Abstract
Studies of the voltage-gated sodium (Nav) channels of extant gnathostomes have made it possible to deduce that ancestral gnathostomes possessed four voltage-gated sodium channel genes derived from a single ancestral chordate gene following two rounds of genome duplication early in vertebrates. We investigated the Nav gene family in two species of lampreys (the Japanese lamprey Lethenteron japonicum and sea lamprey Petromyzon marinus) (jawless vertebrates—agnatha) and compared them with those of basal vertebrates to better understand the origin of Nav genes in vertebrates. We noted six Nav genes in both lamprey species, but orthology with gnathostome (jawed vertebrate) channels was inconclusive. Surprisingly, the Nav2 gene, ubiquitously found in invertebrates and believed to have been lost in vertebrates, is present in lampreys, elephant shark (Callorhinchus milii) and coelacanth (Latimeria chalumnae). Despite repeated duplication of the Nav1 family in vertebrates, Nav2 is only in single copy in those vertebrates in which it is retained, and was independently lost in ray-finned fishes and tetrapods. Of the other five Nav channel genes, most were expressed in brain, one in brain and heart, and one exclusively in skeletal muscle. Invertebrates do not express Nav channel genes in muscle. Thus, early in the vertebrate lineage Nav channels began to diversify and different genes began to express in heart and muscle.
Keywords: voltage-gated sodium channel, lamprey, evolution, agnathans
1. Introduction
Voltage-gated sodium (Na+) channels (Nav) are members of a super-family of four-domain channels that includes voltage-gated calcium (Ca2+) channels (the Cav family) and the NALCN (which stand for Na+ leak channel, non-selective) cation leak channels [1]. The four domains of these channels are arranged around a central pore, and a single amino acid from each of the four domains forms the selectivity filter that determines which ion passes through the channel pore. The Cav family is ancient, evolving in early eukaryotes, and ubiquitous throughout many cell types in most organisms. The NALCN channels, which have secondarily lost voltage-sensitivity, appeared in the opisthokonts. These function as Ca2+ leak channels in single celled eukaryotes [2]; but a branch of this family evolved Na+ permeability in bilateria, hence the channel family's name [1]. Finally, Nav channels evolved from a Cav channel at the origin of the nervous system in metazoans [3]. Nav channels are responsible for the generation and propagation of action potentials in animal nervous systems.
The Nav channel family has two branches: Nav1 and Nav2. Nav2 channels, found in invertebrates including non-vertebrate chordates, are essentially still Ca2+ channels in that they are more permeable to Ca2+ than Na+ [4,5]. Calcium channels have a pore selectivity filter signature of all negatively charged amino acids (typically E/E/E/E). Nav2 family channels have a pore selectivity signature (D/E/E/A) that is intermediate between a calcium channel and a Nav1 channel (D/E/K/A in bilateria, D/K/E/A in cnidaria). The independent evolution of Na+ selectivity in the Nav1 channels of bilateria and cnidaria (conferred by the lysine substitution) and the NALCN channels of bilateria was probably due to strong selective pressures on the early nervous system to use Na+ ions as a charge-carrying ion [6].
Mammals have ten Nav1 channel genes (gene = scnxa; protein = Nav1.x) that are located on four chromosomes, prompting Plummer & Meisler [7] to speculate that an orthologue of the single Nav1 channel then known in tunicates [8] duplicated twice during the two rounds of genome duplication (denoted as ‘2R’) of early vertebrates, as presciently predicted by Ohno [9]. They further suggested that the other Nav channel genes were formed by tandem duplications along some of those chromosomes. Their views have since been confirmed by analysis of sequences from teleosts and tetrapods [10–13]. Comparison of the amphioxus genome to vertebrate genomes suggested that 2R occurred in the stem vertebrate lineage [14], but the timings of 1R and 2R in relation to the divergence of agnathan and gnathostome lineages are not known. Testing the predicted ‘2R’ pattern for various vertebrate gene families has not been straightforward because agnathans may have had their own genome duplication [15].
Nav1 channels are only expressed in the nervous system of non-vertebrate chordates and other invertebrates, whereas they are also expressed in heart and muscle in vertebrates. Nav1 channels have distinctive expression patterns in common in teleosts and tetrapods. For example, Nav1.4 is expressed in muscle [16,17], Nav1.5 in heart [17–19], and Nav1.6 is the major Nav channel in brain and spinal cord [17,20]. However, it is not known exactly when the vertebrate Nav channel genes duplicated or when they began expressing in different tissue types. In order to fully elucidate the history of the Nav channel family in vertebrates, we obtained the Nav channel repertoires of two species of lampreys (the Japanese lamprey Lethenteron japonicum and the sea lamprey Petromyzon marinus), investigated their relationships to gnathostome Nav channels, and assessed their expression in various lamprey tissues.
