C580Y dependent artemisinin resistance. Nat Commun. 2017;8:15159. doi: 10.1038/ncomms15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H, et al. Structure- and function-based design of Plasmodium-selective proteasome inhibitors. Nature. 2016;530:233–236. doi: 10.1038/nature16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ng CL, Fidock DA, Bogyo M. Protein degradation systems as antimalarial therapeutic targets. Trends Parasitol. doi: 10.1016/j.pt.2017.05.009. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rottmann M, et al. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.White NJ, et al. Spiroindolone KAE609 for falciparum and vivax malaria. N Engl J Med. 2014;371:403–410. doi: 10.1056/NEJMoa1315860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiménez-Díaz MB, et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc Natl Acad Sci USA. 2014;111:E5455–E5462. doi: 10.1073/pnas.1414221111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vaidya AB, et al. Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum. Nat Commun. 2014;5:5521. doi: 10.1038/ncomms6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kirk K. Ion regulation in the malaria parasite. Annu Rev Microbiol. 2015;69:341–359. doi: 10.1146/annurev-micro-091014-104506. [DOI] [PubMed] [Google Scholar]
- 103.Spillman NJ, et al. Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe. 2013;13:227–237. doi: 10.1016/j.chom.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Pelt-Koops JC, et al. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to Anopheles mosquito vector. Antimicrob Agents Chemother. 2012;56:3544–3548. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phillips MA, et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci Transl Med. 2015;7:296ra111. doi: 10.1126/scitranslmed.aaa6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCarthy JS, et al. Safety, tolerability, pharmacokinetics, and activity of the novel long-acting antimalarial DSM265: a two-part first-in-human phase 1a/1b randomised study. Lancet Infect Dis. 2017;17:626–635. doi: 10.1016/S1473-3099(17)30171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ding XC, Ubben D, Wells TN. A framework for assessing the risk of resistance for anti-malarials in development. Malar J. 2012;11:292. doi: 10.1186/1475-2875-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hastings IM, Hodel EM. Pharmacological considerations in the design of anti-malarial drug combination therapies - is matching half-lives enough? Malar J. 2014;13:62. doi: 10.1186/1475-2875-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lukens AK, et al. Harnessing evolutionary fitness in Plasmodium falciparum for drug discovery and suppressing resistance. Proc Natl Acad Sci USA. 2014;111:799–804. doi: 10.1073/pnas.1320886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flannery EL, et al. Mutations in the P-type cation-transporter ATPase 4, PfATP4, mediate resistance to both aminopyrazole and spiroindolone antimalarials. ACS Chem Biol. 2015;10:413–420. doi: 10.1021/cb500616x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meister S, et al. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science. 2011;334:1372–1377. doi: 10.1126/science.1211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuhen KL, et al. KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment, and prevention of disease transmission. Antimicrob Agents Chemother. 2014;58:5060–5067. doi: 10.1128/AAC.02727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.White NJ, et al. Antimalarial activity of KAF156 in falciparum and vivax malaria. N Engl J Med. 2016;375:1152–1160. doi: 10.1056/NEJMoa1602250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.LaMonte G, et al. Mutations in the Plasmodium falciparum cyclic amine resistance locus (PfCARL) confer multidrug resistance. MBio. 2016;7:e00696–16. doi: 10.1128/mBio.00696-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lim MY, et al. UDP-galactose and acetyl-CoA transporters as Plasmodium multidrug resistance genes. Nat Microbiol. 2016;1:16166. doi: 10.1038/nmicrobiol.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Magistrado PA, et al. Plasmodium falciparum cyclic amine resistance locus (PfCARL), a resistance mechanism for two distinct compound classes. ACS Infect Dis. 2016;2:816–826. doi: 10.1021/acsinfecdis.6b00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paquet T, et al. Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase. Sci Transl Med. 2017;9:eaad9735. doi: 10.1126/scitranslmed.aad9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNamara CW, et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature. 