Abstract
Two self-assembled redox-active cages are presented. They are obtained by coordination-driven self-assembly of a tetra-pyridile tetrathiafulvalene ligand with cis-M(dppf)(OTf)2 (M = Pd or Pt; dppf = 1,1′-bis(diphenylphosphino)ferrocene; OTf = trifluoromethane-sulfonate) complexes. Both species are fully characterized and are constituted of 12 electro-active subunits that can be reversibly oxidized.
Keywords: self-assembly, metalla-cages, metal-driven, tetrathiafulvalene, redox
1. Introduction
The coordination-driven self-assembly methodology has proven during the last decades to be very efficient for the preparation of discrete polygons (2D) or polyhedra (3D) otherwise challenging to synthesize through conventional covalent multi-step synthesis [1–13]. This approach involves a coordination process between a rigid organic ligand and a metal center of complementary geometry, to provide thermodynamically stable discrete assemblies in high yields. In particular, nitrogen-based binding sites such as substituted pyridines are often used in presence of square planar Palladium (II) or Platinum (II) salts.
The corresponding host cavities offer new promising opportunities for applications in molecular recognition or even in guest transport [14–16]. In this context, very few examples of coordination-driven redox-active self-assembled discrete structures have been described so far. Their constituting ligands are usually based on electro-deficient skeletons such as triazine [17,18] or perylene diimide [19–25] units and more rarely on oxidable fragments [26,27]. It has to be noted that the electro-activity can also be located on the metal complex [28–38] or on pendant units [39–43] instead of being centered on the side-walls. In the course of our studies related to the preparation of electron-rich functional metallosupramolecular discrete architectures, we recently described the first metalla-cycles [44,45] and metalla-cages [46,47] constructed from derivatives of the π-donating tetrathiafulvelene unit [i.e., bispyrrolo(tetrathiafulvalene) (BPTTF) or the so-called extended-tetrathiafulvalene (exTTF)]. The π-donating ability of tetrathiafulvalene (TTF) derivatives is well established and is responsible for the success of this redox-unit which is used in various molecular and supramolecular switchable systems [48–51].
Herein, we present an original example of coordination-driven self-assembled architectures involving the parent TTF redox-active framework and cis-blocked Pd(II) and Pt(II) salts.
2. Results and Discussion
2.1. Ligand L1: Synthesis and Characterization
New ligand L1 was obtained in one step from the pristine TTF through a Palladium-catalyzed C–H cross-coupling reaction [52,53] with 4-iodopyrine (Scheme 1). This reaction is run in good yields (71%) when taking into account that four C–C bonds are simultaneously formed.
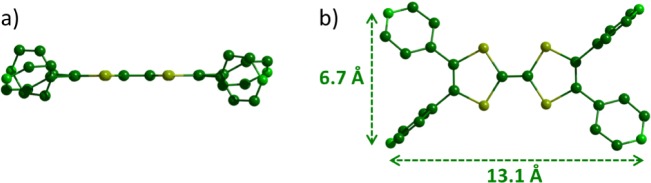
Single crystals of ligand L1 were obtained by slow diffusion of hexane in a solution of L1 in dichloromethane. The XRD analysis (Tables S1) revealed that the TTF skeleton and the four nitrogen atoms are coplanar (Figure 1a) and set in a rectangle defined by a length of 13.1 Å and a width of 6.7 Å (Figure 1b). The electro-deficient pyridile plans are highly rotated related to the TTF backbone (i.e., between 31° and 77°) and are therefore poorly conjugated to the donating part of the molecule. Consequently, the π-donating ability of ligand L1 should be only moderately altered compared to the parent TTF.
Figure 1.

X-Ray structure of ligand L1: (a) side view and (b) top view.
