Abstract
Pannonibacter phragmitetus is a bioremediation reagent for the detoxification of heavy metals and polycyclic aromatic compounds (PAHs) while it rarely infects healthy populations. However, infection by the opportunistic pathogen P. phragmitetus complicates diagnosis and treatments, and poses a serious threat to immunocompromised patients owing to its multidrug resistance. Unfortunately, genome features, antimicrobial resistance, and virulence potentials in P. phragmitetus have not been reported before. A predominant colony (31801) was isolated from a liver abscess patient, indicating that it accounted for the infection. To investigate its infection mechanism(s) in depth, we sequenced this bacterial genome and tested its antimicrobial resistance. Average nucleotide identity (ANI) analysis assigned the bacterium to the species P. phragmitetus (ANI, >95%). Comparative genomics analyses among Pannonibacter spp. representing the different living niches were used to describe the Pannonibacter pan‐genomes and to examine virulence factors, prophages, CRISPR arrays, and genomic islands. Pannonibacter phragmitetus 31801 consisted of one chromosome and one plasmid, while the plasmid was absent in other Pannonibacter isolates. Pannonibacter phragmitetus 31801 may have a great infection potential because a lot of genes encoding toxins, flagellum formation, iron uptake, and virulence factor secretion systems in its genome. Moreover, the genome has 24 genomic islands and 2 prophages. A combination of antimicrobial susceptibility tests and the detailed antibiotic resistance gene analysis provide useful information about the drug resistance mechanisms and therefore can be used to guide the treatment strategy for the bacterial infection.
Keywords: antibiotic resistance mechanism, comparative genomic analysis, genomic sequencing, Pannonibacter phragmitetus, pathogenesis
1. INTRODUCTION
Pannonibacter phragmitetus was first found in human blood cultures in the United Kingdom in 1975 (Holmes, Segers, Coenye, Vancanneyt, & Vandamme, 2006). As a novel genus and species, the nomenclature P. phragmitetus was given to an alkalitolerant strain C6/19T (=DSM 14782T = NCAIM B02025T) isolated from decomposing reed rhizomes in a Hungarian soda lake in 2003 (Borsodi et al., 2003). Pannonibacter phragmitetus is a Gram‐negative, facultative anaerobic, chemoorganotrophic, and motile rod (Borsodi et al., 2003). Before 2006, it was misclassified as Achromobacter groups B and E. With 16S rRNA gene sequencing, DNA–DNA hybridization, G+C content determination, cellular fatty acid evaluation, and biochemical experiments, Holmes et al. (2006) verified that P. phragmitetus and Achromobacter groups (B and E) belonged to same taxon (Holmes et al., 2006). The same group had shown that “Achromobacter groups B and E” are the single genus and species in biochemical characteristics (Holmes et al., 1993).
Pannonibacter phragmitetus survives in extreme environments including hot springs (Bandyopadhyay, Schumann, & Das, 2013; Coman, Druga, Hegedus, Sicora, & Dragos, 2013), alkaline environments (pH 7.0–11.0), zero to high salinity (no NaCl as well as up to 5% [w/v] NaCl) (Borsodi et al., 2003). Currently, the studies on P. phragmitetus mainly focused on its bioremediation potentials including reduction of heavy metal chromium and detoxification of polycyclic aromatic compounds (PAHs) under extreme conditions (Borsodi et al., 2005; Xu et al., 2011a; Shi et al., 2012; Wang et al., 2013; Wang, Jin, Zhou, & Zhang, 2016). For example, Shi et al. (2012) isolated a strain that could be used for the bioremediation of chromate‐polluted soil and water (Shi et al., 2012). This strain displayed the complete Cr(VI) reduction capability under anaerobic conditions (Shi et al., 2012). Furthermore, Xu et al. (2012) demonstrated that reduction capacity of hexavalent chromium by P. phragmitetus LSSE‐09 was not impaired under alkaline conditions (up to pH 9.0) (Xu et al., 2011b). Chai et al. (2009) showed that P. phragmitetus BB could maintain an intact cell surface with a strong chromium reduction capacity under a high concentration of Cr(VI) [500 mg L(−1)] (Chai et al., 2009). Bandyopadhyay et al. (2013) isolated a strain resistant to arsenate in a hot‐spring sediment sample that was also alkalitolerant (Bandyopadhyay et al., 2013). However, the metabolism pathways involving chromium were unclear.
Until now, only four cases of P. phragmitetus infections in patients have been reported, including a case of replacement valve endocarditis (McKinley, Laundy, & Masterton, 1990), two cases of septicemia (Holmes, Lewis, & Trevett, 1992), and one case of recurrent septicemia (Jenks & Shaw, 1997). With BD BACTEC 9240 automated blood culture system, we isolated P. phragmitetus 31801 from the blood sample of a patient with liver abscess (Wang et al., 2017). The patient made a full clinical recovery after 20 days of antibiotic therapy (aerosol inhalation of 1.5 g cefodizime sodium b.i.d and intravenous injection of 0.5 g metronidazole b.i.d) and percutaneous abscess drainage (Wang et al., 2017). Our previous study demonstrated that P. phragmitetus was possibly an opportunistic pathogenic bacterium (Wang et al., 2017). However, the pathogenesis and antibiotic resistance mechanisms in P. phragmitetus remain unexplored.
Only two draft genomes in P. phragmitetus were available in GenBank (CGMCC9175, accession number: LGSQ01000001.1; DSM 14782, accession number: NZ_KB908215.1). There was not enough information available to fully understand the metabolism pathway of heavy metals, the pathogenesis and antibiotic resistance mechanisms. In this article, we first reported the genome sequences from a clinical isolate P. phragmitetus strain 31801. Antibiotic resistance genes and virulence factors were investigated. Furthermore, we also provided a genomic insight into the mechanisms of hexavalent chromium reduction at the genomic level.
2. MATERIALS and METHODS
2.1. Strain and culture conditions
Pannonibacter phragmitetus was isolated on Columbia blood agar plates at 37°C. After overnight incubation, a single colony was purified, subcultured in Luria–Bertani (LB) broth, and stored at −80°C for further study.
2.2. DNA extraction and whole genome sequencing
Genomic DNA was extracted using Qiagen DNA mini Kit (Qiagen, Germany). The strain identity was first confirmed by 16S rRNA sequencing (GenBank No. FJ882624.1). Genome sequencing was performed with PacBio single‐molecule real‐time (SMRT) RS II technique (Pacific Biosciences, Menlo Park, CA, USA).
2.3. Assembly, annotation, and bioinformatics analysis
Raw sequence data processing and genome assembly were performed by SMART portal V2.3. RS_HGAP_Assembly.3 (Pacific Biosciences). Gene calling was finished by using GeneMarkS and Glimmer 3 (Besemer & Borodovsky, 2005; Delcher, Bratke, Powers, & Salzberg, 2007). Initial prediction and annotation of open reading frames (ORF) prediction were carried out with Glimmer 3 using the Rapid Annotation in Subsystem Technology server (RAST) (Delcher, Harmon, Kasif, White, & Salzberg, 1999; Aziz et al., 2008). The quality was further evaluated by GeneMarkS ORF prediction (Besemer & Borodovsky, 2005). DNA sequence visualization and annotation were conducted by Artemis 16 (Rutherford et al., 2000; Carver, Harris, Berriman, Parkhill, & McQuillan, 2012) when necessary. The functional categorization and classification for predicted ORFs were performed by IMG/IMG ER, RAST server‐based SEED viewer, Clusters of Orthologous Groups, and WebMGA programs (Markowitz et al., 2009; Wu, Zhu, Fu, Niu, & Li, 2011; Markowitz et al., 2014; Overbeek et al., 2014; Galperin, Makarova, Wolf, & Koonin, 2015). A circular genome map was generated using GCView based on all predicted CDS information, tRNAs/rRNAs, GC content, and gene cluster information (Grant & Stothard, 2008). The multidrug resistance genes were predicted using both CARD database (McArthur et al., 2013) and RAST (Delcher et al., 1999; Aziz et al., 2008). Prophage and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) were predicted by using PHAST (Zhou, Liang, Lynch, Dennis, & Wishart, 2011) and CRISPRfinder (Grissa, Vergnaud, & Pourcel, 2008). Detection and identification of virulent factors were carried out using VFDB database (Chen, Xiong, Sun, Yang, & Jin, 2012). The pan‐genome analysis was conducted by EDGAR 2.0 (Blom et al., 2016). Secondary metabolic analysis was conducted using the server antiSMASH (Weber et al., 2015) version 3.0.5.
