Abstract
Background
Streptococcus agalactiae (group B Streptococcus [GBS]) is an important neonatal pathogen and emerging cause of disease in adults. The major risk factor for neonatal disease is maternal vaginal colonization. However, little is known about the relationship between GBS and vaginal microbiota.
Methods
Vaginal lavage samples from nonpregnant women were tested for GBS, and amplicon-based sequencing targeting the 16S ribosomal RNA V3–V4 region was performed.
Results
Four hundred twenty-eight of 432 samples met the high-quality read threshold. There was no relationship between GBS carriage and demographic characteristics, α-diversity, or overall vaginal microbiota community state type (CST). Within the non-Lactobacillus-dominant CST IV, GBS positive status was significantly more prevalent in CST IV-A than CST IV-B. Significant clustering by GBS status was noted on principal coordinates analysis, and 18 individual taxa were found to be significantly associated with GBS carriage by linear discriminant analysis. After adjusting for race/ethnicity, 4 taxa were positively associated with GBS, and 6 were negatively associated.
Conclusions
Vaginal microbiota CST and α-diversity are not related to GBS status. However, specific microbial taxa are associated with colonization of this important human pathogen, highlighting a potential role for the microbiota in promotion or inhibition of GBS colonization.
Keywords: group B Streptococcus, vaginal microbiome, neonatal pathogens
Streptococcus agalactiae (group B Streptococcus [GBS]) is a β-hemolytic, gram-positive bacterium that emerged as a major cause of neonatal sepsis in the 1960s [1]. GBS generally colonizes asymptomatically as a member of the gastrointestinal or vaginal microbiota but can cause life-threatening infection in infants and, to an increasing degree, in adults [2, 3]. Maternal colonization is the most important risk factor for neonatal GBS infection [4], and estimates of rectovaginal GBS colonization rates range from 12% to 36% in populations around the world [5]. In the United States, approximately 25% of pregnant women carry GBS, with higher rates among African American women than other groups [6]. Current guidelines from the Centers for Disease Control and Prevention state that pregnant women should undergo screening for GBS carriage by polymerase chain reaction (PCR) or culture in late pregnancy, with intrapartum antibiotic prophylaxis recommended for GBS carriers to prevent early-onset (first week of life) neonatal disease [7].
Previous studies have mainly examined the relationship between GBS colonization and other constituents of the vaginal microbiota using culture-based methods. One large study from Japan demonstrated that detection of GBS was correlated with decreased α-diversity, significantly higher probability of detection of Klebsiella pneumoniae and methicillin-sensitive Staphylococcus aureus, and significantly lower likelihood of coagulase-negative Staphylococcus, Prevotella, and Lactobacillus [8]. A study of healthy pregnant women demonstrated that GBS detection was correlated with decreased detection of Lactobacillus species [9]. Subsequently, another study in pregnancy demonstrated that there was no significant difference in the number of Lactobacillus or Bifidobacterium based on GBS colonization [10]. Most recently, a 16S gene amplification study in pregnant Guatemalan women demonstrated no difference between the overall microbiota of GBS-colonized and GBS-uncolonized women, while finding that increased Corynebacterium species and Aerococcus species were associated with GBS noncolonization and GBS colonization, respectively [11].
Culture-independent assessments of the human vaginal microbiota have revealed 5 distinct community state types (CSTs), 4 of which are often dominated by 1 of 4 species of Lactobacillus (L. crispatus [CST I], L. gasseri [CST II], L. iners [CST III], and L. jensenii [CST V]) and 1 (CST IV) characterized by a paucity of Lactobacillus species and comprised of a diverse group of facultative and strict anaerobes, including species associated with bacterial vaginosis (BV), a common vaginal dysbiosis [12, 13]. We investigated the relationship between GBS colonization and characteristics of the vaginal microbiota, including community ecological metrics, CST, and species-level predictors.