2.2. Assemblies 1 and 2: Synthesis and Characterization
Ligand L1 was engaged in a self-assembly process with cis-M(dppf)(OTf)2 (M = Pt or Pd) complexes in nitromethane at 50 °C and the reaction was monitored by 1H NMR (Figures 2, S1). Several discrete architectures are potentially expected from this reaction, according to the number of ligand L1 involved in the final structures. In both cases, the reaction converged to one unique species 1 (Pt complex) or 2 (Pd complex) in 2 h and 5 min respectively. The self-assembled discrete structures could be isolated by precipitation from Et2O. Compared to ligand L1 (Figure 2a) and starting metal complexes (Figure 2b), assemblies 1 (Figure 2d) and 2 (Figure 2c) present pyridile signals which are upfield-shifted (Hα ≈ 8.5 ppm and Hβ ≈ 7.1 ppm) and one singlet for each proton corresponding to the cyclopentadienyl units (≈4.8 ppm). This behavior is expected and has already been reported for similar compounds [46]. One singlet is observed for each of the 19F and 31P NMR spectra of 1 and 2 (Figures S4, S5, S9, S10), and the corresponding DOSY NMR spectra exhibit a single alignment of signals (Figures 2e, S11). All these data are in agreement with the formation of a unique species during the self-assembly reaction.
Figure 2.
1H NMR (CD3NO2): (a) L1 (*); (b) cis-Pt(dppf)(OTf)2; (c) 2; (d) 1; and (e) DOSY NMR of 1. (*) 1H NMR of ligand L1 was recorded in a mixture of CD3NO2/CDCl3 (2/1).
Remarkably, both self-assemblies present the same diffusion coefficient in solution (D ≈ 2.2 × 10−10 m2·s−1) extracted from the DOSY experiments. This result indicates that both species are of similar size with an estimated hydrodynamic radius of ca. 16.5 Å calculated from the Stokes-Einstein equation [54]. This value is in line with the formation of a large discrete self-assembled architecture but does not allow discriminating between a M6L3 or a M8L4 species.
High resolution ESI mass spectrometry experiments were carried out from dichloromethane solutions of 1 (Figure 3 and Figure S13) and 2 (Figure S14) and confirm the formation of M8L4 architectures with peaks corresponding to multi-charged species [M-3TfO−]3+ (m/z = 3327.6752 (1) and 3091.5087 (2)), [M-4TfO−]4+ (m/z = 2459.0238 (1) and 2280.8943 (2)) and [M-5TfO−]5+ (m/z = 1937.0275 (1)), as well as a good matching between the experimental and theoretical isotopic patterns.
Figure 3.
ESI-MS of self-assembly 1 in CH2Cl2 (C = 10−3 M).
Depending on the orientation of the ligands inside the structure, two types of geometry are conceivable for the M4L8 assemblies (i.e., the rectangular ligands are connected to the metal centers through their short (Scheme 2A) or long side (Scheme 2B). Molecular force field (MM+) studies were undertaken to determinate the most probable structure. Geometry optimization could not yield a symmetric stationary point for the B assembly (Scheme 2B) due to a high structural strain. In contrast, a highly symmetric assembly could be reached for the geometry A (Figure 4). As expected, the structural characteristics of the tetra-pyridyl ligand within the metalla-assemblies are close to those of free L1 (Figure 1) and a distance of 16 Å is found between two facing TTF plans. A total diameter of 36 Å was found for complex 1 in reasonable accordance with the value determined from the DOSY NMR experiment.
Figure 4.
Molecular force field (MM+) model of square 1: (a) front view and (b) side view.
2.3. Redox Properties
Redox properties of ligand L1, complexes 1 and 2 were studied by cyclic voltammetry (Figure 5). As usually observed with TTF derivatives, two reversible oxidation waves are observed for ligand L1 and are assigned to the successive generation of the cation-radical and dication states. A lower π-donor ability is found compared to the parent unsubstituted TTF, with a first redox potential which is shifted by +230 mV. This behavior is ascribed to the presence of four electro-deficient pyridile units on the periphery of the TTF backbone. Three reversible redox processes are observed in the case of the self-assembled metalla-cages 1 and 2. The first two are assigned to the TTF side walls and the third one to the corner ferrocene units. Interestingly, the latter can be used as an internal reference to address the number of electrons exchanged along the redox processes. The relative intensities collected from the deconvoluted cyclic voltammogram (Figure 5b) agree with a successive one, one, two electrons oxidation sequence. Since the assembly encompasses four TTF and eight ferrocene redox units, such observation indicates that full oxidation at a potential of 1.0 V vs Fc/Fc+ leads to the reversible generation of sixteen positive charges in addition to those centered on the eight M(II) complexes. Finally, it has to be mentioned that the CV shape remains unchanged upon several scanning between 0.0 V and 1.0 V vs Fc/Fc+. Nevertheless, a repeated cycling to the upper limit of potentials results in a progressive decreasing of the redox waves intensities, in particular with the Pd derivative, which we attribute to a passivation of the electrode. Therefore, this stability and the possibility which is offered by such systems to control the charge (up to sixteen) on the periphery of the cavity, open promising perspectives in terms of guest binding and transport.