2. Material and methods
(a). Nav channel gene sequences: Japanese lamprey (Lethenteron japonicum)
The genome assembly of the Japanese lamprey (http://jlampreygenome.imcb.a-star.edu.sg/) was searched for Nav channel genes with TBLASTN algorithm using human Nav channel protein sequences as query. Scaffold sequences that showed similarity to Nav channel sequences were searched against non-redundant protein database at NCBI using BLASTX algorithm to confirm the identity of the sequences. In cases where the coding sequences were incomplete, full-length cDNA sequences were generated by comparing with RNA-seq transcripts and/or by doing RT-PCR and RACE using cDNA from appropriate tissues as template using the protocol previously described [21] (GenBank accession numbers KY682689–KY682694). Altogether six Nav channel genes located on the following scaffolds/contigs were identified: (i) Scaf_45 + contig057381; (ii) Scaf_551; (iii) Scaf_28; (iv) Scaf_1; (v) Scaf_103; and (vi) Scaf_304.
(b). RNA-sequence
Total RNA was isolated from the following tissues of adult Japanese lamprey: heart, kidney, muscle, notochord, ovary and testis, using the TRIzol reagent (Invitrogen, Carlsbad, USA). The total RNA was treated with DNase I (TaKaRa Bio Inc., Shiga, Japan) and purified using RNeasy Mini Kit (Qiagen, Hilden). The RNA was then treated with Ribo-Zero Gold reagent to remove rRNA. RNA-seq libraries were constructed using ScriptSeq v2 Library Preparation Kit (Epicentre, Madison, USA). The quality and quantity of the library were analysed on an Agilent 2100 Bioanalyzer. The library was diluted to a concentration of 25 fmol. Cluster generation was performed on a cBOT machine and was sequenced either in two lanes of an Illumina GAIIx platform (76 bp reads) or in a single lane of an Illumina HiSeq2000 platform (only for heart tissue; 101 bp reads). For each tissue 45 million to 75 million read pairs were generated. Sequences were assembled de novo using Trinity v. r2013-08-14 [22]. The RNA-seq reads for heart, kidney, muscle, notochord, ovary and testis have been submitted to GenBank under the Bioproject accession number SRP093856. RNA-seq reads for the brain were downloaded from GenBank (accession number SRP050885).
(c). Expression analysis
To determine the expression levels of Nav channel genes in the Japanese lamprey, we performed abundance estimation of transcripts. Trinity transcripts from all seven tissues and full-length cDNA sequences of the six Nav channel genes were combined together and clustered using CD-HIT v. 4.6.1 at 100% identity [23]. RNA-seq reads from seven tissues were independently aligned to the clustered transcript sequences and abundance in Nav channel gene transcripts was estimated by RSEM v. 1.2.25 [24], which uses Bowtie v. 2.2.6 for aligning [25]. Transcript abundances were measured in terms of transcripts per million (TPM) [24]. TPM is estimated by normalizing the gene length followed by normalizing for sequencing depth.
(d). Nav channel gene sequences: sea lamprey (Petromyzon marinus)
All known mouse sodium channel proteins were retrieved from GenBank and used as queries in standalone BLASTP or TBLASTN searches against the protein and nucleotide sequences of the sea lamprey, respectively. To validate the identified genes, their final protein-coding sequences were used as queries in BLASTP searches against the NCBI non-redundant protein sequence database (nr). The sequences from P. marinus were deposited in GenBank (KY931455-KY931460).
(e). RNA-sequence and expression analysis
The transcriptome libraries constructed with RNA isolated from different tissues of sea lamprey were sequenced using the Illumina GAII platform by the Research Technology Support Facility (RTSF) at Michigan State University. About 20 million reads, 75–100 nucleotides long, were obtained per sample, giving a total of 1.5–2 Gb of mRNA-Seq sequencing reads.
The sodium channel gene sequences identified in lamprey genomes were indexed as the reference sequences with Bowtie v. 1.0.0 [26]. The raw RNA-seq reads were filtered, trimmed using Trimmomatic [27], and then aligned to the reference sequences using TopHat 1.4.1 with default parameters [28]. The number of reads mapped to a specific gene was counted based on the BAM file. The gene expression level was normalized as RPM (the number of reads assigned to a gene per million reads), which was calculated as the number of reads mapped to the specific gene relative to the total number of the filtered reads in each sample.
(f). Phylogenetic analysis
Amino acid and nucleotide sequences were aligned and analysed in a maximum-likelihood format with SeaView (http://doua.prabi.fr/software/seaview) using default parameters [29]. Nucleotide analysis was done both with complete sequences and after poorly aligned portions were trimmed to various extents with Guidance v. 2 (http://guidance.tau.ac.il/ver2/overview.php). One-hundred bootstrap replicates were performed. All sequences used for this analysis are listed in the electronic supplementary material, table S1.
(g). Synteny analysis
The genes flanking the lamprey Nav genes were identified by BlastX search of 25-kb window sequences of flanking region against NCBI NR protein database. Navigation to the NCBI gene page for each of these genes allowed us to identify the scaffold or chromosome on which each gene resides in gnathostome target species.
3. Results
(a). Sequence analysis
Six Nav channel genes were retrieved from each species of lamprey and orthologues were reliably determined across the two species (figure 1). For both species of lamprey, each gene was found in the genome as well as in tissue transcriptomes. Two Nav channel genes had been cloned previously from sea lamprey brain and skeletal muscle [11], and these were identical to genes mined from the sea lamprey genome and transcriptomes. Thus, we believe that this is the complete Nav channel repertoire of Japanese lamprey and sea lamprey.