2.4. The antimicrobial susceptibility test
The antimicrobial susceptibility test (AST) was conducted with the Kirby–Bauer disk diffusion test (K‐B) method (Oxoid, England) (Jorgensen & Ferraro, 2009) and was verified with BD's Phoenix™ 100 Automated Microbiology System with the NMIC/ID‐109 identification/antibiotic susceptibility cards (Becton, Dickinson and Company), according to the NCCLS (Standards, 2006) and CLSI Performance Standards (Institute, 2013). The bacteria was cultured with AST MH broth at 35°C. The positive control was Pseudomonas aeruginosa ATCC27853. The threshold used to determine a strain resistant or sensitive was established according to the CLSI Performance Standards (Standards, 2013). AST tests were replicated three times.
2.5. Deposition of genome sequences
The genome and plasmid sequences were deposited in the GenBank database (accession no. CP013068 and CP013069). The BioProject designation for this project is PRJNA298840. BioSample accession number is SAMN04158710.
3. RESULTS and DISCUSSION
3.1. Genomic properties
From the sequencing libraries, 1,245,556,320 bp reads were obtained. It was estimated to be at least 163‐fold coverage of the genome. After genome assembly, a complete circular chromosome and a circular plasmid were identified with a size of 5,318,696 and 351,005 bp, respectively (Figure 1). The average GC contents are 63.3% and 63.9% for the chromosome and plasmid, respectively, consistent with those in other Pannonibacter genomes (Table 1). The genome size of P. phragmitetus strain 31801 is slightly smaller than that in P. phragmitetus CGMCC9175, but is much larger than those in P. phragmitetus DSM 14782 and Pannonibacter indicus 2340 (Table 1). The chromosome contains at least 4,997 protein‐coding genes (Table 1). It has the most rRNA and tRNA gene numbers (9 rRNAs and 54 tRNAs) among the selected genomes (Table 1). It is interesting that all Pannonibacter genomes have CRISPRs with the copies ranging from 3 to 6 (Table 1). Furthermore, P. phragmitetus strain 31801 possesses a plasmid (p.p‐1) which contains at least 308 genes (see below).
Figure 1.
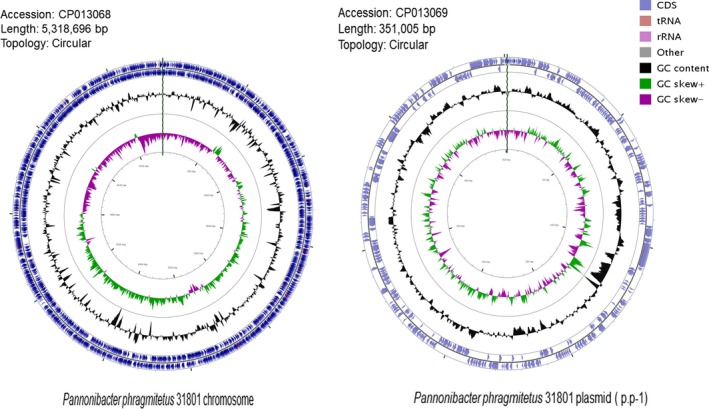
Representation of the completed chromosome and plasmid of Pannonibacter phragmitetus 31801. Concentric rings, from outer to inner rings, represent the following features: in the clockwise or counterclockwise direction, the coding sequences (CDS) in light blue, rRNAs in pink, tRNAs brown; GC content (percentage) as a peak to valley profile in black; GC‐skew graph in purple and green
Table 1.
Genomic features of selected Pannonibacter spp. (chromosome)
| Strains | P. phragmitetus | P. indicus | ||
|---|---|---|---|---|
| 31801 | CGMCC9175 | DSM 14782 | 2340 | |
| Accession | CP013068 | LGSQ01000001.1 | NZ_KB908215.1 | NZ_LIPT01000001.1 |
| Size (Mb) | 5.32 | 5.58 | 4.78 | 4.17 |
| GC% | 63.3 | 63.6 | 63.1 | 63.5 |
| Protein | 4,997 | 4,936 | 4,122 | 3,703 |
| rRNA | 9 | 3 | 4 | 7 |
| tRNA | 54 | 50 | 49 | 50 |
| Gene | 5,150 | 5,060 | 4,232 | 3,845 |
| Pseudo gene | 81 | 70 | 65 | 84 |
| CRISPR | 4 | 6 | 3 | 3 |
| Plasmid | 1 | 0 | 0 | 0 |
3.2. Gene repertoire of Pannonibacter and phylogenetic placement
Placement of the 16s rDNA sequence from P. phragmitetus 31801 into a phylogenetic tree revealed a close relationship with several P. phragmitetus isolates from various sources (Figure 2a). However, the 16S rRNA sequence in P. phragmitetus 31801 was 100% identical to that in P. phragmitetus CGMCC9175, 99% identical to that in P. phragmitetus DSM 14782, and 99% identical to that in P. indicus DSM 23407, indicating that the 16s rDNA sequence analysis did not provide enough resolution to differentiate P. phragmitetus from P. indicus (97%, a cut‐off value for the same species; Tindall, Rossello‐Mora, Busse, Ludwig, & Kampfer, 2010). Pannonibacter phragmitetus strain 31801 and P. phragmitetus CGMCC9175 have an ANI value of 97.67%, agreeing that they belong to the same species according to the microbial taxonomy for species delineation (cut‐off for ANI, 96%; Goris, Konstantinidis, Klappenbach, Coenye, & Vandamme Pand Tiedje, 2007) (Figure 3a). Furthermore, the ANI values between P. phragmitetus 31801 genome and P. phragmitetus DSM 14782 or P. indicus DSM 23407 are below 96%, indicating that they are different species. Overall, Pannonibacter genomes are distinctly different from those in Labrenzia aggregata and Polymorphum gilvum (Figure 2b). An alignment of single‐copy orthologs shared among these selected genomes also shows that P. phragmitetus 31801 and P. phragmitetus CGMCC9175 clusters more closely, forming a clade. Instead, they branch from P. phragmitetus DSM 14782 and P. indicus DSM 23407 (Figure 2b).
Figure 2.
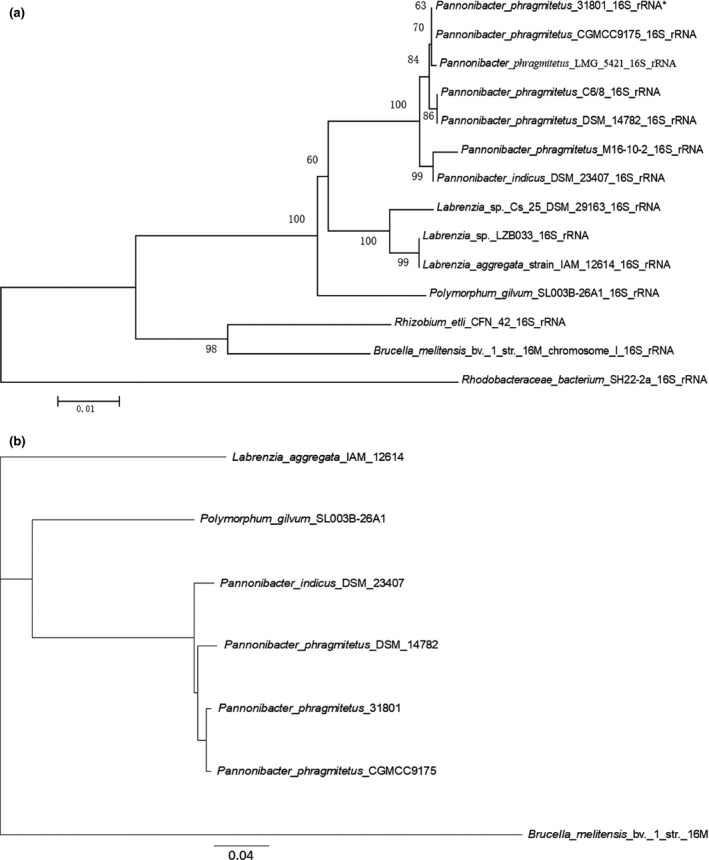
Phylogenetic placement of Pannonibacter phragmitetus strain 31801. (a) The genetic placement of P. phragmitetus strain 31801 (identity with *) based on 16S rRNA. Alignment of 16S rRNA sequences was conducted using ClustalW (Thompson, Higgins, & Gibson, 1994), and the tree was generated using the neighbor‐joining algorithm with 1,000 bootstraps, using MEGA 6.0. The corresponding GenBank accession numbers were indicated in parentheses. Rhodobacteraceae bacterium SH22‐2a was used as out‐group. (b) Phylogenetic tree inferred from concatenated genes. The tree is calculated from 1,125 core amino acids sequences per genome (15,750 core amino acid sequences). The accession numbers for selected genomes are P. phragmitetus 31801 (CP013068), P. phragmitetus CGMCC9175 (LGSQ01000001.1), P. phragmitetus DSM 14782 (NZ_KB908215.1), P. indicus 23407 (NZ_LIPT01000001.1), Polymorphum gilvum SL003B‐26A1 (NC_015259), Labrenzia aggregata IAM 12614 (NZ_AAUW00000000.1), and Brucella melitensis bv. 1 str. 16M (NZ_AHWC01000000)
Figure 3.