MATERIALS AND METHODS
Sample Collection, Processing, 16S Ribosomal RNA Gene Amplification, and Amplicon Sequencing
Human subjects research was performed in accordance with the standards of the Helsinki Declaration and approved by the institutional review boards of Columbia University Medical Center and Weill Cornell Medical College. Written informed consent was obtained from all subjects. Samples used in this work come from the Bacterial Vaginosis–Improved Diagnosis by ELISA and Sequencing (BV-IDEAS) study, in which vaginal lavage specimens were collected from a racially and ethnically diverse group of nonpregnant women aged 18–55 years seeking primary gynecologic care at a site in New York City between July 2010 and June 2012. In brief, 5-mL sterile saline vaginal lavage samples were aliquoted immediately after collection, refrigerated for transport (<6 hours), and stored at –80°C until extraction. Vaginal pH was determined at the time of sample collection using pH-sensitive paper and was recorded as normal (≤4.5) or abnormal (>4.5). Subjects provided demographic information by self-report including age, race/ethnicity (recorded as white/black/Hispanic/other), highest level of education completed (no high school [HS]; some HS; HS graduate; HS equivalency degree; some college; college graduate), and annual household income (by $5000 increments from <$5000 to >$20000). DNA extraction was performed on 500 µL of lavage specimen using the MoBio PowerSoil kit, and PCR amplification and sequencing of the V3–V4 regions of the 16S ribosomal RNA (rRNA) gene were performed at the Institute for Genome Sciences (University of Maryland School of Medicine) on an Illumina MiSeq instrument using the 250-bp paired-end read protocol [14]. Race/ethnicity identification was determined by self-report. Quality control of the BV-IDEAS sequence dataset was performed as previously described [14]. In brief, after trimming the amplification primer sequences, sequence reads not having average quality of 20 over a 30-bp sliding window based on the phred algorithm were truncated, and trimmed reads having <75% of their original length were removed. Then, QIIME software (version 1.6.0) [15] was used to truncate sequences before 3 consecutive low-quality bases, remove ambiguous bases, and remove sequences <150 bp after trimming. Sequence data have been deposited in the National Center for Biotechnology Information Short Read Archive database under accession number SRP110138.
Taxonomic and CST Assignment
Taxonomic assignment was performed using PECAN [16], a Markov chain model–based classifier. CSTs were assigned using a support vector machines–based machine learning algorithm trained with a dataset comprised of 4000 samples to 1 of several previously described CSTs [12].
GBS Colonization Determination
For the BV-IDEAS samples, GBS colonization was determined using a combined PCR and culture approach. Extracted DNA (as above) was subjected to real-time polymerase chain reaction (RT-PCR) targeting the sip gene as previously described [17]. A positive RT-PCR was recorded for cycle threshold (Ct) ≤40. For samples with Ct 41–50, culture on CHROMagar StrepB was performed to confirm GBS colonization, and those samples were only recorded as positive if typical (mauve) colonies were present. All other samples were recorded as negative.
Statistical Analyses
Statistical analyses were performed in R version 3.1.2 for Linux [18] and QIIME version 1.9.1 [15]. A heatmap with CST and GBS status data was generated using the NMF package in R [19]. The α-diversity (within-sample) was calculated using the chao1 statistic [20] in QIIME and was compared using an unpaired t test with 10000 Monte Carlo simulations. β-Diversity (between-sample) was calculated using the Bray-Curtis statistic [21]. Adonis [22] was calculated on the β-diversity distance matrix to compare the groups. Principal coordinates analysis was performed and figures were generated using the 2 principal coordinate axes that explained the most variance.
To evaluate the association between GBS presence and specific bacterial taxa, linear discriminant analysis effect size (LEfSe) was used [23]. This tool first performs a Kruskal–Wallis (KW) test, then calculates the logarithmic linear discriminant analysis (log LDA) score of each feature. The α value for the KW test was 0.05, and we only considered any feature with a log LDA score that was ≤ –2.5 or ≥2.5. Given that colonization of many taxa [24] and GBS [25] have been correlated with race/ethnicity, binomial models were constructed for each LEfSe-identified taxon with independent variables of normalized taxon abundance and categorical race/ethnicity and dependent variable of GBS status. In the subset of taxa identified by LEfSe, ccrepe [26] was used with 1000 bootstrap iterations to look for Spearman correlation between the LEfSe-identified taxa. The Benjamini–Hochberg–Yekutieli false discovery rate correction was used on the output P values from ccrepe.
