Abstract
We report here a pediatric patient whose Cushing’s Disease was diagnosed late because of her cyclical presentation, presumably due to subclinical pituitary apoplexy. Starting at age 8, she presented with observable signs of Cushing’s but was not clinically assessed for Cushing’s Syndrome until the age of 15. Initial tests at age 15 were consistent with Cushing’s Disease, however, the patient presented with spontaneous remission of hypercortisolemia just a few short months later. Her cushingoid features never subsided, and at age 17, her MRI showed a partially empty sella; this finding of an empty sella contributed evidence to our suspicion of asymptomatic apoplexy, especially since the patient never reported an episode of acute headache. Pituitary apoplexy in corticotroph adenomas is very uncommon, but even more rare in microadenomas, making this case very unusual. Lost to follow-up, she was not reevaluated for Cushing’s Disease until age 25, and her laboratory tests were consistent with an adrenocorticotrophic-dependent pituitary tumor; Pituitary magnetic resonance imaging revealed a 9 mm X 6 mm X 8 mm mass projecting on the superior aspect of pituitary and abutting the wall of the right cavernous sinus. The patient had a transsphenoidal surgery to remove the microadenoma and is planned to undergo radiation therapy. To the best of our knowledge, this is the first report of subclinical apoplexy of a microadenoma in a pediatric patient with Cushing’s Disease. It brings to light the importance of long term follow up for pediatric patients presenting with clinical symptoms of Cushing’s Syndrome.
Keywords: Hypercortisolemia, pituitary tumor, Cushing’s Syndrome, cyclical Cushing’s, adolescent
1.1 Introduction
Pituitary adenomas occasionally undergo infarction or hemorrhage, otherwise known as apoplexy, that may destroy part of or the entire tumor. Most pituitary apoplexy occurs in the absence of a recognized precipitating event, such as surgery, bleeding disorder, or trauma. These tumors are thought to be susceptible to spontaneous infarction because of their relatively high metabolic demand and reduced blood vessel density compared with the normal pituitary gland [1]. Pituitary apoplexy may present with a classic combination of acute headache, change in mental status, and decreased visual acuity. The clinical presentation of pituitary apoplexy ranges from relatively minor symptoms to adrenal crisis, loss of consciousness, or even sudden death. Pituitary apoplexy may occur in the absence of characteristic symptoms, as evidenced by histological features consistent with apoplexy in as many as 25% of surgically removed microadenomas [2,3]. The majority of information regarding pituitary apoplexy comes from the adult literature [4]. Apoplexy of an adrenocorticotrophic (ACTH)-secreting pituitary tumor is not indicative of cure; corticotropinomas that undergo apoplexy often recur; this cyclical presentation of CD may present as a diagnostic challenge [5–7]. Gaps remain in our understanding of the prevalence and clinical course of apoplexy in the pediatric patient population, highlighting the importance of its study and description in the literature. Here we describe a pediatric patient with clinical evidence of Cushing’s Disease (CD) that went into remission due to presumed spontaneous apoplexy followed by delayed recurrence.
1.2 Case Presentation
A 25 year old female was admitted to the Clinical Center at the National Institutes of Health (NIH) in 2015 for evaluation of Cushing’s Syndrome (CS). She exhibited the classic clinical features of CS, including obesity, round facies, hirsutism, diabetes, hypertension, purple striae, and oligomenorrhea. Review of her medical history indicated that her CS had been longstanding. At age 8, the patient’s mother noticed an increase in weight and a “chubby” face. The patient was diagnosed with type 2 diabetes at age 14 and was initiated on metformin. She was concurrently noted to have hirsutism and oligomenorrhea, however, the patient was not evaluated for CS until 2005, at the age of 15.
At that time, her lab results confirmed hypercortisolemia with elevated urinary free cortisol (UFC) and lack of diurnal variation of serum cortisol (Table 1). A non-contrast magnetic resonance imaging (MRI) of the pituitary and computed tomography (CT) scan of the abdomen and adrenals were performed in March 2005, and both were read as normal. The patient had repeat lab evaluation in June 2005 (Table 1) and a repeat pituitary MRI with and without contrast in August 2005. The MRI showed a 3 millimeter focal lesion in the left aspect of the sella, suspicious for microadenoma. However, the UFC and serum cortisol did not show evidence of hypercortisolemia; in fact on the contrary, her AM cortisol was low.