Figure 5.
(a) Cyclic voltammogram and (b) deconvoluted cyclic voltammogram of ligand L1 (C = 10−3 M, CH3CN/CH2Cl2, 0.1 M nBu4NPF6, 100 mV·s−1, Cgr) and of squares 1 and 2 (C = 5 × 10−4 M, CH3CN, 0.1 M nBu4PF6, 20 mV·s−1, Cgr), V vs Fc/Fc+.
3. Experimental Section
3.1. Chemicals
All reagents were of commercial reagent grade and were used without further purification. Complexes cis-Pd(dppf)(OTf)2 and cisPt(dppf)(OTf)2 [55], (dppf = 1,1′-bis(diphenylphosphino) ferrocene; OTf = trifluoromethane-sulfonate) were synthesized as described in literature. Silica gel chromatography was performed with a SIGMA Aldrich Chemistry SiO2 (pore size 60 Å, 40–63 μm technical grades).
3.2. Instrumentation
The 300.3 (1H), 75.5 (13C), 121.6 (31P) and 282.6 MHz (19F) NMR spectra were recorded at room temperature using perdeuterated solvents as internal standards (1H), external H3PO4 solution (31P) and CFCl3 (19F), on a NMR Bruker Avance III 300 spectrometer (Bruker, Rheinstetten, Germany). MALDI-TOF-MS spectra were recorded on a MALDI-TOF Bruker Biflex III instrument using a positive-ion mode. ESI-MS spectra were achieved on a Bruker MicrO-Tof-Q 2 spectrometer in CH2Cl2. Cyclic voltammetry experiments were carried out on an ALS electrochemical analyzer model 660 and the conditions were the following: 0.1 M nBu4NPF6 in acetonitrile or acetronitrile/methylene chloride (1/1 v/v), Ag/Ag+ reference electrode, GC or Pt working electrode, and Pt counter electrode, calibrated using internal ferrocene. Elemental analyses were achieved on a Thermo Electron analyzer.
3.3. Single-crystal X-ray Crystallography
X-ray single-crystal diffraction data were collected at low temperature on a Bruker KappaCCD diffractometer, equipped with a graphite monochromator utilizing MoKα radiation (λ = 0.71073 Å). The structure was solved by direct method and refined on F2 by full matrix least-squares techniques using SHELX97 (Programs for Crystal Structure Analysis (Release 97-2). Sheldrick, G.M. [56]) package. All non-hydrogen atoms were refined anisotropically. Absorption was corrected by SADABS program (Sheldrick, Bruker, 2008). The H atoms were found by Fourier difference map. CCDC reference number CCDC 951195 (L1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre [57].
3.4. Molecular Modeling
Molecular modeling was performed by using the molecular mechanics force field MM+ method from the HyperChem 8.0.3 program (Hypercube, Inc., Waterloo, ON, Canada,) configured in vacuo, with a RMS of 10−5 kcal/mole, a number of maximum cycles of 32500, and a Polak-Ribiere algorithm. Counter anions were omitted to simplify the calculation.
