ABSTRACT
Indole is a molecule of considerable biochemical significance, acting as both an interspecies signal molecule and a building block of biological elements. Bacterial indole degradation has been demonstrated for a number of cases; however, very little is known about genes and proteins involved in this process. This study reports the cloning and initial functional characterization of genes (iif and ant cluster) responsible for indole biodegradation in Acinetobacter sp. strain O153. The catabolic cascade was reconstituted in vitro with recombinant proteins, and each protein was assigned an enzymatic function. Degradation starts with oxidation, mediated by the IifC and IifD flavin-dependent two-component oxygenase system. Formation of indigo is prevented by IifB, and the final product, anthranilic acid, is formed by IifA, an enzyme which is both structurally and functionally comparable to cofactor-independent oxygenases. Moreover, the iif cluster was identified in the genomes of a wide range of bacteria, suggesting the potential of widespread Iif-mediated indole degradation. This work provides novel insights into the genetic background of microbial indole biodegradation.
IMPORTANCE The key finding of this research is identification of the genes responsible for microbial biodegradation of indole, a toxic N-heterocyclic compound. A large amount of indole is present in urban wastewater and sewage sludge, creating a demand for an efficient and eco-friendly means to eliminate this pollutant. A common strategy of oxidizing indole to indigo has the major drawback of producing insoluble material. Genes and proteins of Acinetobacter sp. strain O153 (DSM 103907) reported here pave the way for effective and indigo-free indole removal. In addition, this work suggests possible novel means of indole-mediated bacterial interactions and provides the basis for future research on indole metabolism.
KEYWORDS: indole, biodegradation, bacterial metabolism, Acinetobacter, bacterial signaling, cofactor-independent oxygenases
INTRODUCTION
Indole is an N-heterocyclic aromatic compound derived mainly by TnaA tryptophanase from l-tryptophan in Escherichia coli (1) and is widely found in natural environments. Indole acts as cell-to-cell signaling molecule that regulates the expression of several virulence genes (2–4), promotes biofilm formation (5–7), and mediates complex predator-prey interactions (8, 9). At high concentrations, indole and its derivatives exhibit toxic activity to both prokaryotic cells and animals and are even considered mutagens (10). Toxic indole concentrations reportedly vary for different microorganisms in the range of 0.5 to 5 mM (11). The main mechanisms of indole toxicity are reported to be an alteration of membrane potential with subsequent inhibition of cell division (12), depletion of ATP levels (13), and an inhibition of acyl-homoserine lactone (AHL)-based quorum sensing by regulator misfolding (14).
In order to utilize aromatic compounds as an energy source, microorganisms have to cope with the problem of high-resonance energy that stabilizes the aromatic ring system (15). A common strategy is the use of oxygenases and O2, which itself requires the activation of dioxygen. In addition to being thermodynamically unfavorable, reactions between dioxygen (triple state) and most of the organic compounds (singlet state) are not possible due to a spin barrier (16). Diverse elements, including transition metals (iron, manganese, and copper) or organic cofactors (flavins and pterin), are used extensively by oxygenases to form a superoxide, a reactive singlet state form of dioxygen (17). A remarkable group of cofactor-independent oxygenases have been described which require neither an organic cofactor nor a metal to catalyze the incorporation of (di)oxygen into a single molecule of an organic substrate (18, 19). Establishment of the catalytic mechanisms for this group of enzymes provides interesting mechanistic insights into substrate-assisted oxygen activation (20, 21).
To defend against the toxicity of indole, bacteria that encounter indole have established enzymatic detoxification systems, notably the oxidation of indole to insoluble nontoxic indigoid pigments (22, 23), and biodegradation mechanisms (24, 25). A number of indole-degrading bacterial microorganisms (26–28) as well as bacterial consortia (29) were reported previously, but no genetic background in these reports has been specified. Several possible intermediates in bacterial indole degradation are also known, but proteins with specific enzyme activities that drive the degradation cascade have not been identified in this context. In this paper, the identification of an indole degradation gene cluster (iif) in indole-degrading Acinetobacter sp. strain O153 is reported. The catabolic pathway was reconstituted in vitro with the recombinant proteins encoded by the iif genes, and the function of each enzyme was identified by analyzing the reaction products.
RESULTS
Screening for indole-degrading bacteria.
Numerous microbiome samples obtained from invertebrates as well as soil samples were used for the screening of indole-degrading bacteria as these environments potentially contain indole. Due to the known toxicity of indole, this compound was not used as a sole carbon source for selection of indole-mineralizing bacteria in this study. Instead, a derivative of indole was hypothesized to form a dead end pigment in the indole-degrading microorganisms. Such a strategy enabled the selection of desirable bacteria on nutrient-rich medium supplemented with the chromogenic substrate 5-bromoindoline.
A bacterial colony isolated from the intestine of the crustacean Orconectes limosus, which is a widespread invasive species in Europe, was able to convert 5-bromoindoline to insoluble indigoid pigment. The sequence of the 16S rRNA gene of this strain showed 99% similarity to the 16S rRNA gene sequence of Acinetobacter guillouiae strain NBRC 110550, and therefore strain O153 was designated Acinetobacter sp. O153. Notably, this strain did not produce indigoid pigment when grown on Luria-Bertani (LB) agar plates with indole (data not shown).
To test the ability of Acinetobacter sp. O153 to assimilate indole as a carbon (C) and/or nitrogen (N) source, cells of strain O153 were cultured in minimal medium with succinate or indole as a C source and NH4Cl or indole as an N source. No growth was observed with indole as the only C source or the only source for both N and C (Fig. 1A). However, moderate growth (up to an optical density at 600 nm [OD600] of 0.3 in 12 h) was achieved with indole (1 mM) as a nitrogen source and succinate as the sole carbon source. While this effect was observed with indole concentrations ranging from 0.5 mM to 1.5 mM, no growth was recorded with concentrations of 0.1 mM and 2 mM (Fig. 1B), which were most likely insufficient and toxic, respectively.
FIG 1.
Growth kinetics of Acinetobacter sp. strain O153. (A) Growth in minimal medium with different carbon and nitrogen sources. (B) Growth in minimal medium supplemented with different concentrations of indole as a nitrogen source and succinate as a carbon source. Filled squares, NH4Cl (5 g/liter) and succinate (5 mM) (positive control); filled triangles, indole (1 mM) and succinate; filled rhombus, NH4Cl and indole (1 mM); filled circles, indole (1 mM); open squares, no N source and succinate (negative control); open circles, 0.1 mM indole; crosses, 0.5 mM indole; open triangles, 0.75 mM indole; open rhombus, 1.5 mM indole; striped squares, 2 mM indole. Dashed lines represent trend lines using a moving average data approximation (period = 2).
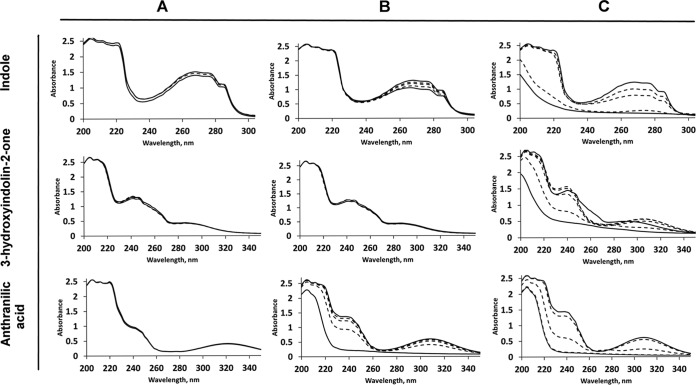
Whole-cell experiments were performed to determine whether the expression of enzymes involved in indole metabolism are inducible or constitutive. After overnight growth in a minimal medium with succinate and with or without indole, cells of strain O153 were resuspended in buffer containing 1 mM indole. Indole was transformed immediately by indole-induced O153 cells (Fig. 2). In contrast, uninduced cells consumed indole significantly more slowly and with a lag period. These results indicated that indole mineralization (biodegradation) was an inducible process.
FIG 2.
Whole-cell bioconversion assays using different substrates and cells of Acinetobacter sp. O153. (A) Substrates without cells (negative control). (B) Bioconversion with uninduced (overnight culture grown without indole) cells. (C) Bioconversion with induced (overnight culture grown in the presence of 1 mM indole) cells. Solid lines indicate spectra of initial and final products; dashed lines indicate spectra of intermediate products during a 6-h bioconversion cycle.
