Highlights
-
•
The first case in literature of urogenital quadruple primary malignancies.
-
•
MPMs affect the GI tract, respiratory and urogenital system, head and neck region.
-
•
One urogenital tumor can influence the development of another one.
-
•
MPMs are caused by (epi)genetic modifications, environmental + behavioral factors.
Abbreviations: PMs, primary malignancies; MPMs, multiple primary malignancies; PCDH17, protocadherin involved in cell adhesion functions; TCF21, transcription factor involved in tissue differentiation
Keywords: Urogenital, Multiple primary, Cancers, Case report
Abstract
Introduction
Urogenital cancers are not an uncommon occurrence in daily practice. Prostate cancer is the second most frequent cancer in men, kidney cancer accounts for 2.4% of all cancers and bladder cancers represent 3.1% of cancers in both men and women [1]. However, the cases of a simultaneous development of all three cancers, including one with a neuroendocrine component, are very few and far between.
Presentation of case
Our case report involves a case of a patient with prostate adenocarcinoma, clear-cell renal carcinoma, papillary renal carcinoma and small-cell bladder cancer. The patient was treated as if he had separate pathologies by a multidisciplinary team: surgical and oncological, performing radical cystoprostatectomy with left perifascial nephroureterectomy, right ureterostomy and adjuvant chemotherapy, with excellent outcome even four years after the initial diagnosis.
Discussion
The distinct features of this case are the occurence of four different malignancies of the urogenital system, the family history of colon cancer, the development of small-cell carcinoma of the bladder, which is extremely rare and the good outcome, despite the quadruple malignancies and the aggresivity of the small-cell carcinoma.
Conclusion
Mutiple primary malignancies are a relatively rare pathology, but should be considered as a possibility in patients who already had a second malignancy. Cases of patients with MPMs should be supervised by a multidisciplinary team and should be followed closely.
1. Introduction
Multiple primary malignancies are a rather rare occurence in clinical practice and are defined by the development of a second pathology within 6 months after the discovery of the first cancer. As fas as we can tell, this is the first case published of a quadruple primary malignancy of the urogenital tract, even though urogenital cancers are common [1].
Unfortunately, due to the scarcity of such cases and the unique combination of malignancies, the medical corps has not yet found a definitive answer on how to optimally tackle these cases. Thus, we treated the malignancies as if they had occured separately, following their individual guidelines. The case was handled by two university hospitals in an academic environment.
2. Presentation of case
We present the case of a 78-year-old male, known with BPH for two years, which he has treated with alpha-blocker, who presents himself at the urology department with asymptomatic gross hematuria and weight loss (∼5 kg) in the past 3 months. His personal history also includes high blood pressure treated by his cardiologist. Regarding his family history, his mother and brother had colon cancer.
Blood tests showed a grade II anemia [2], and the prostate-specific antigen (PSA) was 1.41 ng/mL, and the urine sample revealed 300 red blood cells/μL. The rectal examination revealed an enlarged prostate, mildly irregular, with well defined margins, no tenderness, and no indurations on the posterior bladder wall. The rest of the clinical examination was normal.
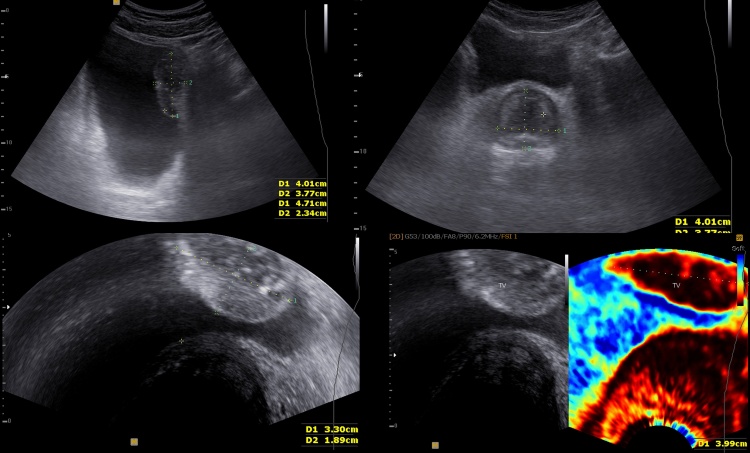
An abdominal ultrasound was performed and it revealed a tumor of the left postero-lateral bladder wall (4.64/3.14 cm). The tumor had a large base of implantation, a hyperechoic capsule and a mixed echogenicity structure. The transabdominal and transrectal Doppler ultrasound examination showed mild increase of the tumoral vascularization, predominantly in the central part. The fibroelastography showed decreased elasticity of the bladder wall.
