Abstract
Background
Arterial stiffness and wall shear stress are powerful determinants of cardiovascular health, and arterial stiffness is associated with increased cardiovascular mortality. Low and oscillatory wall shear stress, termed disturbed flow (d-flow), promotes atherosclerotic arterial remodeling, but the relationship between d-flow and arterial stiffness is not well understood. The objective of this study was to define the role of d-flow on arterial stiffening and discover the relevant signaling pathways by which d-flow stiffens arteries.
Methods
D-flow was induced in the carotid arteries of young and old mice of both sexes. Arterial stiffness was quantified ex vivo with cylindrical biaxial mechanical testing and in vivo from duplex ultrasound and compared to unmanipulated carotid arteries from 80-week-old mice. Gene expression and pathway analysis was performed on endothelial cell-enriched RNA and validated by immunohistochemistry. In vitro testing of signaling pathways was performed under oscillatory and laminar wall shear stress conditions. Human arteries from regions of d-flow and stable flow (s-flow) were tested ex vivo to validate critical results from the animal model.
Results
D-flow induced arterial stiffening through collagen deposition after partial carotid ligation, and the degree of stiffening was similar to that of unmanipulated carotid arteries from 80-week-old mice. Intimal gene pathway analyses identified that transforming growth factor-beta (TGF-β) pathways having a prominent role in this stiffened arterial response, but that this was due to thrombospondin-1 (TSP-1) stimulation of profibrotic genes and not changes to TGF- β. In vitro and in vivo testing under d-flow conditions identified a possible role for TSP-1 activation of TGF-β in the upregulation of these genes. TSP-1 knockout animals had significantly less arterial stiffening in response to d-flow than wild type carotid arteries. Human arteries exposed to d-flow had similar increases TSP-1 and collagen gene expression as seen in our model.
Conclusions
TSP-1 has a critical role in shear-mediated arterial stiffening that is mediated in part through TSP-1’s activation of TGF-β’s profibrotic signaling pathways. Molecular targets in this pathway may lead to novel therapies to limit arterial stiffening and the progression of disease in arteries exposed to d-flow.
Keywords: arterial stiffening, Thrombospondin-1, disturbed-flow
Journal Subject Codes: Animal models of human diseases, fibrosis, peripheral vascular disease
Introduction
Arterial stiffening is a complex age-related remodeling process that independently doubles patient mortality and may contribute to the onset and progression of atherosclerotic cardiovascular disease1–3. Material changes to the elastin and collagen content of the vessel wall mediate arterial stiffness4. Mechanistically, transforming growth factor-beta (TGF-β) has been implicated in arterial stiffening via pro-fibrotic collagen deposition into the adventitia of the arterial wall during aging5, 6, but its role in this process appears more complicated than simple upregulation of TGF-β7. This is not surprising because TGF-β activation and signaling is complex, regulated by TGF-β receptor (TGFBR) expression and matricellular cues. Since the signaling pathways that initiate and sustain arterial stiffening are not well understood8, 9, there is a critical need to develop and test novel translational models of arterial stiffening.
Fluid shear stress is known to be an important mediator of arterial remodeling10. In areas of disturbed flow (d-flow), low and oscillatory wall shear stress (WSS) stimulates pro-inflammatory responses from endothelial cells (EC) and promotes atherosclerotic remodeling of affected arteries through lipid accumulation and elastin degradation11, 12. Excitingly the EC response to d-flow can be used to discover novel shear sensitive genes important to the regulation of vascular remodeling13. One plausible pathway that links d-flow with TGF-β mediated arterial stiffening is thrombospondin-1 (TSP-1), which we and others have identified as a shear sensitive protein that is upregulated under disturbed flow (d-flow) conditions13, 14. TSP-1 activation of TGF-β is thought to be mediated through TSP-1 binding to TGF-β’ latent activating factor [or peptide] (LAF)15. Importantly this pathway can be modified by pharmacologically inhibiting TSP-1 binding to LAF via peptide inhibitors (e.g. LSKL)16, 17. TSP-1 has also been implicated in dysregulation of blood flow, impaired vessel dilation and increased vascular tone18, 19. However these activities appear to be mainly mediated through its interaction with cell receptors CD36 and CD47 and guanylate cyclase pathways20, 21, as opposed to profibrotic signaling and collagen deposition characteristic of stiffened arteries.
Previous studies have shown a relationship between d-flow and arterial stiffening. D-flow induces extracellular matrix stiffening under diabetic conditions22, and by MRI, d-flow portions of human coronary arteries are stiffer than s-flow locations23. However, the causal link between d-flow and arterial stiffening has not been established. Thus, the objective of this study was to determine whether d-flow promoted arterial stiffness in the absence of atherosclerosis, and then to discover the critical signaling pathways involved. We hypothesize that d-flow will promote arterial stiffening through TSP-1 upregulation. Here we test arterial stiffening ex vivo by cylindrical biaxial testing and in vivo by strain analysis. The reversibility of this pathway is tested by disrupting TSP-1 activation of TGF-β with LSKL. Finally, the effect of d-flow on arterial stiffening is then tested in a TSP-1 knockout (KO) mouse, and human arteries exposed to d-flow are used to validate the linking of d-flow with TSP-1 expression and profibrotic signaling pathways.
Methods
Partial Carotid Ligation Model of d-flow
Partial carotid ligation studies24 were performed on young and old (12–20-week-old; 80-week-old) mice [C57BL/6 x 129/SvEv (S129); C57BL/6J (C57) (Jackson Lab); TSP-1 knockout (TSP-1 KO) [C57Bl/6 x 129S2/SvPas] (Jackson Lab) as approved by Emory University’s IACUC. Initial mechanical characterization was performed on young male and female S129 mice. Supplemental Figure 1 provides an overview of experimental methodology and time points.
Quantification of Wall Shear Stress (WSS) Values
The in vivo hemodynamic environment was characterized in the mouse carotid arteries and human peripheral arteries using ultrasound data. In order to describe the WSS disturbances in arteries with and without inline flow, a representative patient’s duplex ultrasound data was analyzed. Here the superficial femoral artery represented s-flow (above occlusion=unimpeded inline flow) and the posterior tibial artery (below occlusion where flow is not inline but enters the vessel via collateral pathways) represented d-flow. Temporal WSS waveforms were quantified by equation 1:
Mechanical Testing of Arterial Stiffness and Biochemical Analysis of Arteries
Biaxial testing: Murine common carotid arteries underwent cylindrical biaxial biomechanical testing with collagen and elastin quantification as published25,26. Unpaired, two-tailed t-test was used to compare mean values with statistical significance corrected for multiple comparisons (P < 0.0031 for compliance curves and P<.0029; this is signified in figures by §).
EC-enriched RNA Isolation from Carotid Arteries
Total RNA from intima was collected from the LCA and RCA at designated time points as published24. EC enriched RNA was then tested for TSP1, TGF-β1-3, TGF-β receptors (TGFBR1-3), TSP-1 receptors CD36 and CD47, profibrotic genes [connective tissue growth factor (CTGF) and plasminogen activator inhibitor-1 (PAI1)] and collagen genes (Col1a1; Col4a1; Col16a1). We used the naturally occurring differences in WSS between the greater (s-flow) and lesser (d-flow) curvature of the aortic arch to test for TSP-1 expression in response to chronic d-flow. Paired or unpaired, two-tailed t-test were used to compare mean values as appropriate with statistical significance set at P<.05.