RESULTS
Samples
Four hundred forty-six vaginal lavage samples were originally collected, of which 432 were eligible for the analysis. These had a mean of 14187 (standard deviation, 6878) assembled paired reads after quality control that were successfully assigned to taxonomy per sample. The subset used for analysis consisted of all those samples with ≥1000 high-quality assembled reads assigned to taxonomy per sample. This set consisted of 428 samples with high-quality reads assigned to 1 of 99 detected bacterial taxa. Of this set, 21% (92/428) of samples were positive for GBS (Table 1). The mean age of the cohort was 32.4 years (GBS-negative group, 32.3; GBS-positive group, 33.1; Student t test P > .05). There were no significant relationships between GBS colonization and demographic information including race/ethnicity, educational level, or income (Fisher exact test P > .05 for each).
Table 1.
Distribution of Samples by Group B Streptococcus Status Across Vaginal Community State Types
| CST | GBS Positive | GBS Negative | Prevalence |
|---|---|---|---|
| I | 15 | 56 | 21% |
| II | 4 | 13 | 24% |
| III | 23 | 91 | 20% |
| IV | 48 | 166 | 22% |
| IV-A | 21 | 32 | 40% |
| IV-B | 27 | 134 | 17% |
| V | 2 | 10 | 17% |
| Total | 92 | 336 | 21% |
CST IV-A and IV-B are subcategories of CST-IV and are not included in totals.
Abbreviations: CST, community state type; GBS, group B Streptococcus.
GBS and Community State Types
The samples were assigned to 1 of the 5 CSTs previously identified (Table 1). There was no significant association between CST and GBS status (P = .98, Fisher exact test) (Figure 1A). CST IV represents a vaginal microbiota with higher diversity of strict and facultative anaerobes, with no or low relative abundance of Lactobacillus species (Figure 2). Further division into CST IV-A and CST IV-B [27] demonstrated a significantly higher proportion of GBS-positive samples in CST IV-A (P = .001, Fisher exact test) (Figure 1B). There was no significant effect of pH on GBS colonization status (pH > 4.5 in 47.3% of GBS-negative and 55.4% of GBS-positive subjects; P = .20, Fisher exact test).
Figure 1.
Proportion of samples positive for group B Streptococcus (GBS) by community state type (CST). A, There was no evidence of difference of proportion GBS colonized when comparing CSTs (P = .96, Fisher exact test). B, Subgroup analysis within CST IV demonstrated significantly higher prevalence of GBS colonization in CST IV-A than in CST IV-B (P = .001, Fisher exact test).
Figure 2.
Heatmap showing top 25 taxa by community state type (CST) and group B Streptococcus (GBS) status. The samples are organized first by CST (top key), then by GBS status (bottom key).
Alpha Diversity and Principal Coordinates Analysis
There was no difference in α-diversity as defined by chao1 based on GBS status (Figure 3A, nonparametric t test with 10000 Monte Carlo permutations, P = .81). While there was no obvious clustering based on GBS status in principal coordinates analysis (Figure 3B), there was statistically significant separation of GBS-positive and GBS-negative groups (Adonis P < .005), though this clustering explained little of the overall variance (R2 = .009).
Figure 3.
Population statistics by group B Streptococcus (GBS) status. A, There was no evidence of difference in α-diversity between GBS-positive and GBS-negative samples based on the chao1 statistic (P = .81) A, Principal coordinates analysis (PCoA) showing the 2 principal coordinates that explained the most variance. B, There was statistically significant clustering of GBS-positive and -negative samples (Adonis P < .001, R2 = 0.009).