3.5. Experimental Procedure and Characterization Data
3.5.1. 4,4′,5,5′-tetra(pyridin-4-yl)-2,2′-bi(1,3-dithiolylidene) (L1)
To a suspension of palladium acetate (82 mg, 0.36 mmol), tri-tert-butylphosphine tetrafluoroborate (320 mg, 1.10 mmol) and cesium carbonate (2.40 g, 7.30 mmol) stirred for 10 min at 90 °C under argon in distilled dioxane (20 mL) was added an argon degassed solution of tetrathiafulvalene (300 mg, 1.46 mmol) and 4-iodopyridine (1.50 g, 7.34 mmol) in dioxane (20 mL). The reaction was stirred under reflux for 24 h. After cooling, a large excess of dichloromethane and water were added. The aqueous phase was extracted and the organic extracts were washed with brine, dried over magnesium sulfate, filtered and concentrated. The residue was purified by chromatography on silica gel (deactivated with triethylamine 1%) eluting from dichloromethane to dichloromethane/methanol (97/3 v/v) to give a red powder (530 mg, 1.03 mmol, 71%). Crystals (dark red needles) were obtained by slow diffusion of hexanes in dichloromethane. Melting Point: >260 °C. 1H NMR [300 MHz, CDCl3]: δ (ppm) = 8.55 (dd, 3J = 4.5 Hz, 4J = 1.6 Hz, 8H, Hα), 7.09 (dd, 3J = 4.5 Hz, 4J = 1.6 Hz, 8H, Hβ). 13C NMR [75 MHz, CDCl3]: δ (ppm) = 150.6, 139.4, 129.6, 123.1, 109.0. Calculated [C26H16N4S4]: 512.69; Observed (MALDI-TOF): 512.5.
3.5.2. Complex 1
A mixture of ligand L1 (10.0 mg, 19.5 μmol) and cis-Pt(dppf)(OTf)2 (40.9 mg, 39.1 μmol) in anhydrous nitromethane (2 mL) was heated at 50 °C for 2 h under argon. After cooling, diethyl ether (10 mL) was added and the mixture was centrifuged. The residue was washed with diethyl ether and dried under vacuum to give complex 1 (46.3 mg, 4.4 μmol, 91%) as a dark orange solid. 1H NMR (CD3NO2) δ = 8.53 (d, 3J = 5.9 Hz, 32 H), 8.01 (m, 32 H), 7.80–7.61 (m, 128 H), 7.12 (d, 3J = 5.9 Hz, 32 H), 4.95 (brs, 16 H), 4.77 (brs, 16 H), 4.67 (m, 32 H); 19F NMR (CD3NO2) δ −81.30; 31P NMR (CD3NO2) δ = 1.64; ESI-MS m/z: 1937.0275 ([1-11OTf]5+), 2459.0238 ([1-12OTf]4+), 3327.6752 ([1-13OTf]3+); mp > 260 °C.
3.5.3. Complex 2
A mixture of ligand L1 (10.0 mg, 19.5 μmol) and cis-Pd(dppf)(OTf)2 (37.4 mg, 39.1 μmol) in anhydrous nitromethane (2 mL) was heated at 50 °C for 5 min under argon. After cooling, diethyl ether (10 mL) was added and the mixture was centrifuged. The residue was washed with diethyl ether and dried under vacuum to give complex 2 (41.2 mg, 4.2 μmol, 87%) as a dark red solid. 1H NMR (CD3NO2) δ = 8.51 (d, 3J = 5.9 Hz, 16 H), 8.04 (m, 32 H), 7.81–7.62 (m, 128 H), 7.07 (d, 3J = 5.9 Hz, 16 H), 4.99 (brs, 16 H), 4.81 (brs, 16 H), 4.71 (brs, 16 H), 4.67 (brs, 16 H); 19F NMR (CD3NO2) δ = 83.77; 31P NMR (CD3NO2) δ = 31.81; ESI-MS m/z: 2280.8943 ([2-12OTf]4+), 3091.5087 ([2-13OTf]3+); mp > 260 °C.
4. Conclusions
The synthesis and the characterization of two coordination-driven self-assembled cages is depicted. They are constructed from an electron-rich TTF based ligand L1 and an electro-active cis-M(dppf)(OTf)2 (M = Pd or Pt) complex. They exhibit an internal cavity of 16 Å in diameter, which is surrounded by four redox-active TTF and eight ferrocene units. All of them can be oxidized, offering the possibility to reversibly generate up to sixteen positive charges on the assembly. This full control over the charge state of the cavity opens very promising perspectives, in particular for answering the key question of the triggering of the guest binding.
Scheme 1.
Synthesis of ligand L1: 4-Iodopyrine (5 equiv.), Pd(OAc)2 (0.25 equiv.), P(tBu)3·HBF4 (0.75 equiv.) Cs2CO3 (5 equiv.), Dioxane, reflux, 24 h, 71%.
Scheme 2.