Identification and characterization of genes involved in indole degradation.
Screening of an Acinetobacter sp. O153 genomic library using E. coli DH5α as a host strain revealed one clone (O153H14) which was able to convert 5-bromoindoline to a purple pigment, a characteristic feature of the wild-type strain O153. Clone O153H14 was bearing a pUC19 vector with a 12-kb genomic DNA fragment. The DNA sequencing of this fragment revealed the presence of nine open reading frames (ORFs) (Fig. 3). Bioinformatic analysis of the ORFs in this fragment using NCBI's blastn algorithm and the Conserved Domain Database (CCD) (30) allowed the identification of two different sets of genes (Table 1). The iif cluster comprised the first set, and the second set encoded a multisubunit anthranilate dioxygenase. The two sets were separated by a putative araC transcription regulator. Analysis of the iif cluster suggested both high sequence identity and structural similarity to iif genes, which were shown to comprise a functional operon induced by indole in Acinetobacter baumannii ATCC 19606 (23). Hence, genes and proteins presented here were given the same nomenclature and were called Iif homologs. Five genes of the iif operon encoded putative enzymes, as described by conserved domain analysis: iifA encodes a dienelactone hydrolase family protein, iifB encodes a short-chain dehydrogenase, iifC encodes an oxygenase, iifD encodes a flavin reductase, and iifE encodes a putative phenol degradation protein. The ant cluster was composed of three genes encoding different subunits of the anthranilate dioxygenase and appeared to be of typical composition: antA and antB encoded large and small subunits of the anthranilate dioxygenase oxygenase component, respectively, while antC coded for the electron transfer component protein.
FIG 3.
Organization and distribution of iif and ant genes in different microbial genomes. Genes are represented by arrows (drawn to scale as indicated). Homologous genes are highlighted in the same pattern according to the scheme in the top line for Acinetobacter sp. O153. White arrows, indole degradation unrelated/unknown. Strains and genomic fragments boxed in solid lines indicate reported activity of certain Iif-homologous proteins; dashed boxes highlight strains for which indole biodegradation activity was reported at the genus level. Identities (percent) of amino acid sequences between Iif proteins of strain O153 and homologs are indicated under the corresponding ORFs.
TABLE 1.
Predicted conserved domains and putative functions of proteins encoded by genes identified in a 12-kb genomic fragment of Acinetobacter sp. O153
| Protein | Conserved domain (identifier) | E value | Closest homolog with known function |
||||
|---|---|---|---|---|---|---|---|
| Proteina | GenBank accession no. or identifier | Function | Identity (%) | Reference | |||
| IifA | α/β hydrolase (COG0412) | 2.3e−53 | ClcD from P. knackmussii B13 | AAB71539.1 | Dienelactone hydrolase | 34 | 66 |
| SnoaL-like polyketide cyclase (pfam07366) | 0.04 | ||||||
| IifB | Short-chain dehydrogenase (cd05233) | 1.2e−56 | FabG from V. cholerae N16961 | VC2021 | Reduction of β-ketoacyl-ACP to 3-hydroxyacyl-ACP | 33 | 67 |
| IifC | IifC from A. baumannii ATCC 19606 | F911_02004 | Monooxygenase | 82 | 23 | ||
| IifD | Flavin reductase (COG1853) | 6.8e−40 | IifD from A. baumannii ATCC 19606 | F911_02003 | Flavin reductase | 81 | 23 |
| IifE | MetA-pathway of phenol degradation (cl01768) | 1.3e−57 | Pput2725 from P. putida F1 | 4RL8 | Outer membrane channel of unknown function | 21 | 68 |
| IifR | AraC-binding-like domain (pfam14525) | 4e−39 | IifR from A. baumannii ATCC 19606 | F911_02001 | Transcriptional regulator | 74 | 23 |
| AntA | Rieske non-heme iron oxygenase (cd03469) | 9e−46 | AntA from Acinetobacter sp. ADP1 | AAC34813.1 | Oxygenase component of anthranilate dioxygenase | 93 | 31 |
| C-terminal catalytic domain of the oxygenase alpha subunit (cd08879) | 9e−83 | ||||||
| AntB | Ring hydroxylating beta subunit (cd00667) | 2e−51 | AntB from Acinetobacter sp. ADP1 | AAC34814.1 | Oxygenase component of anthranilate dioxygenase | 94 | 31 |
| AntC | Benzoate dioxygenase reductase FAD/NAD binding domain (cd06209) | 3e−107 | AntC from Acinetobacter sp. ADP1 | AAC34815.1 | Reductase component of anthranilate dioxygenase | 87 | 31 |
| 2Fe-2S cluster (cd00207) | 3e−21 | ||||||
P. knackmussii, Pseudomonas knackmussii; V. cholerae, Vibrio cholerae.
Distribution of iif homologs among bacterial genomes.
The prevalence of iif operon genes as well as genes of anthranilate dioxygenase among available microbial genomes was analyzed. Mainly organisms from the genera Acinetobacter, Pseudomonas, and Burkholderia were identified, including some notoriously pathogenic strains of A. baumannii, Pseudomonas fluorescens, and Pseudomonas syringae (Fig. 3). Spatial separation of the two operons was found to be a common indication outside the genus Acinetobacter, stretching to as long as 200 kb (Burkholderia cepacia ATCC 25416 and Alcaligenes faecalis ZD02) or even separate replicons (Burkholderia contaminans MS14, Cupriavidus metallidurans CH34, and Cupriavidus basilensis 4G11). Also, aberrant composition of the iif operon was predicted in several cases with four genomes (P. fluorescens NCIMB 11764, C. metallidurans CH34, C. basilensis 4G11, and Ralstonia solanacearum PSI07) lacking the iifE homolog.
Enzyme activity of recombinant Iif proteins.
To identify the enzymatic functions of Iif proteins, iifA, iifB, iifC, iifD, and iifE genes were expressed in E. coli BL21(DE3) with an N-terminal His tag, purified, and tested for activity toward indole, which was monitored by high-performance liquid chromatography–mass spectrometry (HPLC-MS). Molecular masses of the purified proteins observed in SDS-PAGE gels corresponded well to the theoretical masses (46 kDa for IifA, 28.1 kDa for IifB, 46.3 kDa for IifC, and 19.9 kDa for IifD) (see Fig. S1 in the supplemental material).
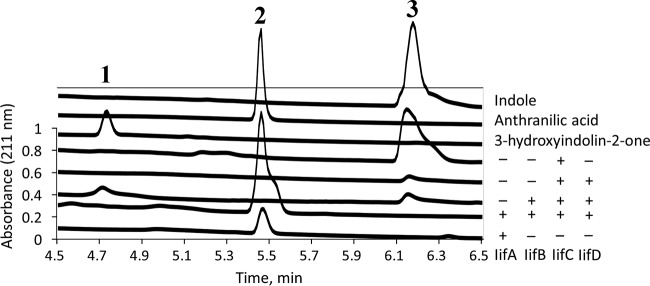
IifC was found to be capable of using indole as a substrate only in the presence of IifD flavin reductase, flavin adenine dinucleotide (FAD) cofactor, and NAD(P)H (Fig. 4, peak 3). The product of this reaction was a blue insoluble indigoid pigment (Fig. S2). Next, when IifB was added to the reaction mixture (IifC, IifD, FAD, NADPH, and indole), indigo formation was abolished, and a product with a retention time (4.75 min) identical to that of 3-hydroxyindolin-2-one was observed (Fig. 4, peak 1). The UV-visibile light (UV-Vis) absorption spectra of this compound and 3-hydroxyindolin-2-one were very similar, with peaks at 211 nm, 253, and 291 nm (Fig. S3E and F). Also, both of these compounds demonstrated identical molecular masses: 150 (3-hydroxyindolin-2-one + H+) and 132 (3-hydroxyindolin-2-one − H2O + H+) (Fig. S3E and F). Moreover, 3-hydroxyindolin-2-one was consumed by wild-type indole-induced Acinetobacter sp. O153 cells in a similar way as indole (Fig. 2). 3-Hydroxyindolin-2-one was rapidly transformed into anthranilic acid, which was then slowly consumed by indole-induced cells. Meanwhile, no consumption was observed with uninduced cells. These results indicated that 3-hydroxyindolin-2-one was the reaction product of an IifB-catalyzed reaction and did not act as an inducer for indole-degrading proteins.