Microarray procedure, data analysis, and bioinformatics
EC enriched RNA from 9 LCAs and 9 RCAs were pooled for microarray analysis (n=3 using total of 9 mice used per each group). RNA sample quality was confirmed and the normalized microarray data was screened for statistically significant genes that were differentially expressed (LCA vs. RCA) with greater than 1.5 fold increase13. Pathway enrichment analysis was performed by mapping these differentially expressed genes to GeneGO’s MetaCore™. This program functions as an integrated software suite for functional analysis of signaling pathways. GeneGO pathways were enriched by the 0.01 gene list derived from the EC-enriched microarray data. The gene array data along with the meta-data was uploaded to the GEO Repository at NCBI (Accession #GSE87199).
Quantitative PCR validation
Total RNA of each sample was transcribed into cDNA24, and qPCR results were normalized for 18S RNA expression in each sample13. Human artery samples were collected from amputated limbs after informed consent under an IRB approved protocol (Emory University approved IRB protocol number: 51432). Arterial specimens were segregated prior to testing into d-flow and s-flow conditions depending on the presence or absence of inline flow to the artery. All d-flow arteries (N=6) were in patients with proximal inflow occlusion and distal reconstitution through collateral pathways. All s-flow arteries (N=4) had inline flow without obstructive PAD. Unpaired, two-tailed t-test was used to compare mean values with statistical significance at P<.05.
Histologic and Immunohistochemical Analysis
Arteries were stained for histologic analyses, and myointimal hyperplasia was quantified in a blinded fashion as published27. Elastin architecture was visualized by autofluorescence. Immunohistochemistry was performed as published13, 24. Unpaired, two-tailed t-test was used to compare mean values with statistical significance at P<.05.
In Vitro EC response to Oscillatory and Laminar WSS
Human aortic endothelial cells (HAEC) were commercially obtained (Genlantis) and grown to confluence on 10cm Petri dishes. They were then exposed to oscillatory shear (OS) [±5 dyn/cm2 at 1Hz] to mimic d-flow or laminar shear (LS) [15 dyn/cm2] to mimic s-flow. Static culture (ST) served as a no shear control. After 48 hours, HAEC RNA was collected and analyzed. Multiple comparisons between treatment groups were statistically tested with analysis of variance with Tukey’s post-hoc test was performed for statistical significance of P<.05.
TSP-1 activation of TGF-β is mediated by latent activating factor (LAF)28. To test the role of TSP-1 on TGF-β activation, we utilized 5μM LSKL (Anaspec) as a competitive inhibitor of LAF and equimolar SLLK (Anaspec) as a nonsense peptide control. For in vivo testing, LSKL or SLLK was administered 4mg/Kg IP every other day as published29. IP treatments began 5 days before partial carotid ligation and were continued through terminal endpoint of 3 days. Paired or unpaired, two-tailed t-test were used to compare mean values as appropriate with statistical significance set at P<.05.
In vivo Strain Measurements
Serial B-mode ultrasound data were acquired in the mid-LCA and RCA at 3 days and 1,2,4 weeks post-partial carotid artery ligation (N=9). The lumen boundaries were manually segmented in the middle 3-mm of each vessel, at least 1-mm proximal to the carotid bifurcation, across one cardiac cycle (approximately 60 images). A spline was fit to the segmented contours, and diameter values were quantified at 10 equally spaced spatial intervals. The resulting diameter waveforms were averaged, generating a global diameter waveform for the vessel, and the circumferential deformation (i.e., change in diameter) across the cardiac cycle was quantified. The maximum circumferential (Green’s) strain (Eθθ), was calculated as a surrogate for arterial stiffness for each vessel. Unpaired, two-tailed t-test was used to compare mean values with statistical significance of P<.05.
Statistical Analysis of Data
Statistical analyses were carried out using Graph-Pad Prism and Microsoft Excel statistical packages. For in vitro PCR testing, analysis of variance with Tukey post hoc analysis was performed. For single comparisons, paired or unpaired two-tailed Student’s t-test, with significance set at P<0.05 was used (*P<0.05; †<.01; ‡<.001). Compliance and pressure diameter measurements were tested by unpaired two-tailed Student’s t-tests with statistical significance corrected for multiple comparisons (§: P<.0031; P<.0029, respectively).
Results
Partial carotid ligation leads to d-flow in young mouse arteries
Partial carotid ligation induces d-flow (Figure 1A, B) in 12-week-old S129 mice similar to that published in ApoE −/− mice24. EC purity of intimal RNA was determined by qPCR for PECAM-1 (an EC marker), α-SMA (a smooth muscle cell marker) and CD11b (a leukocyte marker) (Figure 1C left and middle). D-flow parameters were internally validated by quantifying the EC response partial carotid ligation via down regulation of well-known shear sensitive genes (KLK10; KLF2) in the LCA (Figure 1C right). We then modeled WSS at the initial time point (24 hours) and out to the latest time point of these studies (6 weeks). WSS in the RCA demonstrated physiologic WSS or s-flow out to 6 weeks post partial ligation of the LCA. In contrast, the LCA demonstrated low and oscillatory WSS (d-flow) early and persistently low WSS out to 6 weeks after partial carotid ligation (Figure 1D).
Figure 1. Partial carotid ligation invokes disturbed flow to the intima and down-regulates shear sensitive genes.
A. Carotid artery duplex ultrasound demonstrates flow reversal and depressed velocities in the left carotid artery (LCA) that underwent partial ligation. Right carotid artery (RCA) was not manipulated and demonstrates unidirectional flow. (Note differences in the scale bars on the y axis [LCA: −50, 0, +50mm/s; RCA: −150, 0, +150 mm/s at 1 d post-ligation and −200, 0, +200 mm/s at 42 d post-ligation, respectively]). B. Using velocity data points from the ultrasound images, the LCA had significantly diminished flow rates compared to the RCA through 6 weeks post partial carotid ligation. C. (left panel) Intimal RNA demonstrates EC-enrichment by the selective and segregated PECAM-1 expression from that of α-SMA or CD11b at 24 hours. (middle panel) RNA from the media/adventitia of these arteries demonstrate increased α-SMA. (right panel) D-flow conditions were then internally validated 24 hours after partial ligation by the significant down-regulation of the shear sensitive genes KLK10 and KLF2 in the LCA. n=3; mean ± standard error of the mean (sem); unpaired Student’s t-test with significance at P<.05. D. WSS in the RCA is consistent with stable flow (s-flow) throughout the experiment. However, the LCA manifests disturbed flow (d-flow) with low and oscillatory WSS acutely and low WSS throughout the latest time point of experiments in this work (6 weeks). *P<0.05; †<.01; ‡<.001
D-flow causes arterial stiffening and increased collagen deposition
12-week-old S129 mice underwent partial carotid ligation to induce d-flow in the LCA. After 4 weeks of d-flow, LCAs demonstrated significant arterial stiffening compared to RCAs by pressure diameter and compliance curves generated from ex vivo cylindrical biaxial testing (Figure 2A, B). Stiffened arterial remodeling was induced by d-flow in both male and female mice and over a range of 12–20 weeks (Supplemental Figures 2 and 3). In order to compare the arterial stiffening induced by d-flow in our model to that which occurs naturally with aging, we measured the arterial stiffening occurring in response to d-flow in 12-week-old mice to that of unmanipulated carotid arteries of 80- week-old mice. Despite the 80-week carotid arteries having a larger diameter than the young LCAs yielding differences at higher pressures in the pressure-diameter curve (Figure 2C), the compliance in the unmanipulated aged carotid arteries was remarkably similar to that of young arteries under d-flow (Figure 2D). To examine the material cause of stiffening under d-flow in our model, the collagen and elastin content were quantified. Collagen content in the LCA was increased within two weeks of d-flow (Supplementary Figure 3E) and significantly increased over that of the RCA at 4 weeks, while elastin content was not affected (Figure 2E).