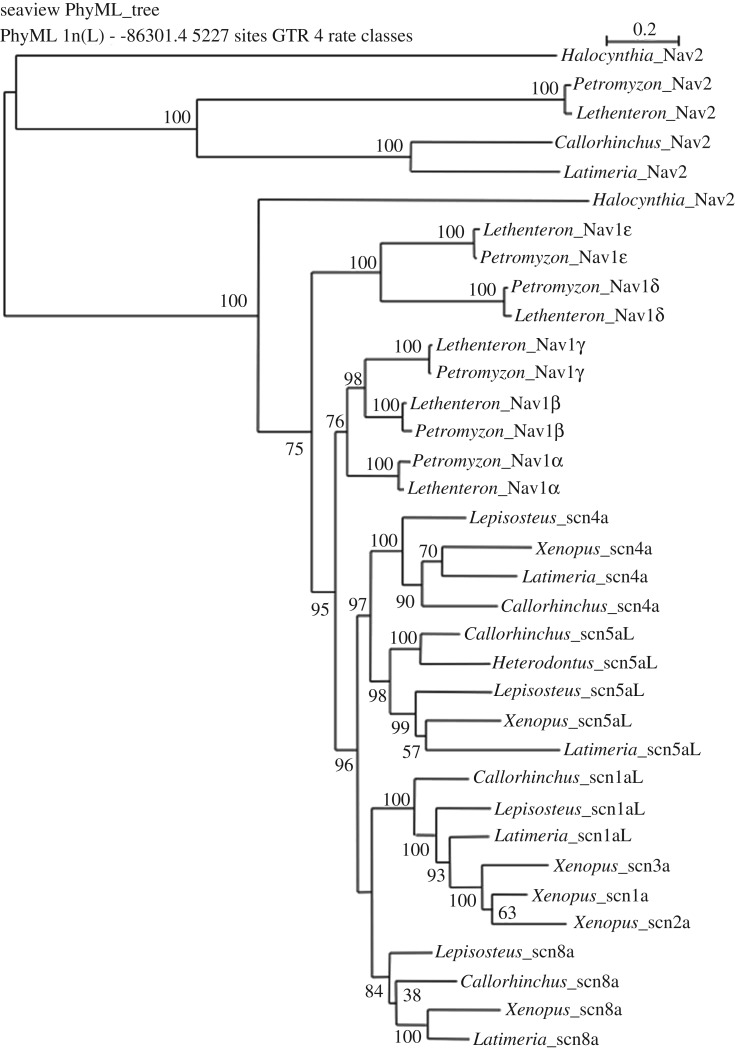
Figure 1.
Maximum-likelihood tree (100 bootstrap replicates) for chordate voltage-gated sodium (Nav) channel genes. Nav2, an invertebrate Nav channel gene believed to be absent in chordates, was observed in two lamprey species, the elephant shark and coelacanth. Nav1 channel genes duplicated in chordates.
In order to assess orthology of lamprey and gnathostome Nav channels, we aligned lamprey Nav channels with those of the tunicate (Halocynthia roretzi) as an outgroup, and with those of the gnathostomes: the elephant shark (Callorhinchus milii), coelacanth (Latimeria chalumnae), spotted gar (Lepisosteus oculatus) and western clawed frog (Xenopus tropicalis). Using SeaView, we generated phylogenetic trees in a maximum-likelihood format. Analysis of amino acid sequences and unedited or trimmed nucleotide sequences all generated trees with identical topologies. The orthology of lamprey Nav1 channel genes to gnathostome Nav1 channel genes could not be unambiguously assigned, so we refer to lamprey channels with Greek letters.
One lamprey gene belongs to the Nav2 family based on its placement in the gene tree, and the characteristic amino acids in the pore selectivity filters (figures 1 and 2). While typical Nav2 channels have a D/E/E/A selectivity filter motif, lamprey Nav2 from both species has D/E/E/G. Surprisingly, we also found Nav2 channel genes in the elephant shark and coelacanth that clustered with the Nav2 channels of tunicate and lampreys. As expected, we did not find a Nav2 gene in Xenopus or Lepisosteus. This suggests that rather than being lost at the base of the vertebrates, Nav2 persisted well into vertebrate evolution and was lost independently in the ancestors of teleosts and tetrapods.
Figure 2.

The selectivity filter of Nav2 and Nav1 voltage-gated sodium channels. Each domain of the four-domain channel contributes to the selectivity filter. Key amino acids for sodium selectivity are highlighted (red). Note difference in domain III (K in Nav1, E in Nav2). (Online version in colour.)
