Abstract
Many preclinical treatment strategies for stroke have failed when tested in human trials. Although the reasons for these translation failures are multifactorial, one potential concern is the statistical analysis of the preclinical data. One way to rigorously evaluate new therapies is to use an intention-to-treat analysis in preclinical studies. Therefore, in this study, we set out to evaluate the treatment efficacy of a potential clinically relevant therapeutic agent for stroke, i.e., anti-Nogo-A immunotherapy, using an intention-to-treat analysis. Adult rats were trained on the skilled forelimb reaching task and subsequently underwent an ischemic stroke. Nine weeks later, the rats either received intracerebroventricular anti-Nogo-A antibody, control antibody, or no treatment. Skilled reaching performance was assessed by a non-linear model using both an intention-to-treat and per-protocol analysis. Following testing, dendritic complexity was evaluated in the contralesional and perilesional sensorimotor cortex. Both intention-to-treat and per-protocol analysis showed that anti-Nogo-A immunotherapy resulted in statistically significant improved recovery on the skilled forelimb reaching task, although treatment effect was less (though statistically significant) in the intention-to-treat group. Improved functional performance was not shown to be associated with dendritic changes. In conclusion, this study provides evidence for the importance of using intention-to-treat paradigms in testing preclinical therapeutic strategies.
Keywords: Anti-Nogo-A, chronic, recovery, sensorimotor, stroke
Introduction
Neuronal damage following ischemic and hemorrhagic stroke often leaves patients with serious and long-term disabilities affecting many facets of their lives including cognition, language, vision, and mobility.1,2 Presently, over six million stroke survivors are living within the United States alone and it is estimated that an additional 795,000 new or recurrent strokes occur each year.2 Over recent years, the treatment of acute ischemic stroke has improved with the ability to use tissue plasminogen activator (t-PA) for clot dissolution3 and endovascular thrombectomy for clot retrieval4–8 in appropriate patients and certain stroke subtypes. However, in both instances, therapy is limited by the amount of time that has passed since the onset of symptoms and often patients are still left with considerable deficits. As such, the development of strategies to enhance neural repair following stroke is of utmost clinical importance.
To date, there have been many therapeutic agents and strategies found to be efficacious in animal models of stroke but have failed the translation to humans. Multiple factors contribute to the treatment failure of these preclinical agents,9,10 and it is likely that experimental design plays an important role. A meta-analysis evaluating treatment efficacy and preclinical study design found that larger estimates of treatment efficacy were associated with poor methodological study design quality.11 Furthermore, unlike human trials, very few preclinical animal studies use an intention-to-treat (ITT) design where data from all subjects are analyzed as randomized regardless of any protocol or treatment deviations.12 Therefore, one way to improve the quality of preclinical studies is to use an ITT paradigm in order to increase the validity of preclinical testing.13–16
Therefore, in this study, we used an ITT paradigm to evaluate the effectiveness of anti-Nogo-A immunotherapy in promoting sensorimotor recovery in animals with a large variety of stroke deficits ranging from moderate to very severe and given at the time point of nine weeks post-stroke. This promising new approach for neural repair after stroke involves blocking the function of Nogo-A, a protein that restricts neuronal plasticity in the adult central nervous system (CNS).17 Nogo-A is expressed on many cell types including the membrane of oligodendrocytes and restricts the outgrowth of neurites after CNS injury through intracellular signaling cascades involving two inhibitory domains, Nogo-66 and Nogo-A-Δ20, and their corresponding receptors.18–23 Based on preclinical testing, we and other investigators have reported that anti-Nogo-A therapy resulted in improved functional outcome, enhanced axonal and dendritic remodeling and increased dendritic spine density in the contralesional sensorimotor cortex after stroke17,24–29 and as reviewed by Kumar and Moon.30 Importantly, we set out to rigorously test this therapy using an ITT paradigm in order to improve the validity of these results and to hopefully improve the likelihood that this treatment could be successfully translated from preclinical to human trials.
Methods
The animal studies were approved by the Institutional Animal Care and Use Committee and conducted in the AAALAC accredited animal facility of Edward Hines Jr. Veterans Affairs Hospital. The studies were performed in compliance with the U.S. Department of Agriculture Animal Welfare Act regulations and the National Institutes of Health's Public Health Service Policy. The ARRIVE guidelines were followed for the preparation of this manuscript.
Animal subjects
A total of 68 adult male Long Evans black-hooded rats of 4 cohorts (2 months of age at the start of the study, HsdBLU:LE, Harlan Laboratories IN, Barrier 217) were used. Rats were housed in standard cages (2 rats per cage) with a 12-h light/dark cycle. Rats had access to fresh water and were fed standard laboratory chow (Harlan 2018 Rodent Diet). Rats were food restricted to 95% of their predicted weight by age starting one week after arrival in the animal facility. All animals and treatments were number coded, prepared by a laboratory member not participating in the study. Investigators conducting treatment surgeries, behavioral testing and neuroanatomical analyses were blind to the treatment conditions throughout the experiments. Codes were broken after all the individual data points were analyzed.
