Abstract
In cooperative breeders, aggression from dominant breeders directed at subordinates may raise subordinate stress hormone (glucocorticoid) concentrations. This may benefit dominants by suppressing subordinate reproduction but it is uncertain whether aggression from dominants can elevate subordinate cooperative behaviour, or how resulting changes in subordinate glucocorticoid concentrations affect their cooperative behaviour. We show here that the effects of manipulating glucocorticoid concentrations in wild meerkats (Suricata suricatta) on cooperative behaviour varied between cooperative activities as well as between the sexes. Subordinates of both sexes treated with a glucocorticoid receptor antagonist (mifepristone) exhibited significantly more pup protection behaviour (babysitting) compared to those treated with glucocorticoids (cortisol) or controls. Females treated with mifepristone had a higher probability of exhibiting pup food provisioning (pup-feeding) compared to those treated with cortisol. In males, there were no treatment effects on the probability of pup-feeding, but those treated with cortisol gave a higher proportion of the food they found to pups than those treated with mifepristone. Using 19 years of behavioural data, we also show that dominant females did not increase the frequency with which they directed aggression at subordinates at times when the need for assistance was highest. Our results suggest that it is unlikely that dominant females manipulate the cooperative behaviour of subordinates through the effects of aggression on their glucocorticoid levels and that the function of aggression directed at subordinates is probably to reduce the probability they will breed.
Keywords: aggression, behavioural plasticity, cooperation, glucocorticoids, sociality, stress
1. Introduction
In mammals that breed cooperatively, where group members raise young produced by dominant breeders, social rank can influence stress-related hormone (glucocorticoid) concentrations of group members [1–3]. In some species, dominant individuals direct aggression towards subordinates, raising their glucocorticoid concentrations (GCs [4–7]). The elevation of GCs in subordinates can suppress their reproductive activity [4–6,8] allowing dominants to monopolize reproductive opportunities [6]. However, such changes in subordinate GCs could also carry additional benefits or costs for dominants by increasing or decreasing subordinate cooperative behaviour, respectively [9–16].
Studies of the effects of GCs on cooperative behaviour in wild animals are rare, but some have identified positive or negative associations between GCs and cooperative behaviour [12–15]. In social mammals including humans, elevated GCs are associated with decreased expression of different social behaviours [17–19] and reductions in the formation of social attachments between parents and offspring [20,21] or opposite-sex conspecifics [22]. The lack of congruency in the direction of relationships between GCs and these different types of social behaviours is not surprising given the observational nature of these previous studies. This is because correlations between GCs and cooperation [12–15] may occur because increased energetic expenditure on cooperative activities raises respective GCs. Experimental studies are, therefore, needed to determine whether changes in GCs cause variation in cooperation.
Fluctuations in GCs can cause changes in behaviour by binding to central and peripheral glucocorticoid (GRs) or mineralocorticoid (MRs) receptors, subsequently altering the activity of those receptors that in turn regulate gene transcription, or through non-genomic effects that do not involve GRs or MRs [23,24]. However, there is at least some evidence that the effects of GCs on social behaviours are mediated by changes in the activity of GRs. For instance, mifepristone is a selective GR antagonist that can lower the expression of behaviours influenced by GCs such as reducing the expression of anti-social behaviours in humans (social withdrawal behaviour) that are associated with major depression, anxiety or post-traumatic stress [25]. How exactly changes in GCs or their genomic (binding to MRs or GRs) or non-genomic consequences affect behaviour is an active area of research. Previous studies suggest the possibility that increased GCs can reduce social behaviour by affecting the activity of neural circuits in the mesolimbic pathway such as promoting fear of conspecifics [26] or altering the reward value of social interactions [27,28]. Regardless of the mechanism, a growing number of studies suggest that changes in GCs can alter the expression of a variety of social behaviours.
Although there is evidence that aggression directed at subordinates can increase their GCs and reduce the probability that they will breed, how changes in subordinate GCs affect their cooperative behaviour has rarely been investigated. Here, we describe the results of manipulations of circulating GC concentrations and GR activity in wild meerkats to determine if they cause changes in alloparental care behaviours (babysitting and pup-feeding) as well as analyses of the distribution of aggression directed by dominant females at subordinates. We provisioned subordinates of both sexes with either exogenous GCs (cortisol), a GR antagonist (mifepristone) or an oil vehicle (controls) for 10 consecutive days during either babysitting or pup-feeding periods. The goal of these treatments was to produce a range of variation in GR activity with those treated with cortisol predicted to have the highest, those treated with mifepristone having the lowest (because mifepristone antagonizes the GR), and the controls in between the cortisol and mifepristone treatments. We confirmed that mifepristone was in fact antagonizing the GR (see electronic supplementary material, figure S1). To investigate whether variation in rates of aggression directed by dominant females at subordinates were related to their need for increased assistance, we used 19 years of long-term behavioural data.
Meerkats are obligate cooperative breeders that live in social groups containing 2–49 individuals [29], many of which are close relatives [30]. Each group contains a monogamous breeding pair that is socially dominant to all same-sex subordinates in their group [31–35] and sire approximately 90% of all offspring produced within the group [31,32]. Subordinate females but not males are known to be infanticidal especially if the former are pregnant [36,37]. Probably as a consequence, dominant females commonly evict older subordinate females from their group before they (the dominants) give birth [6,36], though they do not evict males. Aggression from dominant females can alter GCs in subordinate females, and subordinates (both females and males) that receive more aggression from the dominant female tend to have higher plasma GCs [7]. Moreover, dominant females are more aggressive towards subordinate females when they are pregnant [6,38] and, subordinate females (regardless of their pregnancy status) have higher GCs when the dominant female is pregnant while males show no similar increase in GCs [7].
Subordinates of both sexes provide alloparental care to offspring produced by dominants [33,34,39]. During their first month of life, meerkat pups stay at their natal burrow with an older subordinate (‘babysitter’ [34]) while the rest of the social group goes foraging away from the burrow for the entire day. From 1 to 3 months of age while the pups are foraging with the group, they are provisioned with food items that are found by older subordinates and dominant breeders in their social group [40]. After around 3 months, meerkats obtain most of their food by foraging independently [41].
Previous correlative studies of the relationship between GCs and cooperative behaviour in subordinate male meerkats showed that elevated GCs were positively related to some forms of cooperative behaviour and negatively with others. Specifically, they showed that increased GCs in males were positively associated with pup-feeding [12] but negatively associated with babysitting [15]. Our initial expectation was consequently that subordinates of both sexes treated with cortisol (presumably those with the highest GR activity) would have the lowest levels of babysitting, but the highest levels of pup-feeding. Secondly, we predicted that subordinates of both sexes treated with mifepristone (presumably those with the lowest GR activity) would have the highest levels of babysitting [15], but lowest levels of pup-feeding [12].