FIG 4.
Stacked HPLC chromatograms (211 nm) of enzymatic reaction products and standards. Top three curves, standards; middle four curves, reaction products of indole and Iif proteins as indicated on the right; bottom curve, reaction product of 3-hydroxyindolin-2-one and IifA. Numbers indicate peaks of three major compounds: peak 1, 3-hydroxyindolin-2-one; peak 2, anthranilic acid; peak 3, indole.
When four Iif proteins (IifC, IifD, IifB, and IifA) were used in a reaction with indole, a compound with m/z 138 [M+H+] was identified, corresponding to [137+H+], with the molecular mass of anthranilic acid (Fig. S3A and B). In addition, this reaction product and anthranilic acid shared identical retention times (Fig. 4, peak 2) and UV-Vis absorption spectra (Fig. S3A and B). Importantly, IifA protein was also capable of using 3-hydroxyindolin-2-one as the substrate to form anthranilic acid (Fig. 4, bottom curve). Notably, conversion of 3-hydroxyindolin-2-one to anthranilic acid by IifA did not require any cofactor or metal ion and did not occur with other substrates of similar structure (isatin or N-formyl-anthranilic acid). The presence of IifE protein in any of the above-mentioned reaction mixtures did not change the outcomes of the reactions. Thus, analysis of reaction products formed by IifA both supported the involvement of 3-hydroxyindolin-2-one as an intermediate in indole degradation and showed the formation of anthranilic acid, a possible end product of IifCDBA-catalyzed indole biodegradation.
The kinetic parameters of IifA, IifC, and IifD proteins were determined (Table 2). IifD flavin reductase showed a preference for flavin mononucleotide (FMN) and NADH. In spite of that, FAD, but not FMN or riboflavin, was identified as the only flavin cofactor suitable for indole oxidation performed by IifC (data not shown).
TABLE 2.
Kinetic parameters of IifD, IifC and IifA proteinsa
| Protein and substrate or cofactor | Km (μM) | Kcat (min−1) | Kcat/Km (min−1 μM−1) |
|---|---|---|---|
| IifD | |||
| NADH | 29 ± 0.1 | 1.21 ± 0.15 | 0.041 |
| NADPH | 7.9 ± 0.4 | 0.13 | 0.017 |
| FMN | 2.85 ± 0.19 | 2.7 ± 0.45 | 0.94 |
| FAD | 7.07 ± 0.12 | 7.02 ± 0.64 | 0.99 |
| Riboflavin | 8.35 ± 0.83 | 3.1 ± 0.43 | 0.38 |
| IifC | |||
| Indole | 250 ± 13 | 0.11 ± 0.01 | 0.00047 |
| IifA | |||
| 3-Hydroxyindolin-2-one | 281.7 ± 13.4 | 2476 ± 310 | 17.52 |
Values are means ± standard deviations.
Identification of IifA as a possible cofactor-independent oxygenase.
Structural and functional approaches were used to analyze the enzymatic mechanism of IifA. The conversion of 3-hydroxyindolin-2-one to anthranilic acid by purified recombinant IifA did not require the addition of any cofactor or metal ions. No cofactor-indicating peaks were observed in the UV-Vis spectrum of the purified IifA protein (Fig. S4). Addition of 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.5 mM HgCl2, or 3 mM EDTA did not inhibit the reaction, suggesting a nonhydrolytic metal ion-independent enzymatic conversion. Moreover, oxygen was consumed in the above-mentioned reaction in a substrate-dependent manner (Fig. 5A); i.e., addition of 3-hydroxyindolin-2-one equivalent to the oxygen concentration resulted in complete consumption of oxygen, while a 1:2 molar ratio of substrate/oxygen consumed half of the oxygen present in an air-restricted reaction cell. Notably, the reaction did not occur in the absence of dioxygen (Fig. S5). Oxidation of 3-hydroxyindolin-2-one to anthranilic acid by IifA was monitored spectrophotometrically, and a decrease in absorbance at 260 nm as well as increases in absorbance at 225 nm and 315 nm was observed (Fig. 5B). The presence of two isosbestic points indicated that no intermediates were formed during this reaction.
FIG 5.
Functional characterization of IifA. (A) Oxygen consumption by IifA. Red, green, and blue indicate different substrate concentrations as indicated. Reactions were initiated at 90 s. (B) UV spectra of the transition between 3-hydroxyindolin-2-one and anthranilic acid, catalyzed by IifA. The inset shows the spectral difference between the substrate and the product.
Protein sequence analysis of IifA performed using the CDD indicated the presence of two domains: an N-terminal dienelactone hydrolase (DLH)-like domain and C-terminal SnoaL-like domain (Fig. S6B). Attempts to obtain separate recombinant domains were unsuccessful due to insolubility, suggesting a major structural role of both domains during protein folding. A three-dimensional model of IifA was generated with the I-TASSER platform. A standard α/β hydrolase fold was located in the N-terminal domain and was composed of a β-sheet of seven strands, with the first strand antiparallel to the others. A typical catalytic triad, common to the majority of hydrolases, was located in this domain and was composed of Cys124, Asp173, and His204 (Fig. S6A). Residues of this putative catalytic triad were located close to each other in the three-dimensional model. Although the overall sequence identity between the N-terminal domain of IifA and well-described cofactor-independent dioxygenases Hod and Qdo was low (17.9% with Hod and 19.1% with Qdo), the structural resemblance was clearly evident (Fig. S7).
DISCUSSION
The present article deals with identification of genes in Acinetobacter sp. strain O153 that are required for degradation of the N-heterocyclic compound indole. These genes are clustered into an Iif operon, with five genes coding for potential enzymes. The initial description of proteins encoded by genes of the Iif operon clearly showed the ability of IifC to oxidize indole leading to formation of indigo pigment (23). Here, the ability of IifC to oxidize indole to indigo was confirmed, but no appearance of indigo was observed when three proteins, namely, IifC, IifD, and IifB, were incubated with indole in vitro although the concentration of indole readily decreased. Incorporation of all four proteins (IifC, IifD, IifB, and IifA) into the reaction mixture resulted in the formation of anthranilic acid. Importantly, genes encoding a multisubunit anthranilate dioxygenase are located close to the iif operon in most of the organisms potentially capable of degrading indole or located in separate chromosomes, as in the case of Cupriavidus metallidurans CH34 and C. basilensis 4G11 (Fig. 3). Anthranilate dioxygenase converts anthranilic acid to catechol (31), which is then catabolized through a well-established β-ketoadipate pathway (32). Such a degradation pathway where indole is oxidized to metabolites, directly reaching the tricarboxylic acid (TCA) cycle, might enable microorganisms to both counteract the toxic effect of indole and use it as a carbon and energy source. Although this may be true for some other microorganisms with reported indole utilization as a sole carbon source and an iif-ant combination identified, strain O153 did not grow with indole as the only carbon source. This suggests difficulties in coping with the toxic effects of indole (see the introduction and references cited therein) or insufficient metabolic activity to shuttle the indole degradation products into the TCA cycle, for example, as a result of low expression levels or relatively poor catalytic efficiency of catechol dioxygenase (33, 34). Still, anthranilic acid was consumed by the cells of the strain O153 with no product-indicating spectra suggesting utilization of anthranilate in other cellular processes, possibly as a substrate for anthranilate phosphoribosyltransferase during the synthesis of tryptophan (35). Strain O153 used indole (at concentrations ranging from 0.5 to 1.5 mM) as the sole nitrogen source, indicating a physiological benefit when the bacteria occupy indole-containing environments.
Based on the results presented above, a scheme for indole biodegradation in Acinetobacter sp. strain O153 is proposed (Fig. 6). Degradation starts with indole oxidation at the C-2 and C-3 positions, forming indole-2,3-dihydrodiol. This compound is known to be rather unstable (22) and therefore was not detected. A spontaneous dehydration of indole-2,3-dihydrodiol results in the formation of indoxyl, which is prone to auto-oxidation and forms an insoluble indigo pigment. However, the loss of a water molecule could be prevented by IifB, forming a stable intermediate. The hypothetical short-chain dehydrogenase IifB performs oxidation at the C-2 position to obtain 3-hydroxyindolin-2-one. Anthranilic acid, formed by IifA, is then the end product of IifCDBA-catalyzed indole biodegradation.