Experimental timeline and overview
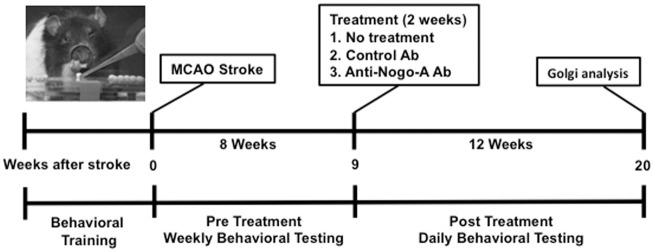
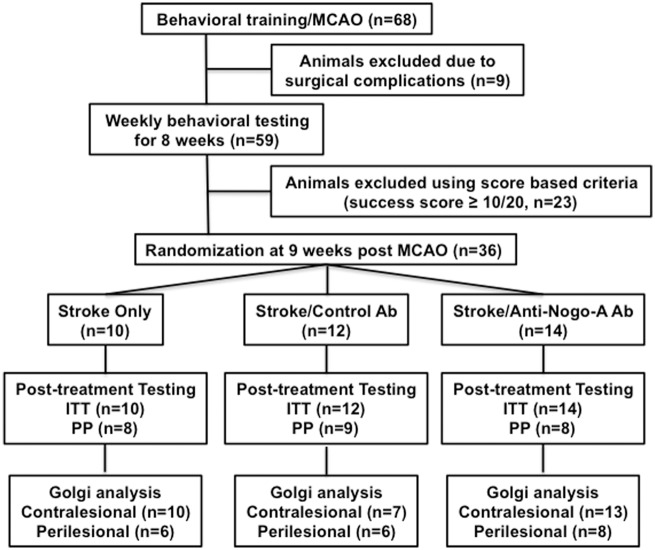
The experimental timeline for various stages of this study is shown in Figure 1, while Figure 2 displays the flowchart and number of animals at each stage.
Figure 1.
Experimental design and timeline.
Figure 2.
Experimental overview. Flow chart showing the experimental design and the number of animals at every stage of the study.
Skilled forelimb reaching task
The skilled forelimb reaching task is a sensitive skilled motor task designed to test voluntary skilled forelimb function in normal as well as brain damaged rats31,32 and has been validated in various forms of neurological injury, including focal ischemic stroke in the rat as done in our previous studies.17,25–28,33,34 Rats were placed in a Plexiglas chamber (30 × 36 × 30 cm) and trained to reach for a round sucrose pellet (45 mg; Bilaney Consultants, Frenchtown, NJ) through a rectangular opening (height by width = 30 by 15 mm). Rats were trained daily, until a success score of 15 or better out of 20 pellets was obtained for three consecutive days using their self-selected preferred forelimb. The final location of the pellet placement was 3 mm lateral to the midline opposite to the preferred limb and 10 mm from the window opening. A successful reach was defined as a pellet that had been grasped by the rat with the preferred forelimb, pulled back inside the reaching apparatus, and placed in the mouth without being dropped.
Stroke surgery
A focal ischemic stroke was induced by using the middle cerebral artery occlusion (MCAO) method as described in Chen et al.35 and as in our previous work.17,25–28,33,34 Rats were anesthetized with isoflurane (3% in oxygen) and core body temperature was maintained during the entire procedure between 37 to 38 ℃. The bilateral common carotid arteries (CCA) were first isolated from an anterior cervical neck incision and the common carotid artery ipsilateral to the hemisphere corresponding to the preferred forelimb was permanently ligated using a 4–0 silk suture. Rats were then placed in a stereotaxic frame and a 2 cm vertical incision was made between the eye and ear. Bupivacaine (1.5 mg/kg) was injected subcutaneously along incision lines before the incision and also applied on the skull before burr hole drilling. The temporalis muscle was retracted and a burr hole was made to expose the middle cerebral artery (MCA) that was ligated with a 10–0 suture and then transected with microscissors. The contralateral CCA was temporarily occluded for 45 min using an aneurysm clip. After the aneurysm clip was removed, all wounds were closed and animals were kept warmed until awake. Animals received 2 ml of normal saline (i.p.) after surgery and were monitored during the post-op period until fully awake and able to eat and drink.
A priori inclusion/exclusion criteria
All rats (n = 68) were trained to successfully reach at least 15 out of 20 pellets for three consecutive days and then underwent an MCAO stroke procedure to result in ischemia in the forelimb cortex associated with the preferred forelimb. After stroke, all surviving rats (n = 59) were tested on the skilled reaching task once a week to assess for spontaneous recovery. At the end of week eight, rats were assessed for a new post-stroke baseline for three consecutive days. Rats were randomized at week nine to undergo treatment if they were able to reach with their impaired forelimb through the window in the skilled reaching apparatus and at least touch the pellets on some attempts out of 20 total. Deficit severity was based on the success score attained during the skilled reaching task as follows: moderate (5 < × < 10), severe (0 < × ≤ 5), very severe ( = 0). Rats (n = 23) that scored 10 or more out of 20 successful pellets (mild deficit) at any time during post-stroke skilled reaching testing were excluded from the study.
ITT dataset
The ITT dataset initially included all 68 animals that underwent focal ischemic stroke. Nine animals died following stroke and post-stroke reaching data were collected from 59 animals. During the post-stroke period of 8 weeks waiting for treatment, a total of 23 animals were excluded due to obtaining 10 or more pellets out of 20 (thought due to spontaneous recovery). Therefore, a total of 36 animals from 4 cohorts were randomized into three treatment groups, taking into account the severity of their stroke deficit. The three experimental groups treated at nine weeks post-stroke consisted of: stroke/11C7 Ab (n = 14), stroke/control Ab (n = 12), and stroke only (n = 10).