2013;504:248–253. doi: 10.1038/nature12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goodman CD, Pasaje CF, Kennedy K, McFadden GI, Ralph SA. Targeting protein translation in organelles of the Apicomplexa. Trends Parasitol. 2016;32:953–965. doi: 10.1016/j.pt.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 120.Sonoiki E, et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat Commun. 2017;8:14574. doi: 10.1038/ncomms14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wong W, et al. Mefloquine targets the Plasmodium falciparum 80S ribosome to inhibit protein synthesis. Nat Microbiol. 2017;2:17031. doi: 10.1038/nmicrobiol.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baragaña B, et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature. 2015;522:315–320. doi: 10.1038/nature14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kato N, et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature. 2016;538:344–349. doi: 10.1038/nature19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.White NJ. Pharmacokinetic and pharmacodynamic considerations in antimalarial dose optimization. Antimicrob Agents Chemother. 2013;57:5792–5807. doi: 10.1128/AAC.00287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Daher W, et al. Assessment of Plasmodium falciparum resistance to ferroquine (SSR97193) in field isolates and in W2 strain under pressure. Malar J. 2006;5:11. doi: 10.1186/1475-2875-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wells TN, Hooft van Huijsduijnen R. Ferroquine: welcome to the next generation of antimalarials. Lancet Infect Dis. 2015;15:1365–1366. doi: 10.1016/S1473-3099(15)00148-6. [DOI] [PubMed] [Google Scholar]
- 127.Held J, et al. Ferroquine and artesunate in African adults and children with Plasmodium falciparum malaria: a phase 2, multicentre, randomised, double-blind, dose-ranging, non-inferiority study. Lancet Infect Dis. 2015;15:1409–1419. doi: 10.1016/S1473-3099(15)00079-1. [DOI] [PubMed] [Google Scholar]
- 128.McCarthy JS, et al. A Phase II pilot trial to evaluate safety and efficacy of ferroquine against early Plasmodium falciparum in an induced blood-stage malaria infection study. Malar J. 2016;15:469. doi: 10.1186/s12936-016-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leroy D. How to tackle antimalarial resistance? EMBO Mol Med. 2017;9:133–134. doi: 10.15252/emmm.201607295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Plucinski MM, et al. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for treatment of uncomplicated malaria in children in Zaire and Uíge Provinces, angola. Antimicrob Agents Chemother. 2015;59:437–443. doi: 10.1128/AAC.04181-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Corey VC, et al. A broad analysis of resistance development in the malaria parasite. Nat Commun. 2016;7:11901. doi: 10.1038/ncomms11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adjalley SH, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci USA. 2011;108:E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siciliano G, et al. A high susceptibility to redox imbalance of the transmissible stages of Plasmodium falciparum revealed with a luciferase-based mature gametocyte assay. Mol Microbiol. 2017;104:306–318. doi: 10.1111/mmi.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelly JX, et al. Discovery of dual function acridones as a new antimalarial chemotype. Nature. 2009;459:270–273. doi: 10.1038/nature07937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gunsaru B, et al. Simplified reversed chloroquines to overcome malaria resistance to quinoline-based drugs. Antimicrob Agents Chemother. 2017;61:e01913–16. doi: 10.1128/AAC.01913-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vial H, et al. CRIMALDDI: platform technologies and novel anti-malarial drug targets. Malar J. 2013;12:396. doi: 10.1186/1475-2875-12-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith CM, et al. Red cells from ferrochelatase-deficient erythropoietic protoporphyria patients are resistant to growth of malarial parasites. Blood. 2015;125:534–541. doi: 10.1182/blood-2014-04-567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kesely KR, Pantaleo A, Turrini FM, Olupot-Olupot P, Low PS. Inhibition of an erythrocyte tyrosine kinase with imatinib prevents Plasmodium falciparum egress and terminates parasitemia. PLoS One. 2016;11:e0164895. doi: 10.1371/journal.pone.0164895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zumla A, et al. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis. 2016;16:e47–e63. doi: 10.1016/S1473-3099(16)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Plouffe DM, et al. High-throughput assay and discovery of small molecules that interrupt malaria transmission. Cell Host Microbe. 2016;19:114–126. doi: 10.1016/j.chom.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.D’Alessandro S, et al. A chemical susceptibility profile of the Plasmodium falciparum transmission stages by complementary cell-based gametocyte assays. J Antimicrob Chemother. 2016;71:1148–1158. doi: 10.1093/jac/dkv493. [DOI] [PubMed] [Google Scholar]