The prostate measured 4.8/3.77/3 cm with a central nodule with a benign aspect and peripheral calcifications (Fig. 1).
Fig. 1.
Ultrasonography and elastography captures of the bladder and prostate cancers.
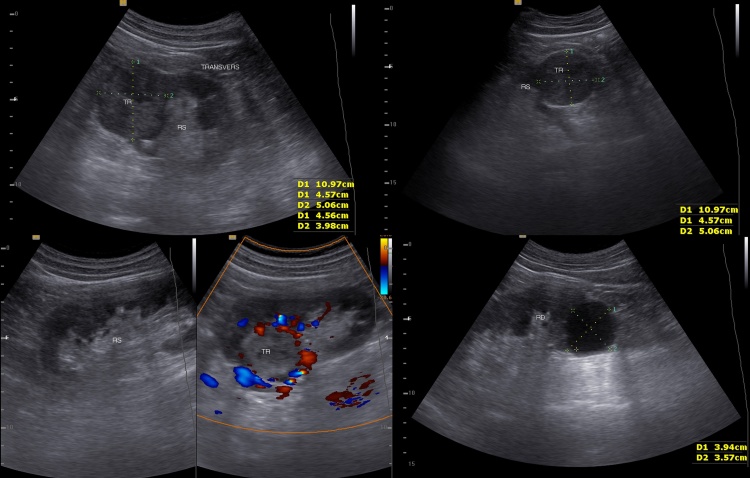
In the superior pole of the left kidney, a tumor of 4/5/4 cm was present. The Doppler ultrasound examination showed increased vascularization in the periphery of the tumor. The right kidney was normal, except for a small inferior pole cyst (Fig. 2).
Fig 2.
Ultrasonography captures of renal cancer and simple renal cyst.
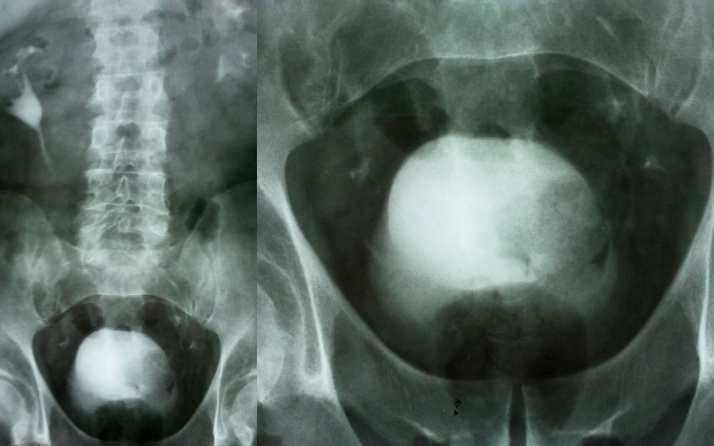
The intravenous urography showed delayed nephrogram and pyelogram of the left kidney at 5–10–15 min and an irregular filling defect on the lateral wall of the urinary bladder (Fig. 3).
Fig. 3.
Intravenous urography.
During the cystoscopy, we identified a bullous tumor infiltrating the lateral bladder wall and the dome. A transurethral resection of bladder tumor was performed.
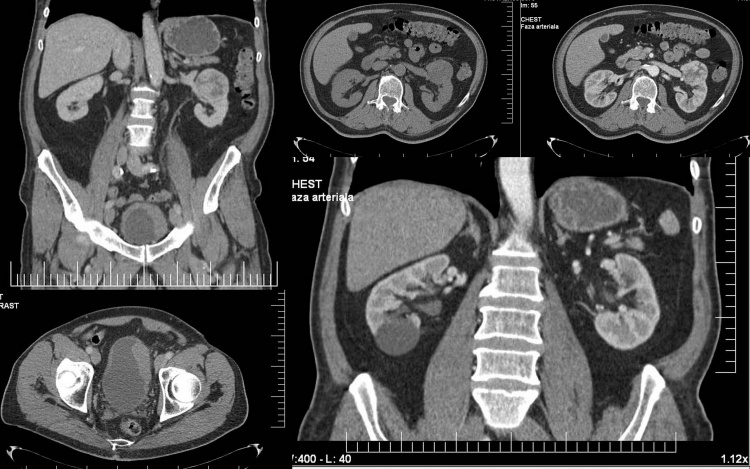
These findings imposed the necessity of a pre-operative CT of the thorax, abdomen and pelvis (Fig. 4). The left renal tumor had a distinct margin, without thickening of the perirenal fascia and it was located on the mediorenal side, extended anteriorly and invaded the sinus. Precontrast, the tumor was isodense with the renal parenchyma and postcontrast (arterial phase), the tumor enhances mainly in the periphery, sparing the central part, suggesting central necrosis. The tumor did not extend in the perinephric fat or to the adrenal glands. The left renal vein had no signs of thrombosis and there were no lombo-aortic lymph nodes involvement. Hence, the pre-operative TNM staging was T1bN0M0–stage I renal cell carcinoma. Posterior and superior in the left kidney there is another 7 mm tumor, T1aN0Mo. In these conditions, nephron-sparring surgery was not a viable therapeutic option. In the lower pole of the right kidney, there is a 42 mm hypodense round, well defined, capsulated tumor, with no contrast enhancement, interpreted as a simple polar cyst − Bosniak I.