Co-occurrence of Bacterial Taxa and GBS
A total of 23 taxa were found to be significantly associated with GBS status by LEfSe. After filtering for only those with an absolute value of the logarithmic discriminant analysis score ≥2.5, 18 taxa remained (Figure 4). Of these, 8 were associated with GBS-positive status and 10 were associated with GBS-negative status. The genus Streptococcus (which includes GBS) and BV-associated bacterium 1 (BVAB1) were the taxa most strongly associated with GBS-positive and GBS-negative status, respectively (Streptococcus absolute value log LDA score and KW P value: 4.617 and 7.58 × 10–19; BVAB1: 4.137 and 0.0205). Prevotella bivia and the genus Staphylococcus were also strongly associated with GBS-positive status, whereas Prevotella genogroup 3 and Megasphaera species type 1 were strongly associated with GBS-negative status.
Figure 4.
Association of specific taxa with group B Streptococcus (GBS) status. linear discriminant analysis effect size output with scores ≥2.5 or ≤ –2.5.
Binomial modeling of GBS status based on relative taxon count and race/ethnicity demonstrated continued significant positive correlations between the genus Streptococcus (P < .01), P. bivia (P = .02), the genus Veillonella (P = .05), and Eubacterium siraeum (P = .03) and GBS-positive status. There were continued negative correlations between BVAB1 (P = .01), Prevotella genogroup 3 (P < .01), Megasphaera species type 1 (P = .02), BV-associated bacterium 2 (BVAB2; P = .03), Prevotella genogroup 4 (P = .02), and Dialister species type 2 (P = .02) and GBS-positive status.
Correlation of Candidate Bacterial Taxa With Each Other
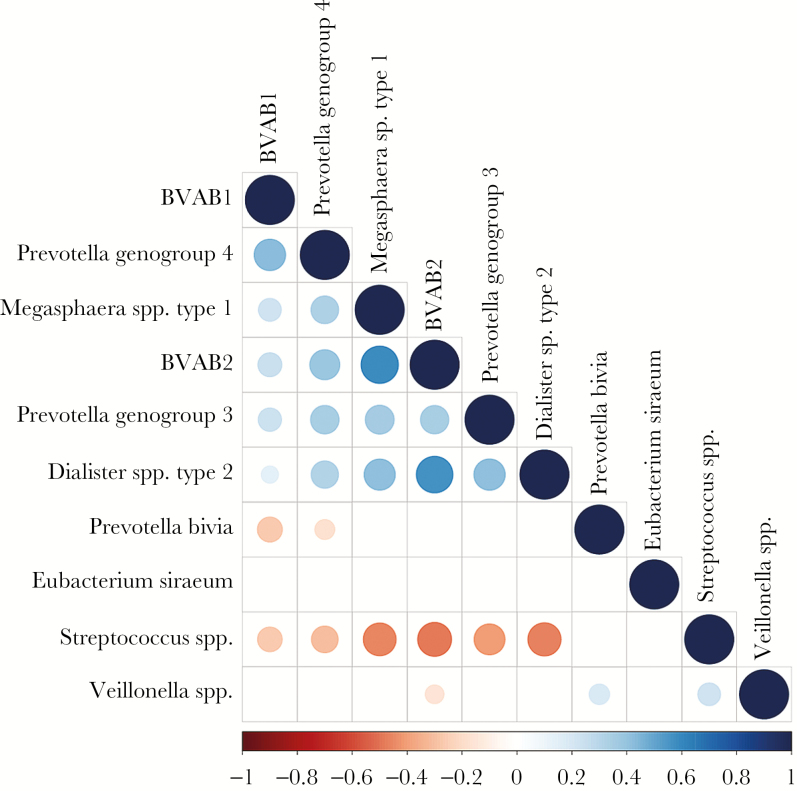
The taxa that met the threshold for significance after modeling with race/ethnicity were considered candidate bacterial taxa. Correlations between each of these candidate bacterial taxa were examined (Figure 5). There were statistically significant positive correlations between each of the candidate taxa associated with GBS-negative status. There were statistically significant positive correlations between the genus Veillonella and P. bivia and the genus Streptococcus.
