Abstract
We present the case of an HIV-2 infected patient who developed progressive multifocal leukoencephalopathy (PML) in the setting of immune reconstitution inflammatory syndrome (IRIS) presenting as Bell’s palsy. The brain MRI showed a single lesion in the facial colliculus considered initially to be ischemic in nature. This case report should alert clinicians that PML can occur in the setting of HIV-2 infection. It also illustrates the difficulty of establishing the diagnosis of PML.
Introduction
Of all people living with the human deficiency virus (HIV), approximately 39 million are infected with HIV type-1 and 1–2 million with HIV type-2(Campbell-Yesufu and Gandhi 2011, Wang, Wolock et al. 2016). HIV-2 is mostly endemic in West Africa and is less efficiently transmitted than HIV-1. Although AIDS may develop in HIV-2 carriers, it takes longer than in HIV-1-infected patients suggesting that immune factors limit viral pathogenesis.(Chan, Wakeman et al. 2008) In HIV-2 infection, humoral, innate and cellular responses are more robust and polyfunctional (Nyamweya, Hegedus et al. 2013). Progressive multifocal leukoencephalopathy (PML) is caused by JC virus (JCV), a ubiquitous polyomavirus which infects more than 50% of the adult population without causing any disease. PML usually occurs in immunocompromised individuals, such as those with advanced HIV-1 infection.(Berger, Aksamit et al. 2013) There are only few published cases of PML in HIV-2 infected patients. (Stoner, Agostini et al. 1998, Bienaime, Colson et al. 2006, Duque, Matos et al. 2010, Descamps, Peytavin et al. 2015) We report an atypical case of infratentorial PML in an HIV-2-infected patient from Cape Verde and reviewed the clinical features of all the reported cases.
Case report
A 65-year-old man with 13 years history of HIV-2 infection and chronic tremors presented with left facial weakness for 48 hours. His initial neurologic exam showed left facial weakness with involvement of the forehead and incomplete left eye closure. He had action tremors involving the right arm greater than the left one and were unchanged from previous neurologic exams. His serum HIV-2 viral load at symptom onset was 311 copies/ml and his CD4+ T-cell count was 361/ul, on elvitegravir, cobicistat, emtricitabine, tenofovir, and atazanavir treatment, up from 254/ul two months prior.
His facial weakness, which was considered to be caused by a peripheral seventh nerve palsy (Bell’s palsy), worsened over 48 hours after admission. However, an MRI of the brain showed a non-enhancing lesion in the left facial colliculus, which was initially interpreted as a subacute infarct. There was also a region of hazy enhancement in the right pons that was unchanged from a study done 7 years prior to his current presentation. This finding was compatible with a capillary telangiectasia.
Of note, he had an episode of varicella zoster virus (VZV) thoracic radiculopathy four months prior to symptom onset. He was discharged home on 1 week p.o steroids and valacyclovir treatment for Bell’s palsy.
Six weeks later, the patient developed progressive dysphagia to solid food. His neurologic exam showed left facial palsy and a weak gag reflex. A repeat MRI showed slight interval increase in the left facial colliculus lesion with new faint peripheral enhancement (Figure 1). He underwent a video oropharyngeal swallow study which revealed difficulty with initiation of swallowing, early spilling and delayed initiation. A lumbar puncture was performed to investigate possible infectious etiologies. CSF analyses showed 2 WBC/ul, elevated protein at 58 mg/dL, normal glucose, negative bacterial cultures and positive CSF JCV PCR at 34 copies/ml, establishing the diagnosis of PML. CSF PCR for TB, VZV, Toxoplasma Gondii, HSV, cytomegalovirus, and EBV were negative and the CSF VDRL was non-reactive and Cryptococcal antigen was not detected.
Figure 1. MRI findings after symptom onset.