Table 1.
| February 2005 Lab Results: | ||
| Patient Value | Normal range | |
| Urinary free cortisol | 556.2 μg/24 h | 2.1 – 38.0 μg/24 hr |
| 8 AM Cortisol | 31.2 μg/dL | 4.0–22.0 μg/dL |
| 3 PM Cortisol | 33.4 μg/dL | 3.0–17.0 μg/dL |
| June 2005 Lab Results: | ||
| Patient Value | Normal range | |
| Urinary free cortisol | 4.7 μg/24 h | 2.1 – 38.0 μg/24 hr |
| 8 AM Cortisol | 2.5 μg/dL | 4.0–22.0 μg /dL |
| EVENING PM Cortisol | 1.7 μg/dL | 3.0–17.0 μg /dL |
| Plasma ACTH | 29.0 pg/mL | 2.0–49.0 pg/mL |
| June 2008 Lab Results: | ||
| Patient Value | Normal range | |
| Urinary free cortisol | 180.0 μg/24 h | 3.0 – 43.0 μg/24 hr |
| April 2015 Lab Results: | ||
| Patient Value | Normal range | |
| Urinary free cortisol | 457.3 μg/24 h | 3.5 – 45.0 μg/24 h |
| 8 AM Cortisol | 20.4 | 5.0–25.0.0 μg/dL |
| Midnight Cortisol | 8.2 | <4.4 μg/dL |
| 8 AM ACTH | 58 | 5.0–46.0 pg/mL |
| Midnight ACTH | 32 | 5.0–46.0 pg/mL |
| CRH Test: suppression from baseline to peak values |
||
| Cortisol | 46% | >20% suggestive of CD |
| ACTH | 169% | >35% consistent with CD |
| High Dose Dexamethasone | >80% suppression | Consistent with pituitary etiology |
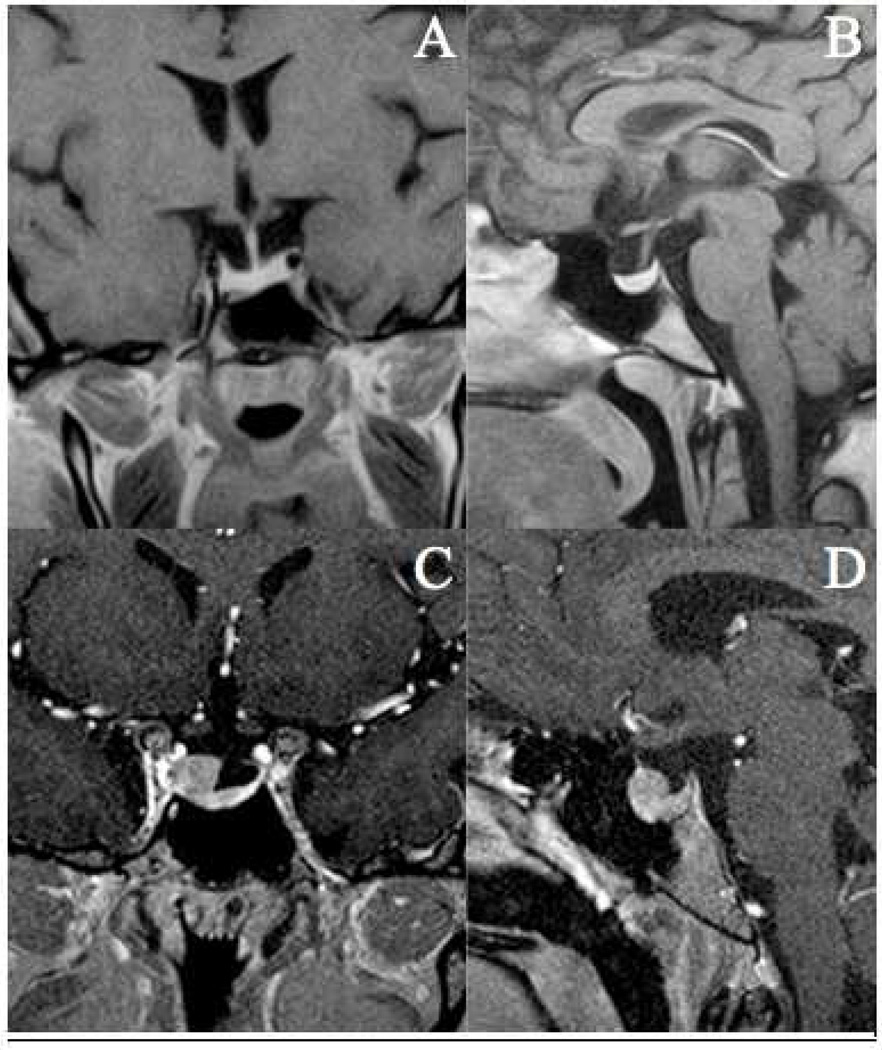
In September 2006, the patient’s repeat MRI of the pituitary was read as unchanged. However, these films were destroyed and we were unable to corroborate the results. In June 2008, the patient’s UFC was again elevated (Table 1) and repeat MRI of pituitary showed a partially empty sella and a small pituitary with no filling defects (Figure 1). In retrospect, in combination with the period of hypocortisolemia, this MRI finding raises the suspicion of apoplexy, although the patient did not recall a severe headache, visual changes, nausea, and/or vomiting [8]. Additionally, the patient, nor her family, did not remember any change in her physical appearance at the time of the suspected apoplexy.
Figure 1.