Fig 4.
Computed-tomography of the abdomen and pelvis showing unenhanced and enhanced characteristics of the renal and bladder tumor, as well as the simple cyst.
The urinary bladder had an elongated 57/42 mm tumor on the left lateral and superior walls that infiltrated the perivesical fat and protruded in the bladder lumen. Precontrast, the tumor was hypodense and post contrast it showed no enhancement. The CT scan revealed 5–7 mm multiple pelvic lymph nodes. The staging of the urinary bladder was T3bN2M0–stage IV.
The prostate was 53 mm in diameter with a few infracentimetric calcifications and preserved rectoprostatic angle.
Considering the radiological findings, the urological team decided to perform radical cystoprostatectomy with left perifascial nephroureterectomy and right ureterostomy, with no long or short term complications.
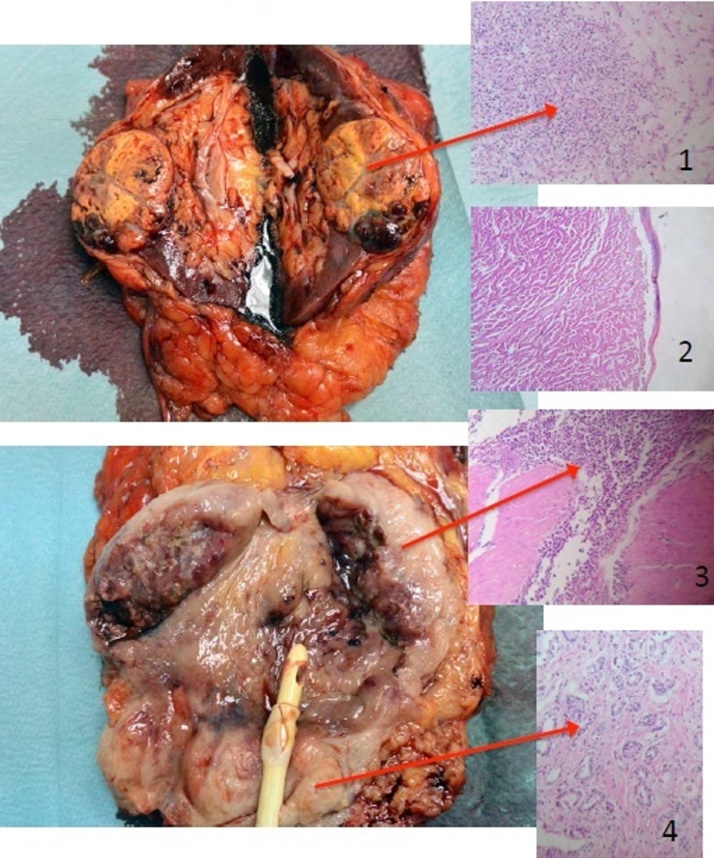
The histopathological findings revealed the following (Fig. 5, Fig 6, Fig. 7):
-
1.
Prostate adenocarcinoma Gleason 6 (3 + 3), limited to one lobe − pT2aN0;
-
2.
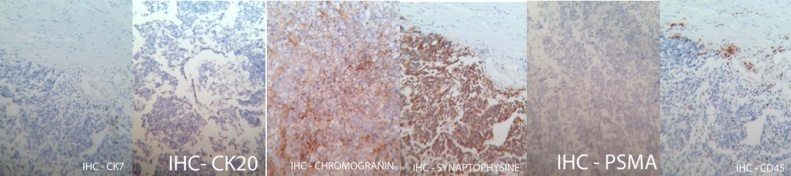
Small cell carcinoma of the urinary bladder and an area of large cell carcinoma, invading the perivesical fat, but not the seminal vesicles, the prostate or the lymph nodes; negative for CD45, CK7, CK20 and PSMA, and positive for chromogranin-A and synaptophisin − pT3bN0G3;
-
3.