Of the remaining five genes, all in the Nav1 family, the tree topology suggests that two pairs of genes are duplicates (Nav1β, Nav1γ) and (Nav1δ, Nav1ε), and one gene (Nav1α) is a singleton. All lamprey Nav1 channels have conserved Nav channel characteristics such as voltage-sensors, inactivation loops (domain III–IV loop) and acceptor site for the inactivation loop (domain IV S4–S5 linker). The lamprey and gnathostome Nav1 channel genes formed parallel clusters in the tree (figure 1). The gnathostome Nav1 channel gene topology showed the expected relationships clearly indicating a 2R origin (Nav1.4 and Nav1.5 L originating from an original 1R gene, and Nav1.1 L and Nav1.6 resulting from a second 1R gene) based on previous studies.
(b). Synteny analysis
We compared the flanking genes on the scaffolds of the two lamprey species (electronic supplementary material, figures S1–S5). Most scaffolds show good synteny across the two species, although there was discordance between the flanking genes of Nav1β and too little sequence around Nav1γ so we were unable to determine synteny for the scaffolds around these two genes. Navi1δ and Nav1ε are flanked by three genes (pde1c; gorasp2; and cdk5r) with paralogues on both scaffolds (electronic supplementary material, figure S4), suggesting that these two regions resulted from a recent, probably lamprey-specific, duplication. This result is consistent with the phylogenetic tree (figure 1).
We then compared the genes flanking the Nav channel genes in lampreys with those in C. milii (elephant shark), Le. oculatus (spotted gar), L. chalumnae (coelacanth) and Homo sapiens. The gene synteny of the scaffold in which Nav2 resides is conserved in all these vertebrates (see electronic supplementary material, figure S1; itag1; itag2; mocs2; fst and arl15). In those gnathostomes that have lost Nav2, the surrounding genes remain syntenic.
Owing to the poor reconstruction of some lamprey scaffolds (electronic supplementary material, figures S3, S5), the divergent histories of gene gain and loss, and chromosomal rearrangements in the agnathan and gnathostome lineages, it was difficult to make a one to one correspondence between most lamprey and gnathostome scaffolds/chromosomes. However, lamprey Nav1α may be homologous with the cluster of five Nav1 channel genes on human chromosome 2 (electronic supplementary material, figure S2) by virtue of its association with a few flanking genes (galnt3; ttc21b and slc38a11) in a block.
(c). Expression analysis
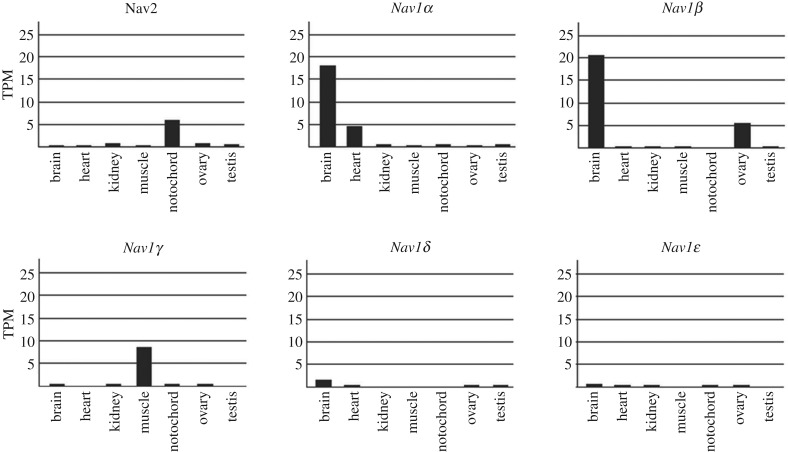
Transcript abundance was measured in various tissues in both species (figures 3 and 4). This was done independently for the two species in two laboratories, so while there was overlap in sampling some tissues (brain, muscle, kidney) other tissues were assessed in only one species. Nevertheless, a clear pattern is evident. Nav1γ is highly expressed in muscle in both species. Nav1α is highly expressed in brain in both species (as well as heart in Japanese lamprey, heart not tested in sea lamprey), and Nav1β is also highly expressed in brain in both species (and in ovary in Japanese lamprey). Nav1δ shows low expression in brain and Nav1ɛ minimal expression in all measured tissues. Interestingly, Japanese lamprey Nav2 is expressed in notochord, kidney and ovary, but not in the nervous system.
Figure 3.
Expression analysis (RNA-seq. TPM) of Nav2 and Nav1 voltage-gated sodium channel genes in brain, heart, kidney, muscle, notochord, ovary and testis of the Japanese lamprey.
Figure 4.
Expression analysis (RNA-seq, RPM) of Nav2 and Nav1 voltage-gated sodium channel genes in brain, gill, rope (male-specific adipose tissue), lips, muscle and larval kidney of the sea lamprey.
4. Discussion
(a). Nav2 genes in vertebrates
The Nav2 family is the ‘poor cousin’ of the Nav channel family. The founding member was discovered in Drosophila and named DSC1 (Drosophila sodium channel 1) [30]. DSC1 gene knock-outs have only small effects in Drosophila [31–33], unlike the Nav1 paralogue, para, so named because temperature-sensitive mutations cause total paralysis; knock-outs of para are lethal [34]. The selectivity filter of Nav2 family members differs from the Nav1 family and recent biophysical studies show that Nav2 family channels are more permeable to Ca2+ than to Na+ [4,5,35,36]. Thus, they are perhaps better considered functionally as Ca2+ channels. Indeed, a recent study has suggested renaming them as a new family of Cav channels [36].