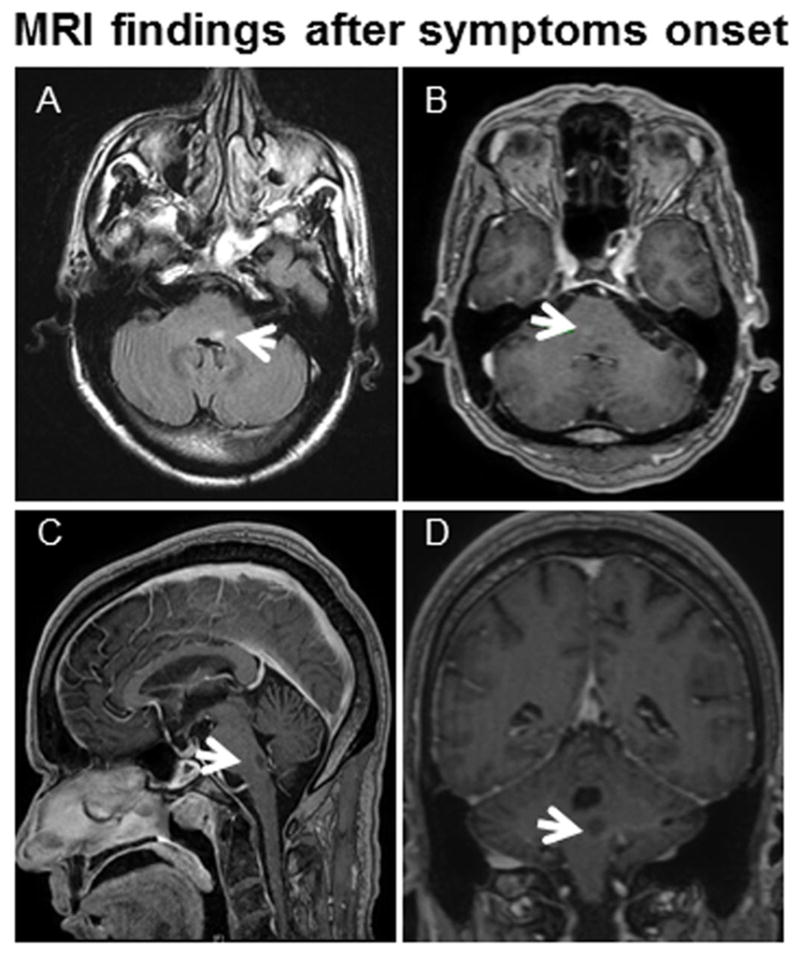
MRI of left facial colliculus PML lesion 6 weeks after symptom onset. (A) Axial fluid-attenuated inversion recovery (FLAIR) image shows a lesion in the left facial colliculus (arrow). (B) Axial post-contrast T1 weighted images demonstrates faint peripheral enhancement in the lesion and hazy enhancement in the right pons (arrow) (the latter was compatible with a capillary telangiectasia that was unchanged from a study done 7 years prior to his current presentation). Sagittal and coronal post-contrast T1 weighted images (C and D) demonstrate faint peripheral enhancement in the lesion (arrows) of the left facial colliculus.
He remained on the same cART regimen and his dysphagia and facial weakness improved mildly three months after symptom onset.
Discussion
We describe a case of PML in a patient with HIV-2 infection. PML is usually a subacute disease with progressive focal neurological deficits related to the location of the lesions. The clinical manifestations are very diverse including motor weakness, gait abnormalities, visual field defects, cognitive and language dysfunction.(Berger, Aksamit et al. 2013) The presentation of PML in this patient is atypical in several aspects: 1) A single lesion was present in the left facial colliculus, affecting the seventh nerve nucleus and causing a neurologic deficit undistinguishable from a peripheral facial palsy. 2) The symptomatology occurred in the setting of immune recovery with CD4+ T-cell count going from 254/ul up to 361/ul on cART. This is consistent with PML-IRIS. Indeed, faint contrast enhancement was present in the PML lesion (Fig 1 B–D), which is one of the features of PML/IRIS. Compared to the other 4 published HIV-2/PML cases, (Stoner, Agostini et al. 1998, Bienaime, Colson et al. 2006, Duque, Matos et al. 2010, Descamps, Peytavin et al. 2015) this is the only one presenting with a single lesion and in the context of IRIS. Of note, HIV-1 can also be the target of the recovered immune system in IRIS, causing fulminant HIV encephalitis.(Nath 2015) To our knowledge, there are no reports of HIV-2 being the sole target of IRIS in the CNS.