Papillary renal cell carcinoma − 1 cm, type 2, Fuhrman 3, pT1a;
-
4.
Clear cell carcinoma − 4.5 cm, Fuhrman 1, without invasion of the renal sinus, perinephric fat or the renal vessels, pT1b.
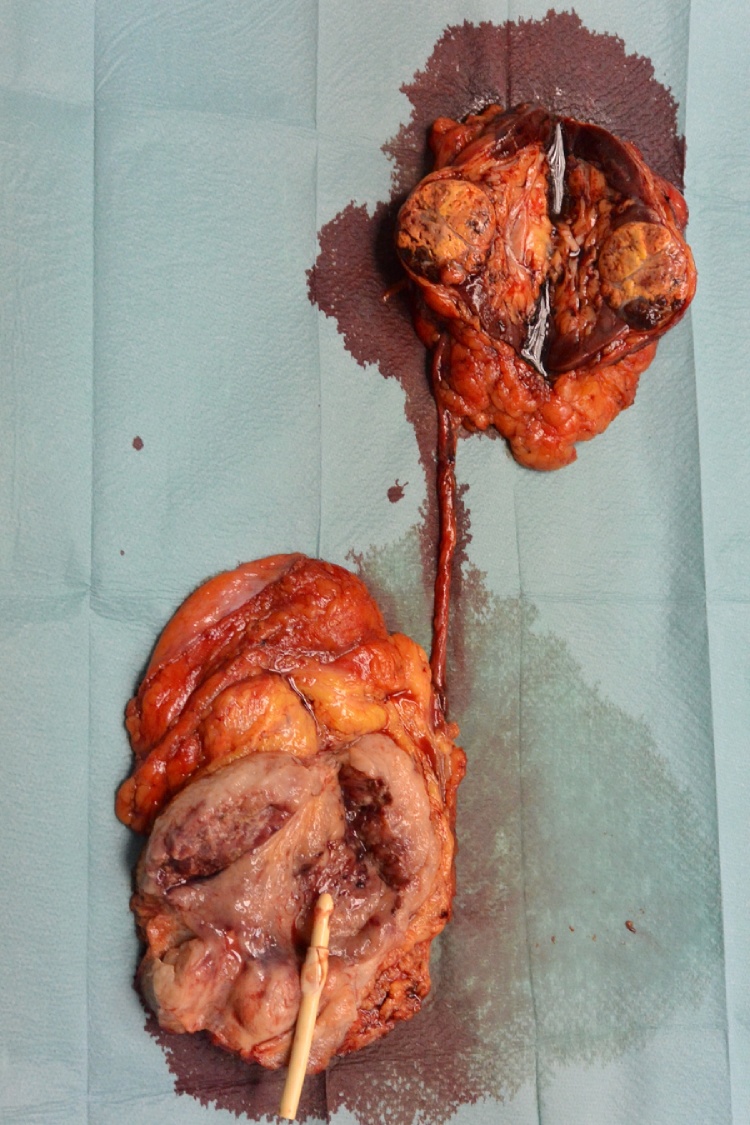
Fig. 5.
Surgical specimen.
Fig 6.
Surgical specimen with the corresponding histopathological slides.
Fig. 7.
Immunohistochemical staining.
The patient was refered to the oncologist, where he started treatment with lanreotide, bicalutamide and leuprolide. At the same hospital, a bone scintigraphy showed no bone metastasis. Four years after the diagnosis, the patient is cancer-free with an active life and excellent quality of life (self-reported).
3. Discussion
Multiple primary malignancies (MPMs) are a rare occurence in clinical practice and are defined by the development of a second pathology within 6 months after the discovery of the first cancer. Theodore Billroth in the 19th century first described this phenomenon [3]. Warren and Gates in 1932 refined the definition to bring it to the shape that we still use today: „each tumor must present a definite picture of malignancy, each must be distinct, and the probability of one being the metastasis of the other must be excluded [4].”
MPMs tend to appear frequently in the upper digestive tract, respiratory system, head and neck region and urogenital system [5], the latter accounting for 13.5% of cases [6]. Patients with prostate cancer are eighteen times more prone to develop bladder cancer and patients with bladder cancer develop prostate cancer nineteen times more frequently.