Figure 2. D-flow and aging stiffen arteries similarly.
A/B. Pressure diameter and compliance curves demonstrate that the carotid arteries of 12-week-old animals who had their LCA exposed to d-flow (solid black circles) were significantly stiffer than the contralateral RCA (open circle), which were not manipulated and exposed to s-flow. C/D. We then compared carotid arteries from 12-week-old mice exposed to d-flow to un-manipulated (s-flow) carotid arteries from aged (80-week-old animals) animals. Due to the enlarged diameter of the older arteries, the pressure diameter curve of the aged carotid arteries demonstrated greater dilation than the 12-week-old mice exposed to d-flow beginning at 60mmHg. However, the compliance curves were very similar without any statistically significant differences in compliance between the 12-week-old d-flow carotid arteries and that of the unmanipulated aged murine carotid arteries. N=6; mean ± sem; unpaired Student’s t-test with significance (§) at P<.0029 and P<.0031 respectively. E. Structurally, the arterial stiffening from d-flow was due to increased collagen deposition, which was found in the LCA (d-flow) compared to RCA (s-flow), and not due to changes in elastin, which were similar between groups. n=12 RCA; n=14 LCA; mean ± sem; unpaired students t-test with significance set at P<.05. *P<0.05; †<.01; ‡<.001
Pathway analysis identified prominent role for TGF-β pathways in d-flow model of arterial stiffening
To determine potential mechanisms by which d–flow causes arterial stiffening, we carried out gene array studies using EC-enriched RNA. Differentially expressed genes from the endothelial-enriched RNA samples obtained from the intima of d-flow LCA and s-flow RCA underwent pathway analysis using MetaCore GeneGo software. Here we identified a prominent role for TGF-β pathways in our model (Table 1).
Table 1.
Pathway analysis of EC-enriched RNA under d-flow versus s-flow conditions.
| S. No. | Pathways | Enrichment Ratio (Genes changed/total genes in the pathway) | False Discovery Rate | P-Value |
|---|---|---|---|---|
| 1 | Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling | 107/111 | 2.4 × 10−22 | 2.71 × 10−25 |
| 2 | Cytoskeletal Remodeling | 96/102 | 5.6 × 10−18 | 1.6 × 10−20 |
| 3 | Endothelial to mesenchymal transition | 48/51 | 1.7 × 10−9 | 8.8 × 10−11 |
| 4 | TGF-beta receptor signaling | 48/50 | 3.2 × 10−10 | 1.06 × 10−11 |
| 5 | Development TGF-beta dependent induction of EMT via MAPK | 46/47 | 1.6 × 10−10 | 3.05 × 10−12 |
Pathway analysis of EC-enriched RNA from murine carotid arteries exposed to disturbed flow demonstrates a prominent role for TGF-β pathways in this model.
Thrombospondin-1 (TSP-1) but not TGF-β was upregulated in vivo by d-flow
Next, we queried TGF-β/TGFβR expression. Surprisingly TGF-β expression was not upregulated by d-flow in vivo, but TGF-β1 was initially decreased 24 hours after partial ligation in the EC enriched LCA RNA (Figure 3A). There were not any other significant differences at 24 hours in TGF-β subtypes or TGFBR in either the EC enriched or media/adventitia RNA (Figure 3B–L).
Figure 3. TGF-β and TGFβR expression in the carotid arterial wall in response to d-flow in vivo.
A/D. TGF-β1 is downregulated in the endothelium (but not media/adventitia) in response to d-flow. B–F. TGF-β2 and TGF-β3 are not changed in the endothelium or media/adventitia under d-flow. G–L. TGFBR1-3 are not changed by d-flow in the endothelium or media/adventitia in vivo. N=3; mean ± sem; paired Students t-test with significance set at P<.05. *P<0.05; †<.01; ‡<.001
However, there was a significant increase in TSP1 in both the EC enriched intimal RNA and the RNA from the media/adventitia in the d-flow left carotid arteries (LCA) versus s-flow right carotid arteries (RCA) (Figure 4A–B). Similarly, IHC staining of the carotid arteries demonstrated increased TSP-1 in the intima and whole artery of the LCA compared to RCA, validating the qPCR expression (Figure 4C). In order to validate the role of chronic d-flow on TSP-1 upregulation, we used the lesser curvature (LC) and greater curvature (GC) of the aortic arch. Again, there was significantly greater expression of TSP1 in the d-flow LC than the s-flow GC (Figure 4D–E). These results demonstrate that TSP-1 is upregulated by d-flow under both surgically induced acute changes in the carotid artery and chronic exposure in the lesser curvature of the aortic arch, where d-flow naturally occurs. The TSP-1 receptors CD36 and CD47 were not significantly elevated in the LCA at 24 hours (Figure 4F–G).
Figure 4. Acute and chronic exposure to d-flow induces TSP-1 expression in vivo.
A/B. TSP-1 expression was increased in the d-flow LCA versus s-flow RCA in the endothelium and media/adventitia. n=3; mean ± sem; paired students t-test; *p<0.05. C. TSP-1 staining (Red: TSP-1; Blue: DAPI) in the mouse carotid artery (L: lumen of artery) is increased under d-flow. C1 and C2: Cross section IHC staining for TSP-1 in the RCA and LCA demonstrate increased TSP-1 in the LCA (d-flow) at day 3 post partial carotid ligation. C3 and C4: Enface IHC staining in the endothelium of the RCA and LCA demonstrates increased TSP-1 in the LCA at day 3 post partial carotid ligation. D/E. Increased TSP-1 expression in the endothelium and media/adventitia under chronic d-flow conditions in the lesser curvature (LC) compared to the greater curvature (GC) of the aortic arch, which is s-flow naturally. F/G. CD36 and CD47 expression were not significantly changed in the endothelium under d-flow conditions. N=4; mean ± sem; paired Student’s t-test with significance set at P<.05. Scale: 20 μm. *P<0.05; †<.01; ‡<.001
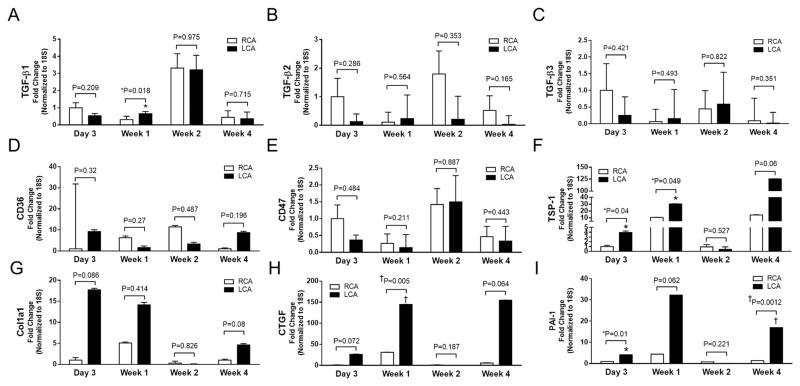
In order to better understand which genes were changed by d-flow over the 4 weeks of the experiment, we analyzed changes in gene expression at day 3 and weeks 1, 2, and 4. Here, we found TGF-β1 (decreased in the LCA at 24 hours) was increased in the LCA at 1 week (Figure 5A) without further differences in TGF-β1-3 (Figure 5A–C). Neither were there any significant differences in CD36 or CD47 expression identified (Figure 5D–E). However, there was sustained TSP-1 upregulation in the LCA that was significant at day 3 and week 1 (Figure 5F). Similarly, there was upregulation of the pro-fibrotic genes in the LCAs with CTGF being statistically significant at 1 week, and PAI1 being significantly elevated at 3 days and 4 weeks (Figure 5G–I).