2. Material and methods
(a). Documenting characteristics of social groups
We studied habituated meerkats at the Kuruman River Reserve (26°58′ S, 21°49′ E) in the Northern Cape, South Africa from 1997 to 2016. Individuals were uniquely and permanently marked with microchips (Identipet®, Johannesburg, South Africa) and small dye marks so that they could be identified visually. Groups were visited for 4–8 h per day once every 2–3 days throughout the year to collect ad libitum or focal behavioural observations during which we recorded the identity of all meerkats present in the group to quantify the group size and the sex ratio of the group. We tracked the identity of all individuals and determined the dominant female and male in each group (via behavioural observations, see electronic supplementary material). Pregnancy status of all females was determined by noting steady mass gain and visible swelling of the abdomen and nipples. Parturition was identified by changes in the appearance of females, dramatic overnight mass loss and the presence of subordinate individuals babysitting [42] at the sleeping burrow while the rest of the group went foraging. Presence of pups <90 days of age was determined either by direct observation or by the presence of babysitters at the burrow.
(b). Manipulating subordinate glucocorticoid (cortisol) concentrations
We provisioned subordinate meerkats with either cortisol (10 mg kg−1, hydrocortisone, Sigma H4126), mifepristone (40 mg kg−1, Sigma M8046) or oil vehicle (100% coconut oil). Dosages were chosen based on previous studies and our own pilot experiments (see electronic supplementary material). We confirmed that our cortisol treatments significantly elevated plasma GCs (cortisol treatment: electronic supplementary material, figure S2) and faecal glucocorticoid metabolites (electronic supplementary material, figure S3) within a biologically relevant range and mifepristone altered the activity of the GR (electronic supplementary material, figures S1 and S4), and that these treatments would influence the behaviour of meerkats (electronic supplementary material, figures S1 and S4) without causing abnormal behavioural changes (electronic supplementary material, figure S5). Treatments (cortisol, mifepristone, control) were randomly assigned to three adult subordinates (>12 months of age) within the same group that were of the same sex and of the same or similar age. When these conditions could not be met, we used another adult subordinate of the opposite sex but of a similar age as a subject. None of the subordinate females were pregnant during treatment (pregnancy status determined as described above). Subjects were first treated continuously for a 10-day period immediately following the birth of the litter (babysitting period) and then again for a second 10-day period (with a different treatment) during the peak pup-feeding period ([40], mean age of pups during treatment = 45.3 d, range = 39–60 d). Subjects who consumed less than 50% of their treatments (n = 4) were excluded from our analyses about the treatment effects on babysitting. However, in our analyses on pup-feeding, we included a covariate for whether the focal observations occurred on the same day of provisioning with the treatment or not (all individuals had consumed their treatment that day, see Statistical analyses).
(c). Body mass and foraging success
We measured subordinate body mass and foraging success to assess their effects on subordinate cooperative behaviour and to assess the influence of our treatments on foraging success (see electronic supplementary material). Meerkats were weighed (to the nearest gram) in the morning immediately after emergence from their sleeping burrow, but before foraging had commenced, after 1–4 h of foraging, and again in the evening after foraging was completed but before they entered their sleeping burrow [43,44]. Birth dates of meerkats were known so we calculated age-corrected body mass as a measure of body condition. We controlled for short-term fluctuations in body mass by averaging the morning body mass of individuals over the 30 days prior to capture or behavioural observations and then used the residuals from a general linear model (response variable was average morning body mass, predictor variable was age in days) to estimate age-corrected body mass. We estimated daily foraging success as the evening body mass minus the morning body mass within the same day and took the average daily foraging success over the previous 30 days prior to capture. We estimated mass-corrected foraging success as the residuals from a general linear model (the response variable was daily foraging success; the predictor variable was morning body mass).
(d). Quantifying aggression that subordinates received from dominants
We used ad libitum sampling [45] to quantify how much aggression subordinates received from dominants. During each visit to the group, we recorded all dominance assertions exhibited by dominant females towards subordinates. Dominance assertions that were collected include aggressive behaviours or signs of dominance exhibited by the dominant female towards subordinates such as charging, chasing or hitting them (see [38,46]). The total number of dominance assertions was summed for each day of behavioural observations for each individual subordinate and the total amount of time of the session was recorded. In total, we examined the distribution of variation in rates of aggression directed by dominant females at subordinate group members using greater than 110 483 h of behavioural data collected over 19 years that provided us with rates of aggression by 98 dominant females towards 1520 subordinates (713 females, 807 males) in 40 different groups. From this same dataset, we also had observations on all the babysitting and pup-feeding contributions of subordinates for each litter that survived to emergence from the natal burrow.
(e). Measuring babysitting behaviour
We measured the relative contributions of each subordinate male or female to babysitting, as we have done previously [34,39,47]. When pups were born but not yet foraging with the group, we visited the natal burrow every day in the morning to record the identity of the babysitter [34,47]. Relative babysitting contributions of each individual for each litter was estimated by dividing the total number of days an individual babysat a litter over the total number of days that this specific litter had a babysitter. If a babysitter was replaced during morning observations, they were both assigned 0.5 d of babysitting. For the subordinates receiving treatments, we measured relative babysitting contributions of the three treated subordinates only during the treatment period. For the long-term correlative data analyses, the proportion of babysitting for a litter relative to all other individuals was used.
(f). Measuring pup-feeding behaviour
Pup-feeding in meerkats is a highly conspicuous event as pups emit distinctive begging vocalizations and subordinates engage in obvious behaviours where they bring prey items to pups [41]. To assess how our experimental treatments affected pup-feeding behaviour in treated subordinate meerkats, we conducted 20 min continuous behavioural focal observation sessions on treated subordinates. Focal observations (focals) began in the morning after the group had left their sleeping burrow and were foraging for at least 10–15 min. Focals were alternated among the treated subjects (first subject focalled was randomly selected) and focals on the same individual were not consecutive and were separated by 20–30 min. During each focal, we recorded all food items found, their size (tiny, small, medium, large, extra-large [48]), and the number and size of prey items that were found during the focal and fed to pups. Prey biomass was estimated as previously [48]. This allowed us to assess the effects of our experimental treatments on (1) whether the subordinates exhibited pup-feeding at all during the focal session (probability of pup-feeding) and (2) the total proportion of prey biomass that was found during the focal session that was fed to pups that were foraging with the group (generosity). We analysed the effects of our treatments on both of these behaviours because they are different measures where the probability of pub-feeding reflects motivation to exhibit this type of alloparental care and generosity controls for its condition-dependence [33].