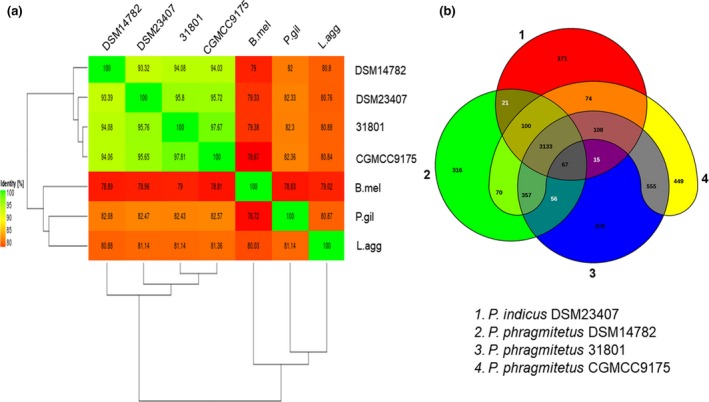
Venn diagram and ANI analysis of representative Pannonibacter spp. and their relatives. (a) Heat map of ANI values among Pannonibacter spp. and their relatives. 31801 represents P. phragmitetus 31801 (CP013068), CGMCC9175 represents P. phragmitetus CGMCC9175 (LGSQ01000001.1), DSM 14782 represents P. phragmitetus DSM 14782 (NZ_KB908215.1), and DSM 23407 represents P. indicus 23407 (NZ_LIPT01000001.1), P.gil represents Polymorphum gilvum SL003B‐26A1 (NC_015259), L.agg represents Labrenzia aggregata IAM 12614 (NZ_AAUW00000000.1), and B.mel represents Brucella melitensis bv. 1 str. 16M (NZ_AHWC01000000). (b) Four representative genomes, P. phragmitetus 31801 (CP013068) and P. phragmitetus CGMCC9175 (LGSQ01000001.1), P. phragmitetus DSM 14782 (NZ_KB908215.1) and P. indicus (NZ_LIPT01000001.1) were selected to illustrate the Venn diagram. The Venn diagram was not drawn in proportion; its sole purpose is to illustrate the shared CDSs between the selected strains. The overlapping regions represent CDSs shared with respective strains. The number outside the overlapping regions indicates the number of CDSs in each genome without homologs in other genomes. ANI, average nucleotide identity
Comparative genomics analysis was conducted by aligning four available Pannonibacter genomes with Progressive Mauve using default parameters (see Figure S1) (Darling, Mau, & Perna, 2010). The genome homology between strain P. phragmitetus 31801 and P. phragmitetus CGMCC9175 was high, though there were also some rearrangements and sequence elements specific to a particular genome, respectively (see Figure S1). However, the genome synteny between P. phragmitetus 31801 and P. phragmitetus 23407 was much lower. The gene repertoire of the selected Pannonibacter genomes was further analyzed using their ubiquitous genes (core genome) and different homologous gene families (pan‐genome) among the selected Pannonibacter and its closest relatives by using EDGAR (Blom et al., 2016). All the four Pannonibacter genomes shared a highly conserved genomic architecture as inferred from synteny of protein‐coding orthologs, tRNA genes, rRNA modules, and their origins of replication. An additional 4,153 protein‐coding genes were shared by P. phragmitetus 31801 and P. phragmitetus CGMCC9175 (Figure 3b). The P. phragmitetus 31801 and P. phragmitetus CGMCC9175, P. phragmitetus DSM 14782, and P. indicus genomes had 370, 449, 316, and 171 unique genes, respectively (Figure 3b). Because only four Pannonibacter genome sequences are available in GenBank, we added a few bacterial genomes (close relatives) to a “pan vs. core development” curve. The core genome is quite stable at ~3,000 genes for Pannonibacter spp. It drops to ~2,400 and ~1,400 when Po. gilvum, L. aggregata, and Brucella melitensis genomes were added (see Figure S2).
3.3. Correlation of antibiotic susceptibilities with antibiotic resistance genes
Among the antibiotics tested, P. phragmitetus 31801 was susceptible to tazobactam, aztreonam, imipenem, ceftazidime, cefepime, amikacin, gatifloxacin, and levofloxacin (Table 2). Under the same conditions, P. phragmitetus 31801 was found to be nonsusceptible to piperacillin (>64 μg/ml), gentamicin (>8 μg/ml), tobramycin (>8 μg/ml), sulfonamides (>2/38 μg/ml), tetracycline (>8 μg/ml), and nitrofurantoin (>16 μg/ml).
Table 2.
The antimicrobial susceptibility test of Pannonibacter phragmitetus strain 31801
| Drug class | Antibiotics | MIC (μg/ml) | SIR | |
|---|---|---|---|---|
| β‐lactam antibiotics | Piperacillin/tazobactam | ≤2/4 | S | |
| Penicillin class | Piperacillin | >64 | R | |
| Monocyclic β‐lactam | Aztreonam | 2 | S | |
| Carbapenems | Imipenem | ≤0.5 | S | |
| Cephalosporins | Ceftazidime | 4 | S | |
| Cephalosporins | Cefepime | ≤1 | S | |
| Cephalosporins | Ceftriaxone | 16 | I | |
| Cephalosporins | Cefotaxime | 16 | I | |
| Aminoglycoside antibiotics | Amikacin | ≤8 | S | |
| Gentamicin | >8 | R | ||
| Tobramycin | >8 | R | ||
| Fluoroquinolone antibiotics | Gatifloxacin | ≤1 | S | |
| Levofloxacin | ≤1 | S | ||
| Sulfonamides antibiotics | Compound sulfanomides | >2/38 | R | |
| Tetracycline antibiotics | Tetracycline | >8 | R | |
| Nitrofurans antibiotics | Nitrofurantoin | ≤16 | R | |
MIC, minimum inhibitory concentration; SIR, sensitive (S), intermediate (I), resistant (R).
The subsystems based on RAST indicates that the annotated genome has up to 110 genes that are potentially involved in virulence, disease, defense, and antimicrobial resistance (Figure 4). They were classified into β‐lactams, fluoroquinolones, fosfomycin, and multidrug resistance efflux pumps (Table S1). Further analysis of the resistance genes in the chromosome genome of P. phragmitetus 31801 through the CARD database showed only one β‐lactam resistance gene, NPS β‐lactamase, and more resistance genes involved in multidrug resistance efflux pumps (Table 3). For the latter classification, only cmeB, macA, macB, and acrB genes were consistent between the annotated genome (Table S1) and the CARD database (Table 3). However, the fluoroquinolones and fosfomycin resistance genes identified by RAST annotation were not verified through the CARD database.
Figure 4.
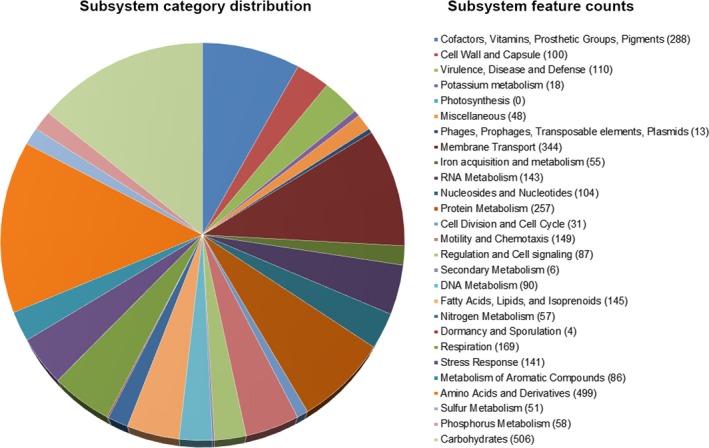
Subsystem distribution in different categories of Pannonibacter phragmitetus 31801. Subsystem coverage shows the total genes in the subsystems (49% in subsystems and 51% not in subsystems). Each part of the pie graph indicates different functions and proportions of genes. The numbers in parentheses show the counts of genes with specific functions
Table 3.