Figure 5.
Correlation between candidate taxa. There were statistically significant positive pairwise correlations between all candidate taxa associated with group B Streptococcus (GBS)–negative status and between the genus Veillonella and Prevotella bivia and the genus Veillonella and the genus Streptococcus (associated with GBS-positive status). Blank boxes indicate no significant pairwise correlation.
Subgroup Analysis of CST IV
We performed post hoc exploratory analyses by subdividing CST IV (Supplementary Figure 1) into CST IV-A and CST IV-B (Table 1). There was no difference in α-diversity based on GBS status (Supplementary Figure 2A; P = .99) between the 2 subgroups of CST IV. There was clustering on principal coordinates analysis based on GBS status (Supplementary Figure 2B, Adonis P < .001) that explained 3.6% of the variance. Assignment to CST IV-A and CST IV-B explained 19% of the variance (Supplementary Figure 2C).
DISCUSSION
Vaginal bacterial community structure is largely driven by the dominant Lactobacillus species in the community or the absence thereof [12]. Prior culture-based studies of the vaginal microbiota have demonstrated inconsistent relationships between Lactobacillus species and GBS. Here we show no relationship between GBS and CST generally, making it unlikely that the Lactobacillus species that drive CST assignment—namely, L. iners, L. gasseri, L. crispatus, and L. jensenii, and the more general community adaptations that follow—play a direct significant role in GBS colonization in vivo. Furthermore, we demonstrate that in this sample set, measures of diversity and community structure are not meaningfully associated with GBS colonization. It has been demonstrated in vitro that GBS adherence [28] and biofilm formation [29] are regulated by pH. We found no significant association between GBS colonization and pH. Lactobacillus-dominated vaginal microbiota (non-CST IV) have lower pH [30] but are not directly associated with GBS status in our analysis. Within CST IV there was clustering, with a higher prevalence of GBS-positive samples in CST IV-A than CST IV-B. CST IV-A has been associated with a microbiota dominated by Streptococcus and Prevotella and clinically associated with vulvovaginal atrophy in postmenopausal women [31].
Individual bacterial taxa associated with bacterial vaginosis had mixed associations with GBS colonization. Veillonella species are commonly identified in the vagina, have been associated with BV [32, 33], and were associated with GBS-positive status. BVAB1 was strongly associated with GBS-negative status. It was recently shown that the Mobiluncus species frequently identified on Gram stain of vaginal samples is, in many cases, actually BVAB1 [34]. BVAB1 is known to be present in high density in a subset of those with BV [35, 36]. Mageeibacillus indolicus (previously BVAB3) [37], was also associated with GBS-negative status. Its presence has been shown to be highly correlated with BV [36]. This complicated picture, combined with α-diversity analysis, and additional studies suggest that GBS colonization is not related to BV state, but to the presence or absence of specific bacterial members of the vaginal microbiota.
Lactobacillus acidophilus and Lactobacillus paracasei are thought to either be able to prevent GBS adherence or to outcompete GBS for adherence to vaginal epithelial cells [38] in vitro, thus affecting directly or through a host-mediated mechanism, GBS colonization. Lactobacillus reuteri was shown to be effective in preventing GBS colonization in a mouse model [39]. Oral L. reuteri and Lactobacillus rhamnosus have been shown in a randomized, placebo-controlled trial to significantly decrease the rectovaginal GBS colonization rates of women determined to be GBS positive between 35 and 37 weeks by the time of delivery [40]. Lactobacillus rhamnosus did not reach the significance requirements of the KW test in LEfSe. Sequences consistent with L. reuteri, L. acidophilus, and L. paracasei (which are generally at low abundance even when present as part of the vaginal microbiota) would have been binned into the genus Lactobacillus in this study, which did not reach the significance requirements of the KW test, though it is possible by bundling Lactobacillus species that could not be resolved to the species level, a type II error may have occurred. These species, when found, are typically in very low abundance in the vaginal microbiota. Therefore, it is unlikely that they would have a significant influence on GBS colonization.