FIG 6.
Proposed indole biodegradation pathway in Acinetobacter sp. O153: step 1, indole; step 2, indole-2,3-dihydrodiol; step 3, 3-hydroxyindolin-2-one; step 4, anthranilic acid; step 5, isatin; step 6, 1H-indol-3-ol (indoxyl); step 7, indigo. Brackets indicate an intermediate not experimentally detected.
Proteins with notable sequence similarity to Iif proteins were described earlier, leading to coherent concepts and hypotheses. First, the MoxY (65% sequence identity to IifC) and MoxZ (38% sequence identity to IifD) proteins with indole oxidation activity were suggested to be involved in the production of a quorum-sensing-inducing compound (36). Later, a genomic fragment of Rhodococcus opacus 1CP containing several genes with high structural similarity to the Iif operon was published (37), emphasizing its role in styrene biodegradation. Monooxygenase activity of this fragment was detected by indole oxidation to indigo and was attributed specifically to StyA2B, a styrene-epoxidizing fusion protein containing both oxidation and flavin-reduction activities (47% sequence identity between the N-terminal segment of StyA2B and IifC; 52% sequence identity between IifD and the C-terminal segment of StyA2B). ORFs 6 and 9, comprising a possible structural homolog of IifA of this fragment and forming a hypothetical dienelactone hydrolase, were interrupted by transposase, most likely rendering this protein inactive. A complete set of Iif-homologous genes was then found in R. opacus MR11, including an adjacent multisubunit anthranilate dioxygenase, leading to the hypothesis that the locus described therein might be involved in the degradation of heteroaromatic compounds (38). Finally, the indole detoxification ability of the IifC-IifD system as well as the flavin oxidoreductase activity of IifD was demonstrated in representatives of the genus Acinetobacter (23, 39). From the current perspective, it appears that a link between high metabolic versatility of acinetobacters and indole as the substrate for Iif proteins is clearly evident, suggesting biodegradation as the fate of indole when exposed to Iif-containing bacteria.
In the proposed model of indole biodegradation, IifC should act as a dioxygenase, incorporating oxygen atoms at both the C-2 and C-3 positions. However, IifC appears to have typical structural elements of flavin-containing monooxygenases (23). Although difficult to detect experimentally, indole dioxygenation was reported for a number of bacterial dioxygenases and monooxygenases (25, 40). Indole-2,3-dihydrodiol, a product of possible dioxygenase attack, was observed in the biotransformation of indole by Cupriavidus sp. strain KK10 (41). Notably, several species of Cupriavidus were identified to possess the iif-ant cluster (Fig. 3). Indole-2,3-dihydrodiol was not observed directly as a reaction intermediate during the indole degradation described here, which might be attributed to the instability of this compound (22). Importantly, the formation of indigo from indole-2,3-dihydrodiol was detected in an indole oxidation reaction catalyzed by naphthalene dioxygenase (22). A possible mechanism of indole-2,3-dihydrodiol formation might be hydrolysis of indole 2,3-epoxide, which was identified as an intermediate during indole epoxidation performed by a styrene monooxygenase (42) and was suggested to form 2,3-trans diol spontaneously or enzymatically (43). The opening of the epoxide to a diol was presumed to be a plausible pathway for indole-2,3-dihydrodiol formation (44). Moreover, the oxidation of several N-heterocyclic compounds catalyzed by monooxygenases when two oxygen atoms (one from oxygen, another from water) were inserted was described recently (45–47). A similar mechanism for bacterial degradation of indole acetic acid was described (48) involving the intermediate dioxindole-3-acetic acid (DOAA), a structural analogue of 3-hydroxyindolin-2-one in indole degradation. Formation of DOAA was not attributed to a single enzymatic step, and either a second hydroxylation or hydroxy/keto oxidoreduction mechanisms were suggested. Given these points, deciphering the mechanism of IifC requires further structural studies.
The role of IifE in the degradation of indole remains poorly understood and might even be ruled out based on experimental evidence. First, no changes were observed when recombinant IifE protein was added to a reaction mixture consisting of indole and other Iif proteins; i.e., such a reaction still yielded anthranilic acid with complete substrate consumption. Next, the expression levels of IifE did not change upon addition of indole, according to quantitative reverse transcription-PCR (qRT-PCR) results (23). Finally, the gene coding for IifE appears to be absent in genomes of several microorganisms that possess the iif operon. Interestingly, the presence of a putative signal sequence was detected in this protein, with a cleavage site between residues 24 and 25 (AQA-YD), using SignalP (49). Also, this protein showed low sequence identity to the Pput2725 membrane channel from Pseudomonas putida F1. Taken together, such inconsistent insights provide no precise functional description for this protein, but they suggest possible functions, such as facilitation of indole uptake or other metabolic activities, that are worth exploring.
As already noted, a distinct group of extraordinary dioxygenases catalyzes the incorporation of oxygen atoms into organic substrates with no requirements for cofactors. Structural and functional properties of IifA appear to be very similar to those of the best described cofactor-independent dioxygenases such as 1H-3-hydroxy-4-oxoquinoline 2,4-dioxygenase (Qdo) from Pseudomonas putida 33/1 and 1H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase (Hod) from Arthrobacter ilicis Rü61a (50). All of these enzymes share an α/β structural fold and a speculative catalytic triad, which was demonstrated to act rather as a dyad as mutants of catalytic Ser retained substantial activity (18, 50). Catalytic properties of IifA also resemble those of cofactor-free dioxygenase from an aerobic Gram-positive coccus participating in indole biodegradation described by Fujioka and Wada (51). This cofactor-free dioxygenase was able to convert 2-oxo-3-hydroxyindoline to stoichiometric amounts of anthranilic acid and CO2 with the consumption of equivalent amounts of dioxygen (51). As it stands, the establishment of IifA as an oxygenase catalyzing the ring-opening reaction requires additional experiments to elucidate the fate of the C-2 carbon atom of 3-hydroxyindolin-2-one during the oxidation process as well as site-directed mutagenesis to prove the role of putative catalytic amino acid residues, and these experiments are under way.
Notably, a number of bacterial genera with iif and ant operons identified in this work have already been described as indole-degrading microorganisms. B. unamae strain CK43B was found to degrade indole under aerobic conditions when supplemented with gallic acid or pyrogallol (27), showing a similar cometabolic effect of indole degradation as in the case of strain O153. Importantly, indoxyl was detected as the reaction intermediate as well as compounds of the β-ketoadipate pathway, anthranilic acid, and catechol, signifying the possible relationship between Iif proteins and indole biodegradation in the genus Burkholderia. Cupriavidus sp. strain SHE was also reported to be able to perform indole biodegradation (28); however, no intermediates were identified, except for isatin. A compound with an m/z corresponding to that of isatin was also observed during Iif-catalyzed indole biodegradation. Nonetheless, based on results presented here indicating (i) that, in contrast to indole, isatin was not consumed by resting cells during an uptake assay and (ii) that isatin was not a substrate for any of the Iif proteins described here, we suggest that isatin might not be an intermediate during Iif-catalyzed indole degradation but, rather, appeared as a dead end product of spontaneous oxidation or an experimental artifact. While indole biodegradation characteristics were also evaluated for representatives of the genera Pseudomonas (P. aeruginosa strain Gs) and Alcaligenes (Alcaligenes sp. strain In3) (26, 52), no data are available regarding the biodegradation of indole in the genus Acinetobacter to date. Such a high prevalence of the iif operon might be explained by a dual beneficial effect of indole degradation. Indole, produced mainly by E. coli in the intestine, can decrease the virulence of P. aeruginosa (53). This effect was later attributed to the indole-caused inhibition of AHL-based quorum-sensing signaling by altering the folding of the AqsR regulator (14, 54). Such an effect was reported for a number of Gram-negative bacteria, including Chromobacterium violaceum, Pseudomonas chlororaphis, Serratia marcescens (55), and Acinetobacter oleivorans DR1 (14), a strain which was identified here to possess the iif-ant operon combination (Fig. 3). In a complex and competition-prone environment such as the gastrointestinal tract, indole production might become an advantage if the producer does not utilize AHL-based signaling, which is the case for E. coli (56). The degradation of indole and its utilization as an additional carbon and energy source using the Iif-AntABC enzymatic system described here might be a possible solution for AHL producers, including members of the genus Acinetobacter (57), to counteract the toxic indole effect and retain fitness. Taken together, the concordance between de facto indole-degrading microbial genera and the prevalence of the iif and ant operon combination suggests that proteins encoded by the iif operon might be responsible for indole biodegradation in the majority of microorganisms described to date as indole degraders.