The antibiotic resistance genes in Pannonibacter phragmitetus 31801 predicted by CARD
| Type | Antibiotic resistance ontology | Locus tag | Description/ definition |
|---|---|---|---|
| β‐lactams | NPS β‐lactamase | APZ00_22055 | Class A β lactamase found in Rhodopseudomonas capsulata. |
| Multidrug resistance efflux pumps | acrB | APZ00_03520, APZ00_24680 | Protein subunit of acrA–acrB–tolC multidrug efflux complex. AcrB functions as a heterotrimer which forms the inner membrane component and is primarily responsible for substrate recognition and energy transduction by acting as a drug/proton antiporter. RND transporter system. |
| acrD | APZ00_03520, APZ00_24680 | AcrD is an aminoglycoside efflux pump expressed in Escherichia coli. Its expression can be induced by indole, and is regulated by baeRS and cpxAR. | |
| acrF | APZ00_03520, APZ00_24680 | AcrF is an inner membrane transporter, similar to acrB. | |
| adeA | APZ00_03515 | AdeA is the membrane fusion protein of the multidrug efflux complex adeABC. | |
| adeB | APZ00_03520, APZ00_24680 | AdeB is the multidrug transporter of the adeABC efflux system. | |
| adeG | APZ00_03520, APZ00_24680 | AdeG is the inner membrane transporter of the adeFGH multidrug efflux complex. | |
| adeJ | APZ00_03520, APZ00_24680 | AdeJ is a RND efflux protein that acts as the inner membrane transporter of the adeIJK efflux complex. | |
| amrB | APZ00_03520, APZ00_24680 | AmrB is the membrane fusion protein of the amrAB–oprM multidrug efflux complex. | |
| ceoB | APZ00_03520, APZ00_24680 | CeoB is a cytoplasmic membrane component of the ceoAB–opcM efflux pump | |
| cmeB | APZ00_03520, APZ00_24680 | CmeB is the inner membrane transporter the cmeABC multidrug efflux complex. | |
| macA | APZ00_05170 | MacA is a membrane fusion protein that forms an antibiotic efflux complex with macB and tolC. | |
| macB | APZ00_05165 | MacB is an ATP‐binding cassette (ABC) transporter that exports macrolides with 14‐ or 15‐membered lactones. It forms an antibiotic efflux complex with macA and tolC. | |
| mdsB | APZ00_03520, APZ00_24680 | MdsB is the inner membrane transporter of the multidrug and metal efflux complex mdsABC. | |
| mdtF | APZ00_03520, APZ00_24680 | MdtF is the multidrug inner membrane transporter for the mdtEF–tolC efflux complex. | |
| mexB | APZ00_01630, APZ00_03520, APZ00_24680 | MexB is the inner membrane multidrug exporter of the efflux complex mexAB–oprM. | |
| mexD | APZ00_03520, APZ00_24680 | MexD is the multidrug inner membrane transporter of the mexCD–oprJ complex. | |
| mexF | APZ00_03520, APZ00_24680 | MexF is the multidrug inner membrane transporter of the mexEF–oprN complex. | |
| mexI | APZ00_01630 | MexI is the inner membrane transporter of the efflux complex mexGHI–opmD. | |
| mexY | APZ00_03520, APZ00_24680 | MexY is the RND‐type membrane protein of the efflux complex mexXY–oprM. | |
| mtrD | APZ00_03520, APZ00_24680 | MtrD is the inner membrane multidrug transporter of the mtrCDE efflux complex. | |
| smeB | APZ00_03520, APZ00_24680 | SmeB is the inner membrane multidrug exporter of the efflux complex smeABC in Stenotrophomonas maltophilia. | |
| smeE | APZ00_03520, APZ00_24680 | SmeE is the RND protein of the efflux complex smeDEF in Stenotrophomonas maltophilia. | |
| tcmA | APZ00_10885, APZ00_18375 | Major facilitator superfamily transporter. Resistance to tetracenomycin C by an active tetracenomycin C efflux system which is probably energized by transmembrane electrochemical gradients. | |
| Mac/lin/phe/str/lin | cfrA | APZ00_07870 | Cfr enzyme adds an additional methyl group at position 8 of A2503 in 23S rRNA, resulting in resistance to florfenicol. |
The identity for the resistance genes was above 27.4%. RND, resistance–nodulation–cell division.
AST results showed that P. phragmitetus 31801 was susceptible to monocyclic β‐lactam, carbapenems, and cephalosporins, while it was intermediately resistant to cefotaxime (Table 2). The only β‐lactam resistance gene in P. phragmitetus 31801 belongs to a class A β‐lactamase, which is known to hydrolyze penicillin (Table 3). It may account for the resistance to piperacillin in our AST test (Table 2). As a matter of fact, we should mention that our patient's liver abscess was cured by abscess draining combined with cefodizime and metronidazole treatment (Wang et al., 2017), the two antibiotics were not included in the AST. It was reported that cefodizime has immunomodulatory properties in stimulating the phagocyte bactericidal function and increasing lymphocyte responses (Labro, 1992). We could not give a clear answer about which strategy(s) played a critical role in curing our patient's infection.
Efflux pumps in Gram‐negative bacteria are exporters of various antimicrobial compounds and biological metabolites (May, 2014). For the genes encoding for the multidrug resistance efflux pumps in P. phragmitetus 31801 obtained with CARD analysis, they could confer the resistance to four types of antibiotics: (1) tetracycline: acrB, adeA, adeB, adeG, adeJ, mexB, mexY, smeE; (2) fluoroquinolone: acrB, acrF, adeG, adeJ, ceoB, cmeB, mdtF, mexB, mexD, mexF, mexI, mexY, smeB, smeE; (3) aminoglycoside: acrD, amrB, ceoB, mexY, smeB; (4) macrolide: adeJ, cmeB, macA, macB, mdtF, mexB, mexD, mexY, smeE, cfrA. All of them encoded the subunits of the efflux pump.
It was reported that the tripartite efflux system (acrA‐acrB‐tolC) led to tetracycline resistance in Escherichia coli (Piddock, 2006), while overproduction of acrA‐acrB‐tolC contributed to clinical fluoroquinolone resistance (Swick, Morgan‐Linnell, Carlson, & Zechiedrich, 2011). acrB, as an inner membrane component of the resistance nodulation cell division (RND) family, was verified to be the major site for substrate recognition and energy transduction in the tripartite efflux system (Pos, 2009). The tripartite efflux system acrA‐acrB‐tolC in P. phragmitetus 31801 is incomplete because only acrB and tolC are identified in the genome. We assume that other genes including adeA, adeB, adeG, adeJ, mexB, mexY, or smeE might contribute to the tetracycline resistance.
Our strain 31801 was sensitive to amikacin, but resistant to gentamicin and tobramycin (Table 2). The quinolones' resistance generally requires overexpression of acrA‐acrB‐tolC. The sensitivity to the fluoroquinolone antibiotics can thus be explained by the fact that acrA is not identified by the RAST and CARD database. The adeABC system was shown to pump out amikacin, chloramphenicol, cefotaxime, erythromycin, gentamicin, kanamycin, norfloxacin, netilmicin, ofloxacin, pefloxacin, sparfloxacin, tetracycline, tobramycin, and trimethoprim (Magnet, Courvalin, & Lambert, 2001). The strain 31801 contains only gene adeA, adeB, which gives less clues for explaining the AST in strain 31801. The adeIJK pump efflux is involved in the resistance to β‐lactams, chloramphenicol, tetracycline, erythromycin, lincosamides, fluoroquinolones, fusidic acid, novobiocin, rifampicin, trimethoprim, acridine, pyronin, safranin, and sodium dodecyl sulfate (SDS) (Damier‐Piolle, Magnet, Bremont, Lambert, & Courvalin, 2008). There was a synergistic interplay between adeIJK and adeABC for resistance to chloramphenicol, fluoroquinolones, and tetracyclines (Coyne, Courvalin, & Perichon, 2011). The gene adeJ in strain 31801 has 57% identity with that in E. coli acrB (Damier‐Piolle et al., 2008). Deletion of the adeFGH pump in a mutant strain of Acinetobacter baumannii lacking the adeABC and adeIJK pumps was further shown to confer hypersusceptibility to chloramphenicol, trimethoprim, ciprofloxacin and clindamycin (Coyne, Rosenfeld, Lambert, Courvalin, & Perichon, 2010). Only adeG was found in strain 31801 (Table 3). These data could not help to clarify why strain 31801 was sensitive to amikacin while was resistant to gentamicin and tobramycin.