Previous work has shown that some strains of Bifidobacterium breve and Bifidobacterium longum have direct inhibitory effects on GBS in vitro [41]. In the current study, neither B. breve nor B. longum reached the significance requirements of the KW test. Species of Prevotella were associated with GBS-positive and GBS-negative status. Prevotella bivia is a gram-negative anaerobic bacillus [42] that was shown to be strongly associated with GBS-positive status in our study. Previous work has shown a mutualistic relationship between GBS and P. bivia in a rat model [43]. The same work showed a similar relationship between GBS and Bacteroides fragilis; however, B. fragilis was not specifically detected in our samples and is rarely seen in the vaginal microbiota. Vaginal P. bivia colonization is correlated with increased risk of human immunodeficiency virus acquisition, possibly mediated by interleukin 6 and interleukin 8 induction in cervical epithelial cells, with corresponding increase in the number of local CD4+ T cells [44]. Here we show that that P. bivia may play a role in human GBS colonization, through a mechanism that remains to be elucidated.
The study population consisted of nonpregnant women, and although nonpregnant individuals may be important in maintenance and transmission of GBS within the population, our findings may not be directly applicable to the vaginal microbiota during pregnancy. The vaginal microbiota has been reported to have a marked increase in α-diversity and gram-negative rods (including specifically Prevotella) along with a decrease in the abundance of Lactobacillus at or around the time of birth that can last for up to 1 year after pregnancy [45–48]. There may be other significant changes during pregnancy that are relevant to the interaction between GBS and the vaginal microbiota. Such interactions would require study in pregnant cohorts, ideally with longitudinal sampling. Previously, the relationship between specific microbial groups (including lactobacilli) and GBS has been examined in pregnancy using culture-based methods, with results showing no correlation [10]. More recently, 16S gene amplification has been used in a study of Guatemalan women, showing no correlation based on principal coordinates analysis between GBS and microbiota composition [11]. There were associations with Corynebacterium species and Aerococcus species and GBS colonization. This study did not look at individual species–level differences. Two species of Aerococcus and 1 species of Corynebacterium were identified in our sample. None of these met the threshold of the KW test in LEfSe. Further study is warranted to see if changes in the prevalence of any candidate putative microbes associated with GBS colonization described here would be also correlated with or affect GBS colonization in pregnancy.
Postpartum maternal colonization may be influenced by the increase in abundance of Prevotella species after pregnancy [45]. The recent study of Guatemalan women showed no difference in abundance of the genus Prevotella between GBS-colonized and GBS-uncolonized women. We have shown that subtaxa of Prevotella have varying correlations with GBS colonization, so future work examining the dynamics of specific Prevotella species in the peripartum period may better inform this work. Some cases of GBS may be acquired from nonmaternal sources in the first year of life. This study does not directly address nonvaginal environments as a source of the GBS reservoir, though further understanding of these nonvaginal environments might have important implications on the dynamics of vaginal GBS colonization and disease.
The cross-sectional design of this study limits the conclusions that can be drawn. The vaginal microbiota is dynamic, and GBS colonization may be transient; none of these factors can be addressed in a single time-point study. In addition, the use of PCR as a primary screening modality may have limited the sensitivity of our assay strategy. There are 10 known serotypes of GBS, with variable importance in human disease [49]. It has been previously shown that GBS colonization is not constant during pregnancy and that colonization with different serotypes may occur during pregnancy [50]. This work was not designed to look at specific GBS serotypes and did not examine temporal relationships. Further studies may help to define whether some of the interactions seen are GBS serotype specific, and study of temporal dynamics may further enhance the understanding of the relationship between GBS colonization and the microbiota.