The clinical features of 4 previously reported cases of PML among HIV-2-infected patients are summarized in Table 1. CD4+ T-lymphocyte count and viral load were available in 3 of them: CD4 count was <100 cells/ul and plasma HIV-2 viral load ranged from 250–23,120 copies/mL. One patient had faint and irregular contrast enhancement in PML lesions on MRI, but there was no evidence of immune recovery (Table 1, case 1). Of note, none of these patients survived more than four months after symptom onset. Conversely our patient demonstrated clinical improvement at 3 months follow up. One possible explanation is his higher number of CD4+ T- cells at symptom onset and the presence of IRIS, which has been associated with better prognosis in PML patients. (Gheuens, Ngo et al. 2012)
Table 1.
Characteristics of Previously Reported Progressive Multifocal Leukoencephalopathy (PML) Cases in HIV-2-Infected Patients
| Case | Reference | Age at PML diagnosis, | Gender | Initial presentation | CD4 count (cells/ul), HIV-2 plasma viral load (copies/ml) | Initial MRI findings | PML diagnosis | Treatment | IRIS | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Duque, Matos et al. 2010 | 51 | F | Left hemiparesis | 9/20,600 | Multiple lesions in the brain and cerebellum. Faint and irregular contrast enhancement | CSF JCV PCR + | cART | No | Death after 4 months |
| 2 | Bienaime, Colson et al. 2006 | 60 | M | Dysarthria, ataxia and gait dysfunction | 83/250 | Multiple lesions in the right cerebellum, pons inferior and middle peduncles | CSF JCV PCR + Typical histopathology / In situ hybridization + | cART | No | Death after 2 months |
| 3 | Descamps, Peytavin et al. 2015 | 43 | M | MR | 85/23,120 | NR | NR | cART | NR | Death after 4 months |
| 4 | Stoner, Agostini et al. 1998 | NR | NR | NR | NR | NR | Typical histopathology / In situ hybridization + | NR | NR | Death after 3 months |
| 5 | Sierra Morales et al. 2017 | 65 | M | Facial weakness and dysphagia | 311/361 | One lesion in the left facial colliculus with associated contrast enhancement | CSF JCV PCR + | cART | Yes | Clinical improvement |
PML, progressive multifocal leukoencephalopathy; VL, viral load; CSF, cerebrospinal fluid; JCV, JC virus; cART, combination antiretroviral therapy; IRIS, immune reconstitution inflammatory syndrome; NR, not reported.
Neurological complications can be seen in the setting of HIV-2 infection. Those include neurocognitive disorder, demyelinating encephalomyelitis, CNS toxoplasmosis, cryptococcal meningitis, and spastic paraplegia.(Moulignier, Lascoux et al. 2006, Choi, Townend et al. 2011) In a study of 5401 patients in West Africa, encephalitis was more frequent in HIV-2 infection than in HIV-1 infection.(Lucas, Hounnou et al. 1993)
This case report also illustrates the difficulty of establishing the diagnosis of PML. Indeed, Bell’s palsy and stroke were first considered more likely, and the correct diagnosis of PML was only established at the second hospitalization 5 weeks after symptom onset. This is consistent with our experience with diagnosis delay in PML both in HIV+ and HIV- individuals.(Miskin, Ngo et al. 2016) Finally, this case report should alert clinicians that the CNS complications of HIV-1 and HIV-2 infection overlap, and highlights the need to consider PML in HIV-2 infected patients with progressive focal neurological deficits.