2008 MRI T1-weighted sequences, coronal (A) and sagittal (B) views showing relatively small pituitary with the superior half of the sella filled by spinal fluid. These findings are consistent with a partially empty sella. 2015 MRI T1-weighted sequences, coronal (C) and sagittal (D) views in patient with 9 mm X 6 mm X 8 mm lesion arising from the superior aspect of the pituitary gland on the right side, with extension into the right cavernous sinus.
The patient grew frustrated with the lack of clear diagnosis and was lost to follow up until February 2015. Upon encouragement from a family member, she came to the NIH where she underwent further endocrine evaluation. In April 2015, the patient’s pituitary MRI showed a 9mm X 6 mm X 8 mm hypoenhancing mass within the right sella turcica abutting the wall of the right cavernous sinus (Figure 1). Laboratory evaluation at the NIH was consistent with ACTH-dependent CD: loss of diurnal cortisol variation, cortisol releasing hormone (CRH) stimulation test indicative of pituitary source, and 80% suppression of cortisol with high dose dexamethasone (Table 1). Evaluation of the rest of her pituitary axes revealed a TSH level of 0.94 mcIU/mL (0.27–4.20), free thyroxine of 1.3 ng/dL (0.9–1.7), LH of 5.2 U/L, FSH of 5.6 U/L, prolactin of 15 mcg/L (2–25), and IGF-1 of 326 ng/mL (116–368). The patient successfully underwent transsphenoidal surgery in June 2015. Lack of a distinct pseudocapsule and infiltration of the cavernous sinus wall was noted during surgery. Gross tumor resection and resection of affected portion of the cavernous sinus wall was performed. Histopathology and immunohistochemistry evaluation (sections analyzed with H&E, Reticulin stain-Dako, and ACTH stain, 1:1000-Dako) was consistent with ACTH-secreting pituitary adenoma (Figure 2). Post-operatively, nadir cortisol (2.3 μg/dL) and ACTH (8.6 pg/mL) were consistent with remission, but due to intraoperative findings, focal sellar radiation treatment is planned beginning at three months post-operatively.