Pup-feeding contributions for each subordinate in our long-term data analyses were recorded using ad libitum sampling when there were pups (up to 90 d of age) foraging with the group. In these ad libitium observations, we did not record how much food each subordinate found, so we used the probability of pup-feeding as a response variable.
(g). Statistical analyses
We used an information-theoretic approach (Akaike information criterion corrected for small sample sizes, AICc [49]) to examine the degree of support among different models that contained a range of covariates (described in electronic supplementary material tables S1–S6). Our approach was to develop a list of candidate models, select the model containing biological predictor variables with the lowest AICc and then evaluate the significance of each of the predictor variables using traditional null hypothesis significance testing. Further details of model selection procedures, use of null models and additional model averaging results are shown in the electronic supplementary material.
We first used generalized linear mixed-effects models (GLMM) with binomial or Poisson errors to examine whether our experimental treatments affected babysitting, the probability that pup-feeding occurred during our focal observations, and the proportion of biomass that was found by subordinates and subsequently fed to pups foraging with the group. We included random intercept terms for individual and litter identity as well as observer in both the pup-feeding models and a random intercept term for litter identity in the babysitting models because of repeated observations. The babysitting model included an observation level random effect due to some evidence of overdispersion. Our model selection procedures revealed that the best model (with lowest AICc shown in electronic supplementary material, tables S1–S3) for babysitting and pup-feeding was the null model that did not contain any biological predictor variables. Because we were interested in the effects of the experimental treatments on babysitting and pup-feeding, we only report results from models (electronic supplementary material, tables S1–S3) containing biological predictor variables with the lowest AICc (model in bold face font in electronic supplementary material, tables S1–S3) in the main text and use these results for our interpretations. Because a second model containing biological predictor variables (i.e. not the null model) was within ΔAICc < 2 (in electronic supplementary material, tables S1–S3), we also include results from model averaging [50] from these top models in electronic supplementary material, tables S10–S12 (using the zero method: [51]). However, our interpretations are focused on the one model with the lowest AICc containing biological predictor variables.
We next examined potential causes of variation in the amount of aggression by dominant females directed at subordinate meerkats (all models in electronic supplementary material, table S4, model with lowest AICc in electronic supplementary material, table S7). Aggression from the dominant female directed at subordinates was infrequently observed so we conducted a GLMM with a Poisson error structure that contained random intercept terms for individual, group, identity of the dominant female and year. Because of variation in the amount of time dominant females were observed, we included an offset for the total time (ln transformed) each meerkat group was observed.
Finally, we examined associations between the amount of aggression subordinates received from dominant females and their (1) babysitting contributions (electronic supplementary material, table S5) and (2) the probability that pup-feeding occurred during our ad libitum behavioural observations (electronic supplementary material, table S6) using a correlative approach. Models assessing how aggression received from the dominant female affected subordinate babysitting contributions (electronic supplementary material, table S5), or the probability of pup-feeding (electronic supplementary material, table S6), are in the electronic supplementary material and we only present models with the lowest AICc (electronic supplementary material, tables S8–S9). The response variable in these models was either the relative proportion of babysitting contributions provided by that individual or a binary response variable to indicate whether pup-feeding did or did not occur in the behavioural observation sessions. All these models were GLMMs (binomial error structure) that contained random intercept terms for individual, identity of the litter and dominant female, and year because of repeated samples on the same individuals, litters, dominant females exhibiting the aggression or within different years. The pup-feeding model included an offset for time we observed the group.
All analyses were conducted in R (v. 3.21: [52]). AICc was calculated using maximum-likelihood (R package MuMin, v. 1.15–1 [50]). We used a graphical approach to confirm the normality and homoscedasticity of the residuals from the models. All continuous variables were standardized (mean of 0, s.d. of 1). All GLMMs were run with the package lme4 (v. 1.1–12: [53]) in R. There was no or limited evidence of overdispersion in our GLMMs (dispersion parameters ranged from 0.7 to 1.08). P-values were generated using the R package lmerTest (v. 2.0–3: [54]). There was little evidence of strong collinearity among the covariates (variance inflation factors were consistently less than 2 [55]) except for terms where we included both a linear- and second-order term in the model or for covariates that were included in an interaction term with others. Unless otherwise indicated, we present mean ± s.d. in the following section.
3. Results
(a). Effects of treatments on cooperative behaviour
Contrary to our prediction, treating subordinates with cortisol (n = 9), which should increase GR activity, did not affect their contributions to babysitting relative to those of controls in either sex (n = 8, z = −0.2, p = 0.84, figure 1a). However, as predicted, mifepristone treatment (which should decrease GR activity), caused an increase in babysitting in both sexes: subordinate females and males treated with mifepristone (n = 9) provided 64% more babysitting than did those treated with cortisol (z = 2.6, p = 0.009) and 55% more than the controls (z = 2.34, p = 0.019, figure 1a). Model selection indicated that there was no evidence that the effects of the treatments on babysitting were sex-specific (electronic supplementary material, table S1).
Figure 1.