Acknowledgments
This work was supported in part by grants NIH R01 NS 047029 and R01 NS 074995 to IJK.
Footnotes
Conflict of Interest:
Fabian Sierra Morales, Carlos Illingworth, Kathie Lin, Ivia Rivera Agosto, Chloe Powell, Jacob A. Sloane, and Igor J. Koralnik declare that they have no conflict of interest.
References
- Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, Bartt R, Major EO, Nath A. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienaime A, Colson P, Moreau J, Zandotti C, Pellissier JF, Brouqui P. Progressive multifocal leukoencephalopathy in HIV-2-infected patient. Aids. 2006;20(9):1342–1343. doi: 10.1097/01.aids.0000232249.89404.d6. [DOI] [PubMed] [Google Scholar]
- Campbell-Yesufu OT, Gandhi RT. Update on human immunodeficiency virus (HIV)-2 infection. Clin Infect Dis. 2011;52(6):780–787. doi: 10.1093/cid/ciq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PA, Wakeman SE, Flanigan T, Cu-Uvin S, Kojic E, Kantor R. HIV-2 diagnosis and quantification in high-risk patients. AIDS Res Ther. 2008;5:18. doi: 10.1186/1742-6405-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Townend J, Vincent T, Zaidi I, Sarge-Njie R, Jaye A, Clifford DB. Neurologic manifestations of human immunodeficiency virus-2: dementia, myelopathy, and neuropathy in West Africa. J Neurovirol. 2011;17(2):166–175. doi: 10.1007/s13365-011-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps D, Peytavin G, Visseaux B, Tubiana R, Damond F, Campa P, Charpentier C, Khuong-Josses MA, Duvivier C, Karmochkine M, Lukiana T, Matheron S. Dolutegravir in HIV-2-Infected Patients With Resistant Virus to First-line Integrase Inhibitors From the French Named Patient Program. Clin Infect Dis. 2015;60(10):1521–1527. doi: 10.1093/cid/civ124. [DOI] [PubMed] [Google Scholar]
- Duque V, Matos AM, da Cunha JS, Melico-Sivestre A. Progressive multifocal leukoencephalopathy in HIV-2 infection. Case report. J Clin Virol. 2010;48(3):215–217. doi: 10.1016/j.jcv.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Gheuens S, Ngo L, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Metabolic profile of PML lesions in patients with and without IRIS: an observational study. Neurology. 2012;79(10):1041–1048. doi: 10.1212/WNL.0b013e318268465b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G, N’Gbichi JM, Yeboue K, Honde M, Diomande M, Giordano C, et al. The mortality and pathology of HIV infection in a west African city. Aids. 1993;7(12):1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- Miskin DP, Ngo LH, Koralnik IJ. Diagnostic delay in progressive multifocal leukoencephalopathy. Ann Clin Transl Neurol. 2016;3(5):386–391. doi: 10.1002/acn3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulignier A, Lascoux C, Bourgarit A. HIV type 2 demyelinating encephalomyelitis. Clin Infect Dis. 2006;42(11):e89–91. doi: 10.1086/503909. [DOI] [PubMed] [Google Scholar]