MATERIALS AND METHODS
Bacterial strains, chemicals, and standard techniques.
Escherichia coli DH5α and BL21(DE3) strains were used as cloning and protein expression hosts, respectively. Transformation of bacteria by electroporation was performed as described previously (58). E. coli strains carrying plasmids were cultivated in Luria-Bertani (LB) medium supplemented with antibiotics (100 μg/ml ampicillin or 50 μg/ml kanamycin). Plasmid DNA was isolated using a ZR Plasmid Miniprep Classic kit (Zymo Research). Indole, isatin, and anthranilic acid were purchased from Sigma-Aldrich; 5-bromoindoline was obtained from Combi-Blocks, Inc. All other chemicals used in this study were of analytical grade. All media and reagent solutions were prepared with Milli-Q water (Merck Millipore).
Isolation and identification of an indole-oxidizing bacterial strain.
Indole-oxidizing microorganisms were isolated from the intestine of Orconectes limosus. Crustacean samples were collected at Lake Ruzis, Alytus district, Lithuania. Intestines were suspended in 0.9% NaCl solution and plated on LB agar plates supplemented with 1 mM 5-bromoindoline. Following an overnight incubation at 30°C, purple pigment-forming colonies were isolated and maintained as pure cultures. The 16S rRNA gene of the isolate strain was amplified with universal bacterial primers 27F and 1492R (Table 3) according to the method of Frank et al. (59) and sequenced. Phylogenetic analysis was performed with the blastn algorithm (https://blast.ncbi.nlm.nih.gov) (58) against the database of 16S rRNA sequences.
TABLE 3.
Primers used in this studya
| Primer name | Sequence (5′–3′) | Purpose | Reference or source |
|---|---|---|---|
| CMBFNdeI | GATCATATGTCAGGCCAAGATATTGAA | Amplification and cloning of iifA gene | This work |
| CMBRXhoI | CTCTCGAGTTAAAACGATTTGCCTTCA | ||
| SCDFNdeI | GAGTGGCATATGGATATTGAATTGAATCAG | Amplification and cloning of iifB gene | This work |
| SCDRXhoI | ATCTCGAGTCACGCTTCATCGGCTA | ||
| APRLF | GATCATATGCGTCGTATCGCTATAG | Amplification and cloning of iifC gene | This work |
| APRLR | CTACTCGAGTTAAGCCACTTTTTGAC | ||
| iifDFNheI | TGGCTAGCATGAATATCAACACATC | Amplification and cloning of iifD gene | This work |
| iifDRXhoI | CTGCTCGAGTTAAATACTCAGTTC | ||
| MetAFNcoI | CTCCATGGCCAATAATGTGATCAAAAG | Amplification and cloning of iifE gene | This work |
| MetARHindIII | GAAGCTTTCAGAAGTGATGAATATAAC | ||
| 27F | AGAGTTTGATCMTGGCTCAG | Amplification and sequencing of 16S rRNA gene | 59 |
| 1492R | TACGGYTACCTTGTTACGACTT |
Underlined nucleotide sequences are introduced sequences for recognition of restriction endonucleases.
Genomic DNA of strain O153 was extracted as described previously (60), digested with HindIII (ThermoFisher Scientific) to obtain 5- to 20-kb DNA fragments, which were cloned into pUC19 (ThermoFisher Scientific). The resulting library was transformed into E. coli DH5α; cells were plated on LB agar with 5-bromoindoline, incubated overnight at 37°C, and screened for purple pigment-producing colonies.
Whole-cell bioconversion assays and growth conditions.
For growth kinetics experiments, an overnight culture of strain O153 grown in LB medium was centrifuged, washed with potassium phosphate buffer, and resuspended in 0.9% NaCl. Cells were diluted in fresh M9 medium with different carbon (5 mM succinate or 1 mM indole) and nitrogen (5 g/liter NH4Cl or different concentrations of indole) sources. Cell suspensions were incubated at 30°C with shaking (180 rpm). Growth was monitored by measuring the absorbance at 600 nm. At least three independent experiments were performed.
For whole-cell experiments, an overnight culture of Acinetobacter sp. O153 was grown in M9 medium (3.5 g/liter Na2HPO4, 1.5 g/liter KH2PO4, 2.5 g/liter NaCl, 0.2 g/liter MgSO4, 0.01 g/liter CaCl2) supplemented with 5 mM succinate and 1 mM indole (induced cells) or with succinate and 5 g/liter NH4Cl (uninduced cells). Cells were pelleted by centrifugation, washed with 20 mM potassium phosphate buffer, pH 7.8, and resuspended in M9 minimal medium to a final OD600 of 0.8 in a volume of 1 ml containing a 1 mM concentration of the appropriate substrate. Cells were incubated at 30°C with shaking. A sample without cells was used as an evaporation (negative) control. Absorbance spectra of the cell-free supernatants were recorded every hour to monitor substrate consumption.
Cloning, expression, and purification of proteins involved in indole degradation.
All reagents used for the cloning experiments were purchased from ThermoFisher Scientific (Lithuania). Target genes were amplified by PCR in a Mastercycler ep Gradient S (Eppendorf) instrument using the primer pairs listed in Table 3, Maxima Hot Start Green PCR master mix, and a colony of Acinetobacter sp. O153 cells. PCR conditions were as follows: initial denaturation at 98°C for 4 min, followed by 30 cycles of denaturation at 98°C for 30s, annealing at various temperatures for 30s, elongation at 72°C for 90s, and a final elongation at 72°C for 10 min. DNA fragments obtained were cloned into pTZ57R/T vector according to the manufacturer's protocol and sequenced. Resulting plasmids were digested with restriction endonucleases that recognize the primer-introduced sequences (Tables 3 and 4), and fragments were ligated into pET28-c(+) (Novagen) previously digested with the same restriction endonucleases. Expression plasmids with N-terminally encoded 6×His tags were transformed into E. coli BL21(DE3) for protein expression. Protein expression and purification were carried out as described earlier (61). Protein concentration was determined with a Coomassie protein assay kit (Pierce) using bovine serum albumin as the standard.
TABLE 4.
Plasmids used in this study
| Plasmid | Description and/or purpose | Source |
|---|---|---|
| pTZ57R/T | TA cloning of PCR products | ThermoFisher Scientific (Lithuania) |
| pUC19 | Construction of genomic library | ThermoFisher Scientific (Lithuania) |
| pET28-c(+) | Expression of recombinant proteins | Novagen (Germany) |
| pET28-iifA | iifA gene, amplified by PCR with CMBFNdeI and CMBRXhoI primers, digested with NdeI/XhoI, and cloned into pET28-c(+) for expression of recombinant N-terminally 6×His-tagged IifA | This work |
| pET28-iifB | iifB gene, amplified by PCR with SCDFNdeI and SCDRXhoI primers, digested with NdeI/XhoI, and cloned into pET28-c(+) for expression of recombinant N-terminally 6×His-tagged IifB | This work |
| pET28-iifC | iifC gene, amplified by PCR with CMBFNdeI and CMBRXhoI primers, digested with NdeI/XhoI, and cloned into pET28-c(+) for expression of recombinant N-terminally 6×His-tagged IifC | This work |
| pET28-iifD | iifD gene, amplified by PCR with iifDFNheI and iifDRXhoI primers, digested with NheI/XhoI, and cloned into pET28-c(+) for expression of recombinant N-terminally 6×His-tagged IifD | This work |
| pET21-iifE | iifE gene, amplified by PCR with MetAFNcoI and MetARHindIII primers, digested with NcoI/HindIII, and cloned into pET28-c(+) for expression of recombinant IifE | This work |
Enzyme assays and detection of reaction products.