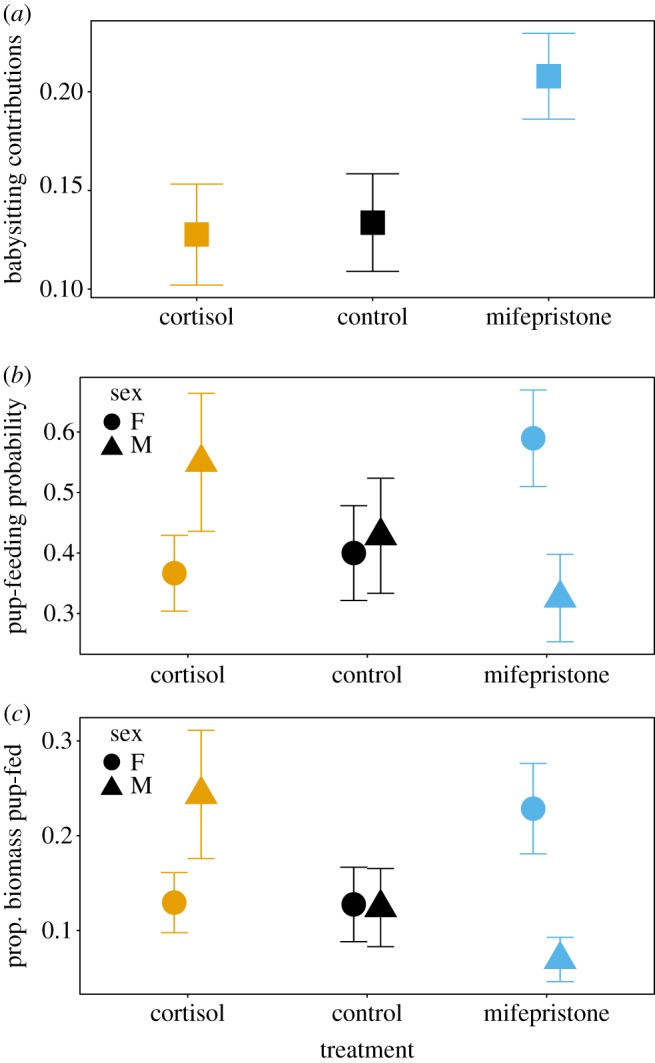
(a) Subordinate female and male meerkats fed mifepristone (n = 9) exhibited significantly more babysitting (proportion of total babysitting provided by subordinate) than did those fed cortisol (n = 9) or the controls (n = 8). (b,c) Unlike babysitting, the effects of the treatments on pup-feeding were sex-specific (electronic supplementary material, tables S2–S3). Subordinate females treated with mifespristone (n = 8) were significantly more likely to feed pups compared to those treated with cortisol (n = 10) but not the controls (n = 7). However, the amount of prey biomass found by subordinate females and fed to pups did not differ among the treatment groups. The probability of pup-feeding by subordinate males was not affected by the treatments but males treated with cortisol (n = 4) fed significantly more of the prey biomass they found to pups compared to those fed mifepristone (n = 7) but not controls (n = 7). (Online version in colour.)
Cortisol and mifepristone treatments affected the likelihood of pup-feeding during our focal behavioural observations (the probability that subordinates would feed pups during 20 min focal observations) though their effects differed between the sexes (electronic supplementary material, table S2). Contrary to our initial predictions, subordinate females treated with cortisol were significantly less likely to exhibit pup-feeding (1200 min observation on 10 females) compared to those treated with mifepristone (780 min observation on eight females, z = 2.02, p = 0.044), but did not differ from control females (800 min observation on seven females, z = 0.26, p = 0.79, figure 1b). Subordinate males treated with cortisol were not more likely to feed food items they found to pups (400 min observation on four males) compared to males treated with mifepristone (860 min observation on seven males, z = −1.59, p = 0.11) and also did not differ from the controls (560 min observation on seven males, z = −1.01, p = 0.31, figure 1b). Females and males treated with mifepristone were equally likely to exhibit pup-feeding during the focals compared to the controls (females: z = 0.48, p = 0.63; males: z = −1.63, p = 0.1, figure 1b).
The effects of cortisol and mifepristone on a subordinate's generosity towards pups (the proportion of total food biomass found that they then fed to pups) also differed between the sexes (electronic supplementary material, table S3). Subordinate females treated with cortisol were not more generous compared to females treated with mifepristone (z = −0.97, p = 0.33) or compared with the controls (z = −0.83, p = 0.41, figure 1c). However, subordinate males treated with cortisol were more generous compared to those treated with mifepristone (z = −2.41, p = 0.016), but did not differ from the controls (z = −1.66, p = 0.096, figure 1c). Females and males treated with mifepristone and the controls did not differ in their generosity (females: z = −0.13, p = 0.89; males: z = 0.92, p = 0.36, figure 1c).
(b). The distribution and effects of aggression from dominant females
Our experimental results showed that mifepristone (which should decrease GR activity) elevated babysitting (figure 1a). If dominant females adjust the frequency with which they direct aggression at subordinates to maximize the contributions of subordinates to babysitting, they would be expected to reduce the amount of aggression directed at subordinates during babysitting to lower their GCs and consequently their GR activity. Our long-term data analyses showed that dominant females directed significantly less aggression at subordinate females during babysitting compared to times when dominant females were pregnant and there were no pups in the group (z = −3.06, p = 0.0022). However, rates of aggression directed at subordinate males did not vary in the same way (z = 0.18, p = 0.86, electronic supplementary material, table S7, figure 2), suggesting that that this difference was not the result of an attempt by dominant females to increase contributions to subordinates to babysitting. In addition, if aggression is used by dominants to stimulate babysitting in subordinates, dominant females might also be expected to reduce rates of aggression directed at individuals that were infrequent babysitters to a greater extent than those directed at more frequent babysitters. However, the amount of aggression subordinates received from dominant females was also not associated with their contributions to babysitting in either sex (females: z = −0.48, p = 0.63; males: z = −1.32, p = 0.19, electronic supplementary material, table S8).
Figure 2.
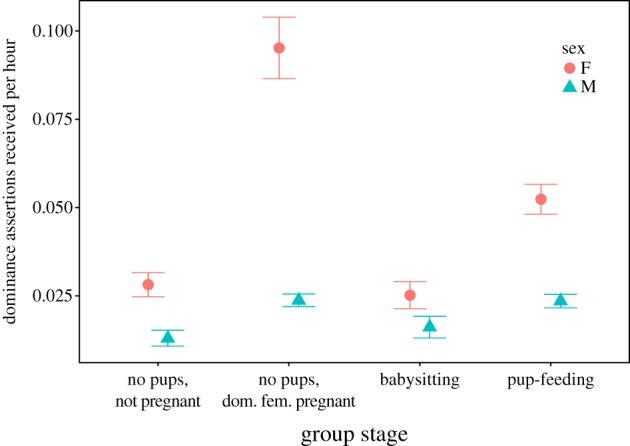
Behavioural data collected over 19 years (greater than 110 483 h) indicated that the amount of aggression subordinate female meerkats (n = 713) received from the dominant female (n = 98 females from 40 groups) varied according to whether the dominant female was pregnant and whether there were offspring in the group that subordinates were taking care of (babysitting and pup-feeding, electronic supplementary material, table S7). Subordinate females received the highest levels of aggression from the dominant female when she was pregnant and there were no pups in the group. The amount of aggression directed at subordinate males (n = 807) was not significantly influenced by the pregnancy status of the dominant female or whether there were offspring in the group being babysat or fed. (Online version in colour.)