Synthesis of polygons 1 and 2: (A) cis-M(dppf)(OTf)2 (2 equiv.), nitromethane, 50 °C; for 1: M = Pt, 2 h, 91%; for 2: M = Pd, 5 min, 87% and (B) Hypothetical alternative structure
Acknowledgments
The authors gratefully acknowledge the CNRS, the Région des Pays de la Loire and the MENRT for PhD grants (Sébastien Bivaud, Vincent Croué), the University of Angers for a post-doctoral fellowship (Vaishali Vajpayee.), as well as the PIAM (Université Angers) and the CRMPO (Université Rennes) for their assistance in spectroscopic analyses, and finally the Johnson-Matthey company for their generous providing of palladium and platinum salts.
Supplementary Materials
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Amouri H., Desmarets C., Moussa J. Confined nanospaces in metallocages: Guest molecules, weakly encapsulated anions, and catalyst sequestration. Chem. Rev. 2012;112 :2015–2041. doi: 10.1021/cr200345v. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarty R., Mukherjee P.S., Stang P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011;111 :6810–6918. doi: 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inokuma Y., Kawano M., Fujita M. Crystalline molecular flasks. Nat. Chem. 2011;3 :349–358. doi: 10.1038/nchem.1031. [DOI] [PubMed] [Google Scholar]
- 4.Han Y.-F., Jia W.-G., Yu W.-B., Jin G.-X. Stepwise formation of organometallic macrocycles, prisms and boxes from Ir, Rh and Ru-based half-sandwich units. Chem. Soc. Rev. 2009;38 :3419–3434. doi: 10.1039/b901649j. [DOI] [PubMed] [Google Scholar]
- 5.Northrop B.H., Zheng Y.R., Chi K.W., Stang P.J. Self-organization in coordination-driven self-assembly. Acc. Chem. Res. 2009;42:1554–1563. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stang P.J. From solvolysis to self-assembly. J. Org. Chem. 2009;74:2–20. doi: 10.1021/jo801682d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therrien B. Arene ruthenium cages: Boxes full of surprises. Eur. J. Inorg. Chem. 2009:2445–2453. [Google Scholar]
- 8.Yoshizawa M., Klosterman J.K., Fujita M. Functional molecular flasks: New properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 2009;48:3418–3438. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]
- 9.Cooke M.W., Chartrand D., Hanan G.S. Self-assembly of discrete metallosupramolecular luminophores. Coord. Chem. Rev. 2008;252:903–921. [Google Scholar]
- 10.Dalgarno S.J., Power N.P., Atwood J.L. Metallo-supramolecular capsules. Coord. Chem. Rev. 2008;252:825–841. [Google Scholar]
- 11.Northrop B.H., Chercka D., Stang P.J. Carbon-rich supramolecular metallacycles and metallacages. Tetrahedron. 2008;64:11495–11503. doi: 10.1016/j.tet.2008.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northrop B.H., Yang H.B., Stang P.J. Coordination-driven self-assembly of functionalized supramolecular metallacycles. Chem. Commun. 2008:5896–5908. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zangrando E., Casanova M., Alessio E. Trinuclear metallacycles: Metallatriangles and much more. Chem. Rev. 2008;108:4979–5013. doi: 10.1021/cr8002449. [DOI] [PubMed] [Google Scholar]
- 14.Therrien B. Transporting and shielding photosensitisers by using water-soluble organometallic cages: A new strategy in drug delivery and photodynamic therapy. Chemistry. 2013;19:8378–8386. doi: 10.1002/chem.201301348. [DOI] [PubMed] [Google Scholar]
- 15.Brinker U.H., Mieusset J.-L., editors. Molecular Encapsulation: Organic Reactions in Constrained Systems. John Wiley & Sons, Ltd; Hoboken, NJ, USA: 2010. [Google Scholar]