Figure 5. Temporal gene changes in the endothelial RNA under d-flow.
A–E. TGF-β1 is increased at 1 week in the LCA, but there were no other significant differences in TGF-β1-3, CD36 or CD47 identified over these time points (day 3, week 1, 2, and 4). F. TSP1 was significantly increased in the LCA at day 3 and week 1 and demonstrated a trend toward significance at week 4. G. Col1a1 was not significantly increased at these time points. H/I. CTGF was significantly increased at one week, and PAI1 was significantly increased at day 3 and week 4. n=3–4; mean ± sem; unpaired Student’s t-test with significance set at P<.05. *P<0.05; †<.01; ‡<.001
Disrupting TSP-1 activation of TGF-β by inhibiting latent activating factor (LAF)
Given the prominent role of TGF-β pathways demonstrated in pathway analyses and the upregulation of TSP-1 by d-flow, we hypothesized that TSP-1 activation of TGF-β was a critical mediator of arterial stiffening in this model. In this pathway, TSP-1 binds to the LAF on TGF-β, leading to TGF-β activation (Figure 6A). In order to test the role of LAF on downstream profibrotic signaling in vitro, we mimicked d-flow with oscillatory shear (OS) and s-flow with laminar shear (LS) in human aortic endothelial cells (HAEC). We used LSKL to inhibit TSP-1 binding to TGF-β’s LAF28; SLLK was the control peptide. Here LSKL treatment did not inhibit the OS increase in TSP1 or TGF-β1 compared to SLLK (Figure 6B–C), but LSKL did significantly decrease the elevations of the profibrotic TGFβ target genes Col1a1, CTGF and PAI1 (Figure 6D–F) compared to the SLLK control under OS. This supports a connection between increased TSP-1 expression and TGF-β activation in the upregulation of these profibrotic target genes.
Figure 6. D-flow increases pro-fibrotic gene expression in human aortic ECs (HAEC) in vitro and LSKL down-regulates these genes in vitro and in vivo.
[Differences between OS and either static (ST) and LS are demonstrated above ST and LS, while differences between OS +LSKL and OS + SLLK are listed above their respective columns.]
A. Putative mechanism by which TSP-1 can activate TGF-β and how the small peptide LSKL competitively inhibits TSP-1 activation of TGF-β. B. ECs under oscillatory shear (OS) have increased TSP-1 expression compared to ECs under static (ST) and laminar shear (LS) conditions. TSP-1 expression is not affected by LSKL treatment or the control peptide (SLLK). C. TGF-β1 was also upregulated in ECs by OS and not affected by LSKL or SLLK. D. Col1a1 was upregulated by OS over ST and LS, and LSKL treatment significantly decreased Col1a1 expression under OS conditions compared to SLLK treatment. E. CTGF is significantly upregulated by OS (compared to ST and LS), and LSKL treatment significantly decreased CTGF expression under OS conditions compared to SLLK. F. PAI-1 is significantly upregulated by OS (compared to ST and LS) and LSKL treatment significantly decreased PAI-1 expression under OS conditions compared to SLLK. [N=5-6; mean±sem.; ANOVA with Tukey’s post-hoc test with significance set at P<.05. *P<0.05; †<.01; ‡<.001
G–I. In vivo, LSKL globally decreased expression of pro-fibrotic genes compared to SLLK control treatment in both the RCA (s-flow) and LCA (d-flow). Here only CTGF was still significantly upregulated in the LSKL treatment group by d-flow. J. TSP-1 expression the LCA of LSKL treated animals was still significantly increased compared to the RCA of LSKL treated animals. K–O. Again, there were no significant differences in TGF-β or TGFβR expression under d-flow or s-flow conditions (LCA versus RCA) in either the LSKL or SLLK groups. However, there were contrasting trends with greater TGF-β1 expression in SLLK treated animals and greater TGF-βR1-3 expression in the LSKL treated animals. P/Q. There were not any flow mediated differences in the expression of CD36 or CD47 identified within groups, but there was a trend toward increased CD47 in the LSKL treated animals compared to SLLK in both the LCA and RCA. n=3–4; mean±sem; paired Student’s t-test with significance set at P<.05. *P<0.05; †<.01; ‡<.001
We then tested the effectiveness of LSKL to decrease these genes in vivo. Here, LSKL globally decreased gene expression of these same profibrotic genes (Col1a1, CTGF, PAI1) compared to SLLK treated mice in both the RCA and LCA (Figure 6G–I). While decreased compared to LCA in SLLK animals, the LCA of LSKL treated mice still had significant increases in TSP-1 and CTGF expression compared to that of LSKL RCA (Figure 6H;J). There were no differences demonstrated in either Col1a1 or Pai1 (Figure 6G;I). TGF-β1-2 and TGF-βR1-3 expression levels were not significantly different between RCA and LCA (Figure 6K–O). TGF-β3 was not sufficiently expressed for analyses in these groups. There was an interesting trend in TGF-β gene regulation with TGF-β1 trending upwards in SLLK-treated animals and TGF-βR1-3 trending upwards in LSKL-treated animals, suggesting a homeostatic interaction between LSKL treatments and TGF-β activity. However, these trends did not appear to be influenced by d-flow or s-flow conditions. Similarly, CD36 and CD47 were not significantly different between RCA and LCA in either group (Figure 6P–Q), but CD47 trended upwards in LSKL treated animals in both the LCA and RCA.
TSP-1 is a critical mediator of d-flow induced stiffened arterial remodeling in vivo
We used TSP-1 KO animals to test whether TSP-1 mediates d-flow-induced arterial stiffening in vivo; C57BL/6J (C57) background mice were used as the WT control. In both the KO and WT mice, the LCA developed arterial stiffening in response to d-flow (figures 7A–D). But when comparing stiffness changes in the LCAs after exposure to d-flow, ex vivo biaxial testing demonstrated that TSP-1 KO mice had significantly more compliant arteries at physiologic mean arterial pressures than the C57 mice (Figure 7E–F). This ex vivo data was then validated in vivo using Green’s strain as a surrogate for arterial stiffness. Here, the LCA of C57, but not of TSP-1 knockout mice, demonstrated increased arterial stiffening within 3 weeks (Figure 7G).