There was also no indication that dominant females adjusted their aggressive behaviour to stimulate pup-feeding by subordinates. Because our experimental results showed that mifepristone treatment increased pup-feeding frequency in females (figure 1b) but decreased pup-feeding generosity in males compared to those treated with cortisol (figure 1c), dominant females that were using aggression to stimulate pup-feeding by subordinates should then have reduced the amount of aggression directed at subordinate females during pup-feeding but increased aggression directed at subordinate males. Although the amount of aggression dominant females directed at subordinate females was lower during pup-feeding compared to when the dominant female was pregnant with no pups in the group (z = −2.01, p = 0.044), it tended to be higher during pup-feeding compared to when subordinate females were babysitting (females: z = −1.84, p = 0.066, electronic supplementary material, table S7, figure 2). Furthermore, during pup-feeding the amount of aggression dominant females directed at subordinate males was not higher compared to periods when they were in groups where the dominant female was pregnant with no pups in the group (z = 0.5, p = 0.61) or when they were babysitting (z = 0.11, p = 0.91, electronic supplementary material, table S7, figure 2). Finally, the amount of aggression received from dominant females by subordinates was not associated with the probability of pup-feeding in either sex (females: z = −0.26, p = 0.8; males: z = 1.53, p = 0.12, electronic supplementary material, table S9).
An alternative interpretation of the distribution of aggression is that the frequency of aggression directed at subordinates by dominant females is adjusted principally to control the frequency that subordinates will attempt to breed and the risk of infanticide [6] and our analyses of the distribution of aggression is consistent with this. Subordinate females received significantly more aggression from the dominant female (z = −3.7, p = 0.0002, electronic supplementary material, table S7) when the dominant female was pregnant compared to when she was not pregnant with no pups in the group (figure 2). No similar changes for aggression received occurred in males (z = 1.52, p = 0.13, electronic supplementary material, table S7, figure 2) supporting the suggestion that the increase in aggression directed at subordinate females was associated with reproductive suppression. In addition, older and heavier subordinate females (age: z = 19.2, p < 0.0001; mass: z = 9.5, p < 0.0001, electronic supplementary material, table S7) and males (age: z = 6.71, p < 0.0001; mass: z = 5.03, p < 0.0001, electronic supplementary material, table S7) received more aggression from dominant females than those that were younger or lighter and the effect of age (sex × age, z = −8.9, p < 0.0001) and body mass (sex × body mass, z = 1.9, p = 0.055, electronic supplementary material, table S7) on aggression received was more pronounced for females than males.
4. Discussion
Our experimental results show that variation in GCs and likely GR activity can influence how much alloparental behaviour subordinates exhibit. Surprisingly, these treatment effects differed both between the sexes and between different forms of cooperative behaviour. Mifepristone (which should decrease GR activity) increased babysitting in subordinates of both sexes, but only elevated pup-feeding behaviour (probability of occurring) in females compared to those treated with cortisol (which should elevate GR activity). Cortisol decreased pup-feeding (probability of occurring) in females but it enhanced pup-feeding behaviour (generosity) in males. Overall, this suggests that elevated GCs and perhaps increased GR activity reduces alloparental care in meerkats except in the case of pup-feeding by subordinate males.
Our results suggest that GCs and the activity of the GR may act as a general mechanism mediating behavioural plasticity in cooperative behaviour across cooperatively breeding species. Factors such as poor body condition [56], harsh environmental conditions [57] or low levels of relatedness [58,59] are associated with decreases in the expression of cooperative behaviour presumably because under such conditions the ratio of costs to benefits of cooperation is increased. Similarly, GCs are highly responsive to fluctuations in the same intrinsic (body condition) and extrinsic (weather, group size) factors [7,33,34,47,59]. Increases in GCs from such intrinsic or extrinsic factors that affect GR activity (or MR activity or have non-genomic effects) could in part trigger these decreases in cooperative behaviour.
We found no evidence to support the hypothesis that dominant females use aggression to elevate the cooperative behaviour of subordinates. The amount of aggression subordinates of both sexes received from the dominant female was not associated with their contributions to babysitting or pup-feeding in our analyses of long-term behavioural data. This matches a previous study in meerkats showing that dominant females do not increase the amount of aggression they exhibit towards subordinates when the need for their help was experimentally increased [60].
Instead of using aggression to stimulate cooperative behaviour, our results are aligned with those of a previous study in meerkats [6] suggesting that dominant females use aggression to control reproduction in subordinate females. Subordinate females, but not males, are often infanticidal and pregnant subordinates are more likely to commit infanticide compared to when they are not pregnant [6,36,37]. Subordinate females but not males received more aggression from the dominant female (this study) and had higher plasma GCs [7] when the dominant female was pregnant. Increased GCs in subordinate females may reduce the probability of them committing infanticide by suppressing their own reproduction or causing abortions [6]. Consequently, antagonistic interactions of social subordinates with dominant breeders and the consequent changes in their GCs likely play a role in the reproductive suppression of same-sex subordinates.
Our results suggest that the effects of GCs and perhaps GR activity on cooperative behaviour have different consequences in males and females. Previous studies looking at alloparental care in meerkats [12,15], African striped mice (Rhabdomys pumilio [13]) and the formation of pair-bonds in prairie voles (Microtus ochrogaster [22]) indicate that in males of these species, increased GCs are associated with increased cooperative behaviour (but see [14]). We found that male meerkats treated with cortisol (presumably resulting in higher GR activity) were more generous when pup-feeding. This result is similar to a previous observational study in male meerkats [12] but differs from a previous short-term study in meerkats that also experimentally increased GCs (using injections of exogenous GCs) and observed no substantial changes in pup-feeding behaviour [61]. This difference may be due to the increases in plasma GCs in the previous study that were higher than the increases in subordinate plasma GCs we induced in this study (electronic supplementary material, figure S2) or because this previous study used manipulations that operated over a shorter timescale.
By contrast, studies of females of the same species as those described above suggest a negative association between GCs and perhaps GR activity and cooperative or social behaviour. Increases in GCs in female prairie voles [22] and African striped mice [13] are associated with reduced cooperative behaviour, suggesting that reduced GR activity elevates cooperation in females. We also found that subordinate female meerkats treated with mifepristone (presumably with lower GR activity) exhibited more babysitting and pup-feeding behaviour. These studies indicate that, during the co-evolution of hormones and social or cooperative behaviours, selection has often favoured sex-specific effects, raising important questions about the function and evolution of these differences that have yet to be investigated. One plausible explanation is that the fitness benefits of alloparental behaviour under stressful environments differ between the sexes. This can occur if there is sex-biased dispersal from the natal group such as in meerkats where there is male-biased dispersal [62] and the average relatedness between subordinate females and dominant females (and consequently the offspring produced in the group) is greater than in males who immigrate into the group [63]. The indirect fitness benefits of staying and helping within the natal group for the sex that is more closely related to the dominant breeders may be greater than emigrating, especially when that sex also queues for the dominant breeding position. We predict that under these conditions, this sex should exhibit higher cooperative behaviour when GCs are elevated. However, our results reject this hypothesis because we did not find that subordinate females exhibited higher cooperative behaviour when their GCs were increased.