- 16.Steed J.W., Atwood J.L. Supramolecular Chemistry. 2nd ed. John Wiley & Sons, Ltd; Hoboken, NJ, USA: 2009. Chapter 6. Molecular Guests in Solution; pp. 307–383. [Google Scholar]
- 17.Bhattacharya D., Chang C.H., Cheng Y.H., Lai L.L., Lu H.Y., Lin C.Y., Lu K.L. Multielectron redox chemistry of a neutral, NIR-active, indigo-pillared ReI-based triangular metalloprism. Chem. Eur. J. 2012;18:5275–5283. doi: 10.1002/chem.201102087. [DOI] [PubMed] [Google Scholar]
- 18.Furutani Y., Kandori H., Kawano M., Nakabayashi K., Yoshizawa M., Fujita M. In situ spectroscopic, electrochemical, and theoretical studies of the photoinduced host-guest electron transfer that precedes unusual host-mediated alkane photooxidation. J. Am. Chem. Soc. 2009;131:4764–4768. doi: 10.1021/ja8089075. [DOI] [PubMed] [Google Scholar]
- 19.Mahata K., Frischmann P.D., Würthner F. Giant electroactive M4L6 tetrahedral host self-assembled with Fe(II) vertices and perylene bisimide dye edges. J. Am. Chem. Soc. 2013;135:15656–15661. doi: 10.1021/ja4083039. [DOI] [PubMed] [Google Scholar]
- 20.Oliva A.I., Ventura B., Wurthner F., Camara-Campos A., Hunter C.A., Ballester P., Flamigni L. Self-assembly of double-decker cages induced by coordination of perylene bisimide with a trimeric Zn porphyrin: Study of the electron transfer dynamics between the two photoactive components. Dalton Trans. 2009;6:4023–4037. doi: 10.1039/b819496c. [DOI] [PubMed] [Google Scholar]
- 21.Wurthner F., You C.-C., Saha-Moller C.R. Metallosupramolecular squares: From structure to function. Chem. Soc. Rev. 2004;33:133–146. doi: 10.1039/b300512g. [DOI] [PubMed] [Google Scholar]
- 22.You C.C., Wurthner F. Self-assembly of ferrocene-functionalized perylene bisimide bridging ligands with Pt(II) corner to electrochemically active molecular squares. J. Am. Chem. Soc. 2003;125:9716–9725. doi: 10.1021/ja029648x. [DOI] [PubMed] [Google Scholar]
- 23.Sautter A., Schmid D.G., Jung G., Würthner F. A triangle–square equilibrium of metallosupramolecular assemblies based on Pd(II) and Pt(II) corners and diazadibenzoperylene bridging ligands. J. Am. Chem. Soc. 2001;123:5424–5430. doi: 10.1021/ja004360y. [DOI] [PubMed] [Google Scholar]
- 24.Würthner F., Sautter A., Schmid D., Weber P.J.A. Fluorescent and electroactive cyclic assemblies from perylene tetracarboxylic acid bisimide ligands and metal phosphane triflates. Chem. Eur. J. 2001;7(1):894–902. doi: 10.1002/1521-3765(20010216)7:4<894::aid-chem894>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Wurthner F., Sautter A. Highly fluorescent and electroactive molecular squares containing perylene bisimide ligands. Chem. Commun. 2000;6:445–446. [Google Scholar]
- 26.Xiong J., Liu W., Wang Y., Cui L., Li Y.-Z., Zuo J.-L. Tricarbonyl mono- and dinuclear Rhenium(I) complexes with redox-active bis(pyrazole)–tetrathiafulvalene ligands: Syntheses, crystal structures, and properties. Organometallics. 2012;31 :3938–3946. [Google Scholar]
- 27.Frank M., Hey J., Balcioglu I., Chen Y-S., Stalke D., Suenobu T., Fukuzumi S., Frauendorf H., Clever GH. Assembly and stepwise oxidation of interpenetrated coordination cages based on phenothiazine. Angew. Chem. Int. Ed. 2013;52:10102–10106. doi: 10.1002/anie.201302536. [DOI] [PubMed] [Google Scholar]
- 28.Chen L.J., Li Q.J., He J.M., Tan H.W., Abliz Z., Yang H.B. Design and construction of endo-functionalized multiferrocenyl hexagons via coordination-driven self-assembly and their electrochemistry. J. Org. Chem. 2012;77:1148–1153. doi: 10.1021/jo201979d. [DOI] [PubMed] [Google Scholar]