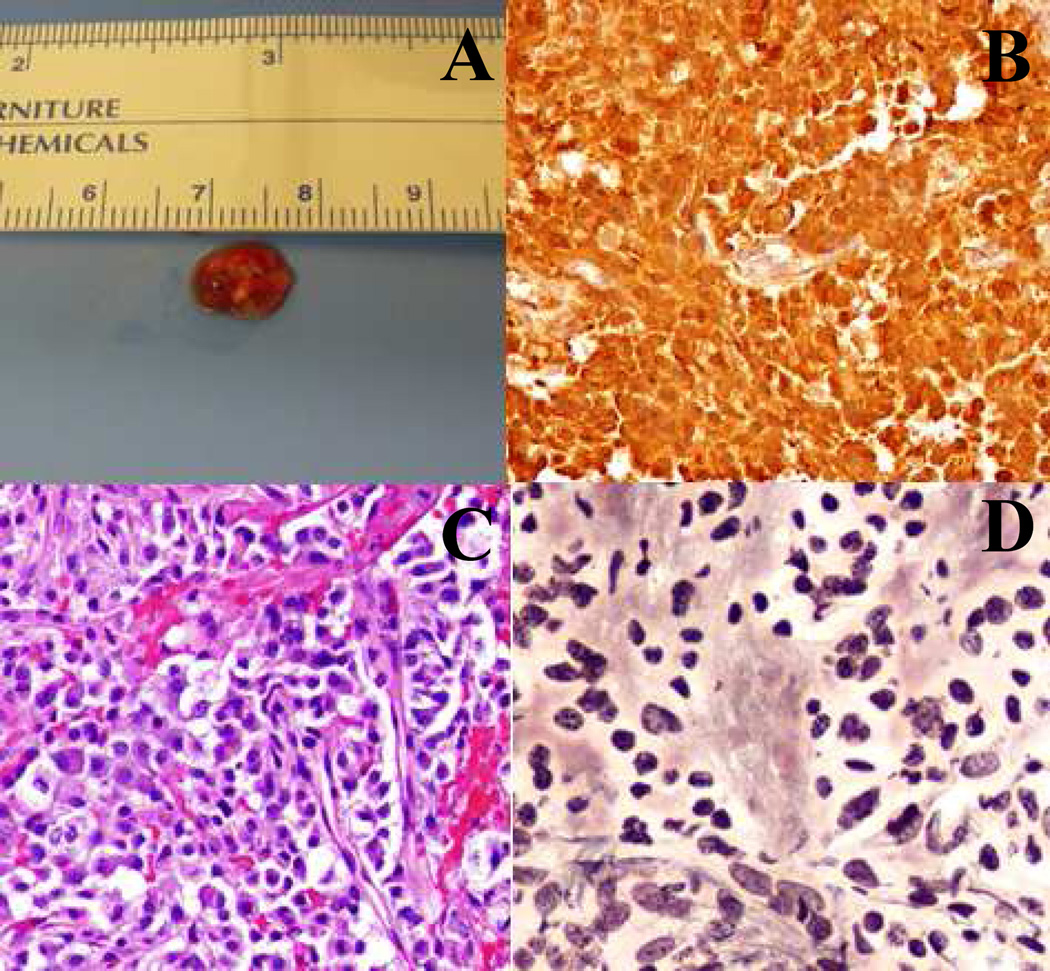
Figure 2.
(A) Macroscopic evaluation of tumor resected June 2015 reveals a soft red-brown tissue fragment measuring 0.8 cm×0.5 cm x0.4 cm. (B) Strong and diffuse ACTH staining is visualized (20X). (C) Hematoxylin and eosin (H&E) staining (20X) shows sheets of large cells with abundant cytoplasm. (D) Reticulin stain (20X) shows lack of normal reticulin framework.
1.3 Discussion
Our case describes a pediatric patient with delayed diagnosis of CD likely secondary to subclinical pituitary apoplexy. Pituitary apoplexy itself is rare, but even more uncommon in corticotroph adenomas [9]. Furthermore, infarction of a pituitary adenoma is more common in macroadenomas, making our case of a presumed infarcted microadenoma unusual [5, 6, 10].
Classically, apoplexy presents with sudden headaches, nausea, vomiting, and/or visual disturbances [5, 8, 11]. These symptoms have been similarly described in adolescents [4]. However, pituitary apoplexy that does not present with these classical symptoms is termed subclinical apoplexy [11]; these asymptomatic apoplexies may have a higher incidence rate than clinical pituitary apoplexies [5,8,11].