Overall, this study demonstrated that specific microbial taxa rather than overall structure are correlated with GBS vaginal colonization. Further studies should use these data to inform investigations of candidate microbe–microbe and microbe–host–microbe interactions involving GBS, with particular attention paid to the relationship between P. bivia and GBS.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank Brent Williams, PhD, for helpful discussion and suggestions.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Financial support. This work was supported by the Doris Duke Charitable Foundation (CSDA 2009–039 to A. J. R.) and the National Institutes of Health (grant numbers R33 AI098654 to A. J. R., K23 HD065844 to T. M. R., and T35 DK093430 to G. H. R.).
Potential conflicts of interest. A. J. R. has served as a consultant to Pfizer. All other authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Eickhoff TC, Klein JO, Daly AK, Ingall D, Finland M. Neonatal sepsis and other infections due to group b beta-hemolytic streptococci. N Engl J Med 1964; 271:1221–8. [DOI] [PubMed] [Google Scholar]
- 2. Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis 2001; 33:556–61. [DOI] [PubMed] [Google Scholar]
- 3. Heath PT, Balfour G, Weisner AM et al. ; PHLS Group B Streptococcus Working Group Group B streptococcal disease in UK and Irish infants younger than 90 days. Lancet 2004; 363:292–4. [DOI] [PubMed] [Google Scholar]
- 4. Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics 1999; 103:e77. [DOI] [PubMed] [Google Scholar]
- 5. Edwards MS, Nizet V, Baker CJ.. Chapter 12: group B streptococcal infections. Infectious diseases of the fetus and newborn. 7th ed. Philadelphia: W.B. Saunders, 2011:419–69. [Google Scholar]
- 6. Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol 2000; 96:498–503. [DOI] [PubMed] [Google Scholar]
- 7. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36. [PubMed] [Google Scholar]
- 8. Kubota T, Nojima M, Itoh S. Vaginal bacterial flora of pregnant women colonized with group B Streptococcus. J Infect Chemother 2002; 8:326–30. [DOI] [PubMed] [Google Scholar]
- 9. Altoparlak U, Kadanali A, Kadanali S. Genital flora in pregnancy and its association with group B streptococcal colonization. Int J Gynaecol Obstet 2004; 87:245–6. [DOI] [PubMed] [Google Scholar]
- 10. Brzychczy-Włoch M, Pabian W, Majewska E et al. Dynamics of colonization with group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol 2014; 37:307–19. [PubMed] [Google Scholar]
- 11. Rick AM, Aguilar A, Cortes R et al. Group B streptococci colonization in pregnant guatemalan women: prevalence, risk factors, and vaginal microbiome. Open Forum Infect Dis 2017; 4:ofx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ravel J, Gajer P, Abdo Z et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011; 108(suppl 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srinivasan S, Hoffman NG, Morgan MT et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 2012; 7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fadrosh DW, Ma B, Gajer P et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014; 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caporaso JG, Kuczynski J, Stombaugh J et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holm JB, Gajer P, Ravel J.. PECAN A fast, novel 16S rRNA gene sequence non- clustering based taxonomic assignment tool. 16th International Symposium on Microbial Ecology (Montreal, Canada). Wageningen, Netherlands: International Society for Microbial Ecology, 2016. [Google Scholar]
- 17. Bergseng H, Bevanger L, Rygg M, Bergh K. Real-time PCR targeting the sip gene for detection of group B Streptococcus colonization in pregnant women at delivery. J Med Microbiol 2007; 56:223–8. [DOI] [PubMed] [Google Scholar]
- 18. R Core Development Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 19. Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics 2010; 11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopman BA, Barnabas R, Hallett TB et al. Assessing adult mortality in HIV-1-afflicted Zimbabwe (1998 -2003). Bull World Health Organ 2006; 84:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bray JR, Curtis JT. An Ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 1957; 27:326–49. [Google Scholar]
- 22. Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology 2001; 26:32–46. [Google Scholar]