The Nav2 channel is widely represented across invertebrates from basal metazoans such as cnidaria, placozoans and ctenophores, to invertebrate chordates such as the tunicates [3,4,8,37,38]. The absence of Nav2 family members in vertebrates to date suggested that the Nav2 gene was lost in the earliest vertebrates. Surprisingly, we noted Nav2 family orthologues in lampreys, elephant shark and coelacanth, proving that the Nav2 lineage continued on in vertebrates. The Nav2 gene presumably duplicated during 2R. While all four of the Nav1 duplicates were retained, two (one loss after 1R and one after 2R) or three (three genes lost after 2R) Nav2 gene duplicates were lost. Finally, the remaining single Nav2 gene was lost independently in the ancestors of ray-finned fishes and tetrapods [12,13]. Synteny analysis shows that Nav2 was excised with almost surgical precision in actinopterygian and sarcopterygian ancestors, leaving the flanking genes intact (electronic supplementary material, figure S1). Meanwhile, the Nav1 family went on to successful independent duplications in teleosts (eight genes resulting from 3R) and tetrapods (6–10 genes from tandem duplications).
Nav2 sodium channel genes are expressed in the nervous systems in invertebrates and tunicates [8,30]. At least in lampreys, Nav2 does not appear to be expressed in brain, heart or muscle. Instead, its main expression is in non-neural structures such as the notochord, kidney and ovary. Its function in these locations is unknown. It will be interesting to determine where Nav2 is expressed in those vertebrates that still possess it.
Finally, it is worth noting that the selectivity filter of Nav2 channels in lamprey (D/E/E/G) differs from all other known Nav2 family members, including from coelacanth and elephant shark, which have the conserved motif (D/E/E/A). We do not know if the A to G substitution alters conductance. Some rare Nav1 channels have a D/E/K/G pore motif and this does not alter the Na+/Li+/K+ permeability ratios [39].
(b). Orthology of Nav1 family genes in lampreys and gnathostomes
The gene tree shows that lampreys have five Nav1 family members (two paired and one singleton). The pairing of Nav1δ and Nav1ε is supported by synteny but too few flanking genes were recovered from the other putative pair (Nav1β and Nav1γ) to ascertain syntenic relationships.
Our analysis of lamprey–gnathostome gene orthology resulted in the lamprey channels being grouped together alongside the gnathostome channels exactly as observed for lamprey myosin, KCNA, Hox, Runx, p53 and somatostatin family genes [15,21,40–43]. While this may suggest that Nav channels as well as these other genes duplicated independently in agnathans and ganthostomes, there are other circumstances that might have led to this clustering pattern. As Qiu et al. [42] and Mehta et al. [15] emphasize, lamprey genomes have a high GC content (48%), affecting the codon usage pattern and amino acid composition of lamprey protein-coding sequences that could artifactually lead to this pattern even if lamprey genes have real gnathostome orthologues. Another possibility is that the Nav1 channel genes were homogenized by gene conversion as is suspected for the scna1 L-related genes in amphibians [13].
Despite the difficulty of assigning orthology by sequence alone, analysis of synteny suggests that the agnathan Nav1α gene is orthologous to gnathostome scn1aL and its expanded family as on human chromosome 2. Further information on the flanking genes of Nav1β and Nav1γ may resolve orthology for the other genes.
Even excluding the thorny question of orthology, reconstructing the history of gene duplication of the lamprey Nav1 gene family is difficult. The most parsimonious pathway is that a single ancestral Nav1 channel gene underwent 2R and then one of those four genes duplicated as a tandem duplication or as part of a chromosome duplication (figure 5a; electronic supplementary material, figure S1). This results in five Nav1 genes with no losses. A less parsimonious possibility is a 2R duplication followed by a single loss, followed by a 3R duplication and another loss (figure 5b). The least parsimonious is a 3R duplication that gave rise to eight Nav1 channel genes. The loss of three Nav1 channel genes would then result in five Nav1 genes (figure 5c). While less parsimonious, the 3R scenarios are supported by an analysis of the lamprey Hox gene clusters [15]. Amniotes have four Hox gene clusters consistent with 2R; teleosts have seven or eight Hox clusters consistent with an additional 3R followed by loss of one Hox cluster [44,45]. Lampreys have six Hox gene clusters, suggesting that they may have undergone 2R (in common with gnathostomes?) followed by (lamprey-specific?) 3R genome duplication. Because the six Hox gene clusters are members of a paralog on the most likely interpretation for the two ‘extra’ Hox clusters (under the 2R scenario) is they result from a large-scale duplication of some sort. With the genomes of two lamprey species now available, the analysis of a number of different gene families will hopefully make it possible to resolve this issue.
Figure 5.
Schematic of possible histories of gene gain and loss for lamprey Nav1 channel genes.
(c). Expression patterns of Nav1 channel genes in lampreys
The single Nav1 channel of tunicates expresses only in the central nervous system and, of course, most vertebrate Nav1 channels do as well. Thus, it is not surprising that two of the Nav1 channel genes of lamprey strongly (Nav1α, Nav1β), and a third channel weakly (Nav1δ), express in the brain.