Per-protocol dataset
Additional exclusion criteria were applied to the PP dataset due to experimental protocol deviations. These exclusions were performed post-randomization. Animals were excluded from the PP analysis due to antibody infusion pump disconnections, behavioral abnormalities that interfered with assessment of the skilled forelimb reaching score, displaying absolute reliance on using the unimpaired forelimb during the skilled reaching task, displaying no functional spontaneous recovery post-stroke prior to treatment initiation, i.e. very severe deficit (success score = 0/20), and severe subcortical striatal damage (total n = 11). After these exclusions, the three experimental groups in the PP dataset consisted of: stroke/11C7 Ab (n = 8), stroke/control Ab (n = 9), and stroke only (n = 8).
Intracerebroventricular antibody infusion
Antibody infusion was initiated at the beginning of week 9 post-stroke as previously reported.28 Rats were placed in a stereotaxic frame and a midline incision was made in the scalp. Bupivacaine (1.5 mg/kg) was injected along the incision line before incision and applied on the bone before burr hole drilling. A burr hole on the same side as the stroke lesion was made to expose the cerebral cortex. A cannula was placed into the lateral cerebral ventricle at coordinates 1.3 mm lateral, 0.8 mm posterior, and 3.8 mm ventral (relative to bregma) and was secured to the skull with cyanoacrylate gel. Through a mid-scapular incision, an Alzet osmotic mini-pump (model 2ML2; Durect Corporation, Cupertino, CA, USA) was implanted subcutaneously posterior to the scapulae and connected to the cannula with polyethylene tubing. Either purified mouse monoclonal anti-Nogo-A antibody (11C7; IgG1) or control antibody (anti-bromodeoxyuridine, IgG1) was infused at a rate of 12.5 µg/h (2.5 mg/ml) for two weeks, after which the animals were again anesthetized and the pumps removed.
Perfusion and Golgi-Cox staining
At the end of the behavioral study, rats were overdosed (pentobarbital 100 mg/kg) and transcardially perfused with 0.9% saline plus heparin (10,000 units/L). The brains were removed and immersed whole in Golgi-Cox solution36 for two weeks. The brains were then coronally sectioned at 200 µm on a vibratome, mounted on 2% gelatinized slides and reacted as described by Gibb and Kolb.37 Slides were coded to blind the experimenters to antibody treatment groups.
Stroke lesion size analysis
All the Golgi-Cox-stained coronal brain sections (ranging from 40–50 sections/rat) were quantitatively analyzed (+4.7 to −5.2 mm from bregma) according to Paxinos and Watson38 using a computer-interfaced imaging system (NIH Image) by the method as described previously.17,39 Stroke size was expressed as a percent of the intact contralateral hemispheric area (total area of the intact contralateral hemisphere minus total area of the intact ipsilesional hemisphere over total area of the intact contralateral hemisphere).
Neuroanatomical analysis
Layer V pyramidal neurons were located within the contralesional and perilesional caudal forelimb area (CFA) with the aid of an atlas38 and according to previous electrophysiological studies.40 Criteria for inclusion in the analyses were that the neuron had to be well impregnated with Golgi-Cox stain, unobstructed by other dendrites, blood vessels or glia, and that the dendritic arborization was intact and visible in the plane of the section. An average of 6 apical and basilar dendritic trees of layer V pyramidal neurons were traced using Neurolucida software (MBF) interfaced with a Leica DM 4000B microscope using a 40 × objective. Dendritic trees were analyzed for dendritic length and number of branch segments using the branched structure analysis tool in Neurolucida. Branches emanating directly from the apical shaft (apical dendrites) or from the cell body (basilar dendrites) were considered first order. Dendritic analysis was performed on the animals in the entire ITT dataset.
Statistical analysis
An alpha value of less than 0.05 was considered statistically significant for all data analyzed. Total stroke lesion size and dendritic complexity were analyzed using a one-way ANOVA (SPSS, Chicago, IL, USA). Skilled reaching data were analyzed using the statistical software SAS (SAS, Institute, Cary, NC, USA) to generate a non-linear mixed effect logistic regression model of the reaching data. This approach to modeling recovery data using a non-linear analysis has been found by us and others to be more accurate and sensitive in modeling the natural recovery curves seen following CNS injury as reported.41–43 The data were fit to a three-parameter binomial logistic exponential function as shown in equation (1) and explained below
| (1) |
P(t) signifies the probability of success at time t (number of successful pellets out of 20), signifies the upper asymptote (final success score reached at plateau), signifies the y-intercept (initial pre-treatment stroke severity), and signifies the rate of the equation (rate of recovery). The success probability, P(t), is a function of time, and it has the usual “S-shape” observed in practical biomedical modelling. The “t-7” in the exponent shifts the origin since in so doing, the parameter is the so-called lower asymptote, or the success probability at baseline (t = 7 weeks). As time progresses (i.e. as t increases), the model for the success probability increases to the ultimate probability parameter, and the parameter controls the rate of increase of the probabilities. Estimates of the model parameters were obtained by standard software using maximum likelihood estimation techniques (see supplemental Figure 1).
A likelihood-based Chi square (χ2) test was used to determine statistical differences between group parameters. The skilled reaching outcome variable is binomial (success or no success) and therefore the dataset was modeled by a χ2 distribution. Fitted models were contrasted by using χ2 likelihood-based ratio tests.