- 29.Li Q.J., Zhao G.Z., Chen L.J., Tan H.W., Wang C.H., Wang D.X., Lehman D.A., Muddiman D.C., Yang H.B. Coordination-driven self-assembly of charged and neutral dendritic tetrakis(ferrocenyl) rhomboids. Organometallics. 2012;31:7241–7247. [Google Scholar]
- 30.Han Q., Li Q.J., He J., Hu B., Tan H., Abliz Z., Wang C.H., Yu Y., Yang H.B. Design and synthesis of 60 degrees dendritic donor ligands and their coordination-driven self-assembly into supramolecular rhomboidal metallodendrimers. J. Org. Chem. 2011;76:9660–9669. doi: 10.1021/jo201594u. [DOI] [PubMed] [Google Scholar]
- 31.Zhao G.Z., Li Q.J., Chen L.J., Tan H.W., Wang C.H., Wang D.X., Yang H.B. Coordination-driven self-assembly of neutral dendritic multiferrocenyl hexagons via oxygen-to-platinum bonds and their electrochemistry. Organometallics. 2011;30:5141–5146. [Google Scholar]
- 32.Zhao G.-Z., Li Q.-J., Chen L.-J., Tan H., Wang C.-H., Lehman D.A., Muddiman D.C., Yang H.-B. Facile self-assembly of supramolecular hexakisferrocenyl triangles via coordination-driven self-assembly and their electrochemical behavior. Organometallics. 2011;30:3637–3642. [Google Scholar]
- 33.Zheng Y.R., Lan W.J., Wang M., Cook T.R., Stang P.J. Designed post-self-assembly structural and functional modifications of a truncated tetrahedron. J. Am. Chem. Soc. 2011;133:17045–17055. doi: 10.1021/ja207217t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao G.-Z., Chen L.-J., Wang C.-H., Yang H.-B., Ghosh K., Zheng Y.-R., Lyndon M.M., Muddiman D.C., Stang P.J. Facile self-assembly of dendritic multiferrocenyl hexagons and their electrochemistry. Organometallics. 2010;29:6137–6140. [Google Scholar]
- 35.Ghosh K., Hu J.M., White H.S., Stang P.J. Construction of multifunctional cuboctahedra via coordination-driven self-assembly. J. Am. Chem. Soc. 2009;131:6695–6697. doi: 10.1021/ja902045q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh K., Hu J.M., Yang H.B., Northrop B.H., White H.S., Stang P.J. Introduction of heterofunctional groups onto molecular hexagons via coordination-driven self-assembly. J. Org. Chem. 2009;74:4828–4833. doi: 10.1021/jo900580w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh K., Zhao Y., Yang H.B., Northrop B.H., White H.S., Stang P.J. Synthesis of a new family of hexakisferrocenyl hexagons and their electrochemical behavior. J. Org. Chem. 2008;73 :8553–8557. doi: 10.1021/jo801692v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H.B., Ghosh K., Zhao Y., Northrop B.H., Lyndon M.M., Muddiman D.C., White H.S., Stang P.J. A new family of multiferrocene complexes with enhanced control of structure and stoichiometry via coordination-driven self-assembly and their electrochemistry. J. Am. Chem. Soc. 2008;130:839–841. doi: 10.1021/ja710349j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanmugaraju S., Samanta D., Mukherjee P.S. Self-assembly of Ru-4 and Ru-8 assemblies by coordination using organometallic Ru(II)(2) precursors: Synthesis, characterization and properties. Beilstein J. Org. Chem. 2012;8 :313–322. doi: 10.3762/bjoc.8.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin P.-C., Chen H.-Y., Chen P.-Y., Chiang M.-H., Chiang M.Y., Kuo T.-S., Hsu S.C.N. Self-assembly and redox modulation of the cavity size of an unusual rectangular iron thiolate aryldiisocyanide metallocyclophane. Inorg Chem. 2011;50:10825–10834. doi: 10.1021/ic201380k. [DOI] [PubMed] [Google Scholar]
- 41.Mattsson J., Govindaswamy P., Renfrew A.K., Dyson P.J., Štěpnička P., Süss-Fink G., Therrien B. Synthesis, molecular structure, and anticancer activity of cationic arene ruthenium metallarectangles. Organometallics. 2009;28 :4350–4357. [Google Scholar]