There are numerous possible mechanisms by which changes in GCs or GR activity can alter behaviour in a sex-specific fashion given the well-documented sex differences in the functioning of the vertebrate neuroendocrine stress axis [64,65]. One hypothesis that could explain our results is that there are sex differences in the central distribution of GRs or receptors for other components involved the neuroendocrine stress axis [66] that in turn modify how GCs alter cooperative behaviour. For example, increased production of corticotropin-releasing factor (CRF) in the mesolimbic system due to elevated GCs could promote fear and anxiety of conspecifics thereby reducing social behaviour [26]. CRF receptors in females may be hypersensitive compared to males [65] such that there is a lower threshold in females than males at which GCs cause a reduction in social behaviour. There are many other possibilities to explain our results given the range of potential mechanisms that contribute to sex differences in the neuroendocrine stress axis [64,65]. Clearly, the mechanisms underlying these sex differences are an area that deserves much more study. Future field-based studies can contribute to this area by examining whether changes in social or environmental factors (group size, weather patterns, food availability) or individual-state (body mass) cause sex-specific changes in the expression of cooperative behaviour.
Our results have general implications for our understanding of both the causes of variation in the expression of cooperative behaviour in cooperatively breeding species and of the possible mechanisms underlying variation in social behaviour across taxa. First, our results reject the hypothesis that dominant breeders in cooperatively breeding species could manipulate the cooperative behaviour of subordinates by strategically increasing or decreasing their GCs and/or affect their GR activity. In both meerkats [60] and in other cooperatively breeding species [16], there is little evidence that dominant breeders use punishment or changes in GCs to alter the cooperative behaviour of subordinates.
Our research adds to the growing number of studies showing that changes in GCs or GR activity can alter the expression of different types of social behaviour ranging from social attachments formed between two opposite-sex individuals [22] or parents and offspring [20,21], anti-social behaviour in humans [17–19] or, in our study and others [12–15], alloparental behaviour. They provide new research avenues by showing that the effects of changes in GCs or GR activity on cooperative behaviour differ not only between the sexes but also between two different types of cooperative behaviour. Why they should vary in this way is still unknown, nor is it clear whether similar differences in the effects of GCs and GR activity occur in other cooperative breeders.
Supplementary Material
Acknowledgements
We thank P. Roth, J. Samson, N. Thavarajah, L. Howell, S. Bischoff-Mattson and staff at the Kalahari Meerkat Project for helping to organize the project, and numerous volunteers who contributed to data collection. Thanks to J. C. Beehner, P. Vullioud and two anonymous reviewers for helpful comments. We thank the Northern Cape Conservation Authority for permission to conduct research and the Kotze family for permission to study meerkats on their property.
Ethics
All protocols used in our experiments were approved by the Animal Ethics Committee at the University of Pretoria (Pretoria, South Africa) and the Northern Cape Conservation Authority (South Africa).
Data accessibility
All data are archived in Dryad: http://dx.doi.org/10.5061/dryad.rb0p7 [67]. All code for statistical analyses is available from B.D.
Authors' contributions
B.D. designed experiments; T.H.C.-B., M.B.M., D.G., B.D. and C.D. coordinated long-term data collection; B.D. T.H.C.-B, M.B.M., D.G., I.B.G., N.B., M.H., A.G., C.D. and H.C.S.-J collected data; B.D. conducted analyses and produced figures; B.D. and T.H.C.-B authored the manuscript with contributions from M.B.M., D.G., I.B.G., N.B., M.H., A.G and C.D.
Competing interests
We have no competing interests.
Funding
This research was supported by grants from the National Environment Research Council (RG53472) and the European Research Council (294494) to T.H.C.-B. I.B.G. and M.B.M. were funded by the Swiss National Science Foundation (grant no. 31003A_13676). The Kalahari Meerkat Project has also been financed by the University of Cambridge (T.H.C.-B.) and University of Zurich (M.B.M.).
References
- 1.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497. ( 10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 2.Creel S, Dantzer B, Goymann W, Rubenstein DR. 2013. The ecology of stress: effects of the social environment. Funct. Ecol. 27, 66–80. ( 10.1111/j.1365-2435.2012.02029.x) [DOI] [Google Scholar]
- 3.Goymann W, Wingfield JC. 2004. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 67, 591–602. ( 10.1016/j.anbehav.2003.08.007) [DOI] [Google Scholar]
- 4.Abbott DH, et al. 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 43, 67–82. ( 10.1016/S0018-506X(02)00037-5) [DOI] [PubMed] [Google Scholar]
- 5.Hackländer K, Möstl E, Arnold W. 2003. Reproductive suppression in female alpine marmots, Marmota marmot. Anim. Behav. 65, 1133–1140. ( 10.1006/anbe.2003.2159) [DOI] [Google Scholar]
- 6.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. 2006. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad Sci. USA 103, 12 005–12 010. ( 10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantzer B, Bennett NC, Clutton-Brock T. 2017. Social conflict and costs of cooperation in meerkats are reflected in measures of stress hormones. Behav. Ecol. 28, 1131–1141. ( 10.1093/beheco/arx077) [DOI] [Google Scholar]
- 8.Sanderson JL, Nichols HJ, Marshall HH, Vitikainen EIK, Thompson FJ, Walker SL, Cant MA, Young AJ. 2015. Elevated glucocorticoid concentrations during gestation predict reduced reproductive success in subordinate female banded mongooses. Biol. Lett. 11, 20150620 ( 10.1098/rsbl.2015.0620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeve HK, Sherman PW. 1991. Intra-colonial aggression and nepotism by the breeding female naked mole-rat. In The biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD), pp. 337–357. Princeton, NJ: Princeton University Press. [Google Scholar]