Figure 7. D-flow induced stiffening is significantly attenuated in TSP-1 KO animals.
A/B. Pressure-diameter and compliance curves from ex vivo cylindrical biaxial mechanical testing of LCA and RCA from C57BL/6 (C57) animals demonstrates that the LCA is significantly stiffer than the RCA in this strain as well (N=5). C/D. Similar but less prominent differences were seen in the TSP-1 KO animals (N=7). E/F. When comparing the pressure-diameter and compliance curves of the LCA (exposed to d-flow) from TSP-1 KO and C57 animals, the TSP-1 KO was significantly less stiff by pressure diameter curves beginning at 70mmHg and by compliance curves at 40, 60, and 70 mmHg (N=5–7). mean ± sem; unpaired Student’s t-test with significance (§) at P<.0029 and P<.0031 respectively. G. For in vivo validation, we calculated Green’s strain Eθθ from ultrasound imaging to identify the time course of stiffening in these animals. Here we identified increased arterial stiffness (compared to TSP-1 KO) in the C57BL/6 arteries exposed to d-flow to occur between weeks 2 and 3 (N=9). H. Similar to young WT arteries in vivo, the LCA of 80-week old animals exposed to d-flow were significantly stiffer than RCA after 4 weeks (N=8). I. H&E staining of C57BL/6 and TSP-1 KO arteries under 4x and 20x magnification demonstrates increased myointimal hyperplasia in the LCA of C57 mice; this was not found in the TSP-1 KO mice. Elastin was visualized by autofluorescence, and we did not demonstrate differences in the elastin orientation or composition in this model. J. Graphical representation of the intima/media ratio demonstrates significant increase in myointimal hyperplasia in the C57 (but not TSP-1) arteries under d-flow (LCA) compared to s-flow conditions (RCA). N=7–8; mean±sem; unpaired Student’s t-test with significance set at P<.05. [scale bar for 20X is 100 microns; scale bar for 40X is 20 microns] *P<0.05; †<.01; ‡<.001
After identifying that the arterial stiffening of young mouse carotid arteries by d-flow closely approximated the mechanical stiffness of unmanipulated 80-week murine carotid arteries, we next tested the impact of stiffened arteries from aging under d-flow. Here we found that the LCA was significantly stiffer than the RCA by strain analysis by 4 weeks after partial carotid ligation (Figure 7H). Interestingly, despite an increase in collagen content from aging in the RCA of 80-week-old animals, the LCA had even greater collagen deposition at week 4 (46% dry weight compared to 24% in RCA). Still there were some unique aspects of the intimal gene regulation in these 80-week-old animals that differed from that seen in young mice. TGF-β 1–2 were significantly down-regulated in these mice, and TSP-1 and CTGF were significantly upregulated in the LCA. Col1a1, PAI1, CD36, and CD47 were not significantly different (Supplementary Figure 4).
While d-flow induced myointimal hyperplasia in the LCA of C57 mice, this was not evident in the TSP-1 KO mice (Figure 7I,J). Similarly, the LCA of 80-week-old mice did not have an increase in myoinimal hyperplasia compared to RCA [I/M ratio of 0.09±.04 versus 0.10±.04, P=.31], but they did have a significant increase in medial thickening [25μm±6 μm versus 22 μm ±6 μm; P=.003].
TSP-1 is upregulated in human arteries exposed to d-flow
In order to validate these findings in patients under clinically relevant conditions, we investigated TSP-1, TGF-β, and collagen gene expression in human arteries exposed to d-flow or s-flow conditions. Validation of d-flow and s-flow conditions in human arteries was performed using ultrasound data from a representative patient. Here we identified s-flow in the artery with unobstructed inline flow and d-flow in the arteries reconstituted distal to an arterial occlusion. The hemodynamic environment in the superficial femoral artery (SFA) was characterized by unidirectional flow throughout the cardiac cycle (i.e., non-reversing) and a time-averaged WSS value of 23.4 ± 17.6 dynes/cm2 (Figure 8A). Conversely, the hemodynamic environment distal to occlusion in the posterior tibial artery (PTA) was observed to be complex with oscillatory WSS from adjacent retrograde (Figure 8B) and antegrade (Figure 8C) flow patterns. D-flow conditions were further supported by time-averaged WSS values in the PTA exhibiting low wall shear stress compared to the SFA with inline flow [9.5 ± 4.7 and 15.6 ± 4.7 dynes/cm2, respectively] (Figure 8D).
Figure 8. TSP-1 and collagen genes are increased in human arteries exposed to d-flow.
A–D. Human peripheral arteries with inline (non-obstructive) flow have s-flow, while those arteries distal to obstruction have d-flow. A. Inline flow is demonstrated in a representative patient’s superficial femoral artery on arterial duplex. B/C. Distal to the occlusion there is flow reversal, which is manifest as retrograde mixed with antegrade filling of the posterior tibial artery and consistent with oscillatory WSS. D. Modeling of WSS in these images during antegrade flow demonstrates low WSS in the posterior tibial artery (distal to occlusion) compared to normal WSS in the superficial femoral artery with inline flow, representing s-flow. E. TSP-1 is significantly increased in human arteries under d-flow conditions compared to s-flow (n=4 for s-flow human arteries, n=6 for d-flow human arteries; mean±sem; unpaired Student’s t-test with significance set at P<.05. F. IHC demonstrates increased TSP-1 expression in d-flow regions of human arteries compared to s-flow regions (L: lumen of artery; scale bar is 500 μm). G–I. Col1a1 is increased under d-flow in our murine model in the endothelium and the media/adventitia of the LCA compared to RCA (s-flow). Col4a1 and Col16a were not significantly affected by d-flow in our murine model. N=3; mean±sem; paired t-test with significance set at P<.05. J–L. In human arteries, both Col1a1 and Col4a1 are upregulated under d-flow compared to s-flow conditions. Col16a1 is not altered by d-flow human arteries. N=4 for s-flow human arteries, N=6 for d-flow human arteries; mean±sem; unpaired Student’s t-test with significance set at P<.05. *P<0.05; †<.01; ‡<.001
Next, we compared TSP-1 expression in human arteries exposed to d-flow or s-flow conditions and found a significant increase in TSP1 expression in human arteries exposed to d-flow compared to s-flow conditions (Figure 8E). IHC confirmed increased TSP-1 expression in the arteries exposed to d-flow (Figure 8F). There was also a significant increase in Col1a1 expression in the d-flow human arteries, similar to that seen in the murine arteries (Figure 8G, J, *p<0.05). In distinction to our murine model, human arteries exposed to d-flow also had elevations in Col4a1 (Figure 8H, K, *p<0.05) and TGF-β1 expression (supplementary figure 5), and decreased expression of TGFβR3. Supplementary Table 1 lists the patient gender, ages, and comorbidities from which the human arteries were collected.