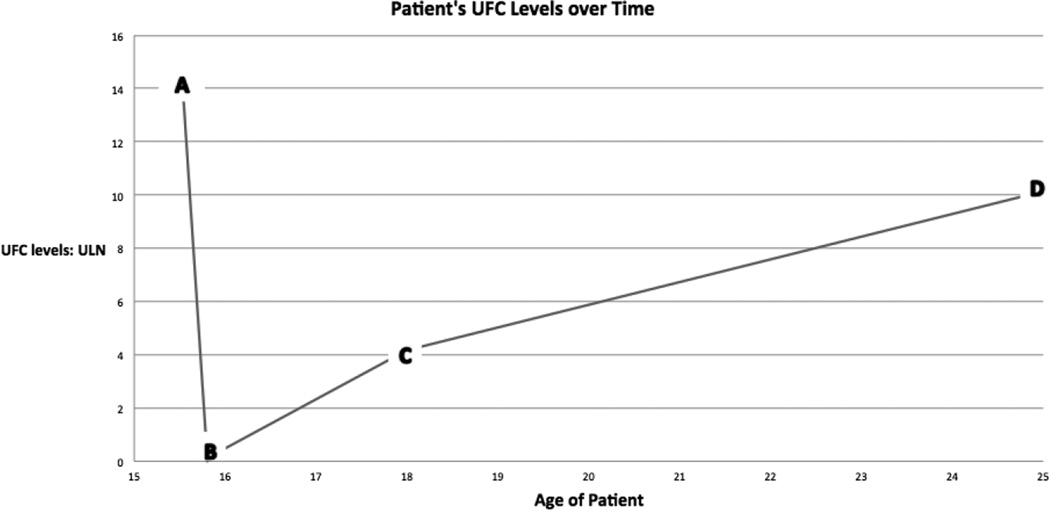
Our patient presented with clear cushingoid features but her laboratory results, though initially showing hypercortisolemia, later showed evidence of cortisol insufficiency, followed by a later resurgence of cortisol levels (Figure 3). Additionally, there have been a number of reports where CD spontaneously resolved because of pituitary apoplexy in adult patients, a consideration that could fit our patient’s early presentation [8–11]. Furthermore, her MRI in 2008 showed an empty sella, which is consistent with pituitary apoplexy (Figure 1) [15, 16]. The timeline for spontaneous regression of pituitary tumors following apoplexy has not been clearly defined, thus empty sella may not be evident on imaging until after the apoplexy has occurred. The patient denied any severe episodes of headache, nausea, vomiting, etc. in her history, which leads us to conclude that she likely had a subclinical apoplexy.
Figure 3.
Urine free cortisol (UFC) levels expressed as ratio of patient’s value to the upper limit of normal of the assay at various ages of the patient. The patient initially showed hypercortisolemia, later showed cortisol insufficiency, followed by a resurgence of cortisol levels. The letters correspond to the lab values reported in Table 1: (A) February 2005. (B) June 2005. (C) June 2008. (D) April 2015.
1.4 Conclusions
All patients who have had an apoplexy should be followed up long term because of the risk of recurrent tumor growths and the rare phenomenon of recurrent pituitary apoplexy; a number of case reports with this cyclical presentation of adult CD due to spontaneous pituitary apoplexy have been described in the literature and mirror our pediatric case [5, 6, 9, 11, 12, 17]. For follow-up, MRI is recommended at 3–6 months after the initial apoplexy, yearly for 5 years, and then 2–3 years subsequently. If regrowth is noted, options include radiotherapy and repeat pituitary surgery [11]. However, the incidence and associated risk factors for pituitary apoplexy has yet to be thoroughly evaluated in pediatric patients, especially in the case of subclinical apoplexy. This may lead to difficulties in the proper diagnosis of CD in pediatric patients. The recurrence of CD after apparent resolution following a pituitary apoplexy occurred in this patient, and it is a reminder that long-term follow up for pediatric patients with clinical symptoms of CS is necessary.
Acknowledgments
We would like to thank the patient and patient’s family for their help with this report. We are grateful for their support.