Flavin reductase activity of the purified IifD protein was determined from the decrease of the absorbance at 340 nm due to oxidation of NADH or NADPH (ε340 = 6,220 M−1 cm−1), using a spectrophotometer and was performed at room temperature. A total reaction volume of 1 ml contained 50 mM Tris-HCl, pH 7.5, variable amounts of NADH or NADPH, and flavins (FAD, FMN, or riboflavin). Kinetic parameters for NADH and NADPH were determined using a constant concentration of FAD (20 μM) and various concentrations of NAD(P)H (5 to 200 μM). In addition, various concentrations of flavin (1 to 40 μM) and a constant concentration of NADH (200 μM) were used for analysis of the enzyme kinetics. Reactions were initiated by adding 0.3 μg of the enzyme. One unit (U) of enzyme activity was defined as the amount of enzyme catalyzing the oxidation of 1 μmol of NAD(P)H per minute.
Indole oxidation activity of IifC was determined as a function of indigo formation in the reaction mixture. An initial indole oxidation rather than spontaneous dimerization was assumed to be a rate-limiting step. A typical reaction mixture contained 50 mM Tris-HCl, pH 7.5, 100 μM flavin, 250 μM NADH or NADPH, and various concentrations of indole (10 to 500 μM) and was initiated by adding 3 μg of IifD as well as 3 μg of IifC. Reaction mixtures were incubated at room temperature for 15 min, and indigo particles were pelleted by centrifugation at 16,000 × g for 5 min. The precipitant was dissolved in 100 μl of dimethylformamide (DMF); dissolving was facilitated by incubating the mixture at 37°C for 15 min. Indigo concentration was determined as described previously (62), using the molar absorption coefficient ε620 = 14,000 M−1 cm−1. One unit of enzyme activity was defined as the amount catalyzing the formation of 1 μmol of indigo per minute.
Oxygenase activity of IifA was measured from the increase of absorbance at 315 nm (formation of anthranilic acid from 3-hydroxyindolin-2-one). A typical assay mixture contained various concentrations (10 to 300 μM) of 3-hydroxyindolin-2-one (synthesized as described below) in 50 mM Tris-HCl, pH 7.5, air-saturated (oxygen concentration, ∼250 μM) buffer. Reactions were initiated by adding 3 μg of IifA, and the concentration of anthranilic acid formed was determined using the molar absorption coefficient ε315 = 1,600 M−1 cm−1 (calculated using the Beer-Lambert law and data on the absorbance of 0.05 to 5 mM anthranilic acid in 50 mM Tris-HCl, pH 7.5). One unit of enzyme activity was defined as the amount catalyzing the formation of 1 μmol of anthranilic acid per minute under the conditions described above. All kinetic parameters were determined by fitting the data to Lineweaver-Burk (double-reciprocal) plots and performing a linear regression. All kinetic experiments were performed in duplicate, and average means were derived.
Enzymatic conversion of indole by Iif proteins was analyzed by monitoring substrate consumption and formation of corresponding reaction products in in vitro reactions. Under standard conditions, a total volume of 250 μl of reaction mixture contained 50 mM Tris-HCl, pH 7.5, 50 μM flavin cofactor, 100 μM NAD(P)H, 1 mM indole, and 3 μg of each purified Iif protein. The combinations of proteins tested were as depicted in Fig. 4. Reaction mixtures were incubated at room temperature for 1 h with shaking (700 rpm; Eppendorf Thermomixer Comfort). Substrate consumption and formation of products were analyzed spectrophotometrically in PowerWave XS plate reader (BioTek Instruments, Inc.) or mixed with an equal volume of acetonitrile, centrifuged at 16,000 × g for 5 min, and analyzed by HPLC-MS.
Oxygen consumption and anaerobic assay.
The measurement of oxygen consumption was performed with a homemade computer-assisted membrane oxygen electrode and 10.0-ml glass cell. The concentration of oxygen was assumed to be 0.25 mM in air-saturated 50 mM Tris-HCl, pH 7.5, buffer solution at 25°C. Different concentrations of 3-hydroxyindolin-2-one (82, 136, and 273 μM) were used, and reactions were initiated by adding 3 μg of IifA.
Anaerobic conditions were generated in an air-restricted cell by argon bubbling of Tris-HCl buffer solution, pH 7.5, at 25°C for 10 min. Prior to bubbling, traces of oxygen were eliminated from argon gas by passage through a solution of alkaline pyrogallol (benzene-1,2,3-triol).
HPLC-MS.
HPLC-MS analyses were performed using a high-performance liquid chromatography system (CBM-20A controller, two LC-2020AD pumps, SIL-30AC auto sampler, and CTO-20AC column oven; Shimadzu, Japan) equipped with a photodiode array (PDA) detector (Shimadzu, Japan) and mass spectrometer (LCMS-2020; Shimadzu, Japan) equipped with an electrospray ionization (ESI) source. The chromatographic separation was conducted using a YMC Pack Pro column (3 by 150 mm; YMC, Japan) at 40°C and a mobile phase that consisted of 0.1% formic acid water solution (solvent A) and acetonitrile (solvent B) delivered in the 0 to 60% gradient elution mode. Mass scans were measured from m/z 10 up to m/z 700 at a 350°C interface temperature, 250°C desolvation line (DL) temperature, ±4,500 V interface voltage, and neutral DL/Qarray, using N2 as nebulizing and drying gas. Mass spectrometry data were acquired in both positive and negative ionization modes. The data were analyzed using LabSolutions liquid chromatography-mass spectrometry (LCMS) software.
Chemical synthesis of 3-hydroxyindolin-2-one.
Reduction of isatin to 3-hydroxyindolin-2-one was performed according to Bergonzini and Melchiorre (63). Isatin (1.5 mmol) was added in small portions to a stirred suspension of sodium borohydride (0.75 mmol; 2 eq) in 20 ml of a 1:1 water-ethanol mixture at room temperature. The mixture was vigorously stirred until the suspension became colorless (about 10 min). The mixture was extracted with chloroform (3 times, 10 ml each). The combined organic extracts were dried (MgSO4), and the solvent was evaporated under reduced pressure. The residual material was dissolved in 5 ml of deionized water and was purified by reverse-phase chromatography (12 g; C18 cartridge) to separate the 3-hydroxyindolin-2-one from the pigments formed during the extraction and evaporation procedures. Prior the purification, the column was equilibrated with water. A mobile phase that consisted of water and methanol was delivered in the gradient elution mode. The collected fractions were analyzed by HPLC-MS. The fractions containing a pure product were combined, and the solvent was removed under reduced pressure.
The structures of the bioconversion products were determined using 1H nuclear magnetic resonance (NMR) and 13C NMR. 1H NMR spectra were recorded in hexadeuterodimethyl sulfoxide (DMSO-D6) or CDCl3 on a Bruker Ascend 400 at 400 MHz, and 13C NMR spectra were recorded on a Bruker Ascend 400 at 100 MHz. All products were dissolved in deuterated dimethyl sulfoxide. Spectra were calibrated with respect to the solvent signal (CDCl3, 1H δ = 7.26, 13C δ = 77.2; DMSO-D6, 1H δ = 2.50, 13C δ = 39.5).
Sequence alignments and structure modeling.
Genome sequences containing genes with high sequence similarity to iif genes were identified with the blastn suite (https://blast.ncbi.nlm.nih.gov) by using each of the five iif genes individually. Hits with E values less than 5e−10 were pooled and used as a database in MultiGeneBlast (64). A homology search was performed using an iif locus of A. guillouiae genomic DNA (ranging from nucleotides 1661941 to 1671563) as a query with default parameters except that the maximum distance between genes in the locus was increased to 100 kb. Positive variants were manually proofread with respect to the structural organization of the iif and ant genes as well as possible orientation in separate DNA fragments.
The model of tertiary structures of the N-terminal (amino acids 1 to 233) and C-terminal (amino acids 252 to 374) domains of the IifA protein were obtained using the I-TASSER platform (http://zhanglab.ccmb.med.umich.edu/I-TASSER) (65). Structures of dienelactone hydrolases (PDB accession numbers 1DIN and 1ZI8) were used as threading templates for modeling of the N-terminal domain, and structures of putative polyketide cyclases (PDB accession numbers 4LGQ and 3F9S) were used as templates for the C-terminal domain. Models with the highest C-scores (1.42 and 0.26 for N- and C-terminal domains, respectively) were selected for further structural analysis and comparison with structures of cofactor-independent oxygenases.
Accession number(s).