Results
Stroke lesion size and location
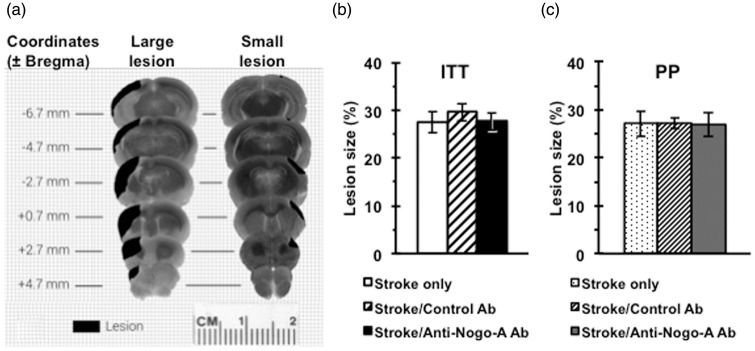
The stroke lesion size varied as shown in Figure 3(a). The largest lesions (∼40–45% stroke size) involved the majority of the unilateral rostral-caudal hemisphere including the rostral forelimb motor area, varying levels of damage to the caudal forelimb motor area, and minimal to severe damage to the dorso-lateral striatum. The smallest lesions (∼15% stroke size) damaged a limited portion of the cortex involving primarily the CFA with minimal damage to the rostral forelimb area and dorso-lateral striatum. Total stroke lesion volume was measured for all animals and expressed as a percentage of the intact contralateral hemisphere. There was no statistical difference in stroke size across groups in either the ITT (F(2, 32) = 0.257, p = 0.78) or PP analysis (F(2,22) = 0.004, p = 0.99). ITT stroke lesion volumes were found to be 27.7 ± 1.9 % in the stroke/anti-Nogo-A antibody group, 29.6 ± 1.9 % in the stroke/control antibody group, and 28.4 ± 2.3 % in the stroke only group (Figure 3(b)). PP stroke lesion volumes were found to be 27.0 ± 2.6 % in the stroke/anti-Nogo-A Ab group, 27.0 ± 1.2 % in the stroke/control Ab group, and 27.1 ± 2.7 % in the stroke only group (Figure 3(c)).
Figure 3.
Stroke lesion size and location. (a) Diagram of stroke lesion topology showing the differences in lesion size and location. There was no statistical difference in the stroke size between treatment groups either in the ITT analysis (F(2, 32) = 0.257, p = 0.78). (b) or the PP analysis (F(2,22) = 0.004, p = 0.99). (c). Data presented as mean ± SEM. one-way ANOVA. ITT: Stroke only (n = 10); Stroke/Control Ab (n = 12); Stroke/Anti-Nogo-A Ab (n = 13), PP: Stroke only (n = 8); Stroke/Control Ab (n = 9); Stroke/Anti-Nogo-A Ab (n = 8).
Skilled reaching performance
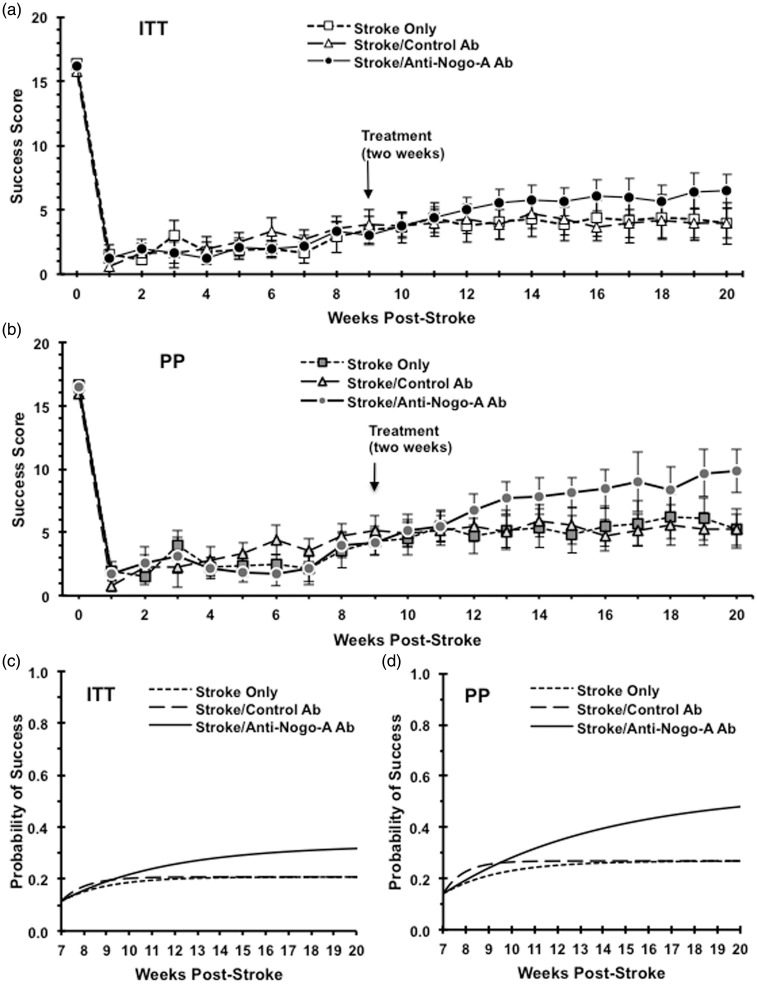
For the skilled forelimb reaching performance, both the raw data set (Figure 4(a) and (b)) and the fitted group-level logistic recovery functions (Figure 4(c) and (d)) for each treatment group is shown. The initial stroke deficit severity (pre-treatment), modeled by the parameter (Figure 4(c) and (d)), was shown to be the same across all three treatment groups in both the ITT (p = 0.33) and the PP (p = 0.30) analysis. Animals in the stroke only and control Ab-treated groups exhibited statistically identical parameter estimates for the final magnitude of recovery ( in both the ITT (p = 0.70) and PP analyses (p = 0.13). Analysis of the final estimated magnitude of recovery between the stroke only and the anti-Nogo-A antibody treated group showed that the animals treated with anti-Nogo-A immunotherapy achieved a statistically significant higher final magnitude of recovery in both the ITT (p = 0.01) and PP (p = 0.02) analyses. Analysis of the final estimated magnitude of recovery between the control antibody-treated group and the anti-Nogo-A antibody-treated group showed that the animals treated with anti-Nogo-A immunotherapy achieved a statistically significant higher final magnitude of recovery in both the ITT (p = 1.76 e−6) and PP (p = 2.58 e−8) analyses. The anti-Nogo-A Ab-treated animals in the ITT dataset reached a final recovery magnitude of 33 % compared to a recovery magnitude of 21% in either control group. The anti-Nogo-A Ab-treated animals in the PP dataset reached a final recovery magnitude of 54% compared to a recovery magnitude of 27% in either control group.