- 42.Berben L.A., Faia M.C., Crawford N.R.M., Long J.R. Angle-dependent electronic effects in 4,4′-bipyridine-bridged Ru-3 triangle and Ru-4 square complexes. Inorg. Chem. 2006;45 :6378–6386. doi: 10.1021/ic060570l. [DOI] [PubMed] [Google Scholar]
- 43.Sun S.-S., Lees A.J. Self-assembly organometallic squares with terpyridyl metal complexes as bridging ligands. Inorg. Chem. 2001;40 :3154–3160. doi: 10.1021/ic0101681. [DOI] [PubMed] [Google Scholar]
- 44.Goeb S., Bivaud S., Dron P.I., Balandier J.-Y., Chas M., Sallé M. A. BPTTF-based self-assembled electron-donating triangle capable of C60 binding. Chem. Commun. 2012;48 :3106–3108. doi: 10.1039/c2cc00065b. [DOI] [PubMed] [Google Scholar]
- 45.Balandier J.-Y., Chas M., Goeb S., Dron P.I., Rondeau D., Belyasmine A., Gallego N., Sallé M. A self-assembled bis(pyrrolo)tetrathiafulvalene-based redox active square. New J. Chem. 2011;35 :165–168. [Google Scholar]
- 46.Bivaud S., Goeb S., Croue V., Dron P.I., Allain M., Sallé M. Self-assembled containers based on extended tetrathiafulvalene. J. Am. Chem. Soc. 2013;135 :10018–10021. doi: 10.1021/ja404072w. [DOI] [PubMed] [Google Scholar]
- 47.Bivaud S., Balandier J.Y., Chas M., Allain M., Goeb S., Sallé M. A metal-directed self-assembled electroactive cage with bis(pyrrolo)tetrathiafulvalene (BPTTF) side walls. J. Am. Chem. Soc. 2012;134 :11968–11970. doi: 10.1021/ja305451v. [DOI] [PubMed] [Google Scholar]
- 48.Canevet D., Sallé M., Zhang G., Zhang D., Zhu D. Tetrathiafulvalene (TTF) derivatives: Key building-blocks for switchable processes. Chem. Commun. 2009:2245–2269. doi: 10.1039/b818607n. [DOI] [PubMed] [Google Scholar]
- 49.Martín N., Segura J.-L. New concepts in tetrathiafulvalene chemistry. Angew. Chem. Int. Ed. 2001;40 :1372–1409. doi: 10.1002/1521-3773(20010417)40:8<1372::aid-anie1372>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 50.Bryce M.R. Functionalised tetrathiafulvalenes: New applications as versatile π-electron systems in materials chemistry. J. Mater. Chem. 2000;10 :589–598. [Google Scholar]
- 51.Nielsen M.B., Lomholt C., Becher J. Tetrathiafulvalenes as building blocks in supramolecular chemistry II. Chem. Soc. Rev. 2000;29 :153–164. [Google Scholar]
- 52.Mitamura Y., Yorimitsu H., Oshima K., Osuka A. Straightforward access to aryl-substituted tetrathiafulvalenes by palladium-catalysed direct C–H arylation and their photophysical and electrochemical properties. Chem. Sci. 2011;2:2017–2021. [Google Scholar]
- 53.Vajpayee V., Bivaud S., Goeb S., Allain M., Popp B.V., Garci A., Therrien B., Sallé M. Electron-rich arene-ruthenium metalla-architectures incorporating tetrapyridyl-tetrathiafulvene donor moieties. 2013 submitted for publication. [Google Scholar]
- 54.Cohen Y., Avram L., Frish L. Diffusion NMR spectroscopy in supramolecular and combinatorial chemistry: An old parameter-new insights. Angew. Chem. Int. Ed. 2005;44:520–554. doi: 10.1002/anie.200300637. [DOI] [PubMed] [Google Scholar]
- 55.Bar A.K., Chakrabarty R., Chi K.-W., Batten S.R., Mukherjee P.S. Synthesis and characterisation of heterometallic molecular triangles using ambidentate linker: Self-selection of a single linkage isomer. Dalton Trans. 2009;17:3222–3229. doi: 10.1039/b900118b. [DOI] [PubMed] [Google Scholar]
- 56.Sheldrick G.M. A short history of. SHELX. Acta Cryst. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 57.CCDC CIF Depository Request Form. [accessed on 14 January 2014]. Available online: www.ccdc.cam.ac.uk/data_request/cif.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.