- 23. Segata N, Izard J, Waldron L et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011; 12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fettweis JM, Brooks JP, Serrano MG et al. ; Vaginal Microbiome Consortium Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014; 160:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stapleton RD, Kahn JM, Evans LE, Critchlow CW, Gardella CM. Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet Gynecol 2005; 106:1246–52. [DOI] [PubMed] [Google Scholar]
- 26. Faust K, Sathirapongsasuti JF, Izard J et al. Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol 2012; 8:e1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gajer P, Brotman RM, Bai G et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park SE, Jiang S, Wessels MR. CsrRS and environmental pH regulate group B Streptococcus adherence to human epithelial cells and extracellular matrix. Infect Immun 2012; 80:3975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D’Urzo N, Martinelli M, Pezzicoli A et al. ; Members of the DEVANI Study Group Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains. Appl Environ Microbiol 2014; 80:2176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 2013; 8:e80074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brotman RM, Shardell MD, Gajer P et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014; 21:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hummelen R, Fernandes AD, Macklaim JM et al. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One 2010; 5:e12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sycuro LK, Fredricks DN.. Microbiota of the genitourinary tract. the human microbiota. Hoboken, NJ: John Wiley & Sons, Inc, 2013:167–210. [Google Scholar]
- 34. Srinivasan S, Morgan MT, Liu C et al. More than meets the eye: associations of vaginal bacteria with Gram stain morphotypes using molecular phylogenetic analysis. PLoS One 2013; 8:e78633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005; 353:1899–911. [DOI] [PubMed] [Google Scholar]
- 36. Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol 2010; 48:1812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Austin MN, Rabe LK, Srinivasan S, Fredricks DN, Wiesenfeld HC, Hillier SL. Mageeibacillus indolicus gen. nov., sp. nov.: a novel bacterium isolated from the female genital tract. Anaerobe 2015; 32:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zárate G, Nader-Macias ME. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett Appl Microbiol 2006; 43:174–80. [DOI] [PubMed] [Google Scholar]
- 39. De Gregorio PR, Juárez Tomás MS, Leccese Terraf MC, Nader-Macías ME. Preventive effect of Lactobacillus reuteri CRL1324 on group B Streptococcus vaginal colonization in an experimental mouse model. J Appl Microbiol 2015; 118:1034–47. [DOI] [PubMed] [Google Scholar]
- 40. Ho M, Chang YY, Chang WC et al. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce group B Streptococcus colonization in pregnant women: a randomized controlled trial. Taiwan J Obstet Gynecol 2016; 55:515–8. [DOI] [PubMed] [Google Scholar]
- 41. Aloisio I, Mazzola G, Corvaglia LT et al. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol 2014; 98:6051–60. [DOI] [PubMed] [Google Scholar]
- 42. Holdeman LV, Johnson JL. Bacteroides disiens sp. nov. and Bacteroides bivius sp. nov. from human clinical infections. Int J Syst Bacteriol 1977; 27:337–45. [Google Scholar]
- 43. Mikamo H, Kawazoe K, Izumi K, Watanabe K, Ueno K, Tamaya T. Studies on the pathogenicity of anaerobes, especially Prevotella bivia, in a rat pyometra model. Infect Dis Obstet Gynecol 1998; 6:61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gosmann C, Anahtar MN, Handley SA et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017; 46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DiGiulio DB, Callahan BJ, McMurdie PJ et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015; 112:11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. MacIntyre DA, Chandiramani M, Lee YS et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 2015; 5:8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bisanz JE, Enos MK, PrayGod G et al. Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of moringa-supplemented probiotic yogurt. Appl Environ Microbiol 2015; 81:4965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walther-António MR, Jeraldo P, Berg Miller ME et al. Pregnancy’s stronghold on the vaginal microbiome. PLoS One 2014; 9:e98514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of group B Streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS One 2011; 6:e17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. Serotype-specific acquisition and loss of group B Streptococcus recto-vaginal colonization in late pregnancy. PLoS One 2014; 9:e98778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.