- Nath A. Neurologic Complications of Human Immunodeficiency Virus Infection. Continuum (Minneap Minn) 2015;21(6 Neuroinfectious Disease):1557–1576. doi: 10.1212/CON.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Nyamweya S, Hegedus A, Jaye A, Rowland-Jones S, Flanagan KL, Macallan DC. Comparing HIV-1 and HIV-2 infection: Lessons for viral immunopathogenesis. Rev Med Virol. 2013;23(4):221–240. doi: 10.1002/rmv.1739. [DOI] [PubMed] [Google Scholar]
- Stoner GL, Agostini HT, Ryschkewitsch CF, Mazlo M, Gullotta F, Wamukota W, Lucas S. Detection of JC virus in two African cases of progressive multifocal leukoencephalopathy including identification of JCV type 3 in a Gambian AIDS patient. J Med Microbiol. 1998;47(8):733–742. doi: 10.1099/00222615-47-8-733. [DOI] [PubMed] [Google Scholar]
- Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, Hay SI, Mills EJ, Trickey A, Msemburi W, Coates MM, Mooney MD, Fraser MS, Sligar A, Salomon J, Larson HJ, Friedman J, Abajobir AA, Abate KH, Abbas KM, Razek MM, Abd-Allah F, Abdulle AM, Abera SF, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Abyu GY, Adebiyi AO, Adedeji IA, Adelekan AL, Adofo K, Adou AK, Ajala ON, Akinyemiju TF, Akseer N, Lami FH, Al-Aly Z, Alam K, Alam NK, Alasfoor D, Aldhahri SF, Aldridge RW, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Ali R, Alkerwi A, Alla F, Mohammad R, Al-Raddadi S, Alsharif U, Alvarez E, Alvis-Guzman N, Amare AT, Amberbir A, Amegah AK, Ammar W, Amrock SM, Antonio CA, Anwari P, Arnlov J, Artaman A, Asayesh H, Asghar RJ, Assadi R, Atique S, Atkins LS, Avokpaho EF, Awasthi A, Quintanilla BP, Bacha U, Badawi A, Barac A, Barnighausen T, Basu A, Bayou TA, Bayou YT, Bazargan-Hejazi S, Beardsley J, Bedi N, Bennett DA, Bensenor IM, Betsu BD, Beyene AS, Bhatia E, Bhutta ZA, Biadgilign S, Bikbov B, Birlik SM, Bisanzio D, Brainin M, Brazinova A, Breitborde NJ, Brown A, Burch M, Butt ZA, Campuzano JC, Cardenas R, Carrero JJ, Castaneda-Orjuela CA, Rivas JC, Catala-Lopez F, Chang HY, Chang JC, Chavan L, Chen W, Chiang PP, Chibalabala M, Chisumpa VH, Choi JY, Christopher DJ, Ciobanu LG, Cooper C, Dahiru T, Damtew SA, Dandona L, Dandona R, das Neves J, de Jager P, De Leo D, Degenhardt L, Dellavalle RP, Deribe K, Deribew A, Des Jarlais DC, Dharmaratne SD, Ding EL, Doshi PP, Driscoll TR, Dubey M, Elshrek YM, Elyazar I, Endries AY, Ermakov SP, Eshrati B, Esteghamati A, Faghmous ID, Farinha CS, Faro A, Farvid MS, Farzadfar F, Fereshtehnejad SM, Fernandes JC, Fischer F, Fitchett JR, Foigt N, Fullman N, Furst T, Gankpe FG, Gebre T, Gebremedhin AT, Gebru AA, Geleijnse JM, Gessner BD, Gething PW, Ghiwot TT, Giroud M, Gishu MD, Glaser E, Goenka S, Goodridge A, Gopalani SV, Goto A, Gugnani HC, Guimaraes MD, Gupta R, Gupta R, Gupta V, Haagsma J, Hafezi-Nejad N, Hagan H, Hailu GB, Hamadeh RR, Hamidi S, Hammami M, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Harun KM, Havmoeller R, Hedayati MT, Heredia-Pi IB, Hoek HW, Horino M, Horita N, Hosgood HD, Hoy DG, Hsairi M, Hu G, Huang H, Huang JJ, Iburg KM, Idrisov