- 10.Mulder RA, Langmore NE. 1993. Dominant males punish helpers for temporary defection in superb fairy-wrens. Anim. Behav. 45, 830–833. ( 10.1006/anbe.1993.1100) [DOI] [Google Scholar]
- 11.Balshine-Earn S, Neat FC, Reid H, Taborsky M. 1998. Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 9, 432–438. ( 10.1093/beheco/9.5.432) [DOI] [Google Scholar]
- 12.Carlson AA, Manser MB, Young AJ, Russell AF, Jordan NR, McNeilly AS, Clutton-Brock T. 2006. Cortisol levels are positively associated with pup-feeding rates in male meerkats. Proc. R. Soc. B 273, 571–577. ( 10.1098/rspb.2005.3087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raynaud J, Schradin C. 2015. Corticosterone levels correlate with alloparental care in a sex-dependent manner in African striped mice, Rhabdomys pumilio. Ethology 121, 57–67. ( 10.1111/eth.12317) [DOI] [Google Scholar]
- 14.Sanderson JL, Young AJ, Hodge SJ, Kyabulima S, Walker SL, Cant MA. 2014. Hormonal mediation of a carry-over effect in a wild cooperative mammal. Func. Ecol. 28, 1377–1386. ( 10.1111/1365-2435.12307) [DOI] [Google Scholar]
- 15.Carlson AA, Russell AF, Young AJ, Jordan NR, McNeilly AS, Parlow AF, Clutton-Brock T. 2006. Elevated prolactin levels immediately precede decisions to babysit by male meerkat helpers. Horm. Behav. 50, 94–100. ( 10.1016/j.yhbeh.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 16.Riehl C, Frederickson ME. 2016. Cheating and punishment in cooperative animal societies. Phil Trans. R. Soc. B 371, 20150090 ( 10.1098/rstb.2015.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kathol RG, Anton R, Noyes R, Gehris T. 1989. Direct comparison of urinary free cortisol excretion in patients with depression and panic disorder. Biol. Psych. 25, 873–878. ( 10.1016/0006-3223(89)90267-9) [DOI] [PubMed] [Google Scholar]
- 18.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. 2005. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 29, 3–38. ( 10.1016/j.neubiorev.2004.08.009) [DOI] [PubMed] [Google Scholar]
- 19.van Goozen SH, Fairchild G. 2006. Neuroendocrine and neurotransmitter correlates in children with antisocial behaviour. Horm. Behav. 50, 647–654. ( 10.1016/j.yhbeh.2006.06.021) [DOI] [PubMed] [Google Scholar]
- 20.Bardi M, French JA, Ramirez SM, Brent L. 2004. The role of the endocrine system in baboon maternal behaviour. Biol. Psych. 55, 724–732. ( 10.1016/j.biopsych.2004.01.002) [DOI] [PubMed] [Google Scholar]
- 21.Saltzman W, Abbott DH. 2009. Effects of elevated circulating cortisol concentrations on maternal behaviour in common marmoset monkeys (Callithrix jacchus). Psychoneuroendocrinology. 34, 1222–1234. ( 10.1016/j.psyneuen.2009.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeVries AC, DeVries MB, Taymans SE, Carter CS. 1996. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc. Natl Acad Sci. USA 93, 11 980–11 984. ( 10.1073/pnas.93.21.11980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller J, Mikics E, Makara GB. 2008. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central nervous system. A critical evaluation of findings. Front Neuroendocrinol. 29, 273–291. ( 10.1016/j.yfrne.2007.10.004) [DOI] [PubMed] [Google Scholar]
- 24.Kumar R, Thompson EB. 2005. Gene regulation by the glucocorticoid receptor: structure: function relationship. J. Steroid Biochem. Mol. Biol. 94, 383–394. ( 10.1016/j.jsbmb.2004.12.046) [DOI] [PubMed] [Google Scholar]
- 25.DeBattista C, Belanoff J. 2006. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol. Metab. 17, 117–121. ( 10.1016/j.tem.2006.02.006) [DOI] [PubMed] [Google Scholar]
- 26.Schulkin J, Morgan MA, Rosen JB. 2005. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 28, 629–635. ( 10.1016/j.tins.2005.09.009) [DOI] [PubMed] [Google Scholar]
- 27.Curtis JT, Liu Y, Aragona BJ, Wang Z. 2006. Dopamine and monogamy. Brain Res. 1126, 76–90. ( 10.1016/j.brainres.2006.07.126) [DOI] [PubMed] [Google Scholar]
- 28.Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. 2012. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490, 402–406. ( 10.1038/nature11436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bateman AW, Ozgul A, Nielsen JF, Coulson T, Clutton-Brock TH. 2013. Social structure mediates environmental effects on group size in an obligate cooperative breeder, Suricata suricatta. Ecology 94, 587–597. ( 10.1890/11-2122.1) [DOI] [PubMed] [Google Scholar]
- 30.Nielsen JF, et al. 2012. Inbreeding and inbreeding depression of early life traits in a cooperative mammal. Molec. Ecol. 21, 2788–2804. ( 10.1111/j.1365-294X.2012.05565.x) [DOI] [PubMed] [Google Scholar]
- 31.Hodge SJ, Manica A, Flower TP, Clutton-Brock TH. 2008. Determinants of reproductive success in dominant female meerkats. J. Anim. Ecol. 77, 92–102. ( 10.1111/j.1365-2656.2007.01318.x) [DOI] [PubMed] [Google Scholar]
- 32.Spong GF, Hodge SJ, Young AJ, Clutton-Brock TH. 2008. Factors affecting the reproductive success of dominant male meerkats. Molec. Ecol. 17, 2287–2299. ( 10.1111/j.1365-294X.2008.03734.x) [DOI] [PubMed] [Google Scholar]
- 33.Clutton-Brock TH, Russell AF, Sharpe LL, Young AJ, Balmforth Z, McIlrath GM. 2002. Evolution and development of sex differences in cooperative behavior in meerkats. Science 297, 253–256. ( 10.1126/science.1071412) [DOI] [PubMed] [Google Scholar]
- 34.Clutton-Brock TH, Brotherton PNM, O'Riain MJ, Griffin AS, Gaynor D, Sharpe L, Kansky R, Manser MB, McIlrath GM. 2000. Individual contributions to babysitting in a cooperative mongoose, Suricata suricatta. Proc. R. Soc. Lond. B 267, 301–305. ( 10.1098/rspb.2000.1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clutton-Brock TH, Hodge SJ, Spong G, Russell AF, Jordan NR, Bennett NC, Sharpe LL, Manser MB. 2006. Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068. ( 10.1038/nature05386) [DOI] [PubMed] [Google Scholar]
- 36.Clutton-Brock TH, Brotherton PNM, Smith R, McIlrath GM, Kansky R, Gaynor D, O'Rian MJ, Skinner JD. 1998. Infanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295. ( 10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young AJ, Clutton-Brock T. 2006. Infanticide by subordinates influences reproductive sharing in cooperatively breeding meerkats. Biol. Lett. 22, 385–387. ( 10.1098/rsbl.2006.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutsukake N, Clutton-Brock TH. 2006. Aggression and submission reflect reproductive conflict between females in cooperatively breeding meerkats Suricata suricatta. Behav. Ecol. Sociobiol. 59, 541–548. ( 10.1007/s00265-005-0079-7) [DOI] [Google Scholar]