Figure 4.
Functional outcome analysis. Upper panels show weekly raw reaching scores starting from week zero (baseline) to the end of study for ITT (a) and PP (b) analyses. Lower panels show the fitted group-level logistic recovery profiles for each treatment group based on the non-linear model for the ITT (c) and PP (d) analyses. Animals treated with anti-Nogo-A antibody after stroke show significant improvement as compared with animals of the stroke only group in both the ITT (p = 0.01) and PP (p = 0.02) analyses. Animals treated with anti-Nogo-A antibody after stroke show significant improvement as compared with animals of the stroke/control antibody in both the ITT (p = 1.76 e−6) and PP (p = 2.58 e−8) analyses. ITT: Stroke Only (n = 10); Stroke/Control Ab (n = 12); Stroke/Anti-Nogo-A Ab (n = 14), PP: Stroke Only (n = 8); Stroke Control Ab (n = 9); Stroke/Anti-Nogo-A Ab (n = 8).
Dendritic complexity
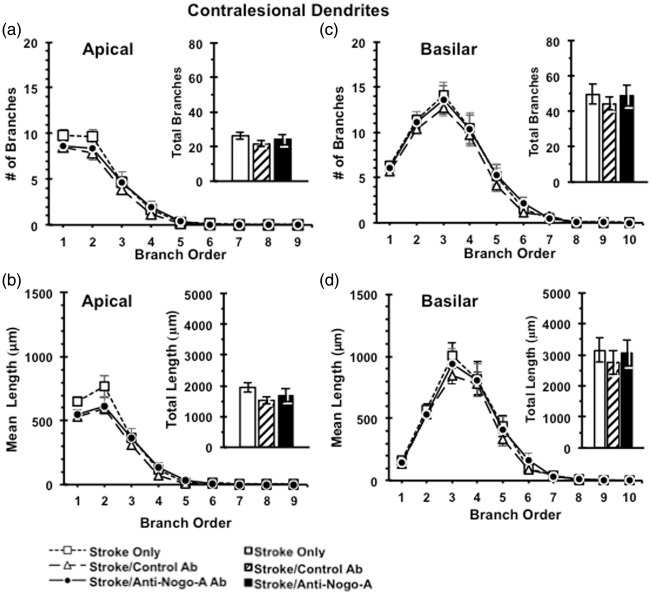
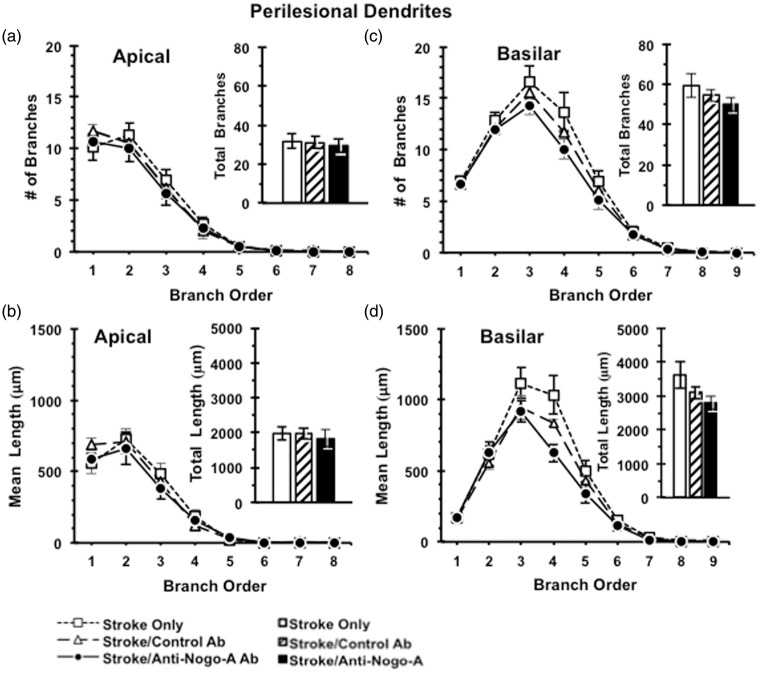
Following the completion of sensorimotor testing, animals were perfused and processed for analysis of neuronal morphology using the Golgi-Cox method. Layer V pyramidal neurons were selected in both the contralesional and perilesional caudal forelimb motor cortex for analysis. Anti-Nogo-A immunotherapy resulted in no discernible effect on the complexity of the apical arbors of contralesional (Figure 5) or perilesional (Figure 6) layer V pyramidal neurons, as evidenced by no significant differences across treatment groups in the number of apical branches across branch order, total number of apical branches of contralesional cortex (F(2,27) = 0.675, p = 0.52) (Figure 5) or perilesional cortex (F(2,17) = 0.112, p = 0.90) (Figures 5(a) and 6(a)), dendritic length across branch order, or total dendritic length in the contralesional cortex (F(2,27) = 1.179, p = 0.32) and the perilesional cortex (F(2,17) = 0.164, p = 0.85) (Figures 5(b) and 6(b)). We also found no effect of anti-Nogo-A immunotherapy on the complexity of the basilar arbors of contralesional or perilesional layer V pyramidal neurons as evidenced by no significant differences across treatment groups in terms of the number of basilar branches across branch order, total number of basilar branches in the contralesional cortex (F(2,27) = 0.195, p = 0.82) and the perilesional cortex (F(2,17) = 1.26, p = 0.31) (Figures 5)(c) and 6(c), dendritic length across branch order, or total dendritic length in the contralesional cortex (F(2,27) = 0.22, p = 0.80) and the perilesional cortex (F(2,17) = 2.51, p = 0.12) (Figures 5(d) and 6(d)).