The genome of strain 31801 contains mexY and acrB (Table 3). It was suggested that, in Ps. aeruginosa, mexY promoted aminoglycoside resistance (Lau, Hughes, & Poole, 2014). In the same study, the proximal binding pocket within mexY was jointed with a periplasm‐linked cleft and was also part of a drug efflux pathway of acrB, which conferred to the resistance (Lau et al., 2014). It was verified that overexpression of mexXY in Ps. aeruginosa promoted resistance to aminoglycoside (Raymond, Dertz, & Kim, 2003) and mexY could be induced by chloramphenicol, tetracycline, macrolides, and aminoglycosides (Jeannot, Sobel, Farid, Keith, & Patrick, 2005). The gene mexY in strain 31801 genome may have the similar functions in conferring resistance to these antibiotics (Table 3). SmeB and mexB in strain 31801 showed 52% identity to mexY (Li, Zhang, & Poole, 2002). In Gram‐negative bacteria, when smeABC multidrug efflux system was overexpressed, the bacteria became more resistant to aminoglycosides, β‐lactams, and fluoroquinolones (Li et al., 2002). In Burkholderia vietnamiensis, the amrAB‐oprM efflux system contributed to clinical and in vitro resistance to aminoglycosides tobramycin and azithromycin (Westbrock‐Wadman et al., 1999). Besides mexY, smeB, and amrB, two other aminoglycoside resistance genes acrD and ceoB were identified in P. phragmitetus 31801. However, these data could not help understand why strain 31801 was sensitive to amikacin while it was resistant to gentamicin and tobramycin.
The tripartite efflux pump macAB–tolC was involved in antibiotic resistance in Gram‐negative bacteria with macB as a basic member in the macrolide transporters family (Lu & Zgurskaya, 2012). Similar efflux pump systems, including macA, macB, and tolC, were found in strain 31801 (Table 3). It was reported that the resistance gene tcmA of tetracenomycin C in Streptomyces glaucescens could be induced by the tetracenomycin C itself (Guilfoile & Hutchinson, 1992). The gene cfrA in a plasmid pSCFS1 mediated the resistance to clindamycin, macrolides, lincosamides, and streptogramin B (Kehrenberg, Aarestrup, & Schwarz, 2007). The macrolide was not tested in AST; however, the above macrolide resistance‐related genes identified with CARD analysis might promote the resistance to the macrolide. The above multidrug resistance genes available in strain 31801 may contribute to the related antimicrobial resistance. However, further experiments are warranted.
3.4. The genes involved in the reduction of hexavalent chromium
RAST annotation shows that there are list of six genes involving in resistance to chromium compounds, including chromate resistance protein chrI, chrB, chromate transport protein chrA, rhodanese‐like protein chrE, superoxide dismutase SodM‐like protein chrF, and superoxide dismutase chrC. Only chrA gene is found in P. phragmitetus 31801 chromosome and plasmid. However, its gene product was not a reductase in P. phragmitetus LSSE‐09 (Xu et al., 2011b). It was reported that the expression of chrB and chrA genes in a chromium‐sensitive Ochrobactrum tritici strain resulted in a high chromium resistance (Branco et al., 2008)
3.5. Virulence factors
The protein‐encoding genes were searched against the virulence factor database (VFDB) and PATRIC (Table 4). Up to 16 putative virulence factors were identified with high identity to those well‐known ones (Table 4). For example, pyochelin synthetase, flagellar M‐ring protein, peptide synthase, and cyclolysin secretion ATP‐binding protein were found. This bacterium was predicted to have mobility ability including flagellum that enables the bacterial movement and chemotaxis (149 genes predicted in RAST subsystem). When pathogens move to target cells and access to receptors, they directly utilize flagellin to adhere to and colonize on the surface of epithelial cells (Haiko & Westerlund‐Wikstrom, 2013). In many pathogenic bacteria, flagellin or the flagellar proteins have been demonstrated to function as adhesins (Haiko & Westerlund‐Wikstrom, 2013). Besides the function involving in motility and invasion organelles, the flagellum has been demonstrated to not only promote innate immunity, but also function as a predominant cue to prime the adaptive immune response (Dingle, Mulvey, & Armstrong, 2011).
Table 4.
Virulence factors in Pannonibacter phragmitetus 31801 chromosome
| Name | Function | Locus tag |
|---|---|---|
| fleQ | Transcriptional regulator FleQ | APZ00_13230 |
| fliF | Flagellar M‐ring protein FliF | APZ00_17970 |
| fecE | ATP‐binding protein FecE | APZ00_03600; APZ00_09395 |
| cyaB | Cyclolysin secretion ATP‐binding protein. | APZ00_22975; APZ00_10075 |
| clpV | Clp‐type ATPase chaperone protein. | APZ00_15745 |
| tagT | Type VI secretion associated protein TagT, ATP‐binding component of ABC transporter. | APZ00_21510 |
| clpV1 | Type VI secretion system AAA+ family ATPase | APZ00_15605; APZ00_22570 |
| bcrD | Type III secretion system LcrD homolog protein BcrD | APZ00_21695; APZ00_22570 |
| pchI | ABC transporter ATP‐binding protein | APZ00_20870 |
| fliI | Flagellum‐specific ATP synthase FliI | APZ00_19800 |
| algB | Two‐component response regulator AlgB | APZ00_12920; APZ00_19550; APZ00_21280 |
| fliP | Flagellar biosynthetic protein FliP | APZ00_09865 |
| pchF | Pyochelin synthetase PchF | APZ00_05615 |
| pchH | ABC transporter ATP‐binding protein | APZ00_02420 |
| fha1 | Type VI secretion system forkhead‐associated protein Fha1 | APZ00_12570 |
| waaG | B‐band O‐antigen polymerase | APZ00_07670 |
Cyab encoded cyclolysin secretion protein which participates in secretion of CyaA (Table 4) (Glaser, Sakamoto, Bellalou, Ullmann, & Danchin, 1988). Toxin CyaA was a bifunctional protein with adenylate cyclase and hemolytic activities, critical for pathogen Bordetella pertussis to colonize in respiratory tract (Glaser et al., 1988; Gross, Au, Smith, & Storm, 1992). Besides CyaB, two different hemolysins (ALV25768.1 and ALV26033.1) may be involved in erythrocyte degradation.
Cyclic di‐GMP phosphodiesterase was predicted here as a virulence factor, participating in synthesis of an important intracellular signaling molecule c‐di‐GMP (Romling, Galperin, & Gomelsky, 2013) (Table 4). Cyclic di‐GMP as the second message was involved in regulation of a variety of cellular functions including motility, autoregulation, flagellum synthesis, biofilm formation, cell invasion, and virulence (Sondermann, Shikuma, & Yildiz, 2012). For example, the disruption of cdpA in Burkholderia pseudomallei led to a threefold reduction in invasion of human lung epithelial cells and a sixfold decrease in cytotoxicity on human macrophage cells, indicating that cdpA contribute to virulence of pathogenic bacteria (Lee, Gu, Ching, Lam, & Chua, 2010).
Some extracellular virulence factors (proteins) require to be secreted through type VI secretion system (T6SS) (Table 4). At least three genes (fha1, clpV1, and tagT) encode virulence proteins that are part of the T6SS secretion machinery. The T6SS mimics the injection apparatus of contractile tailed bacteriophages (Leiman et al., 2009). The type VI secretion system participates in interbacterial competition and animal pathogenesis (Basler, 2015). In Vibrio cholerae, mutants deficient in Hcp and VgrG proteins had decreased virulence and infectivity (Pukatzki, Ma, Revel, Sturtevant, & Mekalanos, 2007). Similarly, Ps. aeruginosa secreted and transferred Hcp into target cells and chronic infection through the T6SS (Hood et al., 2010). The predicted virulence gene bcrD encodes a protein component in type III secretion system (T3SS) which is essential for its pathogenicity (the ability to infect) (Fauconnier et al., 2001). Defects in the T3SS may render a nonpathogenic bacterium (Coburn, Sekirov, & Finlay, 2007). As a matter of fact, there is a type IV secretion system (T4SS) in this genome which may be also important for secretion of virulence factors (see next).
Three genes (PvdL, pchF, and pvdI) involved in siderophore synthesis participate in acquiring iron from the environment (Table 4). They were verified by using the server antiSMASH (Weber et al., 2015) version 3.0.5 (https://antismash.secondarymetabolites.org/). Further examination of P. phragmitetus genome reveals at least 36 genes are related with siderophore synthesis, assembly, and transportation (predicted by RAST subsystem). It seems that P. phragmitetus may secrete both enterobactin (four genes) and aerobactin (nine genes) which have the capability to chelate a very low concentration of environmental ferric ion (Fe3+) with the extreme high affinity (Miethke & Marahiel, 2007). At least 12 ferric ion ABC uptake receptors were identified, which allowed to efficiently transport the chelated and/or free forms of iron from the environment. Furthermore, heme, hemin uptake, and utilization systems were found (32 genes), indicating that P. phragmitetus has a sophisticated iron/heme acquisition system.