Invertebrates, including non-vertebrate chordates such as tunicates, do not express voltage-gated Nav channels in muscle (two possible exceptions may be in rapidly contracting muscles of chaetognaths and squid [46–48]). Instead, L-type Ca2+ channels generate the muscle action potential allowing a rapid influx of Ca2+[8,49,50]. The skeletal muscle of vertebrates including agnathans evolved a novel mechanism whereby the L-type Ca2+ channel in the muscle membrane does not conduct Ca2+ into the cell but, instead, mechanically activates a molecule—the ryanodine receptor—on the surface of the sarcoplasmic reticulum that initiates the release of Ca2+ from this internal reservoir [51,52]. L-type Ca2+ channels do not carry inward depolarizing current in vertebrate skeletal muscle; that is the role of a Nav channel. It is likely that the evolution of mechanical coupling of the vertebrate skeletal muscle L-type channel to the ryanodine receptor could not have occurred without the prior expression of a Nav1 channel in the skeletal muscle of early vertebrates.
In lampreys, one Nav channel gene (Nav1γ) is uniquely expressed in muscle just as in other vertebrates. A second Nav channel (Nav1α) is expressed in heart as well as brain; this channel must underlie the Na+-based action potentials of the lamprey heart [53]. Because of difficulty in establishing orthology, we cannot say whether the compartmentalization of distinct Nav1 channels to muscle and heart occurred independently in agnathans and gnathostomes or just once with the same genes early in vertebrate history. Nevertheless, the duplication of Nav channel genes and the expression of one gene in muscle and another in heart led to major vertebrate innovations in muscle and heart function.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgement
Thanks to Nicole Elmer for artwork.
Data accessibility
All sequences are available from GenBank (see electronic supplementary material, table S1).
Authors' contributions
H.H.Z., B.V. and W.L. designed the study; N.E.P., S.T., B.V., J.R. and W.L. derived data; H.H.Z., B.V. and W.L. analysed the data and drafted the manuscript.
Competing interests
We have no competing interests.
Funding
H.H.Z. was supported by the University of Texas. Work in B.V.'s laboratory was supported by the Biomedical Research Council of A*STAR, Singapore. W.L. was supported by a grant from the US National Institutes of Health (R24GM83982) and by the Great Lakes Fisheries Commission. J.R. was supported by International S&T Cooperation Programs from Shanghai Committee of Science and Technology (15410723300) and the Shanghai Ocean University and Michigan State University Joint Research Center grant no. (A1-0209-15-0806).
References
- 1.Liebeskind BJ, Hillis DM, Zakon HH. 2012. Phylogeny unites animal sodium leak channels with fungal calcium channels in an ancient, voltage-insensitive clade. Mol. Biol. Evol. 29, 3613–3616. ( 10.1093/molbev/mss182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iida K, Tada T, Iida H. 2004. Molecular cloning in yeast by in vivo homologous recombination of the yeast putative α1 subunit of the voltage-gated calcium channel. FEBS Lett. 576, 291–296. ( 10.1016/j.febslet.2004.09.021) [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind BJ, Hillis DM, Zakon HH. 2011. Evolution of sodium channels predates the origin of nervous systems in animals. Proc. Natl Acad. Sci. USA 108, 9154–9159. ( 10.1073/pnas.1106363108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gur Barzilai M, Reitzel AM, Kraus JEM, Gordon D, Technau U, Gurevitz M, Moran Y. 2012. Convergent evolution of sodium ion selectivity in metazoan neuronal signaling. Cell Rep. 2, 242–248. ( 10.1016/j.celrep.2012.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T, Liu Z, Song W, Du Y, Dong K. 2011. Molecular characterization and functional expression of the DSC1 channel. Insect. Biochem. Mol. Biol. 41, 451–458. ( 10.1016/j.ibmb.2011.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hille B. 2001. Ion channels of excitable membranes. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 7.Plummer N, Meisler M. 1999. Evolution and diversity of mammalian sodium channel genes. Genomics 57, 323–331. ( 10.1006/geno.1998.5735) [DOI] [PubMed] [Google Scholar]
- 8.Nagahora H, Okada T, Yahagi N, Chong JA, Mandel G, Okamura Y. 2000. Diversity of voltage-gated sodium channels in the ascidian larval nervous system. Biochem. Biophys. Res. Commun. 275, 558–564. ( 10.1006/bbrc.2000.3290) [DOI] [PubMed] [Google Scholar]
- 9.Ohno S. 1970. Evolution by gene duplication. New York, NY: Springer. [Google Scholar]