Figure 5.
Dendritic analysis of Layer V pyramidal neurons in contralesional caudal forelimb cortex. Dendritic complexity analyses showed no difference between ITT groups in the numbers of dendritic branches (a) and total dendritic length (b) of apical dendrites, as well as in the numbers of dendritic branches (c) and total dendritic length (d) of basilar dendrites in the contralesional caudal forelimb cortex. Data presented as mean ± SEM. p > 0.05, one-way ANOVA. Stroke Only (n = 10); Stroke/Control Ab (n = 7); Stroke/Anti-Nogo-A Ab (n = 13).
Figure 6.
Dendritic analysis of Layer V pyramidal neurons in perilesional caudal forelimb cortex. Dendritic complexity analyses showed no difference between ITT groups in the numbers of dendritic branches (a) and total dendritic length (b) of apical dendrites, as well as in the numbers of dendritic branches (c) and total dendritic length (d) of basilar dendrites in the perilesional caudal forelimb cortex. Data presented as mean ± SEM. p > 0.05, one-way ANOVA. ITT: Stroke Only (n = 6); Stroke/Control Ab (n = 6); Stroke/Anti-Nogo-A Ab (n = 8).
Discussion
The present study demonstrates that there is an extended treatment window for the use of anti-Nogo-A immunotherapy to improve sensorimotor function in adult rats with moderate to severe stroke deficits. Adult rats treated with anti-Nogo-A antibodies at nine weeks post-stroke significantly recovered skilled forelimb reaching ability in both the ITT and per-protocol (PP) analyses when compared to the control groups. To our knowledge, the present study is the first strict ITT analysis of anti-Nogo-A efficacy at a clinically relevant chronic time point and in a population that includes animals with severe and very severe stroke deficits and corresponding large stroke sizes. Based on these results, we can conclude that anti-Nogo-A immunotherapy is effective chronically after stroke and after the lesion-induced neuroplasticity-promoting environment in the brain is thought to have diminished,44,45 thereby validating our previous report.28
For anti-Nogo-A immunotherapy in animals with moderate to very severe stroke deficits, the data were first evaluated using an ITT analysis. Although this approach may lead to conservative estimates of efficacy, it provides the most valid assessment of meaningful differences across groups and is considered the gold standard approach to data analysis in human clinical trials.46,47 Our ITT population treated with anti-Nogo-A immunotherapy included animals with a range of deficit severity including those with moderate deficits (36 %), severe deficits (50 %), and very severe deficits (14 %). Our results showed that only animals with moderate to severe deficits improved after anti-Nogo-A immunotherapy. Animals in the very severe category showed no improvement on the skilled reaching task (see supplemental Figure 2). Therefore, these results show that the effectiveness of anti-Nogo-A immunotherapy (as determined by the skilled forelimb reaching task) may be attenuated if there is no residual ability to grasp with the forelimb digits prior to treatment.
Next, we set out to see how the efficacy of anti-Nogo-A immunotherapy is affected by post-randomization exclusions, i.e. PP analysis. Firstly, animals were excluded if, upon removal, the intracerebroventricular antibody pump was found to be disconnected, thereby potentially supplying a suboptimal therapeutic dose of antibody to the animal. Secondly, animals were excluded when displaying reliance on using the unimpaired forelimb during the skilled reaching task. These animals primarily used their “good” forelimb in reaching, leading to inaccurate success score numbers. Thirdly, animals were excluded if they displayed an “absolute deficit,” when after stroke, no pellets were ever successfully grasped. Fourthly, rats were excluded with very severe subcortical damage (complete damage of dorso-lateral striatum), as damage to the striatum has been shown to result in impairment of initiation of reaching and severe forelimb dysfunction.48,49 It is important to note that these exclusions left the total number of rats remaining in the PP category at n = 25, indicating a large ‘drop-out’ and the need for enough animals at the beginning of the study, typically more than calculated by a power analysis. Our PP results showed that once these animals were excluded from the study, the animals in the anti-Nogo-A immunotherapy group showed an even greater recovery. Although the mean total stroke size per treatment group did not change between the ITT and PP datasets, the distribution of stroke deficit severity at time of treatment in the population was affected. Our PP population with anti-Nogo-A Ab treatment included animals with moderate (50%) and severe deficits (50%), while animals with very severe deficits were excluded. Animals treated with anti-Nogo-A antibody had a final recovery of 54% in the PP analysis compared to 33% in the ITT analysis. These results suggest that when limiting the analysis, we can expect a greater improvement of sensorimotor function than is suggested by the ITT analysis. Therefore, this study supports the perspective that post-randomization exclusions lead to the reporting of greater effects, and preclinical data can best be interpreted with inclusive reporting of all subjects.