3.6. Plasmids, prophages, and genomic islands
At least two prophages were identified in P. phragmitetus 31801 by using PHAST tool. The prophage 1 is moderately large with a size of 18,253 bps (locates between 5071,359 and 5089,611 bp). The GC content (66.19%) was more than that in average genome (63.3%), indicating that the prophage was horizontally transferred from other microorganisms (Figure 5a). The prophage 1 consists of 21 genes encoding 14 proteins with known functions, 7 hypothetical proteins, and 2 bacterial protein (Figure 5a and Table S2). As predicted by PHAST, prophage 1 was possibly a complete one because it consists of phage tail, head, portal, integrase, lysin, and other component proteins involving in phage structure and assembly. It is interesting that prophage 1 are conserved in three other Pannonibacter genomes (>95% identical) and the same prophage also exists in Po. gilvum SL003B‐26A1, Stappia sp. ES.058, and Roseibium hamelinense ATCC BAA‐252 (data not shown), indicating that it is integrated and adopted in the Rhodobacteraceae genomes. Despite that there were no virulence factors encoding genes identified inside the prophage region, we found that some genes encoding penicillin‐binding protein 1A, serine protease, and aminopeptidase immediately up or downstream regions. Not like prophage 1, the second predicted prophage 2 (located between 622,766 and 634,619 bp) seems to be incomplete because many phage structure proteins, lysin, integrase, protease, and transposase genes are absent (Figure 5b, Table S2). It consists of 18 proteins with 9 phage proteins and 9 hypothetical proteins. However, prophage 2 was only found in P. phragmitetus 31801 and P. phragmitetus CGMCC9175 (data now shown).
Figure 5.
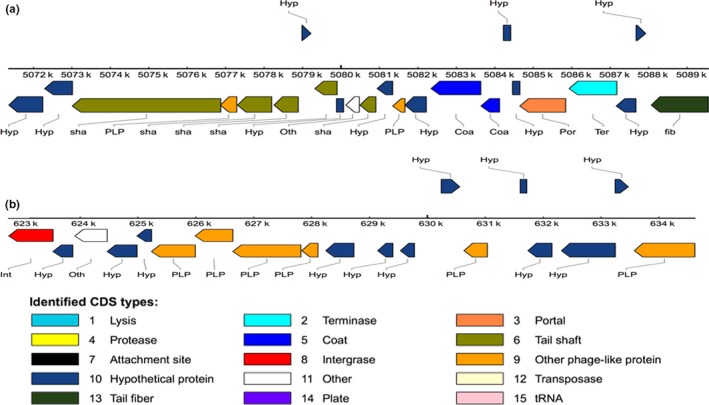
The gene organization of the predicted prophages in Pannonibacter phragmitetus 31801 chromosome. (a) The prophage 1 locates between 5,071,359 and 5,089,611 bp (18,253 bp); the GC content is 66.19%. The different colored rectangles indicate the different phage elements. (b) The prophage 2 locates between 622,766 and 633,573 bp (10,807 bp); the GC content is 64. 09%
Up to 21 genomic islands were found with the sizes ranging from 4,202 to 12,259 bp, sparsely spreading in P. phragmitetus 31801 genome (see Figure 6 and Table S3). Further analysis shows that GI2–GI4 regions have a large “conjugative plasmid”‐like genetic element with an estimated size of 60,989 bp (551,959–612,947) (Figure 6). This conjugative plasmid consists of integrases, transposases, transcriptional regulators, and conjugal transfer proteins (type IV secretion systems). Type IV secretion systems (601,481–612,947) exist in P. phragmitetus CGMCC9175, while they are absent in P. phragmitetus DSM 14782 and P. indicus. The function of The type IV secretion systems (TraI, G, F, L, J, E, virB3, C, B, G, and virD2) is well established in Gram‐negative bacteria. Pathogenic Gram‐negative bacteria utilized type IV secretion systems to translocate effector proteins or oncogenic DNA into eukaryotic host cells. Genetic materials can be exchanged through type IV secretion systems, which mediate horizontal gene transfer (Wallden, Rivera‐Calzada, & Waksman, 2010). Thus, type IV secretion systems significantly facilitate the adaptation to dramatic environmental changes which confer the spread of antibiotic resistance among microorganisms (Zechner, Lang, & Schildbach, 2012). Other highlights from these regions are the presence of several genes that are involved in detoxification of heavy metals including arsenic transporters, manganese transporter, zinc transporter ZitB, lead/cadmium/zinc, and mercury transporting ATPase and copper oxidase, which agrees that P. phragmitetus has good heavy metal resistance (Xu et al., 2011a; Shi et al., 2012).
Figure 6.

Genomic islands predicted by IslandViewer 3 and the type IV secretion system in Pannonibacter phragmitetus 31801 chromosome. (a) Twenty‐four genome islands were predicted with the sizes ranging from 4,202 to 12,259 bp; the “conjugative plasmid”‐like regions (551,959–612,947 bp) span GI and GI4. (b) The putative genetic elements in T4SS shown on the top are Tra I, G, F, L, J, E, C, B, G; virB3 and virD2 are shown below. Their relative positions are shown
The plasmid p.p‐1 has many important functional genes with a total size of 351,005 bp (Figure 1). For example, there are four genes encoding selenate and selenite transporters SomE, F, G, and K, which have been demonstrated to detoxify selenate compounds (based on RAST subsystem analysis). The toxin–antitoxin systems (parD, doc1, and doc2) exists in this p.p‐1, possible participating in stabilization of plasmid and regulation of toxins. Nine siderophore synthesis genes are involved in the formation of enterobactin and aerobactin, indicating that this plasmid is critical for iron uptake. Furthermore, the subsystem analysis (RAST) also showed there are at least 18 genes encoding membrane transporters contributing to the transportation of various nutrient molecules. Therefore, plasmid p.p‐1 in P. phragmitetus is one of the largest ones with many important functions among the sequenced Rhodobacteraceae.
4. CONCLUSIONS
Pannonibacter phragmitetus 31801 is a multidrug‐resistant opportunistic pathogen. From the genomic level, we explained its infection potential by showing that it contained many genes encoding for virulence factors, and its characteristic of multidrug resistance by finding that it contained a list of genes conferring resistance to several classifications of antibiotics. This complete sequenced genome could be the new reference for P. phragmitetus. It contributes to further elucidate antibiotic resistance and infectivity mechanisms. It may help understand the evolution traits from bioremediation reagents to virulence strains.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
CONFLICT OF INTEREST
None declared.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the Huaqiao University Graduate Student Scientific Research Innovation Ability Cultivation Plan Projects, the Fong Shu Fook Tong and Fong Yun Wah Foundations (14X30127), the Technology Planning Projects of Quanzhou Social Development Fields (2014Z24), and the Major Support Research Project of National Key Colleges Construction of Quanzhou Medical College (2013A13). We thank Dr. Hongzhi Gao Clinical Laboratory, Quanzhou Second Hospital affiliated to Fujian Medical University, for providing biosafety facilities.
Zhou Y, Jiang T, Hu S, Wang M, Ming D , Chen S. Genomic insights of Pannonibacter phragmitetus strain 31801 isolated from a patient with a liver abscess. MicrobiologyOpen. 2017;6:e515 https://doi.org/10.1002/mbo3.515
Contributor Information
Mingxi Wang, Email: mxwang@hqu.edu.cn.
Desong Ming, Email: mds6430@126.com.
Shicheng Chen, Email: shicheng@msu.edu.