Sequence data described in this paper have been submitted to GenBank database under the following accession numbers: 16S rRNA gene of strain Acinetobacter sp. O153, KX955254; iifA, KX955255; iifB, KX955256; iifC, KX955257; iifD, KX955258; iifE, KY700688. The strain Acinetobacter sp. O153 has been deposited in the DSMZ under accession number DSM 103907.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Regina Vidziunaite and I. Bratkovskaja for technical assistance with oxygen consumption measurements and A. Laurynenas for generation of anaerobic conditions.
We have no conflicts of interest to declare.
This work was supported by the Research Council of Lithuania (project no. MIP-042/2012).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01453-17.
REFERENCES
- 1.Deeley MC, Yanofsky C. 1981. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J Bacteriol 147:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirakawa H, Inazumi Y, Masaki T, Hirata T, Yamaguchi A. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol Microbiol 55:1113–1126. doi: 10.1111/j.1365-2958.2004.04449.x. [DOI] [PubMed] [Google Scholar]
- 3.Mueller RS, Beyhan S, Saini SG, Yildiz FH, Bartlett DH. 2009. Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J Bacteriol 191:3504–3516. doi: 10.1128/JB.01240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q, Pande GSJ, Lin B, Rubin RA, Vora GJ, Defoirdt T. 2017. Indole signaling and (micro)algal auxins decrease the virulence of Vibrio campbellii, a major pathogen of aquatic organisms. Environ Microbiol 19:1987–2004. doi: 10.1111/1462-2920.13714. [DOI] [PubMed] [Google Scholar]
- 5.Martino PD, Fursy R, Bret L, Sundararaju B, Phillips RS. 2003. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can J Microbiol 49:443–449. doi: 10.1139/w03-056. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki-Imamura T, Yano A, Yoshida Y. 2010. Production of indole from l-tryptophan and effects of these compounds on biofilm formation by Fusobacterium nucleatum ATCC 25586. Appl Environ Microbiol 76:4260–4268. doi: 10.1128/AEM.00166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, Sitepu IR, Hashidoko Y. 2013. Induction of biofilm formation in the Betaproteobacterium Burkholderia unamae CK43B exposed to exogenous indole and gallic acid. Appl Environ Microbiol 79:4845–4852. doi: 10.1128/AEM.01209-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erb M, Veyrat M, Robert CA, Xu H, Frey M, Ton J, Turlings TC. 2015. Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun 6:6273. doi: 10.1038/ncomms7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J-H, Kim Y-G, Kim M, Kim E, Choi H, Kim Y, Lee J. 2017. Indole-associated predator-prey interactions between the nematode Caenorhabditis elegans and bacteria. Environ Microbiol 19:1776–1790. doi: 10.1111/1462-2920.13649. [DOI] [PubMed] [Google Scholar]
- 10.Ochiai M, Wakabayashi K, Sugimura T, Nagao M. 1986. Mutagenicities of indole and 30 derivatives after nitrite treatment. Mutat Res 172:189–197. doi: 10.1016/0165-1218(86)90056-X. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Lee J. 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 12.Chimerel C, Field CM, Pinero-Fernandez S, Keyser UF, Summers DK. 2012. Indole prevents Escherichia coli cell division by modulating membrane potential. Biochim Biophys Acta 1818:1590–1594. doi: 10.1016/j.bbamem.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Hong H, Heo A, Park W. 2013. Indole toxicity involves the inhibition of adenosine triphosphate production and protein folding in Pseudomonas putida. FEMS Microbiol Lett 343:89–99. doi: 10.1111/1574-6968.12135. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Park W. 2013. Indole inhibits bacterial quorum sensing signal transmission by interfering with quorum sensing regulator folding. Microbiology 159:2616–2625. doi: 10.1099/mic.0.070615-0. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs G, Boll M, Heider J. 2011. Microbial degradation of aromatic compounds—from one strategy to four. Nat Rev Microbiol 9:803–816. doi: 10.1038/nrmicro2652. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer DT. 1991. Oxygen chemistry. Oxford University Press, Inc., Oxford, United Kingdom. [Google Scholar]
- 17.Kovaleva EG, Lipscomb JD. 2008. Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nat Chem Biol 4:186–193. doi: 10.1038/nchembio.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner RA, Janssen HJ, Roversi P, Oakley AJ, Fetzner S. 2010. Structural basis for cofactor-independent dioxygenation of N-heteroaromatic compounds at the α/β-hydrolase fold. Proc Natl Acad Sci U S A 107:657–662. doi: 10.1073/pnas.0909033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fetzner S, Steiner RA. 2010. Cofactor-independent oxidases and oxygenases. Appl Microbiol Biotechnol 86:791–804. doi: 10.1007/s00253-010-2455-0. [DOI] [PubMed] [Google Scholar]
- 20.Thierbach S, Bui N, Zapp J, Chhabra SR, Kappl R, Fetzner S. 2014. Substrate-assisted O2 activation in a cofactor-independent dioxygenase. Chem Biol 21:217–225. doi: 10.1016/j.chembiol.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Ortega A, Quesne MG, Bui S, Heyes DJ, Steiner RA, Scrutton NA, de Visser SP. 2015. Catalytic mechanism of cofactor-free dioxygenases and how they circumvent spin-forbidden oxygenation of their substrates. J Am Chem Soc 137:7474–7487. doi: 10.1021/jacs.5b03836. [DOI] [PubMed] [Google Scholar]
- 22.Ensley BD, Ratzkin BJ, Osslund TD, Simon MJ, Wackett LP, Gibson DT. 1983. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 222:167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- 23.Lin GH, Chen HP, Shu HY. 2015. Detoxification of indole by an indole-induced flavoprotein oxygenase from Acinetobacter baumannii. PLoS One 10:e0138798. doi: 10.1371/journal.pone.0138798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fetzner S. 1998. Bacterial degradation of pyridine, indole, quinoline, and their derivatives under different redox conditions. Appl Microbiol Biotechnol 49:237–250. doi: 10.1007/s002530051164. [DOI] [Google Scholar]
- 25.Arora PK, Sharma A, Bae H. 2015. Microbial degradation of indole and its derivatives. J Chem 2015:129159. doi: 10.1155/2015/129159. [DOI] [Google Scholar]
- 26.Yin B, Gu JD, Wan N. 2005. Degradation of indole by enrichment culture and Pseudomonas aeruginosa Gs isolated from mangrove sediment. Int Biodeter Biodegr 56:243–248. doi: 10.1016/j.ibiod.2005.10.001. [DOI] [Google Scholar]
- 27.Kim D, Rahman A, Sitepu IR, Hashidoko Y. 2014. Accelerated degradation of exogenous indole by Burkholderia unamae strain CK43B exposed to pyrogallol-type polyphenols. Biosci Biotech Biochem 77:1722–1727. doi: 10.1271/bbb.130282. [DOI] [PubMed] [Google Scholar]
- 28.Qu Y, Shen E, Ma Q, Zhang Z, Liu Z, Shen W, Wang J, Li D, Li H, Zhou J. 2015. Biodegradation of indole by a newly isolated Cupriavidus sp. SHE. J Environ Sci (China) 34:162–132. [DOI] [PubMed] [Google Scholar]