The improved functional recovery after stroke and anti-Nogo-A immunotherapy in this study is consistent with numerous other studies demonstrating anti-Nogo-A efficacy at various time points after stroke (see reviews Kumar and Moon30 and Schwab and Strittmatter50). We have shown that treatment with anti-Nogo-A immunotherapy when started immediately,17 one week,26,27,33,41 and nine weeks28 post-stroke in adult rats with moderate stroke deficits significantly improved recovery beyond control antibody treatment. In our earlier studies, success scores approached an almost complete return to baseline function in the skilled reaching task. However, in the present study, we found a more modest recovery plateau. The difference in recovery between studies is likely due to a larger mean stroke size in the present study, therefore resulting in more severe stroke deficits.
Given the functional recovery seen in the present study, we evaluated potential neuroanatomical correlates of improvement. Multiple studies by our laboratory and others have shown that anti-Nogo-A treatment leads to axonal and dendritic reorganization (neuroplasticity) that is associated with functional recovery after stroke17,25,26,28 and silencing newly formed axonal connections abolished anti-Nogo-A therapy-induced functional improvement.51 Therefore, we evaluated dendritic structural plasticity in the contralesional and perilesional forelimb motor cortex. However, our study did not show evidence of changes in the complexity of layer V pyramidal neurons in the contralesional and perilesional caudal forelimb motor cortex. These results underscore the difficulty in relating neuroanatomical plasticity with recovery of function and potentially imply other factors that are responsible for the functional recovery seen in these experiments. For example, it is possible that anti-Nogo-A immunotherapy may enhance plasticity in pyramidal neurons found within areas such as the contralesional rostral forelimb motor cortex40 or the contralesional striatum. Earlier studies have shown that Nogo-A plays a role in dendritic structure, as in Nogo-A knockout mice, where cerebellar Purkinje cells were found to have larger and more complex dendritic trees as compared to wild-type mice.52 Furthermore, we previously reported that anti-Nogo-A immunotherapy given immediately post-stroke resulted in increased dendritic complexity in contralesional layer V pyramidal neurons observed at six weeks post-treatment.25 Therefore, in the present study, we may have missed any dendritic changes due to the prolonged delay of treatment (nine weeks) plus the long post-treatment time of 12 weeks. Earlier time points to determine dendritic complexity will be evaluated in future studies.
In conclusion, in this study, we found that anti-Nogo-A immunotherapy was efficacious at nine weeks post-stroke for improving sensorimotor recovery in rats with moderate to severe stroke deficits and is a promising therapeutic strategy for improving disability after stroke, even long after the ischemic injury has taken place. Also important is the finding that the recovery with anti-Nogo-A immunotherapy varies based on post-randomization exclusions and on initial stroke severity. It is suggested that pre-clinical studies testing therapeutic interventions report ITT and PP analyses to increase the likelihood of translating therapies that have the greatest chance of succeeding in human trials.
Supplementary Material
Acknowledgements
We would like to thank the following people for their assistance to this project: Professor Martin Schwab and Dr. Daniel Shepherd for critical reading of the manuscript, Dr. Nitin Bangera for assistance with the figures and graphs, and Mrs. Ana Marinopoulos, Dr. Ian Vaagenes and Dr. Steve Nawara for their assistance with experimental animals. We also thank Professor Martin Schwab for his generous gift of anti-Nogo-A antibody (11C7) and control antibody.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the US Department of Veterans Affairs, Rehabilitation Research and Development Service (grant 5I01RX000828 to G.L.K.).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Katherine M Podraza contributed to the concept and design, animal behavioral training/testing, acquisition/analysis of data, and drafted the article. Vicki A Husak contributed to animal behavioral training/testing, assisting surgeries, and lesion analysis. Yasmin Mehta and Elise Lippmann contributed to acquisition of data in Golgi and lesion analysis. Timothy E O'Brien contributed to data analysis/interpretation. Gwendolyn L Kartje contributed to the concept and design, performed randomization, analysis/interpretation of all data and revised the article for important intellectual content. Shih-Yen Tsai contributed to the concept and design, performed all the surgeries, analysis/interpretation of all data and revised the article for important intellectual content.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Courtney-Long EA, Carroll DD, Zhang QC, et al. Prevalence of disability and disability type among adults – United States, 2013. Morb Mortal Wkly Rep 2015; 64: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Neurological D and Stroke rt PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 7.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 9.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 2010; 1: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nosek BA, Ebersole CR, DeHaven AC, et al. The Preregistration Revolution, osf.io/swz75 (2017). [DOI] [PMC free article] [PubMed]
- 11.Horn J, de Haan RJ, Vermeulen M, et al. Nimodipine in animal model experiments of focal cerebral ischemia: a systematic review. Stroke 2001; 32: 2433–2438. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Sattar M, Krauth D, Anglemyer A, et al. The relationship between risk of bias criteria, research outcomes, and study sponsorship in a cohort of preclinical thiazolidinedione animal studies: a meta-analysis. Evid Based Preclin Med 2014; 1: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012; 10: 28–55. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 2010; 1: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroke Therapy Academic Industry R Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30: 2752–2758. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos CM, Tsai SY, Alsbiei T, et al. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol 2002; 51: 433–441. [DOI] [PubMed] [Google Scholar]