REFERENCES
- Aziz, R. K. , Bartels, D. , Best, A. A. , DeJongh, M. , Disz, T. , Edwards, R. A. , … Zagnitko, O. (2008). The RAST Server: Rapid annotations using subsystems technology. BMC Genomics, 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay, S. , Schumann, P. , & Das, S. K. (2013). Pannonibacter indica sp. nov., a highly arsenate‐tolerant bacterium isolated from a hot spring in India. Archives of Microbiology, 195, 1–8. [DOI] [PubMed] [Google Scholar]
- Basler, M. (2015). Type VI secretion system: Secretion by a contractile nanomachine. Philosophical Transactions of the Royal Society B: Biological Sciences, 370, 20150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besemer, J. , & Borodovsky, M. . (2005). GeneMark: Web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Research, 33(Web Server issue), W451–W454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom, J. , Kreis, J. , Spanig, S. , Juhre, T. , Bertelli, C. , Ernst, C. , & Goesmann, A. (2016). EDGAR 2.0: An enhanced software platform for comparative gene content analyses. Nucleic Acids Research, 44(W1), W22–W28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsodi, A. K. , Micsinai, A. , Kovacs, G. , Toth, E. , Schumann, P. , Kovacs, A. L. , … Marialigeti, K. (2003). Pannonibacter phragmitetus gen. nov., sp. nov., a novel alkalitolerant bacterium isolated from decomposing reed rhizomes in a Hungarian soda lake. International Journal of Systematic and Evolutionary Microbiology, 53(Pt 2), 555–561. [DOI] [PubMed] [Google Scholar]
- Borsodi, A. K. , Micsinai, A. , Rusznyak, A. , Vladar, P. , Kovacs, G. , Toth, E. M. , & Marialigeti, K. (2005). Diversity of alkaliphilic and alkalitolerant bacteria cultivated from decomposing reed rhizomes in a Hungarian soda lake. Microbial Ecology, 50, 9–18. [DOI] [PubMed] [Google Scholar]
- Branco, R. , Chung, A. P. , Johnston, T. , Gurel, V. , Morais, P. , & Zhitkovich, A. (2008). The chromate‐inducible chrBACF operon from the transposable element TnOtChr confers resistance to chromium(VI) and superoxide. Journal of Bacteriology, 190, 6996–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver, T. , Harris, S. R. , Berriman, M. , Parkhill, J. , & McQuillan, J. A. (2012). Artemis: An integrated platform for visualization and analysis of high‐throughput sequence‐based experimental data. Bioinformatics, 28, 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, L. Y. , Huang, S. H. , Yang, Z. H. , Peng, B. , Huang, Y. , & Chen, Y. H. (2009). Hexavalent chromium reduction by Pannonibacter phragmitetus BB isolated from soil under chromium‐containing slag heap. Journal of Environmental Science and Health, 44, 615–622. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Xiong, Z. , Sun, L. , Yang, J. , & Jin, Q. . (2012). VFDB 2012 update: Toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Research, 40(Database issue), D641–D645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn, B. , Sekirov, I. , & Finlay, B. B. (2007). Type III secretion systems and disease. Clinical Microbiology Reviews, 20, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman, C. , Druga, B. , Hegedus, A. , Sicora, C. , & Dragos, N. (2013). Archaeal and bacterial diversity in two hot spring microbial mats from a geothermal region in Romania. Extremophiles, 17, 523–534. [DOI] [PubMed] [Google Scholar]
- Coyne, S. , Courvalin, P. , & Perichon, B. (2011). Efflux‐mediated antibiotic resistance in Acinetobacter spp. Antimicrobial Agents and Chemotherapy, 55, 947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, S. , Rosenfeld, N. , Lambert, T. , Courvalin, P. , & Perichon, B. (2010). Overexpression of resistance‐nodulation‐cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy, 54, 4389–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier‐Piolle, L. , Magnet, S. , Bremont, S. , Lambert, T. , & Courvalin, P. (2008). AdeIJK, a resistance‐nodulation‐cell division pump effluxing multiple antibiotics in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy, 52, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling, A. E. , Mau, B. , & Perna, N. T. (2010). progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE, 5, e11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher, A. L. , Bratke, K. A. , Powers, E. C. , & Salzberg, S. L. (2007). Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics, 23, 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher, A. L. , Harmon, D. , Kasif, S. , White, O. , & Salzberg, S. L. (1999). Improved microbial gene identification with GLIMMER. Nucleic Acids Research, 27, 4636–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle, T. C. , Mulvey, G. L. , & Armstrong, G. D. (2011). Mutagenic analysis of the Clostridium difficile flagellar proteins, FliC and FliD, and their contribution to virulence in hamsters. Infection and Immunity, 79, 4061–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconnier, A. , Veithen, A. , Gueirard, P. , Antoine, R. , Wacheul, L. , Locht, C. , … Godfroid, E. (2001). Characterization of the type III secretion locus of Bordetella pertussis . International Journal of Medical Microbiology, 290, 693–705. [DOI] [PubMed] [Google Scholar]
- Galperin, M. Y. , Makarova, K. S. , Wolf, Y. I. , & Koonin, E. V. . (2015). Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Research, 43(Database issue), D261–D269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, P. , Sakamoto, H. , Bellalou, J. , Ullmann, A. , & Danchin, A. (1988). Secretion of cyclolysin, the calmodulin‐sensitive adenylate cyclase‐haemolysin bifunctional protein of Bordetella pertussis . EMBO Journal, 7, 3997–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris, J. , Konstantinidis, K. T. , Klappenbach, J. A. , Coenye, T. , & Vandamme Pand Tiedje, J. M. (2007). DNA‐DNA hybridization values and their relationship to whole‐genome sequence similarities. International Journal of Systematic and Evolutionary Microbiology, 57(Pt 1), 81–91. [DOI] [PubMed] [Google Scholar]
- Grant, J. R. , & Stothard, P. . (2008). The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Research, 36(Web Server issue), W181–W184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissa, I. , Vergnaud, G. , & Pourcel, C. . (2008). CRISPRcompar: A website to compare clustered regularly interspaced short palindromic repeats. Nucleic Acids Research, 36(Web Server issue), W145–W148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, M. K. , Au, D. C. , Smith, A. L. , & Storm, D. R. (1992). Targeted mutations that ablate either the adenylate cyclase or hemolysin function of the bifunctional cyaA toxin of Bordetella pertussis abolish virulence. Proceedings of the National Academy of Sciences of the United States of America, 89, 4898–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoile, P. G. , & Hutchinson, C. R. (1992). The Streptomyces glaucescens TcmR protein represses transcription of the divergently oriented tcmR and tcmA genes by binding to an intergenic operator region. Journal of Bacteriology, 174, 3659–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko, J. , & Westerlund‐Wikstrom, B. (2013). The role of the bacterial flagellum in adhesion and virulence. Biology (Basel), 2, 1242–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, B. , Lewis, R. , & Trevett, A. (1992). Septicaemia due to Achromobacter group B: A report of two cases. Medical Microbiology Letters, 1, 177–184. [Google Scholar]
- Holmes, B. , Moss, C. W. , & Daneshvar, M. I. (1993). Cellular fatty acid compositions of “Achromobacter groups B and E”. Journal of Clinical Microbiology, 31, 1007–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, B. , Segers, P. , Coenye, T. , Vancanneyt, M. , & Vandamme, P. (2006). Pannonibacter phragmitetus, described from a Hungarian soda lake in 2003, had been recognized several decades earlier from human blood cultures as Achromobacter groups B and E. International Journal of Systematic and Evolutionary Microbiology, 56(Pt 12), 2945–2948. [DOI] [PubMed] [Google Scholar]
- Hood, R. D. , Singh, P. , Hsu, F. , Güvener, T. , Carl, M. A. , Trinidad, R. R. S. , … Mougous, J. D. (2010). A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host & Microbe, 7, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute CLS (2013). CLSI Performance Standards for Antimicrobial Disk Susceptibility Tests. In. NCCLS.