- 10.Lopreato G, Lu Y, Southwell A, Atkinson A, Hillis D, Wilcox T, Zakon HH. 2001. Evolution and divergence of sodium channel genes in vertebrates. Proc. Natl Acad. Sci. USA 98, 7588–7592. ( 10.1073/pnas.131171798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak A, Jost M, Lu Y, Taylor A, Zakon H, Ribera A. 2006. Gene duplications and evolution of vertebrate voltage-gated sodium channels. J. Mol. Evol. 63, 208–221. ( 10.1007/s00239-005-0287-9) [DOI] [PubMed] [Google Scholar]
- 12.Widmark J, Sundström G, Ocampo Daza D, Larhammar D. 2011. Differential evolution of voltage-gated sodium channels in tetrapods and teleost fishes. Mol. Biol. Evol. 28, 859–871. ( 10.1093/molbev/msq257) [DOI] [PubMed] [Google Scholar]
- 13.Zakon H, Lu Y, Jost M. 2011. Expansion of voltage-dependent sodium channel gene family in early tetrapods coincided with the emergence of terrestriality and increased brain complexity. Mol. Biol. Evol. 28, 1415–1424. ( 10.1093/molbev/msq325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putnam NH, et al. 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071. ( 10.1038/nature06967) [DOI] [PubMed] [Google Scholar]
- 15.Mehta TK, et al. 2013. Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum). Proc. Natl Acad. Sci. USA 110, 16 044–16 049. ( 10.1073/pnas.1315760110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnegard ME, Zwickl DJ, Lu Y, Zakon HH. 2010. Old gene duplication facilitates origin and diversification of an innovative communication system--twice. Proc. Natl Acad. Sci. USA 107, 22 172–22 177. ( 10.1073/pnas.1011803107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak AE, Taylor AD, Pineda RH, Lasda EL, Wright MA, Ribera AB. 2006. Embryonic and larval expression of zebrafish voltage-gated sodium channel α-subunit genes. Dev. Dyn. 235, 1962–1973. ( 10.1002/dvdy.20811) [DOI] [PubMed] [Google Scholar]
- 18.Chopra SS, Stroud DM, Watanabe H, Bennett JS, Burns CG, Wells KS, Yang T, Zhong TP, Roden DM. 2010. Voltage-gated sodium channels are required for heart development in zebrafish. Circ. Res. 106, 1342 ( 10.1161/CIRCRESAHA.109.213132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shih Y-H, Zhang Y, Ding Y, Ross CA, Li H, Olson TM, Xu X. et al. 2015. Cardiac transcriptome and dilated cardiomyopathy genes in zebrafish. Circ. Cardiovasc. Genet. 8, 261 ( 10.1161/CIRCGENETICS.114.000702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y-H, Huang F-L, Cheng Y-C, Wu C-J, Yang C-N, Tsay H-J. 2008. Knockdown of zebrafish Nav1.6 sodium channel impairs embryonic locomotor activities. J. Biomed. Sci. 15, 69–78. ( 10.1007/s11373-007-9200-4) [DOI] [PubMed] [Google Scholar]
- 21.Nah GS, Tay B, Brenner S, Osato M, Venkatesh B. 2014. Characterization of the Runx gene family in a jawless vertebrate, the Japanese lamprey (Lethenteron japonicum). PLoS ONE 9, e113445 ( 10.1371/journal.pone.0113445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grabherr MG, et al. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotech. 29, 644–652. ( 10.1038/nbt.1883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659. ( 10.1093/bioinformatics/btl158) [DOI] [PubMed] [Google Scholar]
- 24.Li B, Dewey C. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 ( 10.1186/1471-2105-12-323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langmead B, Salzberg S. 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. ( 10.1038/nmeth.1923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langmead B, Trapnell C, Pop M, Salzberg S. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 ( 10.1186/gb-2009-10-3-r25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolger A, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. ( 10.1093/bioinformatics/btu170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trapnell C, Pachter L, Salzberg S. 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. ( 10.1093/bioinformatics/btp120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouy M, Guindon Sp, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224. ( 10.1093/molbev/msp259) [DOI] [PubMed] [Google Scholar]
- 30.Hong C-S, Ganetzky B. 1994. Spatial and temporal expression patterns of two sodium channel genes in Drosophila. J. Neurosci. 14, 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong K, Du Y, Rinkevich F, Wang L, Xu P. 2015. The Drosophila Sodium Channel 1 (DSC1): the founding member of a new family of voltage-gated cation channels. Pestic. Biochem. Physiol. 120, 36–39. ( 10.1016/j.pestbp.2014.12.005) [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni NH, Yamamoto AH, Robinson KO, Mackay TFC, Anholt RRH. 2002. The DSC1 channel, encoded by the smi60E locus, contributes to odor-guided behavior in Drosophila melanogaster. Genetics 161, 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Wang Z, Wang L, Luo N, Jiang L, Liu Z, Wu C-F, Dong K, Anholt RRH. 2013. Role of the DSC1 channel in regulating neuronal excitability in Drosophila melanogaster: extending nervous system stability under stress. PLoS Genet. 