- 18.Caroni P, Savio T, Schwab ME. Central nervous system regeneration: oligodendrocytes and myelin as non-permissive substrates for neurite growth. Prog Brain Res 1988; 78: 363–370. [DOI] [PubMed] [Google Scholar]
- 19.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol 1988; 106: 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen MS, Huber AB, van der Haar ME, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000; 403: 434–439. [DOI] [PubMed] [Google Scholar]
- 21.GrandPre T, Nakamura F, Vartanian T, et al. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature 2000; 403: 439–444. [DOI] [PubMed] [Google Scholar]
- 22.Kempf A, Tews B, Arzt ME, et al. The sphingolipid receptor S1PR2 is a receptor for Nogo-A repressing synaptic plasticity. PLoS Biol 2014; 12: e1001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prinjha R, Moore SE, Vinson M, et al. Inhibitor of neurite outgrowth in humans. Nature 2000; 403: 383–384. [DOI] [PubMed] [Google Scholar]
- 24.Lindau NT, Banninger BJ, Gullo M, et al. Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy. Brain 2014; 137: 739–756. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos CM, Tsai SY, Cheatwood JL, et al. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-A neutralization. Cereb Cortex 2006; 16: 529–536. [DOI] [PubMed] [Google Scholar]
- 26.Seymour AB, Andrews EM, Tsai SY, et al. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab 2005; 25: 1366–1375. [DOI] [PubMed] [Google Scholar]
- 27.Tsai SY, Markus TM, Andrews EM, et al. Intrathecal treatment with anti-Nogo-A antibody improves functional recovery in adult rats after stroke. Exp Brain Res 2007; 182: 261–266. [DOI] [PubMed] [Google Scholar]
- 28.Tsai SY, Papadopoulos CM, Schwab ME, et al. Delayed anti-Nogo-A therapy improves function after chronic stroke in adult rats. Stroke 2011; 42: 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiessner C, Bareyre FM, Allegrini PR, et al. Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab 2003; 23: 154–165. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P, Moon LD. Therapeutics targeting Nogo-A hold promise for stroke restoration. CNS Neurol Disord Drug Targets 2013; 12: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alaverdashvili M, Whishaw IQ. Motor cortex stroke impairs individual digit movement in skilled reaching by the rat. Eur J Neurosci 2008; 28: 311–322. [DOI] [PubMed] [Google Scholar]
- 32.Castro AJ. The effects of cortical ablations on digital usage in the rat. Brain Res 1972; 37: 173–185. [DOI] [PubMed] [Google Scholar]
- 33.Markus TM, Tsai SY, Bollnow MR, et al. Recovery and brain reorganization after stroke in adult and aged rats. Ann Neurol 2005; 58: 950–953. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd DJ, Tsai SY, O'Brien TE, et al. Anti-Nogo-A immunotherapy does not alter hippocampal neurogenesis after stroke in adult rats. Front Neurosci 2016; 10: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen ST, Hsu CY, Hogan EL, et al. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke 1986; 17: 738–743. [DOI] [PubMed] [Google Scholar]
- 36.Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Meth 1981; 4: 117–125. [DOI] [PubMed] [Google Scholar]
- 37.Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Meth 1998; 79: 1–4. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 5th ed New York: Academic Press, 2005. [Google Scholar]
- 39.Kawamata T, Speliotes EK, Finklestein SP. The role of polypeptide growth factors in recovery from stroke. Adv Neurol 1997; 73: 377–382. [PubMed] [Google Scholar]
- 40.Neafsey EJ, Bold EL, Haas G, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res 1986; 396: 77–96. [DOI] [PubMed] [Google Scholar]
- 41.Gillani RL, Tsai SY, Wallace DG, et al. Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy. Behav Brain Res 2010; 208: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaagenes IC, Tsai SY, Ton ST, et al. Binge ethanol prior to traumatic brain injury worsens sensorimotor functional recovery in rats. PLoS One 2015; 10: e0120356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wainwright PE, Leatherdale ST, Dubin JA. Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol 2007; 49: 664–674. [DOI] [PubMed] [Google Scholar]
- 44.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci 2004; 24: 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Mendoza EH, Hermann DM. Correlates of post-stroke brain plasticity, relationship to pathophysiological settings and implications for human proof-of-concept studies. Front Cell Neurosci 2016; 10: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Day S. Analysis Issues, ITT, Post-Hoc, and Subgroups. http://ocw.jhsph.edu/courses/BiostatMedicalProductRegulation/biomed_lec7_day.pdf.: Johns Hopkins University, 2008 (accessed 25 August 2017).
- 47.Guideline for the Format and Content of the Clinical and Statistical Sections of Applications. Rockville, Md: US Dept. of Health and Human Services, Public Health Service, Food and Drug Admnistration, 1988.
- 48.MacLellan CL, Gyawali S, Colbourne F. Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. Behav Brain Res 2006; 175: 82–89. [DOI] [PubMed] [Google Scholar]
- 49.Pisa M. Motor functions of the striatum in the rat: critical role of the lateral region in tongue and forelimb reaching. Neuroscience 1988; 24: 453–463. [DOI] [PubMed] [Google Scholar]
- 50.Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol 2014; 27: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahl AS, Omlor W, Rubio JC, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 2014; 344: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 52.Petrinovic MM, Hourez R, Aloy EM, et al. Neuronal Nogo-A negatively regulates dendritic morphology and synaptic transmission in the cerebellum. Proc Natl Acad Sci U S A 2013; 110: 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.