Funding
This work was supported by the Division of Intramural Research, Eunice Kennedy Shriver NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: the perfect storm. Journal of neurosurgery. 2015;122(6):1444–1449. doi: 10.3171/2014.10.JNS141720. [DOI] [PubMed] [Google Scholar]
- 2.Mohr G, Hardy J. Hemorrhage, necrosis, and apoplexy in pituitary adenomas. Surgical neurology. 1982;18(3):181–189. doi: 10.1016/0090-3019(82)90388-3. [DOI] [PubMed] [Google Scholar]
- 3.Sibal L, Ball SG, Connolly V, et al. Pituitary apoplexy: a review of clinical presentation, management and outcome in 45 cases. Pituitary. 2004;7(3):157–163. doi: 10.1007/s11102-005-1050-3. [DOI] [PubMed] [Google Scholar]
- 4.Jankowski PP, Crawford JR, Khanna P, et al. Pituitary tumor apoplexy in adolescents. World neurosurgery. 2015;83(4):644–651. doi: 10.1016/j.wneu.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Alarifi A, Alzahrani AS, Salam SA, et al. Repeated remissions of Cushing's disease due to recurrent infarctions of an ACTH-producing pituitary macroadenoma. Pituitary. 2005;8(2):81–87. doi: 10.1007/s11102-005-2961-8. [DOI] [PubMed] [Google Scholar]
- 6.Machado MC, Gadelha PS, Bronstein MD, Fragoso MC. Spontaneous remission of hypercortisolism presumed due to asymptomatic tumor apoplexy in ACTH-producing pituitary macroadenoma. Arquivos brasileiros de endocrinologia e metabologia. 2013;57(6):486–489. doi: 10.1590/s0004-27302013000600012. [DOI] [PubMed] [Google Scholar]
- 7.Pal A, Capatina C, Tenreiro AP, et al. Pituitary apoplexy in non-functioning pituitary adenomas: long term follow up is important because of significant numbers of tumour recurrences. Clinical endocrinology. 2011;75(4):501–504. doi: 10.1111/j.1365-2265.2011.04068.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZH, Chang CN, Pai PC, et al. Clinical features and surgical outcome of clinical and subclinical pituitary apoplexy. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2010;17(6):694–699. doi: 10.1016/j.jocn.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Kamiya Y, Jin-No Y, Tomita K, et al. Recurrence of Cushing's disease after long-term remission due to pituitary apoplexy. Endocrine journal. 2000;47(6):793–797. doi: 10.1507/endocrj.47.793. [DOI] [PubMed] [Google Scholar]
- 10.Sahin SB, Cetinkalp S, Erdogan M, et al. Pituitary apoplexy in an adrenocorticotropin-producing pituitary macroadenoma. Endocrine. 2010;38(2):143–146. doi: 10.1007/s12020-010-9367-8. [DOI] [PubMed] [Google Scholar]
- 11.Johnston PC, Hamrahian AH, Weil RJ, Kennedy L. Pituitary tumor apoplexy. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2015;22(6):939–944. doi: 10.1016/j.jocn.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Pignatta AB, Diaz AG, Gomez RM, Bruno OD. Spontaneous remission of Cushing's disease after disappearance of a microadenoma attached to the pituitary stalk. Pituitary. 2004;7(1):45–49. doi: 10.1023/b:pitu.0000044626.25624.48. [DOI] [PubMed] [Google Scholar]
- 13.Dickstein G, Spindel A, Shechner C, et al. Spontaneous remission in Cushing's disease. Archives of internal medicine. 1991;151(1):185–189. [PubMed] [Google Scholar]
- 14.Roerink SH, van Lindert EJ, van de Ven AC. Spontaneous remission of acromegaly and Cushing’s disease following pituitary apoplexy: Two case reports. The Netherlands journal of medicine. 2015;73(5):242–246. [PubMed] [Google Scholar]
- 15.Lenz AM, Root AW. Empty sella syndrome. Pediatric endocrinology reviews : PER. 2012;9(4):710–715. [PubMed] [Google Scholar]
- 16.Mehta GU, Bakhtian KD, Oldfield EH. Effect of primary empty sella syndrome on pituitary surgery for Cushing's disease. Journal of neurosurgery. 2014;121(3):518–526. doi: 10.3171/2014.3.JNS132012. [DOI] [PubMed] [Google Scholar]
- 17.Briet C, Salenave S, Chanson P. Pituitary apoplexy. Endocrinology and metabolism clinics of North America. 2015;44(1):199–209. doi: 10.1016/j.ecl.2014.10.016. [DOI] [PubMed] [Google Scholar]