Discussion
Flow-mediated murine model of stiffened arterial remodeling
Excitingly, this model demonstrates that d-flow induced arterial stiffening through collagen deposition after partial carotid ligation in 12-week-old mice that is mechanically similar to that of unmanipulated 80-week-old mice. In an a priori fashion, we used pathway analysis of intimal gene expression in this model to identify a critical role for TGF-β signaling pathways. We discovered that TSP-1 stimulated profibrotic genes to promote stiffening. We then validated these findings in human arteries exposed to d-flow. To our knowledge, this is the first report identifying a critical role for TSP-1 in the mechanical stiffening of arteries and the localization of TSP-1 to areas of d-flow in human arteries of PAD patients.
TSP-1 has previously been linked to PAD, but the mechanism of upregulation is not known. Given the direct relationship of arterial stiffness and PAD with aging, our validation of TSP-1 in human arteries under d-flow may explain in part why TSP-1 is upregulated in PAD patients 30, 31. TSP-1 can also influence vascular tone and nitric oxide signaling13, 19. Specifically, it has been reported that TSP-1 KO animals have improved vasodilation through CD4732 that may be protective of the adverse effects of d-flow on EC dysfunction. In our model neither CD36 nor CD47 gene expression was significantly affected by flow conditions in the carotid endothelium and did not appear important to our model. Still it is highly likely that crosstalk does exist in arteries between CD36 and/or CD47 and TSP-1/TGF-β pathways as has been recently reported in pericytes33. Finally, it is likely that there are redundant pathways that affect vascular tone and EC dysfunction that may also contribute to arterial stiffening1, but the focus of this work was to identify the molecular mediators of d-flow induced mechanical stiffening (as opposed to vascular tone).
TSP-1 pathways in fibrosis and arterial remodeling
TSP-1 is a large (450kDa) matricellular protein that is found in the intima and media of atherosclerotic arteries that is important to arterial remodeling due to injury or hypertension30, 34–36. TSP-1 can promote fibrosis in wound healing through both TGF- β dependent and independent mechanisms37. In the TGF-β dependent pathway, TSP-1 activates latent TGF-β through its LAF38, 39. Active TGF-β then binds sequentially to TGFβRs that promote downstream cascades regulating the extracellular matrix40, 41. Excitingly, these pathways have a number of pharmacologic targets including blocking the activation of TGFβ by TSP-1 (LSKL) and TGFβR/Smad protein inhibitiors42, 43. TSP-1 can also promote fibrosis in TGF-β-independent pathways by binding to calreticulin (CRT) in an Akt-dependent manner44, and matrix metalloproteinases are activated by TSP-1 through its binding with low-density lipoprotein receptor-related protein45. Here we demonstrate significant attenuation of arterial stiffening in TSP-1 KO mice and global down-regulation of profibrotic genes in LSKL treated-mice. However, the precise contribution of these TSP-1 pathways in d-flow induced arterial stiffening remains to be discovered.
Arterial stiffening is closely linked to aging, and TGF-β1 has been found to promote adventitial collagen I and III in aged mice6. This is similar to our findings of collagen deposition in response to d-flow in young (12–20 weeks) mice. Thus, the rapid onset in this model may be instructive to mechanisms of aging. Importantly, arterial stiffening may also be a modifiable intermediary condition affecting atherosclerotic plaque formation and progression. Unfortunately to date, the role of stiffness in atherosclerotic arterial remodeling is unclear from the literature. Gotschy et al. identified by MRI that local arterial stiffness preceded atherosclerotic plaque formation in the ApoE−/− mouse model of atherosclerosis46. Van Herck et al. modified ApoE−/− knockout mice to disrupt elastin formation and found that atherosclerosis formed in stiffened arteries and that more arterial stiffness was associated with more atherosclerotic plaque47. However recently, the direct relationship between stiffness and atherosclerosis has been called into question by the Wagensiel group using a combination of elastin deficient and Ldlr knockout mice48. Prior work in the Jo lab has demonstrated that combining partial carotid ligation with a high fat diet in ApoE−/− mice led to rapid atherosclerotic plaque formation in d-flow regions24, and given the model and our findings in this paper, it is highly likely that stiffening preceded these plaques. Interestingly in a double knockout (TSP-1−/−/apoE−/−) mouse model49, Moura et al. found a mixed effect of TSP-1 on atherosclerosis in the aortic root of mice over time. They had a delay to peak plaque volume with no difference in total late plaque volume, but these animals did have greater inflammation and necrotic core formation in their atherosclerotic plaques. It is unclear what effect would be found if we added an atherogenic environment into the TSP-1 KO’s remodeling to d-flow.
Our novel discovery of TSP-1 mediating d-flow induced arterial stiffening may help reconcile these discrepancies in the literature. Specifically, we propose a role for TSP-1 (under d-flow conditions) in the amplification of inflammatory signaling pathways that contribute both to arterial stiffening and the onset and/or progression of focal atherosclerotic plaque formation (Supplemental Figure 6). The modifiability of this pathway seen in the LSKL treatments and TSP-1 KO supports these pathways having promise for PAD therapies and suggest that such therapies may improve cardiovascular health in d-flow arterial regions broadly. Here we used normal S129 and C57Bl/6J mice that did not have their lipids molecularly manipulated in our d-flow model of arterial stiffness. These mice did not develop atherosclerosis, but they did develop myointimal hyperplasia, which is thought to contribute to atherosclerosis12. Interestingly myointimal hyperplasia was not present in the LCA of TSP-1 KO mice, and these arteries were also relatively protected from stiffening under d-flow conditions. Since TGF-β has been implicated in the development of myointimal hyperplasia50, it is not clear if the lack of intimal hyperplasia in TSP-1 KO arteries exposed to d-flow is due to decreased stiffness, or if this too, is mediated by TSP-1 mediated activation of TGF-β. Moura et al. have previously published that the TSP-1 KO animals undergoing complete (not partial) carotid ligation can mount a myointimal response that related to smooth muscle cell activation34. However, even in this model, the absolute myointimal response was limited in the TSP-1 KO animals, which is consistent with our findings in this partial carotid ligation model. Further support of our findings has been demonstrated with TSP-1 antibodies decreasing neointima in a denuded carotid artery model of endothelial cell injury driven arterial remodeling51.