Subhankar reported that solid tumors were the most common type of second malignancy in patients with renal cell carcinoma (90%), and the male genital system was the most common site of occurence (23.6%) [7], [8]. Several papers prove that renal carcinomas influence the development of prostate and bladder cancer and viceversa [9], [10].
One of the most comprehensive studies by Bittorf et al. shows that 3.8% of oncological patients had at least 2 PMs and out of the patients with 2 PMs, 3.8% had at least one more [11], results confirmed by other studies on a large number of patients [12], [13], [14].
Several studies showed an unexpected survival of patients with MPMs than those without a second malignancy [8].
In some cases, the subsequent PMs have been found to be caused by the treatment for the previous cancer(s), especially with alkylating agents, topoisomerase II inhibitors or radiotherapy [15]. In others, the association of cancers can be due to genetic mutations: multiple endocrine neoplasia (MEN) 1, 2a and 2b, Li-Fraumeni, Muir-Torre, Lynch, von Hippel-Lindau, Beckwith-Wiedemann.
Regarding the genetic footprint of urogenital cancers, clear-cell renal carcinoma shows a chromosome 3p loss of heterozygosity, that is not shared by the papillary cell carcinoma [16]. PCDH17 and TCF21 methylation in urogenital cell lines is strongly associated with the same pattern in tumor tissue [17].
Unfortunately, genetic testing currently has a restrictive price and availability. The general consensus is that MPMs are a multifactorial pathology caused by genetic and epigenetic modifications, environmental and behavioral factors (smoking, alchohol, pollution), as well as the improved monitoring and survival of oncological patients, that live long enough to develop other malignancies [18].
Case reports are still of great importance such as to contribute to the pool of existent ones and pave the way to finding clinical or phenotypical associations and even mutations that link these pathologies.
Our own review of the literature and the accounts of other articles confirm that there are 17 reported cases of triple urogenital cancers. As fas as we can tell, this is the first case published of a quadruple PMs of the urogenital tract.
Unfortunately, our patient did not undergo genetic testing, but we suspected that his family line was harboring some sort of genetic instability and we suggested a careful monitoring of his offsprings in the event it has been passed forth.
The main questions that puzzle us about this case are not yet answered by science. Which cancer was first? Did the first one influence or accelerate the development of the others? Had it not appeared, would the others have developed anyway or can a cancer weaken the immune system or release carcinogenic factors in the bloodstream and/or locally and facilitate the development of other cancers? Is there a urogenital MPMs syndrome? And last, but not least: does it matter? Or do we treat them the same as if they were an individual occurence?
4. Conclusion
Mutiple primary malignancies are a relatively rare pathology, but should be considered by clinicians as a possibility in an oncological patient and most of all, in a patient who was already had a second malignancy. In a situation of high clinical suspicion, genetic testing should be ordered, if possible. Cases of patients with multiply primary malignancies should be supervised by a multidisciplinary team and should be followed closely. Our article has been reported in line with the SCARE criteria [19].
Conflicts of interest
None to declare. The authors have no financial, consultative, institutional, and other relationship that might lead to bias or conflict or interest.
Funding
None to declare.
Ethical approval
Decision number 2/2017 of the ethics committee of the Urology and Renal Tranplantation Institute, Cluj-Napoca, Romania.
Consent
Writen and signed consent has been obtained from the patient and is available for submission upon request.
Author contribution
Liviu Ghervan: Design of study, revision, approval of final manuscript.
Florin-Ioan Elec: Design of study, revision, approval of final manuscript.
Andreea Zaharie: Data collection, drafting, revision, approval of final manuscript.
Bogdan-Mihai Ene: Data collection, drafting, revision, approval of final manuscript.
Guarantor
Liviu Ghervan.
Florin-Ioan Elec.
Acknowledgements
All authors have approved the final manuscript.
The authors would like to extend their gratitude to dr. Cristina Cebotaru for managing the oncological treatment, dr. Iacob Gheorghiţă for interpreting the pathological slides and Oana Ţenter for proofreading our article.
References
- 1.World Cancer Research Fund International. Cancer Facts and Figures −Worldwide Data. Available from: http://www.wcrf.org/int/cancer-facts-figures/worldwide-data.