BT, Innos K, Iyer VJ, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Javanbakht M, Jayatilleke AU, Jeemon P, Jha V, Jiang G, Jiang Y, Jibat T, Jonas JB, Kabir Z, Kamal R, Kan H, Karch A, Karema CK, Karletsos D, Kasaeian A, Kaul A, Kawakami N, Kayibanda JF, Keiyoro PN, Kemp AH, Kengne AP, Kesavachandran CN, Khader YS, Khalil I, Khan AR, Khan EA, Khang YH, Khubchandani J, Kim YJ, Kinfu Y, Kivipelto M, Kokubo Y, Kosen S, Koul PA, Koyanagi A, Defo BK, Bicer BK, Kulkarni VS, Kumar GA, Lal DK, Lam H, Lam JO, Langan SM, Lansingh VC, Larsson A, Leigh J, Leung R, Li Y, Lim SS, Lipshultz SE, Liu S, Lloyd BK, Logroscino G, Lotufo PA, Lunevicius R, Razek HM, Mahdavi M, Majdan M, Majeed A, Makhlouf C, Malekzadeh R, Mapoma CC, Marcenes W, Martinez-Raga J, Marzan MB, Masiye F, Mason-Jones AJ, Mayosi BM, McKee M, Meaney PA, Mehndiratta MM, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Miller TR, Mikesell J, Mirarefin M, Mohammad KA, Mohammed S, Mokdad AH, Monasta L, Moradi-Lakeh M, Mori R, Mueller UO, Murimira B, Murthy GV, Naheed A, Naldi L, Nangia V, Nash D, Nawaz H, Nejjari C, Ngalesoni FN, de Dieu Ngirabega J, Nguyen QL, Nisar MI, Norheim OF, Norman RE, Nyakarahuka L, Ogbo FA, Oh IH, Ojelabi FA, Olusanya BO, Olusanya JO, Opio JN, Oren E, Ota E, Padukudru MA, Park HY, Park JH, Patil ST, Patten SB, Paul VK, Pearson K, Peprah EK, Pereira CC, Perico N, Pesudovs K, Petzold M, Phillips MR, Pillay JD, Plass D, Polinder S, Pourmalek F, Prokop DM, Qorbani M, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MH, Rahman SU, Rai RK, Rajsic S, Ram U, Rana SM, Rao PV, Remuzzi G, Rojas-Rueda D, Ronfani L, Roshandel G, Roy A, Ruhago GM, Saeedi MY, Sagar R, Saleh MM, Sanabria JR, Santos IS, Sarmiento-Suarez R, Sartorius B, Sawhney M, Schutte AE, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shaikh MA, Sharma R, She J, Sheikhbahaei S, Shen J, Shibuya K, Shin HH, Sigfusdottir ID, Silpakit N, Silva DA, Silveira DG, Simard EP, Sindi S, Singh JA, Singh OP, Singh PK, Skirbekk V, Sliwa K, Soneji S, Sorensen RJ, Soriano JB, Soti DO, Sreeramareddy CT, Stathopoulou V, Steel N, Sunguya BF, Swaminathan S, Sykes BL, Tabares-Seisdedos R, Talongwa RT, Tavakkoli M, Taye B, Tedla BA, Tekle T, Shifa GT, Temesgen AM, Terkawi AS, Tesfay FH, Tessema GA, Thapa K, Thomson AJ, Thorne-Lyman AL, Tobe-Gai R, Topor-Madry R, Towbin JA, Tran BX, Dimbuene ZT, Tsilimparis N, Tura AK, Ukwaja KN, Uneke CJ, Uthman OA, Venketasubramanian N, Vladimirov SK, Vlassov VV, Vollset SE, Wang L, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, Wijeratne T, Wilkinson JD, Wiysonge CS, Wolfe CD, Won S, Wong JQ, Xu G, Yadav AK, Yakob B, Yalew AZ, Yano Y, Yaseri M, Yebyo HG, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Yu S, Zaidi Z, Zaki Mel S, Zeeb H, Zhang H, Zhao Y, Zodpey S, Zoeckler L, Zuhlke LJ, Lopez AD, Murray CJ. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3(8):e361–387. doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