- 29.Gu JD, Berry DF. 1991. Degradation of substituted indoles by an indole-degrading methanogenic consortium. Appl Environ Microbiol 57:2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Bryant SH. 2015. CDD: NCBI's conserved domain database. Nucleic Acids Res 43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bundy BM, Cambell AL, Neidle EL. 1998. Similarities between the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J Bacteriol 180:4466–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harwood CS, Parales RE. 1996. The beta-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol 50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 33.Neidle EL, Hartnett C, Bonitz S, Ornston LN. 1988. DNA sequence of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA sequence repetitions. J Bacteriol 170:4874–4880. doi: 10.1128/jb.170.10.4874-4880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki K, Ichimura A, Ogawa N, Hasebe A, Miyashita K. 2002. Differential expression of two catechol 1,2-dioxygenases in Burkholderia sp. strain TH2. J Bacteriol 184:5714–5722. doi: 10.1128/JB.184.20.5714-5722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohn W, Crawford IP. 1976. Regulation of enzyme synthesis in the tryptophan pathway of Acinetobacter calcoaceticus. J Bacteriol 127:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan C, Ju J, Borlee BR, Williamson LL, Shen B, Raffa KF, Handelsman J. 2007. Signal mimics derived from a metagenomic analysis of the gypsy moth gut microbiota. Appl Environ Microbiol 73:3669–3676. doi: 10.1128/AEM.02617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tischler D, Eulberg D, Lakner S, Kaschabek SR, van Berkel WJ, Schlömann M. 2009. Identification of a novel self-sufficient styrene monooxygenase from Rhodococcus opacus 1CP. J Bacteriol 191:4996–5009. doi: 10.1128/JB.00307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tischler D, Gröning JAD, Kaschabek SR, Schlömann M. 2012. One-component styrene monooxygenase: an evolutionary view on a rare class of flavoproteins. Appl Biochem Biotechnol 167:931–944. doi: 10.1007/s12010-012-9659-y. [DOI] [PubMed] [Google Scholar]
- 39.Gröning JAD, Kaschabek SR, Schlömann M, Tischler D. 2014. A mechanistic study on SMOB-ADP1: an NADH:flavin oxidoreductase of the two-component styrene monooxygenase of Acinetobacter baylyi ADP1. Arch Microbiol 196:829–845. doi: 10.1007/s00203-014-1022-y. [DOI] [PubMed] [Google Scholar]
- 40.Han X, Wang W, Xiao X. 2008. Microbial biosynthesis and biotransformation of indigo and indigo-like pigments. Sheng Wu Gong Cheng Xue Bao 24:921–926. (In Chinese.) doi: 10.1016/S1872-2075(08)60043-6. [DOI] [PubMed] [Google Scholar]
- 41.Fukuoka K, Tanaka K, Ozeki Y, Kanaly RA. 2015. Biotransformation of indole by Cupriavidus sp. KK10 proceeds through N-heterocyclic and carbocyclic-aromatic ring cleavage and production of indigoids. Int Biodeter Biodegr 97:13–24. [Google Scholar]
- 42.O'Connor KE, Dobson AD, Hartmans S. 1997. Indigo formation by microorganisms expressing styrene monooxygenase activity. Appl Environ Microbiol 63:4287–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyd DR, Larkin MJ, Reid KA, Sharma ND, Wilson K. 1997. Metabolism of naphthalene, 1-naphtol, indene, and indole by Rhodococcus sp. strain NCIMB 12038. Appl Environ Microbiol 63:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linhares M, Rebelo SLH, Simoes MMQ, Silva AMS, Neves MGPMS, Cavaleiro JAS, Freire C. 2014. Biomimetic oxidation of indole by Mn(III)porphyrins. Appl Catal A Gen 470:427–433. doi: 10.1016/j.apcata.2013.11.023. [DOI] [Google Scholar]
- 45.Chaiyen P. 2010. Flavoenzymes catalyzing oxidative aromatic ring-cleavage reactions. Arch Biochem Biophys 493:62–70. doi: 10.1016/j.abb.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Stanislauskienė R, Gasparaviciute R, Vaitekunas J, Meskiene R, Rutkiene R, Casaite V, Meskys R. 2012. Construction of Escherichia coli-Arthrobacter-Rhodococcus shuttle vectors based on a cryptic plasmid from Arthrobacter rhombi and investigation of their application for functional screening. FEMS Microbiol Lett 327:78–86. doi: 10.1111/j.1574-6968.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 47.Kutanovas S, Stankeviciute J, Urbelis G, Tauraite D, Rutkiene R, Meskys R. 2013. Identification and characterization of tetramethylpyrazine catabolic pathway in Rhodococcus jostii TMP1. Appl Environ Microbiol 79:3649–3657. doi: 10.1128/AEM.00011-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donoso R, Leiva-Novoa P, Zúñiga A, Timmermann T, Recabarren-Gajardo G, González B. 21 October 2016. Biochemical and genetic bases of indole-3-acetic acid (auxin phytohormone) degradation by the plant-growth-promoting rhizobacterium Paraburkholderia phytofirmans PsJN. Appl Environ Microbiol doi: 10.1128/AEM.01991-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 50.Fischer F, Kunne S, Fetzner S. 1999. Bacterial 2,4-dioxygenases: new members of the α/β hydrolase-fold superfamily of enzymes functionally related to serine hydrolases. J Bacteriol 18:5725–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujioka M, Wada H. 1968. The bacterial oxidation of indole. Biochim Biophys Acta 158:70–78. doi: 10.1016/0304-4165(68)90073-1. [DOI] [PubMed] [Google Scholar]
- 52.Claus G, Kutzner HJ. 1983. Degradation of indole by Alcaligenes spec. Syst Appl Microbiol 4:169–180. doi: 10.1016/S0723-2020(83)80046-0. [DOI] [PubMed] [Google Scholar]
- 53.Lee J, Attila C, Cirillo SLG, Cirillo JD, Wood TK. 2009. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb Biotechnol 2:75–90. doi: 10.1111/j.1751-7915.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu W, Zere TR, Weber MW, Wood TK, Whiteley M, Hidalgo-Romano B, Valenzuela E, McLean RJC. 2012. Indole production promotes Escherichia coli mixed-culture growth with Pseudomonas aeruginosa by inhibiting quorum signaling. Appl Environ Microbiol 78:411–419. doi: 10.1128/AEM.06396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hidalgo-Romano B, Gollihar J, Brown SA, Whiteley M, Valenzuela E Jr, Kaplan HB, Wood TK, McLean RJ. 2014. Indole inhibition of N-acylated homoserine lactone-mediated quorum signaling is widespread in Gram-negative bacteria. Microbiology 160:2464–2473. doi: 10.1099/mic.0.081729-0. [DOI] [PubMed] [Google Scholar]
- 56.Ahmer BM. 2004. Cell-to-cell signaling in Escherichia coli and Salmonella enterica. Mol Microbiol 52:933–945. doi: 10.1111/j.1365-2958.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 57.Chan KG, Atkinson S, Mathee K, Sam C-K, Chhabra SR, Camara M, Koh C-L, Williams P. 2011. Characterization of N-acetylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: Coexistence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol 11:51. doi: 10.1186/1471-2180-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 59.Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. 2008. Critical evaluation of two primers commonly used for amplification of bacterial 16s rRNA genes. Appl Environ Microbiol 74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambrook J. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 61.Vaitekunas J, Gasparaviciute R, Rutkiene R, Tauraite D, Meskys R. 2015. A novel 2-hydroxypyridine catabolic pathway in Rhodococcus rhodochrous PY11. Appl Environ Microbiol 82:1264–1273. doi: 10.1128/AEM.02975-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Melo JS, Moura AP, Melo MJ. 2004. Photophysical and spectroscopic studies of indigo derivatives in their keto and leuco forms. J Phys Chem 108:6975–6981. doi: 10.1021/jp049076y. [DOI] [Google Scholar]
- 63.Bergonzini G, Melchiorre P. 2012. Dioxindole in asymmetric catalytic synthesis: routes to enantioenriched 3-substituted 3-hydroxyoxindoles and the preparation of maremycin A. Angew Chem Int Ed Engl 51:971–974. doi: 10.1002/anie.201107443. [DOI] [PubMed] [Google Scholar]
- 64.Medema MH, Takano E, Breitling R. 2013. Detecting sequence homology at the gene cluster level with MultiGeneBlast. Mol Biol Evol 30:1218–1223. doi: 10.1093/molbev/mst025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Yan R, Roy A, Xu D, Poisson J, Zhang Y. 2015. The I-TASSER suite: protein structure and function prediction. Nat Methods 12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pathak D, Ollis D. 1990. Refined structure of dienolactone hydrolase at 1.8 Å. J Mol Biol 214:497–525. doi: 10.1016/0022-2836(90)90196-S. [DOI] [PubMed] [Google Scholar]
- 67.Hou J, Zheng H, Chruszcz M, Zimmerman MD, Shumilin IA, Osinski T, Demas M, Grimshaw S, Minor W. 2015. Dissecting the structural elements for the activation of β-ketoacyl-(acyl carrier protein) reductase from Vibrio cholerae. J Bacteriol 198:463–476. doi: 10.1128/JB.00360-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Berg B, Bhamidimarri SP, Winterhalter M. 2015. Crystal structure of a COG4313 outer membrane channel. Sci Rep 5:11927. doi: 10.1038/srep11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.