- 2.World Health Organization. Vitamin and Mineral Nutrition Information System - Haemoglobin Concentrations for the Diagnosis of Anaemia and Assesment of Severity. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
- 3.Billroth T. Die allgemeine chirurgische Pathologie and Therapie. In: Reimer G., editor. 51 Vorlesungen – Ein Handbuch für Studierende and Ärzte. 14th ed. Reimer; Berlin: 1889. [Google Scholar]
- 4.Warren S., Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am. J. Cancer. 1932;16:1358–1414. [Google Scholar]
- 5.Chun T.Y. Coincidence of bladder and prostate cancer. J. Urol. 1997;157:65–67. [PubMed] [Google Scholar]
- 6.Ray P., Sharifi R., Ortolano V., Guinan P. Involvement of the genitourinary system in multiple primary malignant neoplasms: a review. J. Clin. Oncol. 1983;1:574–581. doi: 10.1200/JCO.1983.1.9.574. [DOI] [PubMed] [Google Scholar]
- 7.Beisland C., Talleraas O., Bakke A. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006;97:698–702. doi: 10.1111/j.1464-410X.2006.06004.x. (PubMed:16536756) [DOI] [PubMed] [Google Scholar]
- 8.Subhankar C., Stefano T., Surinder B. Incidence and prognostic significance of secondary primary cancers in renal cell carcinoma. Am. J. Clin. Oncol. 2013;36(April (2)):132–142. doi: 10.1097/COC.0b013e3182438ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabbani F., Reuter V.E., Katz J. Second primary malignancies associated with renal cell carcinoma: influence of histologic type. Urology. 2000;56:399–403. doi: 10.1016/s0090-4295(00)00682-8. (PubMed: 10962302) [DOI] [PubMed] [Google Scholar]
- 10.McCredie M., Macfarlane G.J., Stewart J. Second primary cancers following cancers of the kidney and prostate in New South Wales (Australia), 1972–91. Cancer Causes Control. 1996;7:337–344. doi: 10.1007/BF00052939. (PubMed: 8734827) [DOI] [PubMed] [Google Scholar]
- 11.Bittorf B., Kessler H., Merkel S. Multiple primary malignancies: an epidemiological and pedigree analysis of 57 patients with at least three tumours. Eur. J. Surg. Oncol. 2001;27:302–313. doi: 10.1053/ejso.2001.1112. [DOI] [PubMed] [Google Scholar]
- 12.Rosso S., De Angelis R., Ciccolallo L., Carrani E., Soerjomataram I., Grande E., Zigon G., EUROCARE Working Group Working Group: Multiple tumours in survival estimates. Eur. J. Cancer. 2009;45(April (6)):1080–1094. doi: 10.1016/j.ejca.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Feyerabend T., Richter E., Brandt A. Multiple malignomas–an analysis of 352 patients. Strahlenther. Onkol. 1991;167(April (4)):214–219. (Article in German) [PubMed] [Google Scholar]
- 14.Bauer M., zum Winkel K., Tempel M., Kempf B., Fritz P. Multiple cancers. Onkologie. 1984;7(June (3)):156–166. doi: 10.1159/000215429. (Article in German) [DOI] [PubMed] [Google Scholar]
- 15.Munker R., Hiller E., Melnyk A., Gutjahr P. Second malignancies: clinical relevance and basic research. Int. J. Oncol. 1996:363–776. doi: 10.3892/ijo.9.4.763. [DOI] [PubMed] [Google Scholar]
- 16.Lindor N., Greene M. The concise handbook of family cancer syndromes. J. Natl. Cancer Inst. 1998;90(14):1063–1064. doi: 10.1093/jnci/90.14.1039. [DOI] [PubMed] [Google Scholar]
- 17.Costa V.L., Henrique R., Danielsen S.A. TCF21 and PCDH17 methylation: an innovative panel of biomarkers for a simultaneous detection of urological cancers. Epigenetics. 2011;6(September (9)):1120–1130. doi: 10.4161/epi.6.9.16376. [DOI] [PubMed] [Google Scholar]
- 18.Coleman M.P. Multiple primary malignant neoplasms in England and Wales, 1971–1981. Yale J. Biol. Med. 1986;59:517–531. [PMC free article] [PubMed] [Google Scholar]
- 19.Agha Riaz A., Fowler Alexander J., Saeta Alexander, Barai Ishani, Rajmohan Shivanchan, Orgill Dennis P., for the SCARE Group Erratum to the SCARE guidelines: consensus-based surgical case report guidelines [Int J. Surg. 34 (2016) 180 −186] Int. J. Surg. 2016;36(Part A, December) doi: 10.1016/j.ijsu.2016.11.021. page 396. [DOI] [PubMed] [Google Scholar]