- 142.Lucantoni L, Fidock DA, Avery VM. Luciferase-based, high-throughput assay for screening and profiling transmission-blocking compounds against Plasmodium falciparum gametocytes. Antimicrob Agents Chemother. 2016;60:2097–2107. doi: 10.1128/AAC.01949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sinden RE. Targeting the parasite to suppress malaria transmission. Adv Parasitol. 2017;97:147–185. doi: 10.1016/bs.apar.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 144.Graves PM, Gelband H, Garner P. Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission. Cochrane Database Syst Rev. 2015;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ashley EA, Recht J, White NJ. Primaquine: the risks and the benefits. Malar J. 2014;13:418. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sundararaman SA, et al. Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria. Nat Commun. 2016;7:11078. doi: 10.1038/ncomms11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Douglas RG, Amino R, Sinnis P, Frischknecht F. Active migration and passive transport of malaria parasites. Trends Parasitol. 2015;31:357–362. doi: 10.1016/j.pt.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seydel KB, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–1137. doi: 10.1056/NEJMoa1400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fowkes FJ, Boeuf P, Beeson JG. Immunity to malaria in an era of declining malaria transmission. Parasitology. 2016;143:139–153. doi: 10.1017/S0031182015001249. [DOI] [PubMed] [Google Scholar]
- 150.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bejon P, et al. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J Infect Dis. 2005;191:619–626. doi: 10.1086/427243. [DOI] [PubMed] [Google Scholar]
- 153.Pongtavornpinyo W, et al. Probability of emergence of antimalarial resistance in different stages of the parasite life cycle. Evol Appl. 2009;2:52–61. doi: 10.1111/j.1752-4571.2008.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vanaerschot M, Huijben S, Van den Broeck F, Dujardin JC. Drug resistance in vectorborne parasites: multiple actors and scenarios for an evolutionary arms race. FEMS Microbiol Rev. 2014;38:41–55. doi: 10.1111/1574-6976.12032. [DOI] [PubMed] [Google Scholar]
- 155.World Health Organization. Guidelines for the treatment of malaria. 3. WHO; 2015. available at http://www.who.int/malaria/publications/atoz/9789241549127/en/ [PubMed] [Google Scholar]
- 156.Chang C, Lin-Hua T, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Trans R Soc Trop Med Hyg. 1992;86:7–10. doi: 10.1016/0035-9203(92)90414-8. [DOI] [PubMed] [Google Scholar]
- 157.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77(Suppl 6):181–192. [PubMed] [Google Scholar]
- 158.Leang R, et al. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother. 2013;57:818–826. doi: 10.1128/AAC.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ecker A, Lehane AM, Clain J, Fidock DA. PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 2012;28:504–514. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mita T. Origins and spread of pfdhfr mutant alleles in Plasmodium falciparum. Acta Trop. 2010;114:166–170. doi: 10.1016/j.actatropica.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 161.Corredor V, et al. Origin and dissemination across the Colombian Andes mountain range of sulfadoxine-pyrimethamine resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:3121–3125. doi: 10.1128/AAC.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roper C, et al. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 163.Kheir A, et al. Transmission and cross-mating of high-level resistance Plasmodium falciparum dihydrofolate reductase haplotypes in The Gambia. Am J Trop Med Hyg. 2010;82:535–541. doi: 10.4269/ajtmh.2010.09-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gething PW, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Callaghan PS, Hassett MR, Roepe PD. Functional comparison of 45 naturally occurring isoforms of the Plasmodium falciparum chloroquine resistance transporter (PfCRT) Biochemistry. 