9, e1003327 ( 10.1371/journal.pgen.1003327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganetzky B. 1984. Genetic studies of membrane excitability in Drosophila: lethal interaction between two temperature-sensitive paralytic mutations. Genetics 708, 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou W, Chung I, Liu Z, Goldin AL, Dong K. 2004. A voltage-gated calcium-selective channel encoded by a sodium channel-like gene. Neuron 42, 101–112. ( 10.1016/S0896-6273(04)00148-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gosselin-Badaroudine P, Moreau A, Simard L, Cens T, Rousset M, Collet C, Charnet P, Chahine M. 2016. Biophysical characterization of the honeybee DSC1 orthologue reveals a novel voltage-dependent calcium channel subfamily. J. Gen. Physiol. 148, 133–145. ( 10.1085/jgp.201611614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui Y-J, Yu L-L, Xu H-J, Dong K, Zhang C-X. 2012. Molecular characterization of DSC1 orthologs in invertebrate species. Insect Biochem. Mol. Biol. 42, 353–359. ( 10.1016/j.ibmb.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 38.Okamura Y, et al. 2005. Comprehensive analysis of the ascidian genome reveals novel insights into the molecular evolution of ion channel genes. Physiol. Genomics 22, 269–282. ( 10.1152/physiolgenomics.00229.2004) [DOI] [PubMed] [Google Scholar]
- 39.Wu M, Ye N, Sengupta B, Zakon HH. 2013. A naturally occurring amino acid substitution in the voltage-dependent sodium channel selectivity filter affects channel gating. J. Comp. Physiol. A 199, 829–842. ( 10.1007/s00359-013-0845-3) [DOI] [PubMed] [Google Scholar]
- 40.Coffill CR, et al. 2016. The p53-Mdm2 interaction and the E3 ligase activity of Mdm2/Mdm4 are conserved from lampreys to humans. Genes Dev. 30, 281–292. ( 10.1101/gad.274118.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikeda D, Ono Y, Hirano S, Kan-no N, Watabe S. 2013. Lampreys have a single gene cluster for the fast skeletal myosin heavy chain gene family. PLoS ONE 8, e85500 ( 10.1371/journal.pone.0085500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu H, Hildebrand F, Kuraku S, Meyer A. 2011. Unresolved orthology and peculiar coding sequence properties of lamprey genes: the KCNA gene family as test case. BMC Genomics 12, 325 ( 10.1186/1471-2164-12-325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tostivint H, Dettaï A, Quan FB, Ravi V, Tay B-H, Rodicio MC, Mazan S, Venkatesh B, Kenigfest NB. 2016. Identification of three somatostatin genes in lampreys. Gen. Comp. Endocrinol. 237, 89–97. ( 10.1016/j.ygcen.2016.08.006) [DOI] [PubMed] [Google Scholar]
- 44.Amores A, et al. 1998. zebrafish hox clusters and vertebrate genome evolution. Science 282, 1711–1714. ( 10.1126/science.282.5394.1711) [DOI] [PubMed] [Google Scholar]
- 45.Bian C, et al. 2016. The Asian arowana (Scleropages formosus) genome provides new insights into the evolution of an early lineage of teleosts. Sci. Rep. 6, 24501 ( 10.1038/srep24501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers C, Nelson L, Brown B. 1997. Different excitation-contraction coupling mechanisms exist in squid, cuttlefish and octopod mantle muscle. J. Exp. Biol. 200, 3033–3041. [DOI] [PubMed] [Google Scholar]
- 47.Tsutsui I, Inoue I, Bone Q, Carre C. 2000. Activation of locomotor and grasping spine muscle fibres in chaetognaths: a curious paradox. J. Muscle Res. Cell Motil. 21, 91–97. ( 10.1023/A:1005627918789) [DOI] [PubMed] [Google Scholar]
- 48.Schwartz LM, Stuhmer W. 1984. Voltage-dependent sodium channels in an invertebrate striated muscle. Science 225, 523 ( 10.1126/science.6330898) [DOI] [PubMed] [Google Scholar]
- 49.Inoue I, Tsutsui I, Bone Q. 2002. Excitation-contraction coupling in isolated locomotor muscle fibres from the pelagic tunicate Doliolum which lack both sarcoplasmic reticulum and transverse tubular system. J. Comp. Physiol. B 172, 541–546. ( 10.1007/s00360-002-0280-1) [DOI] [PubMed] [Google Scholar]
- 50.Rokni D, Hochner B. 2002. Ionic currents underlying fast action potentials in the obliquely striated muscle cells of the octopus arm. J. Neurophysiol. 88, 3386–3397. ( 10.1152/jn.00383.2002) [DOI] [PubMed] [Google Scholar]
- 51.Di Biase V, Franzini-Armstrong C. 2005. Evolution of skeletal type e-c coupling. J. Cell Biol. 171, 695–704. ( 10.1083/jcb.200503077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas MJ, Hamman BN, Tibbits GF. 1996. Dihydropyridine and ryanodine binding in ventricles from rat, trout, dogfish and hagfish. J. Exp. Biol. 199, 1999–2009. [DOI] [PubMed] [Google Scholar]
- 53.Haverinen J, Egginton S, Vornanen M. 2014. Electrical excitation of the heart in a basal vertebrate, the European river lamprey (Lampetra fluviatilis). Physiol. Biochem. Zool. 87, 817–828. ( 10.1086/678954) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequences are available from GenBank (see electronic supplementary material, table S1).