Limitations
Still there are limitations to our study that deserve discussion. First, this model has been focused on the EC response to d-flow, but there are known strain forces that are sensed by the arterial media explaining the transmural upregulation of TSP-1 seen in Figure 4. While it is not surprising that stiffened arterial remodeling is a transmural process, it is notable that in vivo and in vitro testing performed here support the hypothesis that the EC response to d-flow is critical to this process. Also, the association of TSP-1 and collagen gene upregulation in human arteries exposed to d-flow supports the clinical relevance of this pathway. Secondly while the mechanical analyses of this model demonstrate similar stiffness in d-flow exposed LCA from young mice with unmanipulated carotid arteries in 80-week-old mice, our d-flow model may not exactly mimic the arterial stiffening manifest by hypertension and aging52. This may account for the slight differences in the EC response to d-flow between the younger and older mice. However, the upregulation of TSP-1, CTGF, and increased collagen deposition remained very similar. It is also important to recognize that the human arteries that were exposed to d-flow necessarily came from patients with PAD who had blockage proximal to the arteries interrogated. The human arteries exposed to s-flow came from patients of similar ages who did not have obstructive PAD. The ideal comparison of arteries would have been to compare arteries from the same patient under s-flow and d-flow conditions. However, since it is rare to perform a major amputation above the level required for healing by the vascular supply, comparable human arteries under both s-flow and d-flow conditions are rare, this was not feasible in this study. Here larger animal models of d-flow such as our porcine model of PAD could be used to definitively determine in a large animal the direct link between d-flow and TSP-153. Finally, hypertension certainly promotes medial thickening and pathologic vascular smooth muscle cell behavior, and its absence in our model (Supplementary Table 2) may underestimate the role of the media (and vascular smooth muscle cells) in stiffened arterial remodeling in hypertensive populations. Nevertheless, this model’s rapid and progressive arterial stiffening with collagen deposition (mimicking aging), may be very useful in determining the role of more and less stiff arteries in the development of focal atherosclerotic plaque formation. Specifically, the relative lack of stiffening under d-flow in TSP-1 KO mice uniquely enables this model to be modified with hypertensive or atherogenic conditions to answer clinically important questions in PAD.
Conclusion
We have developed a flow-dependent in vivo murine model of arterial stiffness that is mechanically similar to that of aged mice and identified a critical role for TSP-1 in d-flow induced arterial stiffening. These findings were validated in arteries from d-flow regions of PAD patients. This model of and the TSP-1 pathways involved in arterial stiffness holds promise for the development of novel therapies that can reverse this arterial pathology before atherosclerotic plaque forms and irreversible damage has occurred. Such therapies may be helpful in promoting arterial health during aging and in patients with cardiovascular disease.
Supplementary Material
Clinical Perspective.
What is new?
The development of a novel murine model of disturbed blood flow to stimulate arterial stiffening through collagen deposition in young male and female mice.
The authors discovered a critical role for thrombospondin-1 (TSP-1) in stimulating d-flow induced arterial stiffening, which was significantly attenuated in the TSP-1 knockout animal.
Blockade of TSP-1 activation of TGF-β decreased the upregulation of profibrotic genes that contribute to arterial stiffening.
TSP-1 localized to regions of disturbed flow in arteries from patients with peripheral arterial disease (PAD) and these arteries had similar increases in collagen gene expression.
What are the clinical implications
Existing literature has linked TSP-1 to PAD, but the mechanism and biologic importance of this relationship was not known.
This work validates the localization of increased TSP-1 to regions of disturbed flow in peripheral arteries, which may provide the mechanism by which TSP-1 is increased in PAD.
This work links TSP-1 upregulation to arterial stiffening and identifies TSP-1 as an important promoter of pathologic arterial remodeling in PAD arteries under d-flow conditions.
The pro-fibrotic molecular pathways stimulated by TSP-1 may provide novel pharmaceutical targets for promoting arterial health and inhibiting pathologic arterial remodeling.
Acknowledgments
Part of this work was recognized with the Jay D. Coffman Early Career Investigator Award by the PVD council of the American Heart Association and presented as an oral presentation at the 2014 American Heart Association’s Scientific Sessions in Chicago, IL, USA. The authors would also like to thank Sebastian Perez from Emory University’s Department of Surgery for his assistance with the statistical analysis of this work.
Research Support: Funding for this work was provided by NIH: KO8HL119592 (LB), HL119798 and HL124879 (HJ), The Society for Vascular Surgery/American College of Surgeons Mentored Clinical Scientist Research Career Development Award (LB), American Heart Association, IRG1470001 (LB), and Departmental Support from Emory University Department of Surgery (Dr. John F. Sweeney) to Luke Brewster and the Emory University School of Medicine and Georgia Institute of Technology to Hanjoong Jo, the John and Jan Portman Professor of Biomedical Engineering.
Footnotes
Disclosures: Dr. Brewster has received significant compensation from his NIH KO8 award discovering the role of thrombospondin-1 in flow mediated arterial remodeling. There are no other relevant disclosures among the authors.
References
- 1.Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res. 2015;116:895–908. doi: 10.1161/CIRCRESAHA.116.305720. [DOI] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26:689–699. doi: 10.1081/ceh-200031982. [DOI] [PubMed] [Google Scholar]
- 5.Rammos C, Hendgen-Cotta UB, Deenen R, Pohl J, Stock P, Hinzmann C, Kelm M, Rassaf T. Age-related vascular gene expression profiling in mice. Mech Ageing Dev. 2014;135:15–23. doi: 10.1016/j.mad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sie MP, Mattace-Raso FU, Uitterlinden AG, Arp PP, Hofman A, Hoeks AP, Reneman RS, Asmar R, van Duijn CM, Witteman JC. TGF-beta1 polymorphisms and arterial stiffness; the Rotterdam Study. J Hum Hypertens. 2007;21:431–437. doi: 10.1038/sj.jhh.1002175. [DOI] [PubMed] [Google Scholar]
- 8.Dudenbostel T, Glasser SP. Effects of Antihypertensive Drugs on Arterial Stiffness. Cardiol Rev. 2012;20:259–263. doi: 10.1097/CRD.0b013e31825d0a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 10.Newcomer SC, Thijssen DH, Green DJ. Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise-induced hemodynamics. J Appl Physiol. 2011;111:311–320. doi: 10.1152/japplphysiol.00033.2011. [DOI] [PubMed] [Google Scholar]
- 11.Jo H, Song H, Mowbray A. Role of NADPH Oxidases in Disturbed Flow- and BMP4- Induced Inflammation and Atherosclerosis. Antioxid Redox Signal. 2006;8:1609–1619. doi: 10.1089/ars.2006.8.1609. [DOI] [PubMed] [Google Scholar]
- 12.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 13.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freyberg MA, Kaiser D, Graf R, Buttenbender J, Friedl P. Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem Biophys Res Commun. 2001;286:141–149. doi: 10.1006/bbrc.2001.5314. [DOI] [PubMed] [Google Scholar]
- 15.Mustonen E, Ruskoaho H, Rysa J. Thrombospondins, potential drug targets for cardiovascular diseases. Basic Clin Pharmacol Toxicol. 2013;112:4–12. doi: 10.1111/bcpt.12026. [DOI] [PubMed] [Google Scholar]