2015;54:5083–5094. doi: 10.1021/acs.biochem.5b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pulcini S, et al. Mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, enlarge the parasite’s food vacuole and alter drug sensitivities. Sci Rep. 2015;5:14552. doi: 10.1038/srep14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pelleau S, et al. Adaptive evolution of malaria parasites in French Guiana: Reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc Natl Acad Sci USA. 2015;112:11672–11677. doi: 10.1073/pnas.1507142112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sá JM, et al. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc Natl Acad Sci USA. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gabryszewski SJ, Modchang C, Musset L, Chookajorn T, Fidock DA. Combinatorial genetic modeling of pfcrt-mediated drug resistance evolution in Plasmodium falciparum. Mol Biol Evol. 2016;33:1554–1570. doi: 10.1093/molbev/msw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sisowath C, et al. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis. 2009;199:750–757. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Conrad MD, et al. Comparative impacts over 5 years of artemisinin-based combination therapies on Plasmodium falciparum polymorphisms that modulate drug sensitivity in Ugandan children. J Infect Dis. 2014;210:344–353. doi: 10.1093/infdis/jiu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Venkatesan M, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am J Trop Med Hyg. 2014;91:833–843. doi: 10.4269/ajtmh.14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lehane AM, Hayward R, Saliba KJ, Kirk K. A verapamil-sensitive chloroquine-associated H+ leak from the digestive vacuole in chloroquine-resistant malaria parasites. J Cell Sci. 2008;121:1624–1632. doi: 10.1242/jcs.016758. [DOI] [PubMed] [Google Scholar]
- 174.Petersen I, Eastman R, Lanzer M. Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett. 2011;585:1551–1562. doi: 10.1016/j.febslet.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 175.Lekostaj JK, Natarajan JK, Paguio MF, Wolf C, Roepe PD. Photoaffinity labeling of the Plasmodium falciparum chloroquine resistance transporter with a novel perfluorophenylazido chloroquine. Biochemistry. 2008;47:10394–10406. doi: 10.1021/bi8010658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Richards SN, et al. Molecular mechanisms for drug hypersensitivity induced by the malaria parasite’s chloroquine resistance transporter. PLoS Pathog. 2016;12:e1005725. doi: 10.1371/journal.ppat.1005725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takala-Harrison S, Laufer MK. Antimalarial drug resistance in Africa: key lessons for the future. Ann NY Acad Sci. 2015;1342:62–67. doi: 10.1111/nyas.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lewis IA, et al. Metabolic QTL analysis links chloroquine resistance in Plasmodium falciparum to impaired hemoglobin catabolism. PLoS Genet. 2014;10:e1004085. doi: 10.1371/journal.pgen.1004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gabryszewski SJ, et al. Evolution of fitness cost-neutral mutant PfCRT conferring P falciparum 4-aminoquinoline drug resistance is accompanied by altered parasite metabolism and digestive vacuole physiology. PLoS Pathog. 2016;12:e1005976. doi: 10.1371/journal.ppat.1005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Veiga MI, et al. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat Commun. 2016;7:11553. doi: 10.1038/ncomms11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheeseman IH, et al. Population structure shapes copy number variation in malaria parasites. Mol Biol Evol. 2016;33:603–620. doi: 10.1093/molbev/msv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rosenthal PJ. The interplay between drug resistance and fitness in malaria parasites. Mol Microbiol. 2013;89:1025–1038. doi: 10.1111/mmi.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sanchez CP, et al. A HECT ubiquitin-protein ligase as a novel candidate gene for altered quinine and quinidine responses in Plasmodium falciparum. PLoS Genet. 2014;10:e1004382. doi: 10.1371/journal.pgen.1004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ferdig MT, et al. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol Microbiol. 2004;52:985–997. doi: 10.1111/j.1365-2958.2004.04035.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.