- 39.English S, Nakagawa S, Clutton-Brock TH. 2010. Consistent individual differences in cooperative behaviour in meerkats (Suricata suricatta). J. Evol. Biol. 23, 1597–1604. ( 10.1111/j.1420-9101.2010.02025.x) [DOI] [PubMed] [Google Scholar]
- 40.Brotherton PNM, Clutton-Brock TH, O'Riain MJ, Gaynor D, Sharpe L, Kansky R, McIlrath GM. 2001. Offspring food allocation by parents and helpers in a cooperative mammal. Behav. Ecol. 12, 590–599. ( 10.1093/beheco/12.5.590) [DOI] [Google Scholar]
- 41.Kunc HP, Madden JR, Manser MB. 2007. Begging signals in a mobile feeding system: the evolution of different call types. Am. Nat. 170, 617–624. ( 10.1086/521233) [DOI] [PubMed] [Google Scholar]
- 42.Clutton-Brock TH, et al. 1998. Costs of cooperative behaviour in suricates (Suricata suricatta). Proc. R. Soc. Lond. B 265, 185–190. ( 10.1098/rspb.1998.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.English S, Bateman AW, Clutton-Brock TH. 2011. Lifetime growth in wild meerkats: incorporating life history and environmental factors into a standard growth model. Oecologia 169, 143–153. ( 10.1007/s00442-011-2192-9) [DOI] [PubMed] [Google Scholar]
- 44.English S, Bateman AW, Mares R, Ozgul A, Clutton-Brock TH. 2014. Maternal, social and abiotic environment effects on growth vary across life stages in a cooperative mammal. J. Anim. Ecol. 83, 332–342. ( 10.1111/1365-2656.12149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altmann J. 1974. Observational study of behaviour: sampling methods. Behaviour 49, 227–265. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 46.Thavarajah NK, Fenkes M, Clutton-Brock TH. 2014. The determinants of dominance relationships among subordinate females in the cooperatively breeding meerkat. Behaviour 151, 89–102. ( 10.1163/1568539X-00003124) [DOI] [Google Scholar]
- 47.Russell AF, Sharpe LL, Brotherton PNM, Clutton-Brock TH. 2003. Cost minimization by helpers in cooperative vertebrates. Proc. Natl Acad. Sci. USA 100, 3333–3338. ( 10.1073/pnas.0636503100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornton A. 2008. Early body condition, time budgets and the acquisition of foraging skills in meerkats. Anim. Behav. 75, 951–962. ( 10.1016/j.anbehav.2007.08.004) [DOI] [Google Scholar]
- 49.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. New York, NY: Springer-Verlag. [Google Scholar]
- 50.Barton K.2015. MuMIn: Multi-model inference. R package version 1.15.1. See http://CRAN.R-project.org/package=MuMIn .
- 51.Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. 2011. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711. ( 10.1111/j.1420-9101.2010.02210.x) [DOI] [PubMed] [Google Scholar]
- 52.R Development Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See www.r-project.org/. [Google Scholar]
- 53.Bates DM, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 54.Kuznetsova A, Brockhoff PB, Christensen RHB.2016. lmerTest: tests in linear mixed effects models. R package version 2.0–30. See http://CRAN.R-project.org/package=lmerTest .
- 55.Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Meth. Ecol. Evol. 1, 3–14. ( 10.1111/j.2041-210X.2009.00001.x) [DOI] [Google Scholar]
- 56.Clutton-Brock TH, O'Riain MJ, Brotherton PNM, Gaynor D, Kansky R, Griffin AS, Manser M. 1999. Selfish sentinels in cooperative mammals. Science 284, 1640–1644. ( 10.1126/science.284.5420.1640) [DOI] [PubMed] [Google Scholar]
- 57.Nichols HJ, Amos W, Bell MBV, Mwanguhya F, Kyabulima Cant MA. 2012. Food availability shapes patterns of helping effort in a cooperative mongoose. Anim. Behav. 83, 1377–1385. ( 10.1016/j.anbehav.2012.03.005) [DOI] [Google Scholar]
- 58.Griffin AS, West SA. 2003. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636. ( 10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- 59.Russell AF, Hatchwell BJ. 2001. Experimental evidence for kin-based helping in a cooperatively breeding vertebrate. Proc. R. Soc. Lond. B 268, 2169–2174. ( 10.1098/rspb.2001.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santema P, Clutton-Brock T. 2012. Dominant female meerkats do not use aggression to elevate work rates of helpers in response to increased brood demand. Anim. Behav. 83, 827–832. ( 10.1016/j.anbehav.2011.12.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santema P, Teitel Z, Manser M, Bennett N, Clutton-Brock T. 2013. Effects of cortisol administration on cooperative behavior in meerkat helpers. Behav. Ecol. 24, 1122–1127. ( 10.1093/beheco/art039) [DOI] [Google Scholar]
- 62.Doolan SP, Macdonald DW. 1996. Dispersal and extra-territorial prospecting by slender-tailed meerkats (Suricata suricatta) in the south-western Kalahari. J. Zool. 240, 59–73. ( 10.1111/j.1469-7998.1996.tb05486.x) [DOI] [Google Scholar]
- 63.Griffin AS, Pemberton JM, Brotherton PNM, McIlrath G, Gaynor D, Kansky R, O'Riain J, Clutton-Brock TH. 2003. A genetic analysis of breeding success in the cooperative meerkat (Suricata suricatta). Behav. Ecol. 14, 472–480. ( 10.1093/beheco/arg040) [DOI] [Google Scholar]
- 64.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. 1994. Gonadal steroid hormone receptors and sex differences in the hypothalmo-pituitary-adrenal axis. Horm. Behav. 28, 464–476. ( 10.1006/hbeh.1994.1044) [DOI] [PubMed] [Google Scholar]
- 65.Bourke CH, Harrell CS, Neigh GN. 2012. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm. Behav. 62, 210–218. ( 10.1016/j.yhbeh.2012.02.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Owen D, Matthews SG. 2003. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinol. 144, 2775–2784. ( 10.1210/en.2002-0145) [DOI] [PubMed] [Google Scholar]
- 67.Dantzer B, et al. 2017. Data from: The influence of stress hormones and aggression on cooperative behaviour in subordinate meerkats Dryad Digital Repository. ( 10.5061/dryad.rb0p7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dantzer B, et al. 2017. Data from: The influence of stress hormones and aggression on cooperative behaviour in subordinate meerkats Dryad Digital Repository. ( 10.5061/dryad.rb0p7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are archived in Dryad: http://dx.doi.org/10.5061/dryad.rb0p7 [67]. All code for statistical analyses is available from B.D.