- Jeannot, K. , Sobel, M. L. , Farid, E. G. , Keith, P. , & Patrick, P. (2005). Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug‐ribosome interaction. Journal of Bacteriology, 187, 5341–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks, P. J. , & Shaw, E. J. (1997). Recurrent Septicaemia due to “Achromobacter Group B”. Journal of Infection, 34, 143–145. [DOI] [PubMed] [Google Scholar]
- Jorgensen, J. H. , & Ferraro, M. J. (2009). Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clinical Infectious Diseases, 49, 1749–1755. [DOI] [PubMed] [Google Scholar]
- Kehrenberg, C. , Aarestrup, F. M. , & Schwarz, S. (2007). IS21‐558 Insertion Sequences Are Involved in the Mobility of the Multiresistance Gene cfr▿. Antimicrobial Agents and Chemotherapy, 51, 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labro, M. T. (1992). Immunological evaluation of cefodizime: A unique molecule among cephalosporins. Infection, 20(Suppl 1), S45–S47. [DOI] [PubMed] [Google Scholar]
- Lau, C. H.‐F. , Hughes, D. , & Poole, K. . (2014). MexY‐promoted aminoglycoside resistance in Pseudomonas aeruginosa: Involvement of a putative proximal binding pocket in aminoglycoside recognition. mBio, 5, e01068–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. S. , Gu, F. , Ching, S. M. , Lam, Y. , & Chua, K. L. (2010). CdpA is a Burkholderia pseudomallei cyclic di‐GMP phosphodiesterase involved in autoaggregation, flagellum synthesis, motility, biofilm formation, cell invasion, and cytotoxicity. Infection and Immunity, 78, 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiman, P. G. , Basler, M. , Ramagopal, U. A. , Bonanno, J. B. , Sauder, J. M. , Pukatzki, S. , … Mekalanos, J. J. (2009). Type VI secretion apparatus and phage tail‐associated protein complexes share a common evolutionary origin. Proceedings of the National Academy of Sciences of the United States of America, 106, 4154–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. Z. , Zhang, L. , & Poole, K. (2002). SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia . Antimicrobial Agents and Chemotherapy, 46, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. , & Zgurskaya, H. I. (2012). Role of ATP binding and hydrolysis in assembly of MacAB‐TolC macrolide transporter. Molecular Microbiology, 86, 1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet, S. , Courvalin, P. , & Lambert, T. (2001). Resistance‐nodulation‐cell division‐type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrobial Agents and Chemotherapy, 45, 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz, V. M. , Chen, I. M. , Chu, K. , Szeto, E. , Palaniappan, K. , Pillay, M. , … Kyrpides, N. C. . (2014). IMG/M 4 version of the integrated metagenome comparative analysis system. Nucleic Acids Research, 42(Database issue), D568–D573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz, V. M. , Mavromatis, K. , Ivanova, N. N. , Chen, I. M. , Chu, K. , & Kyrpides, N. C. (2009). IMG ER: A system for microbial genome annotation expert review and curation. Bioinformatics, 25, 2271–2278. [DOI] [PubMed] [Google Scholar]
- May, M. (2014). Drug development: Time for teamwork. Nature, 509, S4–S5. [DOI] [PubMed] [Google Scholar]
- McArthur, A. G. , Waglechner, N. , Nizam, F. , Yan, A. , Azad, M. A. , Baylay, A. J. , … Wright, G. D. (2013). The comprehensive antibiotic resistance database. Antimicrobial Agents and Chemotherapy, 57, 3348–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley, K. P. , Laundy, T. J. , & Masterton, R. G. (1990). Achromobacter Group B replacement valve endocarditis. Journal of Infection, 20, 262–263. [DOI] [PubMed] [Google Scholar]
- Miethke, M. , & Marahiel, M. A. (2007). Siderophore‐based iron acquisition and pathogen control. Microbiology and Molecular Biology Reviews, 71, 413–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek, R. , Olson, R. , Pusch, G. D. , Olsen, G. J. , Davis, J. J. , Disz, T. , … Stevens, R. . (2014). The SEED and the rapid annotation of microbial genomes using Subsystems technology (RAST) Nucleic Acids Research, 42(Database issue), D206–D214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock, L. J. (2006). Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clinical Microbiology Reviews, 19, 382–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pos, K. M. . (2009). Drug transport mechanism of the AcrB efflux pump. Biochimica et Biophysica Acta (BBA), 1794, 782–793. [DOI] [PubMed] [Google Scholar]
- Pukatzki, S. , Ma, A. T. , Revel, A. T. , Sturtevant, D. , & Mekalanos, J. J. (2007). Type VI secretion system translocates a phage tail spike‐like protein into target cells where it cross‐links actin. Proceedings of the National Academy of Sciences of the United States of America, 104, 15508–15513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, K. N. , Dertz, E. A. , & Kim, S. S. (2003). Enterobactin: An archetype for microbial iron transport. Proceedings of the National Academy of Sciences of the United States of America, 100, 3584–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling, U. , Galperin, M. Y. , & Gomelsky, M. (2013). Cyclic di‐GMP: The first 25 years of a universal bacterial second messenger. Microbiology and Molecular Biology Reviews, 77, 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, K. , Parkhill, J. , Crook, J. , Horsnell, T. , Rice, P. , Rajandream, M. A. , & Barrell, B. (2000). Artemis: Sequence visualization and annotation. Bioinformatics, 16, 944–945. [DOI] [PubMed] [Google Scholar]
- Shi, Y. , Chai, L. , Yang, Z. , Jing, Q. , Chen, R. , & Chen, Y. (2012). Identification and hexavalent chromium reduction characteristics of Pannonibacter phragmitetus . Bioprocess and Biosystems Engineering, 35, 843–850. [DOI] [PubMed] [Google Scholar]
- Sondermann, H. , Shikuma, N. J. , & Yildiz, F. H. (2012). Truncated form of the title: Mechanism of c‐di‐GMP signaling. Current Opinion in Microbiology, 15, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standards , National committee for clinical laboratory standards. (2006). Performance Standards For Antimicrobial Susceptibility Testing. Clinical Laboratory Standards Institute, M100 S16 Ed. 16, 4.
- Standards , National committee for clinical laboratory standards. (2013). CLSI Performance Standards for Antimicrobial Disk Susceptibility Tests. Clinical Laboratory Standards Institute, M100‐S23
- Swick, M. C. , Morgan‐Linnell, S. K. , Carlson, K. M. , & Zechiedrich, L. (2011). Expression of multidrug efflux pump genes acrAB‐tolC, mdfA, and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrobial Agents and Chemotherapy, 55, 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. D. , Higgins, D.G. , Gibson, T. J . (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position‐specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673‐4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall, B. J. , Rossello‐Mora, R. , Busse, H. J. , Ludwig, W. , & Kampfer, P. (2010). Notes on the characterization of prokaryote strains for taxonomic purposes. International Journal of Systematic and Evolutionary Microbiology, 60(Pt 1), 249–266. [DOI] [PubMed] [Google Scholar]
- Wallden, K. , Rivera‐Calzada, A. , & Waksman, G. (2010). Type IV secretion systems: Versatility and diversity in function. Cellular Microbiology, 12, 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Jin, D. , Zhou, L. , & Zhang, Z. (2016). Draft genome sequence of Pannonibacter phragmitetus strain CGMCC9175, a halotolerant polycyclic aromatic hydrocarbon‐degrading bacterium. Genome Announcements, 4, e01675–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Yang, Z. , Peng, B. , Chai, L. , Wu, B. , & Wu, R. (2013). Biotreatment of chromite ore processing residue by Pannonibacter phragmitetus BB. Environmental Science and Pollution Research, 20, 5593–5602. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Zhang, X. , Jiang, T. , Hu, S. , Yi, Z. , Zhou, Y. , … Chen, S. (2017). Liver abscess caused by Pannonibacter phragmitetus: Case report and literature review. Frontiers in Medicine, 4:48 https://doi.org/10.3389/fmed.2017.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, T. , Blin, K. , Duddela, S. , Krug, D. , Kim, H. U. , Bruccoleri, R. , … Medema, M. H. (2015). antiSMASH 3.0‐a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Research, 43(W1), W237–W243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrock‐Wadman, S. , Sherman, D. R. , Hickey, M. J. , Coulter, S. N. , Zhu, Y. Q. , Warrener, P. , … Stover, C. K. (1999). Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrobial Agents and Chemotherapy, 43, 2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Zhu, Z. , Fu, L. , Niu, B. , & Li, W. (2011). WebMGA: A customizable web server for fast metagenomic sequence analysis. BMC Genomics, 12, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Luo, M. , Jiang, C. , Wei, X. , Kong, P. , Liang, X. , … Liu, H. (2012). In vitro reduction of hexavalent chromium by cytoplasmic fractions of Pannonibacter phragmitetus LSSE‐09 under aerobic and anaerobic conditions. Applied Biochemistry and Biotechnology, 166, 933–941. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Luo, M. , Yang, L. , Wei, X. , Lin, X. , & Liu, H. (2011a). Encapsulation of Pannonibacter phragmitetus LSSE‐09 in alginate‐carboxymethyl cellulose capsules for reduction of hexavalent chromium under alkaline conditions. Journal of Industrial Microbiology & Biotechnology, 38, 1709–1718. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Yang, L. , Luo, M. , Liang, X. , Wei, X. , Zhao, J. , & Liu, H. (2011b). Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE‐09 coated with polyethylenimine‐functionalized magnetic nanoparticles under alkaline conditions. Journal of Hazardous Materials, 189, 787–793. [DOI] [PubMed] [Google Scholar]
- Zechner, E. L. , Lang, S. , & Schildbach, J. F. (2012). Assembly and mechanisms of bacterial type IV secretion machines. Philosophical Transactions of The Royal Society of London Series B‐Biological Sciences, 367, 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Liang, Y. , Lynch, K. H. , Dennis, J. J. , & Wishart, D. S. . (2011). PHAST: A fast phage search tool. Nucleic Acids Research, 39(Web Server issue), W347–W352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