- 16.Krishna SM, Seto SW, Jose RJ, Biros E, Moran CS, Wang Y, Clancy P, Golledge J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler Thromb Vasc Biol. 2015;35:389–398. doi: 10.1161/ATVBAHA.114.304732. [DOI] [PubMed] [Google Scholar]
- 17.Rico MC, Rough JJ, Del Carpio-Cano FE, Kunapuli SP, DeLa Cadena RA. The axis of thrombospondin-1, transforming growth factor beta and connective tissue growth factor: an emerging therapeutic target in rheumatoid arthritis. Curr Vasc Pharmacol. 2010;8:338–343. doi: 10.2174/157016110791112296. [DOI] [PubMed] [Google Scholar]
- 18.Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol. 2012;32:2966–2973. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers NM, Sharifi-Sanjani M, Yao M, Ghimire K, Bienes-Martinez R, Mutchler SM, Knupp HE, Baust J, Novelli EM, Ross M, St Croix C, Kutten JC, Czajka CA, Sembrat JC, Rojas M, Labrousse-Arias D, Bachman TN, Vanderpool RR, Zuckerbraun BS, Champion HC, Mora AL, Straub AC, Bilonick RA, Calzada MJ, Isenberg JS. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc Res. 2017;113:15–29. doi: 10.1093/cvr/cvw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers NM, Yao M, Novelli EM, Thomson AW, Roberts DD, Isenberg JS. Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am J Physiol Renal Physiol. 2012;303:F1117–1125. doi: 10.1152/ajprenal.00359.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res. 2012;93:682–693. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green J, Yurdagul A, Jr, McInnis MC, Albert P, Orr AW. Flow patterns regulate hyperglycemia-induced subendothelial matrix remodeling during early atherogenesis. Atherosclerosis. 2014;232:277–284. doi: 10.1016/j.atherosclerosis.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duivenvoorden R, Vanbavel E, de Groot E, Stroes ES, Disselhorst JA, Hutten BA, Lameris JS, Kastelein JJ, Nederveen AJ. Endothelial shear stress: a critical determinant of arterial remodeling and arterial stiffness in humans--a carotid 3.0-T MRI study. Circ Cardiovasc imaging. 2010;3:578–585. doi: 10.1161/CIRCIMAGING.109.916304. [DOI] [PubMed] [Google Scholar]
- 24.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleason RL, Gray SP, Wilson E, Humphrey JD. A multiaxial computer-controlled organ culture and biomechanical device for mouse carotid arteries. J Biomech Eng. 2004;126:787–795. doi: 10.1115/1.1824130. [DOI] [PubMed] [Google Scholar]
- 26.Wang RY, Raykin J, Li HY, Gleason RL, Brewster LP. Differential mechanical response and microstructural organization between non-human primate femoral and carotid arteries. Biomech Model Mechan. 2014;13:1041–1051. doi: 10.1007/s10237-014-0553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewster L, Brey EM, Addis M, Xue L, Husak V, Ellinger J, Haudenschild CC, Greisler HP. Improving endothelial healing with novel chimeric mitogens. Am J Surg. 2006;192:589–593. doi: 10.1016/j.amjsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Meek RL, Cooney SK, Flynn SD, Chouinard RF, Poczatek MH, Murphy-Ullrich JE, Tuttle KR. Amino acids induce indicators of response to injury in glomerular mesangial cells. Am J Physiol Renal Physiol. 2003;285:F79–86. doi: 10.1152/ajprenal.00419.2002. [DOI] [PubMed] [Google Scholar]
- 29.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell’italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riessen R, Kearney M, Lawler J, Isner JM. Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am Heart J. 1998;135:357–364. doi: 10.1016/s0002-8703(98)70105-x. [DOI] [PubMed] [Google Scholar]
- 31.Favier J, Germain S, Emmerich J, Corvol P, Gasc JM. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathology. 2005;207:358–366. doi: 10.1002/path.1833. [DOI] [PubMed] [Google Scholar]
- 32.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010;88:471–481. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, Curtis MA, Park TI, Dragunow M. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. doi: 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moura R, Tjwa M, Vandervoort P, Cludts K, Hoylaerts MF. Thrombospondin-1 activates medial smooth muscle cells and triggers neointima formation upon mouse carotid artery ligation. Arterioscler Thromb Vasc Biol. 2007;27:2163–2169. doi: 10.1161/ATVBAHA.107.151282. [DOI] [PubMed] [Google Scholar]
- 35.Isenberg JS, Roberts DD, Frazier WA. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol. 2008;28:615–621. doi: 10.1161/ATVBAHA.107.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed MJ, Iruela-Arispe L, O’Brien ER, Truong T, LaBell T, Bornstein P, Sage EH. Expression of thrombospondins by endothelial cells. Injury is correlated with TSP-1. Am J Pathol. 1995;147:1068–1080. [PMC free article] [PubMed] [Google Scholar]
- 37.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–468. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 39.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 40.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 41.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26:1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- 43.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 44.Sweetwyne MT, Pallero MA, Lu A, Van Duyn Graham L, Murphy-Ullrich JE. The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am J Pathol. 2010;177:1710–1724. doi: 10.2353/ajpath.2010.090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Sottile J, Strickland DK, Mosher DF. Binding and degradation of thrombospondin-1 mediated through heparan sulphate proteoglycans and low-density-lipoprotein receptor-related protein: localization of the functional activity to the trimeric N-terminal heparin-binding region of thrombospondin-1. Biochem J. 1996;318(Pt 3):959–963. doi: 10.1042/bj3180959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gotschy A, Bauer E, Schrodt C, Lykowsky G, Ye YX, Rommel E, Jakob PM, Bauer WR, Herold V. Local arterial stiffening assessed by MRI precedes atherosclerotic plaque formation. Circ Cardiovasc imaging. 2013;6:916–923. doi: 10.1161/CIRCIMAGING.113.000611. [DOI] [PubMed] [Google Scholar]
- 47.Van Herck JL, De Meyer GR, Martinet W, Van Hove CE, Foubert K, Theunis MH, Apers S, Bult H, Vrints CJ, Herman AG. Impaired fibrillin-1 function promotes features of plaque instability in apolipoprotein E-deficient mice. Circulation. 2009;120:2478–2487. doi: 10.1161/CIRCULATIONAHA.109.872663. [DOI] [PubMed] [Google Scholar]
- 48.Maedeker JA, Stoka KV, Bhayani SA, Gardner WS, Bennett L, Procknow JD, Staiculescu MC, Walji TA, Craft CS, Wagenseil JE. Hypertension and decreased aortic compliance due to reduced elastin amounts do not increase atherosclerotic plaque accumulation in Ldlr−/− mice. Atherosclerosis. 2016;249:22–29. doi: 10.1016/j.atherosclerosis.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moura R, Tjwa M, Vandervoort P, Van Kerckhoven S, Holvoet P, Hoylaerts MF. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in ApoE−/− mice. Circ Res. 2008;103:1181–1189. doi: 10.1161/CIRCRESAHA.108.185645. [DOI] [PubMed] [Google Scholar]
- 50.Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R, Wang C, Liu B, Kent KC. TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am J Physiol Heart Circ Physiol. 2009;297:H540–549. doi: 10.1152/ajpheart.91478.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen D, Asahara T, Krasinski K, Witzenbichler B, Yang J, Magner M, Kearney M, Frazier WA, Isner JM, Andres V. Antibody blockade of thrombospondin accelerates reendothelialization and reduces neointima formation in balloon-injured rat carotid artery. Circulation. 1999;100:849–854. doi: 10.1161/01.cir.100.8.849. [DOI] [PubMed] [Google Scholar]
- 52.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. 2013;4:76–83. [PMC free article] [PubMed] [Google Scholar]
- 53.Long CA, Timmins LH, Koutakis P, Goodchild TT, Lefer DJ, Pipinos II, Casale GP, Brewster LP. An endovascular model of ischemic myopathy from peripheral arterial disease. J Vasc Surg. 2016 doi: 10.1016/j.jvs.2016.07.127. In Press:[Epub ahead of print: http://dx.doi.org/10.1016/j.jvs.2016.07.127] [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.